Abstract
Purpose
To determine the long-term visual outcomes of six eyes of 3 patients up to 13 years following the Laser Anterior Ciliary Excision (LaserACE) procedure.
Methods
Three male patients of ages 59, 59, and 60 presented for evaluation at Storm Eye Institute, Medical University of South Carolina at 8, 10, and 13 years after the LaserACE procedure for presbyopia, respectively. All 3 patients had a history of laser vision correction (LVC) prior to LaserACE treatment. Visual performance was evaluated using ray-tracing aberrometry, specifically higher-order aberrations, visual Strehl of the optical transfer function (VSOTF), depth of focus (DoF), and effective range of focus (EROF). VSOTF was computed as a function of defocus using a through-focus curve. Subjective DoF was overlaid on the VSOTF through-focus curve to establish the best image quality metric threshold value for correlation between subjective and objective DoF. EROF was determined by measuring the difference in diopters between the near and distance DoF curves, at 50% of VSOTF.
Results
Distance-corrected visual acuity, distance-corrected intermediate visual acuity, and distance-corrected near visual acuity for all patients remained at 20/20 or better up to 13 years postoperatively. EROF averaged 1.56 ± 0.36 (D) for all eyes.
Conclusions and Importance
LaserACE provided improvement in near vision functionality in these LVC patients with long-term stability. The LaserACE procedure is not on the visual axis, therefore these patients could still receive correction to their hyperopic regression.
Keywords: Presbyopia, Accommodation, Visual acuity, Laser anterior ciliary excision
1. Introduction
Presbyopia is an age-related loss in accommodative ability, affecting an estimated half a billion people worldwide.1 It has been traditionally described following Helmholtz’ theory of accommodation, wherein the loss of elasticity of the lens substance causes a reduction in accommodation, resulting in presbyopia.2 This cannot be the sole explanation as recent studies have demonstrated the influence that ocular rigidity, the vitreous membrane, peripheral choroid, zonules, and ciliary muscles have on the loss of accommodation.3, 4, 5, 6, 7
In the particular case of ocular rigidity, the human sclera has been shown to lose virtually all its elasticity after 70 years.8 This increased ocular rigidity with age has been correlated with a clinically significant loss of accommodation.3 Laser Anterior Ciliary Excision (LaserACE) is designed to alter the biomechanical properties of the rigid sclera. LaserACE utilizes an excimer laser to create a matrix array of micro-excisions (micropores) in the sclera.9 Within the matrix, there are areas of both positive stiffness (remaining interstitial tissue) and negative stiffness (removed tissue or micropores), which increase the plasticity and compliance of the scleral tissue during contraction of the ciliary muscles, and improve the efficiency of the accommodation apparatus.9
To treat presbyopia, spectacles and contact lenses are the prevailing treatments, however they do not attempt to restore accommodation to the presbyopic eye. Many current presbyopia treatments that do attempt to restore accommodation, only aim to increase the depth of focus (DoF) for patients. This can be done by the use of corneal refractive surgeries or intraocular lens replacement.10 These treatment options may enhance the ‘pseudoaccommodation’ of presbyopic patients, but not their true accommodation. True accommodation is the ability of the eye to modify its focal length to see objects clearly when changing focus from distance to near. LaserACE is one of the only treatment options for presbyopia that aims to restore both true accommodation and pseudoaccommodation.
Wavefront analysis is a widely used method to assess the visual system,11 and is typically used to measure higher-order aberrations (HOA), visual Strehl ratio, and DoF.12 DoF is the variation in defocus that can be tolerated by the eye without a noticeable change in image sharpness.13 Ray-tracing aberrometry can objectively determine DoF by computing near and distance through-focus curves.14 The visual Strehl of the optical transfer function (VSOTF) is a precise method to measure the objective visual performance of patients.15,16 It is an optical wavefront error-derived metric that predicts patient visual acuity,16 and is defined as:15
We have previously reported the improvements in uncorrected near visual acuity (UNVA) and distance-corrected near visual acuity (DCNVA) immediately after the LaserACE procedure and up to 24 months postoperatively.17 In this brief report, we describe the visual outcomes of three patients who were recently examined at 8, 10, and 13 years postoperatively, respectively. We present the long-term visual outcomes for three patients, following LaserACE, including the visual acuities at near and distance, HOA, effective range of focus, and VSOTF.
2. Materials and methods
Three male patients of ages 59, 59, and 60 presented for evaluation at Storm Eye Institute, Medical University of South Carolina at 8, 10, and 13 years after LaserACE procedure for presbyopia, respectively. All 3 patients had a history of laser vision correction (LVC) prior to LaserACE treatment.
