Abstract
Background:
Previous studies have shown that hypertension is an important factor contributing to the occurrence and progression of diabetic kidney damage. However, the relationship between the patterns of blood pressure (BP) trajectory and kidney damage in the diabetic population remains unclear. This prospective study investigated the effect of long-term systolic BP (SBP) trajectory on kidney damage in the diabetic population based on an 8-year follow-up community-based cohort.
Methods:
This study included 4556 diabetic participants among 101,510 participants. BP, estimated glomerular filtration rate (eGFR), and urinary protein were measured every 2 years from 2006 to 2014. SBP trajectory was identified by the censored normal modeling. Five discrete SBP trajectories were identified according to SBP range and the changing pattern over time. Kidney damage was evaluated through eGFR and urinary protein value. A multivariate logistic regression model was used to analyze the influence of different SBP trajectory groups on kidney damage.
Results:
We identified five discrete SBP trajectories: low-stable group (n = 864), moderate-stable group (n = 1980), moderate increasing group (n = 609), elevated decreasing group, (n = 679), and elevated stable group (n = 424). The detection rate of kidney damage in the low-stable group (SBP: 118–124 mmHg) was the lowest among the five groups. The detection rate of each kidney damage index was higher in the elevated stable group (SBP: 159–172 mmHg) compared with the low-stable group. For details, the gap was 4.14 (11.6% vs. 2.8%) in eGFR <60 ml·min−1·1.73 m−2 and 3.66 (17.2% vs. 4.7%), 3.38 (25.0% vs. 7.4%), and 1.8 (10.6% vs. 5.9%) times in positive urinary protein, eGFR <60 ml·min−1·1.73 m−2 and/or positive urinary protein, and eGFR decline ≥30%, respectively (P < 0.01).
Conclusion:
An elevated stable SBP trajectory is an independent risk factor for kidney damage in the diabetic population.
Keywords: Blood Pressure Trajectory, Diabetes with Hypertension, Kidney Damage, Longitudinal Data, Trajectory Model
摘要
背景:
既往研究表明,高血压是糖尿病患者肾损害发生和发展的重要因素。然而,长周期血压轨迹和糖尿病患者肾损害之间 的关系尚不清楚。这是一项基于8年糖尿病随访人群的长期收缩压(SBP)轨迹对肾损伤影响的前瞻性研究。
方法:
本研究包括101,510名受试者,其中4556名糖尿病患者。从2006到2014年,每两年测量该队列人群血压、血糖、肾小球 滤过率(eGFR)、尿蛋白等指标,收缩压轨迹通过截尾正态模型确定。根据SBP范围和随时间变化趋势,建立五组独立的SBP 轨迹。用eGFR和尿蛋白值等实验室检查指标评价肾损伤。采用多元logistic回归模型分析不同收缩压轨迹对肾脏损害的影响。
结果:
我们确定了五组独立的收缩压轨迹:低阶稳定组(n=864),中阶稳定组(n=1980),中阶增高组(n=609),高阶 下降组(n=679)和高阶稳定组(n=424)。低阶稳定组肾损害的检出率(收缩压为118-124mmHg)是五组中最低的。随着收 缩压轨迹的增高,肾脏损伤的检出率逐渐增高。与低阶稳定组相比,高阶稳定组肾损害检出率指标(收缩压为159-172mmHg) 中,EGFR<60 ml·min-1·1.73m-2、尿蛋白阳性,EGFR<60 ml·min-1·1.73m-2或尿蛋白阳性、EGFR=下降30%分别增加了 4.14(11.6% vs 2.8%)、3.66(17.2% vs 4.7%), 3.38(25% vs 7.4%), 和1.8(10.6% vs 5.9%)倍,差异有显著统计学意义 (P<0.01)。
结论:
高阶稳定SBP轨迹是糖尿病患者肾脏损害的独立危险因素。
INTRODUCTION
Diabetes is a serious chronic disease. Both the number of cases and the prevalence of diabetes have been steadily increasing over the past few decades. Globally, an estimated 422 million adults were living with diabetes in 2014, with an estimated prevalence of 8.5% among the adult population.[1] Diabetes endangers several systems in the body and leads to many complications. Kidney damage and diabetic nephropathy are among the most serious complications of diabetes. Previous studies have shown that hypertension is an important factor contributing to the occurrence and progression of diabetic kidney damage. The incidence of chronic kidney disease was significantly higher among hypertensive patients with diabetes mellitus.[2,3]
The effect of blood pressure (BP) on kidney function is long term, so a single BP measurement might not be sufficient for reflecting the BP load experienced by the patient over a prolonged period. The change in BP over time should be considered. BP trajectory shows similar trends as BP. Compared with single BP monitoring and 24 h ambulatory BP monitoring, long-term BP trajectory is helpful in assessing its long-term cumulative effects.[4,5,6] BP trajectory has been associated with atherosclerosis and stroke. In addition, BP trajectory yielded better cardiovascular risk predictions than BP monitoring alone.[7,8] However, little research has been conducted on the correlation between long-term BP trajectory and kidney damage in diabetic populations. This study investigated the effect of long-term systolic BP (SBP) trajectory on kidney damage in the diabetic population, based on the large data analysis of the China Kailuan prospective cohort study.
METHODS
Ethical approval
This study was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the General Hospital of Kailuan.
Study population
This prospective study involving an 8-year follow-up was conducted in a northern Chinese community. The China Kailuan study (registration number: CHiCTR-TNC-11001489) was a community-based longitudinal cohort study evaluating cardiovascular disease risk factors based on a functional community population located in Tangshan city, China. This study evaluated the comprehensive assessment of cardiovascular risk factors including BP, blood sugar, serum creatinine, and urine protein every 2 years from 2006 to 2014.
In total, 101,510 employees participated in examination in 2006, and 9489 cases were diagnosed with diabetes. Wherein 29 cases did not have estimated glomerular filtration rate (eGFR) data, 479 cases lost urinary protein data, 1761 cases eGFR <60 ml·min−1·1.73 m−2, and 641 cases of urinary protein were positive, 6579 cases were included in the study cohort. After the removal of 746 cases that participated in examination less than twice, 1196 cases that did not participate in examination in 2012 and 2014 (594 deaths), 51 cases lost eGFR data, and 30 cases lost urinary protein data, even though they participated in examinations in 2012 and 2014; therefore, 4556 cases remained in the final statistical analysis. A flowchart of the participants included in the current analysis is attached in Figure 1.
Figure 1.
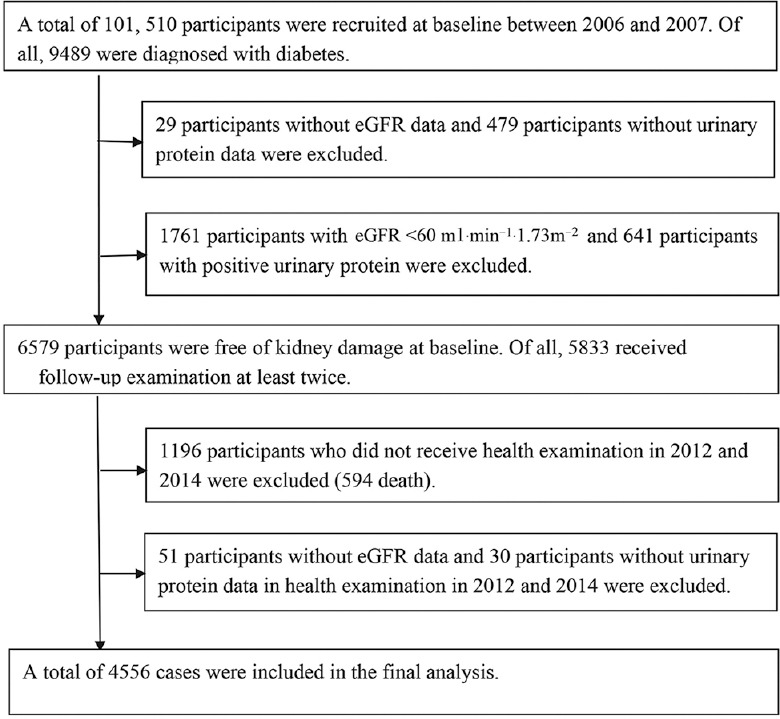
Flowchart of the participants included in the current analysis.
Inclusion and exclusion criteria
Patients were included in the study if they fulfilled the following inclusion criteria: (1) fasting blood glucose (FBG) ≥7.0 mmol/L in 2006 or FBG <7.0 mmol/L with exact history of diabetes or antidiabetic medication use in the meantime; (2) with complete eGFR and proteinuria data in 2006; and (3) agreed to participate in this study and signed informed consent.
Exclusion criteria included (1) eGFR <60 ml·min−1·1.73 m−2 or urine protein was positive; (2) examination times were less than twice; (3) did not participate in examinations in 2012 and 2014; and (4) had participated in examinations in 2012 and 2014 eGFR but proteinuria data were lost.
Data collecting
Epidemiological survey content, anthropometric indicators, and biochemical tests could be found in published literature by our research group.[9]
Participants were forbidden to smoke and drink tea or coffee 30 min before measuring BP and were required to relax for 15 min. The brachial artery BP was measured on the right using a corrected desktop mercury sphygmomanometer for the first four examinations and was measured using a medical electronic sphygmomanometer arm on the 5th visit.
The measurement continued three times and was performed once every 1–2 min, and the mean was used for calculations.
Blood testing
In the morning after 8 h of fasting, the participants donated 5 ml of venous blood. The blood sample was stored in a vacuum and centrifuged at 3000 ×g for 10 min at room temperature below 24°C. We collected the upper serum in 4 h for testing.
Determination of serum creatinine was used by the bitter almond acid method in 2006 and colorimetry was used after 2006 by professional laboratory technicians. Urine protein was measured by the immunoturbidimetric method and operated strictly according to reagent instructions.
Definitions
Diabetes[10] was defined as FBG ≥7.0 mmol/L or FBG <7.0 mmol/L with exact history of diabetes or using antidiabetic medications in the meantime.
eGFR calculation[11] was defined as eGFR calculated by the CKD-EPI method. For females: if serum creatinine ≤0.7 mg/dl (1 mg/dl = 88.4 μmol/L), eGFR = 144 × (serum creatinine/0.7)−0.329 × (0.993)Age; if serum creatinine >0.7 mg/dl, eGFR = 144 × (serum creatinine/0.7)−1.209 × (0.993)Age. For males: if serum creatinine ≤0.9 mg/dl, eGFR = 141 × (serum creatinine/0.9)−0.411 × (0.993)Age; if serum creatinine >0.9 mg/dl, eGFR = 141 × (serum creatinine/0.9)−1.209 × (0.993)Age.
New onset kidney damage: In the current study, kidney damage was evaluated by the status of eGFR and proteinuria. Participants with eGFR <60 ml·min−1·1.73 m−2 in 2014 or eGFR in 2014 decreased ≥30%[12] versus eGFR in 2006 or urinary protein positive in 2014 or eGFR <60 ml·min−1·1.73 m−2 and/or urinary protein positive in 2014 were considered as having kidney damage.
Dyslipidemia[13] was defined as total cholesterol (TC) >5.0 mmol/L, low-density lipoprotein cholesterol (LDL-C) >3.0 mmol/L, triglyceride >1.7 mmol/L, high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L in men, and HDL-C <1.2 mmol/L in women.
Statistical method
SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for analysis. For the characteristics of BP data, the trajectory model was completed by the CNORM (the censored normal model) mode of the SAS 9.3 (SAS Institute Inc., Cary, NC, USA)[14,15,16] which can set the minimum and maximum values to eliminate possible bias. Bayesian Information Criterion and the average probability (AvePP) after grouping were used to select the best trajectory model and assess the degree of fit of the trajectory. To determine the number of groups, the required proportion of each group was <5%. As generally believed, a higher degree of fit was indicated when AvePP values were >0.7.[17,18,19] The AvePP values in the trajectories of this study were >0.8, indicating that the selected trajectory model was of good fit. There were five trajectory groups determined by 4, 4, 4, 4, and 4 as the polynomial order.
The SBP slope of each observed participant was calculated using the SAS PROC REG program. The continuity variables were expressed as a mean ± standard deviation, and single-factor analysis of variance was used to compare the two groups. The Least Significant Difference test was used for variance and Dunnett's T3 test was used for poor variance. Classification variable was represented by n (%), and the Chi-square test was used to compare different groups. The model adjusted for age, gender, baseline SBP (2006), FBG, body mass index (BMI), eGFR, high cholesterol, smoking, alcohol consumption, physical activity, BP medication, and SBP slope. The multivariate logistic regression model was used to analyze different SBP trajectory groups' influence on renal damage. Meanwhile, sensitivity analysis was used to analyze the impact of taking antihypertensive medications on the results. There was a significant difference between the two groups if P < 0.05.
RESULTS
General subject situations
A total of 4556 cases were included in the final statistical analysis. In total, 3683 patients (80.8%) were male and 890 (19.2%) were female. The average age of the 4556 patients was 53.75 ± 9.15 years [Table 1].
Table 1.
General situations of different SBP trajectory groups
| Parameter* | Low-stable group (n = 864) | Moderate-stable group (n = 1980) | Moderate increasing group (n = 609) | Elevated decreasing group (n = 679) | Elevated stable group (n = 424) | Total (n = 4556) | P† |
|---|---|---|---|---|---|---|---|
| Age (years) | 49.25 ± 9.47 | 53.09 ± 8.96a,‡ | 56.86 ± 7.54a,c | 55.82 ± 8.68a,b | 58.18 ± 7.53a,b | 53.75 ± 9.15 | <0.001 |
| Male, n (%) | 657 (76.0) | 1625 (82.1) | 496 (81.4) | 560 (82.5) | 345 (81.4) | 3683 (80.8) | 0.004 |
| SBP_06 (mmHg) | 115.64 ± 11.29 | 131.27 ± 12.30a | 135.76 ± 12.26a,b | 159.65 ± 12.27a,b | 159.23 ± 15.45a,b,d | 135.64 ± 19.36 | <0.001 |
| SBP_14 (mmHg) | 123.18 ± 12.38 | 140.78 ± 12.47a | 167.51 ± 12.60a,b | 143.98 ± 13.06a,b,c | 173.94 ± 13.12a,b,c,d | 144.14 ± 20.12 | <0.001 |
| SBP14–06 (mmHg) | 7.16 ± 16.99 | 9.57 ± 18.11 | 31.67 ± 17.12a,b | −15.17 ± 17.04a,b,c | 13.71 ± 19.78a,b,c,d | 8.76 ± 21.70 | <0.001 |
| DBP_06 (mmHg) | 76.78 ± 8.30 | 84.07 ± 8.83a | 85.24 ± 9.08a | 96.11 ± 11.50a,b,c | 93.57 ± 11.76a,b,c | 85.53 ± 11.34 | <0.001 |
| DBP_14 (mmHg) | 75.90 ± 8.93 | 82.19 ± 9.26a | 87.30 ± 11.15a,b | 83.81 ± 10.06a,b,c | 90.27 ± 12.21a,b,c,d | 82.56 ± 10.73 | <0.001 |
| DBP14–06 (mmHg) | −1.12 ± 11.04 | −1.95 ± 11.40 | 2.12 ± 12.83a,b | −12.49 ± 12.23a,b,c | −3.59 ± 14.60c,d | −2.93 ± 12.84 | <0.001 |
| SBP slope | 1.48 ± 4.26 | 1.69 ± 4.64 | 7.72 ± 4.07a,b | −4.73 ± 4.40a,b,c | 3.03 ± 5.44a,b,c,d | 1.63 ± 5.63 | <0.001 |
| FBG (mmol/L) | 9.24 ± 3.14 | 9.03 ± 2.82 | 9.33 ± 3.01 | 8.71 ± 2.33a,c | 9.31 ± 2.99d | 9.09 ± 2.86 | <0.001 |
| TC (mmol/L) | 5.11 ± 1.14 | 5.20 ± 1.21 | 5.10 ± 1.14 | 5.35 ± 1.20a,b,c | 5.24 ± 1.29a,c | 5.19 ± 1.20 | 0.001 |
| HDL-C (mmol/L) | 1.50 ± 0.38 | 1.53 ± 0.39 | 1.53 ± 0.43 | 1.57 ± 0.45a | 1.55 ± 0.42 | 1.53 ± 0.41 | 0.025 |
| LDL-C (mmol/L) | 2.36 ± 0.90 | 2.41 ± 1.00 | 2.35 ± 0.92 | 2.39 ± 1.10 | 2.51 ± 1.04 | 2.40 ± 0.99 | 0.082 |
| BMI (kg/m2) | 25.08 ± 3.30 | 26.25 ± 3.31a | 26.20 ± 3.16a | 27.15 ± 3.31a,b,c | 26.81 ± 3.50a,b,c | 26.20 ± 3.36 | <0.001 |
| Exercise, n (%) | 128 (14.8) | 351 (17.7) | 143 (23.5) | 145 (21.4) | 110 (25.9) | 877 (19.2) | 0.005 |
| Smoking, n (%) | 275 (31.8) | 578 (29.2) | 185 (30.4) | 202 (29.7) | 114 (26.9) | 1354 (29.7) | 0.382 |
| Drinking, n (%) | 127 (14.7) | 328 (16.6) | 118 (19.4) | 122 (18.0) | 69 (16.3) | 764 (16.8) | 0.061 |
| Taking antihypertensive medications, n (%) | 71 (8.2) | 591 (29.8) | 313 (51.4) | 428 (63.0) | 304 (71.7) | 1707 (37.5) | <0.001 |
| Taking hypoglycemic agents, n (%) | 453 (52.4) | 1045 (52.8) | 371 (60.9) | 349 (51.4) | 250 (59.0) | 2468 (54.2) | 0.019 |
| Sulfonylureas, n (%) | 28 (3.2) | 63 (3.2) | 27 (4.4) | 25 (3.7) | 20 (4.7) | 163 (3.7) | 0.393 |
| Biguanide, n (%) | 66 (7.6) | 182 (9.2) | 68 (11.2) | 55 (8.1) | 41 (9.7) | 412 (9.0) | 0.171 |
| Glucosidase inhibitor, n (%) | 10 (1.2) | 14 (0.7) | 6 (1.0) | 8 (1.2) | 5 (1.2) | 43 (0.9) | 0.686 |
| Thiazolidine diones, n (%) | 3 (0.3) | 6 (0.3) | 4 (0.7) | 2 (0.3) | 3 (0.7) | 18 (0.4) | 0.598 |
| Noninsulin secretagogue, n (%) | 9 (1.0) | 8 (0.4) | 6 (1.0) | 4 (0.6) | 3 (0.7) | 30 (0.7) | 0.292 |
| Insulin, n (%) | 50 (5.8) | 96 (4.8) | 35 (5.7) | 33 (4.9) | 20 (4.7) | 234 (5.1) | 0.770 |
| Traditional Chinese medicine and others, n (%) | 65 (7.5) | 161 (8.1) | 57 (9.4) | 54 (8.0) | 39 (9.2) | 376 (8.3) | 0.697 |
| High cholesterol, n (%) | 489 (56.6) | 1295 (65.4) | 412 (67.7) | 484 (71.3) | 290 (68.4) | 2970 (65.2) | <0.001 |
*SBP: Systolic blood pressure; DBP: Diastolic blood pressure; BMI: Body mass index; FBG: Fasting blood glucose; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; TC: Total cholesterol; SBP14–06: 06 years and 14 years, SBP difference; DBP14–06: 06 years and 14 years, the difference between the DBP; Taking antihypertensive medications: Claimed to take medications in 06, 08, 10 or 12; Taking hypoglycaemic agents: Claimed to take hypoglycaemic agents in 06, 08, 10 or 12; Hypoglycaemic agents species using 2006 data; †P value are for the difference comparison between the five groups; ‡Superscript a, b, c, d refers to the difference in each group. Compared with low-stable group, aP<0.01; Compared with moderate-stable group, bP<0.01; Compared with moderate increasing group, cP<0.01; Compared with elevated decreasing group, dP<0.01.
Basic characteristics of blood pressure trajectory
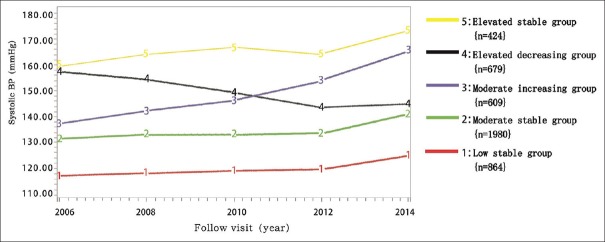
Five SBP trajectory groups were determined according to SBP range and the changing pattern over time [Figure 2].
Figure 2.
Systolic blood pressure trajectories for 4556 diabetics participating in China Kailuan study.
Low-stable group: SBP maintained steady growth in the low level (SBP: 118–124 mmHg, n = 864, 19.0%).
Moderate-stable group: SBP maintained steady growth in the moderate level (SBP: 132–140 mmHg, n = 1980, 43.4%).
Moderate increasing group: SBP grew rapidly to a high level from the moderate level (SBP: 139–164 mmHg, n = 609, 13.4%).
Elevated decreasing group: SBP decreased rapidly to moderate level from high level (SBP: 157–145 mmHg, n = 679, 14.9%).
Elevated stable group: SBP maintained steady growth in elevated level (SBP: 159–172 mmHg, n = 424, 9.3%).
Trend of blood pressure trajectory
This study yielded the following results in 8 years of follow-up.
The initial value of the SBP in the low stable group was low (115.64 ± 11.29 mmHg) and rose by only 7.16 ± 16.99 mmHg during the 8-year study period with a slope of 1.48 ± 4.26.
The initial value of the SBP in the moderate-stable group was 131.27 ± 12.30 mmHg and rose by 9.57 ± 18.11 mmHg, with a slope of 1.69 ± 4.64.
The initial value of the SBP in the moderate increasing group was 135.76 ± 12.26 mmHg and rose rapidly by 31.67 ± 17.12 mmHg, with a slope of 7.72 ± 4.07.
The initial value of the SBP in the elevated decreasing group was 159.65 ± 12.27 mmHg and decreased by 12.49 ± 12.23 mmHg, with a slope of −4.73 ± 4.40.
The initial value of the SBP in the elevated stable group was 159.23 ± 15.45 mmHg, and rose by13.71 ± 19.78 mmHg, with a slope of 3.03 ± 5.44.
Most of the population was in the low-stable group (19.0%) and moderate-stable group (43.4%). In the other three groups (37.6%), the SBP values were above 140 mmHg during the follow-up period and also reflected the relatively high prevalence of hypertension in the diabetic population.
With the rising of SBP trajectory, SBP, diastolic BP, TC, BMI, FBG, HDL-C, and LDL-C showed an upward trend, whereas eGFR showed a downward trend in the same manner. The percent differences in age, drinking, exercise, antihypertensive medications, and high cholesterol of the different groups were statistically significant.
Detection rate of kidney damage
The detection rate of kidney damage in the different trajectory groups is shown in Table 2.
Table 2.
The detection rate of different SBP trajectories to kidney damage
| Parameters | Low-stable group (n = 864) | Moderate-stable group (n = 1980) | Moderate increasing group (n = 609) | Elevated decreasing group (n = 679) | Elevated stable group (n = 424) | Total (n = 4556) | P |
|---|---|---|---|---|---|---|---|
| eGFR <60 ml·min−1·1.73 m−2, n (%) | 24 (2.8) | 105 (5.3) | 40 (6.6) | 57 (8.4) | 49 (11.6) | 275 (6.0) | <0.001 |
| Positive urinary protein, n (%) | 41 (4.7) | 144 (7.3) | 84 (13.8) | 85 (12.5) | 73 (17.2) | 427 (9.4) | <0.001 |
| eGFR <60 ml·min−1·1.73 m−2 and/or positive urinary protein, n (%) | 64 (7.4) | 232 (11.7) | 113 (18.6) | 129 (19.0) | 106 (25.0) | 644 (14.1) | <0.001 |
| eGFR`s decline ≥30%, n (%) | 51 (5.9) | 131 (6.6) | 43 (7.1) | 71 (10.5) | 45 (10.6) | 341 (7.5) | <0.001 |
SBP: Systolic blood pressure; eGFR: Estimated glomerular filtration rate.
The detection rate of kidney damage in the low-stable group was the lowest among the five groups. An increase of SBP trajectory led to a gradual increase in the detection rate of kidney damage.
The detection rate of each kidney damage index of the elevated stable group, compared to that in the low-stable group, which included eGFR <60 ml·min−1·1.73 m−2, positive urinary protein, eGFR <60 ml·min−1·1.73 m−2 and/or positive urinary protein, and eGFR decline ≥30%, increased by 4.14 (11.6% vs. 2.8%), 3.66 (17.2% vs. 4.7%), 3.38 (25% vs. 7.4%), and 1.8 (10.6% vs. 5.9%) times, respectively.
As compared to that, in the low-stable group, the detection rate of each kidney damage index of the moderate-stable group, which was eGFR <60 ml·min−1·1.73 m−2, positive urinary protein, eGFR <60 ml·min−1·1.73 m−2 and/or positive urinary protein, and eGFR decline ≥30%, increased by 1.89 (5.3% vs. 2.8%), 1.55 (7.3% vs. 4.7%), 1.58 (11.7% vs. 7.4%), and 1.12 (6.6 vs. 5.9%) times, respectively. These results indicated that the effect of the moderate-stable SBP on kidney damage increased significantly compared to that of the low-stable SBP, although SBP remained below 140 mmHg.
The detection rate of kidney damage index in the elevated decreasing group was higher than that in the moderate increasing group, except for positive urinary protein result. The kidney damage index between the two groups has not yet reached statistical significance. This may be due to the small difference in the level of exposure between the two groups.
After adjusting the SBP (2006), BMI, eGFR, FBG, smoking, alcohol consumption, physical activity, and high blood lipids, the results showed that compared with the low-stable group, the odds ratios (95% confidence intervals) of eGFR <60 ml·min−1·1.73 m−2, urinary protein positive, eGFR <60 ml·min−1·1.73 m−2, and/or urinary protein positive and eGFR decreased by ≥30% of the elevated stable group were 2.01 (0.90–4.50), 2.39 (1.25–4.56), 1.86 (1.08–3.19), and 2.20 (1.03–4.71), separately. As compared with that, in the low-stable group, the risk of kidney damage also increased in the other four groups [Table S1].
Table S1.
Logistic regression analysis of different SBP trajectory groups to parameters of kidney damage
| Parameters of kidney damage|| | Groups | OR (95% CI) | |||
|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 4§ | ||
| eGFR <60 ml·min−1·1.73 m−2 | Low-stable group | 1 | 1 | 1 | 1 |
| Moderate-stable group | 1.96 (1.24–3.07) | 1.58 (1.01–2.50) | 1.59 (0.98–2.60) | 1.45 (0.86–2.46) | |
| Moderate increasing group | 2.46 (1.46–4.12) | 1.63 (0.96–2.77) | 1.65 (0.90–3.03) | 1.37 (0.84–2.78) | |
| Elevated decreasing group | 3.20 (1.96–5.22) | 2.22 (1.34–3.65) | 2.15 (1.22–3.80) | 1.74 (0.86–3.50) | |
| Elevated stable group | 4.57 (2.76–7.56) | 2.81 (1.68–4.72) | 2.70 (1.51–4.83) | 2.01 (0.90–4.50) | |
| P-trend | <0.001 | 0.001 | 0.009 | 0.446 | |
| Slope of SBP | 0.92 (0.79–1.06) | 0.96 (0.81–1.15) | |||
| SBP_06 | 1.00 (0.99–1.01) | ||||
| Positive urinary protein | Low-stable group | 1 | 1 | 1 | 1 |
| Moderate-stable group | 1.57 (1.10–2.24) | 1.50 (1.04–2.15) | 1.46 (1.00–2.13) | 1.34 (0.89–2.03) | |
| Moderate increasing group | 3.21 (2.17–4.73) | 2.97 (2.00–4.43) | 1.99 (1.26–3.14) | 1.68 (0.98–2.89) | |
| Elevated decreasing group | 2.87 (1.95–4.23) | 2.67 (1.80–3.97) | 3.20 (2.02–5.06) | 2.63 (1.49–4.64) | |
| Elevated stable group | 4.17 (2.79–6.42) | 3.82 (2.52–5.79) | 3.17 (1.99–5.05) | 2.39 (1.25–4.56) | |
| P-trend | <0.001 | <0.001 | <0.001 | 0.007 | |
| Slope of SBP | 1.22 (1.08–1.37) | 1.29 (1.12–1.50) | |||
| SBP_06 | 1.00 (0.99–1.01) | ||||
| eGFR <60 ml·min−1· 1.73 m−2 and/or positive urinary protein | Low-stable group | 1 | 1 | 1 | 1 |
| Moderate-stable group | 1.65 (1.24–2.21) | 1.47 (1.10–1.97) | 1.40 (1.02–1.90) | 1.25 (0.89–1.75) | |
| Moderate increasing group | 2.84 (2.05–3.94) | 2.27 (1.62–3.17) | 1.64 (1.12–2.41) | 1.31 (0.83–2.07) | |
| Elevated decreasing group | 2.93 (2.13–4.03) | 2.40 (1.74–3.33) | 2.59 (1.78–3.77) | 1.98 (1.24–3.17) | |
| Elevated stable group | 4.16 (2.97–5.83) | 3.18 (2.25–4.50) | 2.63 (1.78–3.87) | 1.86 (1.08–3.19) | |
| P-trend | <0.001 | <0.001 | <0.001 | 0.034 | |
| Slope of SBP | 1.11 (1.01–1.23) | 1.19 (1.06–1.35) | |||
| SBP_06 | 1.01 (1.00–1.01) | ||||
| eGFR reduced ≥30% | Low-stable group | 1 | 1 | 1 | 1 |
| Moderate-stable group | 1.12 (0.80–1.57) | 1.06 (0.75–1.48) | 1.09 (0.74–1.62) | 1.13 (0.73–1.75) | |
| Moderate increasing group | 1.21 (0.79–1.84) | 1.07 (0.69–1.65) | 1.36 (0.79–2.35) | 1.40 (0.73–2.69) | |
| Elevated decreasing group | 1.86 (1.28–2.70) | 1.67 (1.14–2.46) | 1.77 (1.09–2.88) | 1.92 (1.01–3.64) | |
| Elevated stable group | 1.89 (1.24–2.87) | 1.64 (1.06–2.53) | 2.10 (1.23–3.58) | 2.20 (1.03–4.71) | |
| P-trend | 0.001 | 0.006 | 0.011 | 0.119 | |
| Slope of SBP | 0.87 (0.76–1.01) | 0.86 (0.73–1.02) | |||
| SBP_06 | 0.99 (0.98–1.01) | ||||
*Model 1: With different parameters as the dependent variable, different BP trajectory group as independent variable, low-stable group as the control group; †Model 2: Adjusting for age, gender on the basis of Model 1; ‡Model 3: Adjusting for FBG, BMI, eGFR, high cholesterol, smoking, alcohol consumption, physical activity, taking antihypertensive medications, slope of SBP on the basis of Model 2; §Model 4: Adjusting for SBP in 2006 on the basis of Model 3; ||Parameters of kidney damage: “eGFR ≥60 ml·min−1·1.73 m−2” = 0, “eGFR <60 ml·min−1 ·1.73 m−2” = 1; “negative urine protein” = 0, “positive urinary protein” = 1; “eGFR ≥60 ml·min−1 ·1.73 m−2 and negative urine protein” = 0, “eGFR <60 ml·min−1 ·1.73 m−2 or urine protein positive” = 1; “eGFR decline <30%” = 0, “eGFR decline ≥30%” = 1; gender: “female” = 0, “male” = 1; high cholesterol, smoking, alcohol consumption, exercise, taking antihypertensive medications: “No” = 0,”Yes” = 1. SBP: Systolic blood pressure; BMI: Body mass index; FBG: Fasting blood glucose; eGFR: Estimated glomerular filtration rate; BP: Blood pressure; OR: Odds ratio; CI: Confidence interval.
Sensitivity analyses
After eliminating the population who took antihypertensive medications, the result of re-statistical analysis showed that the overall trend of the influence of elevated SBP trajectory on kidney damage did not change [Table S2]. This indicated that an elevated stable SBP trajectory was a risk factor for kidney damage.
Table S2.
Multivariate regression logistics analysis of kidney damage parameters of different SBP trajectory groups*
| Parameters of renal damage | Group | OR (95% CI) (n = 2949) |
|---|---|---|
| eGFR <60 ml·min−1·1.73 m−2 | Low-stable group | 1 |
| Moderate-stable group | 1.01 (0.55–1.85) | |
| Moderate increasing group | 1.05 (0.41–2.66) | |
| Elevated decreasing group | 1.05 (0.38–2.88) | |
| Elevated stable group | 3.04 (1.01–9.21) | |
| Positive urinary protein | Low-stable group | 1 |
| Moderate-stable group | 1.41 (0.86–2.29) | |
| Moderate increasing group | 2.23 (1.13–4.41) | |
| Elevated decreasing group | 3.58 (1.60–8.01) | |
| Elevated stable group | 2.16 (0.81–5.77) | |
| eGFR <60 ml·min−1 1.73 m−2 and/or positive urinary protein | Low-stable group | 1 |
| Moderate-stable group | 1.13 (0.76–1.68) | |
| Moderate increasing group | 1.41 (0.79–2.52) | |
| Elevated decreasing group | 2.08 (1.06–4.07) | |
| Elevated stable group | 2.55 (1.15–5.65) | |
| eGFR reduced ≥30% | Low-stable group | 1 |
| Moderate-stable group | 0.95 (0.57–1.57) | |
| Moderate increasing group | 1.27 (0.53–3.05) | |
| Elevated decreasing group | 1.09 (0.43–2.74) | |
| Elevated stable group | 4.32 (1.45–12.83) |
*Sensitivity analysis: Except people taking antihypertensive medications. With different BP trajectories as independent variables, low-stable group as the control group; adjusting for age, gender, SBP in 2006, FBG, BMI, eGFR, high cholesterol, smoking, alcohol consumption, physical activity, antihypertensive medications, slope of SBP. The same assignment as Table S1. BP: Blood pressure; eGFR: Estimated glomerular filtration rate; BMI: Body mass index; FBG: Fasting blood glucose; SBP: Systolic blood pressure; OR: Odds ratio; CI: Confidence interval.
DISCUSSION
This study is a prospective study involving 4556 diabetic participants during an 8-year follow-up in a northern Chinese community. This study investigated the effect of BP trajectory on kidney function in the diabetic population. The trajectory model can evaluate the BP changes over a long period of time. Its advantages are repeated measurements of BP data, grouping people with similar trends and changes in the trajectory of the BP, and the analysis of kidney damage in the different groups.
Previous studies have confirmed that there are three basic BP trajectories for adults. The first is stable BP trajectory, which indicates that BP steadily grows during follow-up. The second is increasing BP trajectory, which means that BP increases rapidly during the follow-up. The third is decreasing BP trajectory, which means that BP decreases rapidly during the follow-up.[20,21] This study advanced the BP trajectory by dividing it into the low-stable group, moderate-stable group, and elevated stable group. In total, five distinct SBP trajectories named as the low-stable group, moderate-stable group, elevated stable group, moderate increasing group, and elevated decreasing group were established in this study, which supplemented and improved the BP trajectories model.
Previous studies have shown a relatively high prevalence of hypertension in the diabetic population.[22,23] The result of this study showed that kidney damage in the diabetic population increased with an increasing SBP trajectory. An elevated stable SBP trajectory was an independent risk factor for kidney damage. Possible mechanisms for this result are as follows: (1) long-term hyperglycemia in the diabetic population led to atherosclerotic changes and fibrous sclerosis, which caused chronic kidney damage as a result;[24] (2) continual SBP trajectory increases lead to glomerular and microvascular wall thickening and hardening and luminal stenosis, which caused a decrease in kidney blood flow and showed a decrease in eGFR;[25] (3) the above two factors together accelerated the process of vascular atherosclerosis, leading to the destruction of vascular endothelial cells and smooth muscle cells, which then caused kidney damage; (4) elevated BP changed the function of the glomerular basement membrane;[26] (5) the vicious cycle of kidney function destruction leads to a BP increase and caused kidney parenchymal necrosis and even kidney failure.[25]
In this study, BP trajectory decreased in the elevated decreasing group. This is the comprehensive effect of antihypertensive medications, diet, genetics, and other factors. Compared with the elevated stable group, the risk of new-onset kidney damage decreased in the other four groups. However, examination of the moderate increasing group and elevated decreasing group showed that decreasing SBP trajectory would not completely reverse the effect of early persistent hypertension on kidney damage. Previous studies have shown that early elevated BP levels were critical to target organ effects, and target organ damage was difficult to reverse.[27,28,29] In this study, it is important to note that the elevated decreasing group of SBP values still presents a relatively high value (157–145 mmHg). This group's BP values did not reach the recommended guidelines standard, which is 130/80 mmHg in China, Japan, and Canada, whereas 140/90 mmHg in the USA and UK and 140/85 mmHg in Europe.[30,31,32]
In combination with this study, we found that the low-stable group (SBP: 118–124 mmHg) and moderate-stable group (SBP: 132–140 mmHg) diabetes patients had less influence on kidney function compared to those in the elevated stable group and the moderate increasing group. In addition, although the BP values in the moderate and low-stable groups were lower than 140 mmHg, kidney damage in the low-stable group was significantly lower than that in the moderate-stable group. This suggests that long-term diabetic kidney function protection should be controlled with lower BP. A more stringent BP control strategy when SBP of the diabetic population reaches 120 mmHg instead of other higher levels may be more beneficial.
Although there was predictive value for kidney damage in the individual analysis of baseline SBP (2006) and SBP slope, the predictive value disappeared after combining these two and the trajectory model. While the predictive value of the SBP trajectory still existed, this result indicated that the predictive value of SBP trajectory for kidney damage was superior to baseline SBP and SBP slope [Table S1, Table S3]. The long-term SBP trajectories provide more insight into the evolving risk. This study extended the results of previous studies, as these cardiovascular disease predictors were superior to single BP measurements.
Table S3.
Multivariate regression logistics analysis of kidney damage parameters of different quartile*
| Parameters | Quartile | OR (95% CI) | |
|---|---|---|---|
| Baseline SBP | Last SBP | ||
| eGFR <60 ml·min−1 1.73 m−2 | Quartile 1 | 1 | 1 |
| Quartile 2 | 0.93 (0.63–1.38) | 1.22 (0.78–1.92) | |
| Quartile 3 | 0.96 (0.65–1.42) | 0.95 (0.60–1.49) | |
| Quartile 4 | 1.61 (1.13–2.30) | 1.06 (0.68–1.64) | |
| P-trend | 0.002 | 0.669 | |
| Positive urine protein | Quartile 1 | 1 | 1 |
| Quartile 2 | 0.87 (0.62–1.21) | 0.98 (0.65–1.50) | |
| Quartile 3 | 1.38 (1.02–1.88) | 1.63 (1.12–2.39) | |
| Quartile 4 | 1.71 (1.27–2.31) | 2.17 (1.49–3.15) | |
| P-trend | <0.001 | <0.001 | |
| eGFR <60 ml·min−1 1.73 m−2 and/or positive urine protein | Quartile 1 | 1 | 1 |
| Quartile 2 | 0.86 (0.66–1.12) | 1.12 (0.81–1.55) | |
| Quartile 3 | 1.13 (0.87–1.46) | 1.33 (0.97–1.81) | |
| Quartile 4 | 1.60 (1.25–2.05) | 1.68 (1.24–2.28) | |
| P-trend | <0.001 | 0.004 | |
| eGFR reduced ≥30% | Quartile 1 | 1 | 1 |
| Quartile 2 | 0.92 (0.65–1.30) | 0.90 (0.61–1.34) | |
| Quartile 3 | 1.00 (0.71–1.41) | 0.94 (0.63–1.39) | |
| Quartile 4 | 1.61 (1.15–2.25) | 1.18 (0.79–1.74) | |
| P-trend | 0.002 | 0.557 | |
*With the exists of different kidney damage as dependent variable, 4 quartiles as independent variable, quartile 1 and 4 as control group. Adjusted for age, gender, fasting glucose, BMI, eGFR, high cholesterol, smoking, alcohol consumption, exercise, BP medication. BP: Blood pressure; eGFR: Estimated glomerular filtration rate; BMI: Body mass index; SBP: Systolic blood pressure; OR: Odds ratio; CI: Confidence interval.
These results indicated that kidney damage progression can be slowed by controlling hypertension and regular follow-up. Monitoring and controlling trajectories of SBP may provide an important approach to identify diabetic patients and help to prevent kidney damage. Basic essential technologies available, such as wearable BP monitoring technology and rehabilitation services, play a fundamental role across the continuum of care for people with diabetes that helps to prevent complications and provides interventions to maintain healthier lives.
Advantages and significance of this study
This trajectory model was used to analyze the effects of SBP on kidney damage during an 8-year follow-up in the diabetic population. We identified five distinct SBP trajectories according to SBP ranges and changing patterns over time and found that an elevated SBP trajectory was significantly associated with the risks of long-term kidney damage in diabetic patients.
Limitations in this study
First, one limitation is that we only included Chinese adults living in the Kailuan community. The trajectories identified in these participants may not be generalizable to other populations. Second, half of the diabetic patients were excluded because of deaths and incomplete data, which may lead to offset results. The rates of cardiovascular events and all-cause mortality of the noninclusion group were higher than the included group, and the difference showed statistical significance [Table S4]. This may underestimate the impact of different BP trajectories on kidney damage in the diabetes population. Third, there may be laboratory bias in SBP data used in this study because BP was determined by mercury sphygmomanometer in 2006–2012, and a medical electronic sphygmomanometer arm in 2014. Future research needs to be conducted to determine the outcome of the BP trajectories, and data from large well-designed randomized controlled trials are needed to further confirm the BP management strategies for the diabetic population.
Table S4.
Situation of noninclusion population and included population in 2006
| Parameters | Noninclusion population (n = 2023) | Included population (n = 4556) | P | |
|---|---|---|---|---|
| Refused (n = 1429) | Dead (n = 594) | |||
| Age (years old) | 57.89 ± 10.51 | 63.96 ± 9.73 | 53.83 ± 9.11 | <0.001 |
| Male, case (%) | 1265 (88.5) | 544 (91.6) | 3683 (80.8) | <0.001 |
| SBP (mmHg) | 139.44 ± 22.70 | 141.10 ± 22.50 | 136.08 ± 20.12 | <0.001 |
| DBP (mmHg) | 85.91 ± 12.48 | 84.82 ± 12.79 | 85.53 ± 11.34 | 0.161 |
| BMI (kg/m2) | 26.00 ± 3.54 | 25.38 ± 3.68 | 26.22 ± 3.36 | <0.001 |
| TC (mmol/L) | 5.21 ± 1.24 | 5.14 ± 1.28 | 5.19 ± 1.20 | 0.563 |
| HDL-C (mmol/L) | 1.56 ± 0.44 | 1.57 ± 0.49 | 1.53 ± 0.41 | 0.030 |
| LDL-C (mmol/L) | 2.41 ± 0.92 | 2.38 ± 1.10 | 2.40 ± 0.99 | 0.795 |
| FBG (mmol/L) | 9.44 ± 3.24 | 9.51 ± 3.16 | 9.09 ± 2.86 | <0.001 |
| eGFR (ml·min−1·1.73 m−2) | 83.92 ± 24.00 | 81.27 ± 26.70 | 85.43 ± 28.27 | <0.001 |
| Physical exercise, case (%) | 214 (15.0) | 143 (24.1) | 877 (19.2) | <0.001 |
| Smoking, case (%) | 418 (29.2) | 159 (26.8) | 1354 (29.7) | 0.330 |
| Drinking, case (%) | 239 (16.7) | 85 (14.3) | 764 (16.8) | 0.310 |
| Taking antihypertensive medications, case (%) | 271 (19.0) | 143 (24.1) | 873 (19.2) | <0.001 |
| Taking hypoglycaemic agents, case (%) | 322 (22.5) | 172 (29.0) | 1174 (25.8) | <0.001 |
SBP: Systolic blood pressure; DBP: Diastolic blood pressure; eGFR: Estimated glomerular filtration rate; BMI: Body mass index; FBG: Fasting blood glucose; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; TC: Total cholesterol.
In conclusion, this study observed different BP trajectories in the diabetic population of Kailuan study participants, and the trajectories were associated with future kidney damage. An elevated SBP trajectory is an independent risk factor for kidney damage. As the BP trajectory decreased, the risk of new-onset kidney damage decreased. A low-stable SBP trajectory, when SBP is 120 mmHg, might be more beneficial in the diabetic population. Monitoring trajectories of BP may provide an important approach to identify diabetic populations and help to prevent kidney damage.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Global Report on Diabetes. World Health Organization. [Last accessed on 18 Mar 18]. Available from: http://www.who.int/diabetes/global-report .
- 2.Konno S, Hozawa A, Miura Y, Ito S, Munakata M. High-normal diastolic blood pressure is a risk for development of microalbuminuria in the general population: The Watari study. J Hypertens. 2013;31:798–804. doi: 10.1097/HJH.0b013e32835e2146. doi: 10.1097/HJH.0b013e32835e2146. [DOI] [PubMed] [Google Scholar]
- 3.Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, Navarro-Pérez J, Pallares V, Valls F, et al. 3A05: Hypertension and risk of events associated to reduced EGFR. The escarval-risk study J Hypertens. 2015;33(Suppl 1):e32–3. doi: 10.1097/01.hjh.0000467435.02925.75. [Google Scholar]
- 4.Munakata M, Konno S, Ohshima M, Ikeda T, Miura Y, Ito S, et al. High-normal blood pressure is associated with microalbuminuria in the general population: The Watari study. Hypertens Res. 2011;34:1135–40. doi: 10.1038/hr.2011.98. doi: 10.1038/hr.2011.98. [DOI] [PubMed] [Google Scholar]
- 5.Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Wang JG, et al. Beat-to-beat, reading-to-reading, and day-to-day blood pressure variability in relation to organ damage in untreated Chinese. Hypertension. 2014;63:790–6. doi: 10.1161/HYPERTENSIONAHA.113.02681. doi: 10.1161/HYPERTENSIONAHA.113.02681. [DOI] [PubMed] [Google Scholar]
- 6.Leehey DJ, Zhang JH, Emanuele NV, Whaley-Connell A, Palevsky PM, Reilly RF, et al. BP and renal outcomes in diabetic kidney disease: The veterans affairs nephropathy in diabetes trial. Clin J Am Soc Nephrol. 2015;10:2159–69. doi: 10.2215/CJN.02850315. doi: 10.2215/CJN.02850315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–7. doi: 10.1001/jama.2013.285122. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, et al. Childhood to early-midlife systolic blood pressure trajectories: Early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108–15. doi: 10.1161/HYPERTENSIONAHA.115.05831. doi: 10.1161/HYPERTENSIONAHA.115.05831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Jiang B, Song L, Yang C, Wu Y, Chen S, et al. Correlation between visit-to-visit and short-term blood pressure variability calculated using different methods and glomerular filtration rate. J Hum Hypertens. 2017;31:132–7. doi: 10.1038/jhh.2016.51. doi: 10.1038/jhh.2016.51. [DOI] [PubMed] [Google Scholar]
- 10.Imam K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Adv Exp Med Biol. 2012;771:340–55. doi: 10.1007/978-1-4614-5441-0_25. doi: 10.1007/978-1-4614-5441-0_25. [DOI] [PubMed] [Google Scholar]
- 11.Rognant N, Lemoine S, Laville M, Hadj-Aïssa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care. 2011;34:1320–2. doi: 10.2337/dc11-0203. doi: 10.2337/dc11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson TW, Komenda P, Tangri N. Change in estimated glomerular filtration rate and outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25:240–4. doi: 10.1097/MNH.0000000000000210. doi: 10.1097/MNH.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 13.Joint Committee on the Development of Guidelines for the Prevention and Control of Dyslipidemia in Chinese adults. Guidelines for the prevention and treatment of dyslipidemia in Chinese adults. Chin J Cardiol. 2007;19:390–419. doi: 10.3760/j.issn:0253-3758.2007.05.003. [Google Scholar]
- 14.Jones BL, Nagin D, Roeder K. A SAS procedure based on mixture model for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–93. doi: 10.1177/0049124101029003005. [Google Scholar]
- 15.Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, et al. Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: A meta-analysis of 16 cohort studies. Circulation. 2009;119:1892–8. doi: 10.1161/CIRCULATIONAHA.108.823112. doi: 10.1161/CIRCULATIONAHA.108.823112. [DOI] [PubMed] [Google Scholar]
- 16.Reinders I, Murphy RA, Martin KR, Brouwer IA, Visser M, White DK, et al. Body mass index trajectories in relation to change in lean mass and physical function: The health, aging and body composition study. J Am Geriatr Soc. 2015;63:1615–21. doi: 10.1111/jgs.13524. doi: 10.1111/jgs.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 18.Nagin DS, Odgers CL. Group-based trajectory modeling (Nearly) two decades later. J Quant Criminol. 2010;26:445–53. doi: 10.1007/s10940-010-9113-7. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagin DS. Group-based trajectory modeling: An overview. Ann Nutr Metab. 2014;65:205–10. doi: 10.1159/000360229. doi: 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- 20.Tielemans SM, Geleijnse JM, Menotti A, Boshuizen HC, Soedamah-Muthu SS, Jacobs DR., Jr Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: The minnesota business and professional men study and the Zutphen study. J Am Heart Assoc. 2015;4:e001378. doi: 10.1161/JAHA.114.001378. doi: 10.1161/JAHA.114.001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. doi: 10.1371/journal.pmed.1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group T H I D S. Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–17. doi: 10.1097/00004872-199303000-00012. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Tashko G, Gabbay RA. Evidence-based approach for managing hypertension in type 2 diabetes. Integr Blood Press Control. 2010;3:31–43. doi: 10.2147/ibpc.s6984. doi: 10.2147/ibpc.s6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halimi JM, Joly D, Combe C, Choukroun G, Dussol B, Fauvel JP, et al. Blood pressure and proteinuria control remains a challenge in patients with type 2 diabetes mellitus and chronic kidney disease: Experience from the prospective observational ALICE-PROTECT study. BMC Nephrol. 2016;17:135. doi: 10.1186/s12882-016-0336-1. doi: 10.1186/s12882-016-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–51. doi: 10.1161/HYPERTENSIONAHA.110.164780. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 26.Boivin JM, Koch C, Vigié L, Meppiel L. Prevalence of target organ damage in patients treated for primary arterial hypertension: Comparison between men and women. ESSENTIELLE study. Ann Cardiol Angeiol (Paris) 2015;64:150–7. doi: 10.1016/j.ancard.2015.04.006. doi: 10.1016/j.ancard.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Petruski-Ivleva N, Viera AJ, Shimbo D, Muntner P, Avery CL, Schneider AL, et al. Longitudinal patterns of change in systolic blood pressure and incidence of cardiovascular disease: The atherosclerosis risk in communities study. Hypertension. 2016;67:1150–6. doi: 10.1161/HYPERTENSIONAHA.115.06769. doi: 10.1161/HYPERTENSIONAHA.115.06769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing-implications in hypertension. J Mol Cell Cardiol. 2015;83:112–21. doi: 10.1016/j.yjmcc.2015.04.011. doi: 10.1016/j.yjmcc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–41. doi: 10.1161/HYPERTENSIONAHA.113.01445. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 31.Lindholm LH, Carlberg B. The new Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014): A giant undertaking. Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.21. doi: 10.1038/hr.2014.18. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16. doi: 10.3109/08037051.2014.868629. doi: 10.3109/08037051.2014.868629. [DOI] [PubMed] [Google Scholar]