An outline of the LaserACE procedure is shown in Fig. 1. In brief, an erbium-doped yttrium aluminum garnet (Er:YAG) laser is utilized to create 9 micropores in the sclera of the eye. Excisions were placed in a matrix pattern from 0.5 mm up to 6.0 mm from the anatomical limbus (AL) over the 3 critical anatomical and physiological zones of significance: 1) the scleral spur at the origin of the ciliary muscle (0.5–1.1 mm from AL); 2) the mid ciliary muscle body (1.1–4.9 mm from AL); and 3) insertion of the longitudinal muscle fibers of the ciliary, just anterior to the ora serrata at the insertion of the posterior vitreous zonules (4.9–5.5 mm from AL).9,18,19 Excision depth was 85–90% the depth of the sclera, to the point that the blue hue of the choroid just became visible. An opaque corneal shield was placed on the cornea, and remained in place until the completion of the procedure. A representative postoperative slit lamp image is shown in Fig. 2.
Fig. 1.
Laser Anterior Ciliary Excision (LaserACE) surgical technique. Photo A. Quadrant marker; B. Matrix marker; C. Corneal Shield; D. LaserACE micropore ablation; E. Subconjunctival Collagen F. Completed 4 quadrants. Reprinted with permission from Hipsley et al.17
Fig. 2.
Representative photo of a postoperative Laser Anterior Ciliary Excision patient eye under a slit lamp.
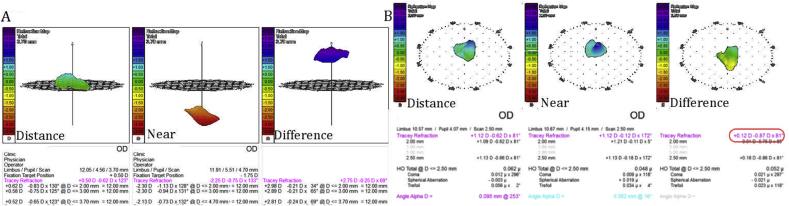
Uncorrected and distance corrected visual acuities were measured using standard Early Treatment Diabetic Retinopathy Study (ETDRS) charts. Measurement of HOAs, VSOTF, DoF, and effective range of focus (EROF) were performed using ray-tracing aberrometry (iTrace, Tracey Technologies, Houston, TX, USA). The iTrace aberrometer is capable of creating corneal and lenticular maps as well as a difference map which can demonstrate independent image quality metrics (IQM) for the lens (Fig. 3). VSOTF was computed as a function of defocus using a through-focus curve, as described previously.14 Subjective DoF was overlaid on the VSOTF through-focus curve to establish the best IQM threshold value for correlation between subjective and objective DoF. The EROF was determined by measuring the difference in diopters between the near and distance through-focus curves, at 50% of VSOTF. The EROF is the range of focus with acceptable blur, and will be a combination of both the true accommodation and the pseudoaccommodation.
Fig. 3.
Representative example of image quality metrics at near, distance, and the difference.
The true accommodative ability of patient eyes was determined by first measuring the difference in distance and near refraction, shown in Fig. 4. The spherical equivalent of the refraction difference will be the true accommodation. For a young eye (Fig. 4A) this was ∼2.65 D in true accommodation, while for a presbyope (Fig. 4B) there was little to no true accommodation.
Fig. 4.
Determining objective accommodation using iTrace aberrometry. Objective accommodation (OA) is measured by determining the spherical equivalent of the difference between distance and near refraction. Examples shown for A. non-presbyopic eye (OA = 2.63 D); B. presbyopic eye (OA = 0.32 D).
This was an IRB monitored and registered international clinical pilot study, which followed the tenets of the Declaration of Helsinki. Patients provided written consent for imaging and publication of personal identifying information including medical record details.
3. Results
Summaries of each patient's visual outcomes prior to the LaserACE procedure are shown in Table 1. Summaries of each patient's visual outcomes after LaserACE are shown in Table 2. Despite the hyperopic regression observed in all eyes, the distance-corrected near visual acuity of the patients were stable following the LaserACE procedure and preserved for 8, 10, and 13 years postoperatively. Corrected distance visual acuity (CDVA), distance-corrected intermediate visual acuity (DCIVA), and DCNVA for all patients remained at 20/20 or better following the LaserACE procedure. These are large improvements compared to preoperative DCIVA and DCNVA, which ranged from 20/40 to 20/60 (OD) and 20/40 to 20/400 (OS), respectively. Additionally, both DCIVA and DCNVA maintained these improvements of 1–15 lines and 2–15 lines, respectively, up to 13 years postoperatively.
Table 1.
Patient visual outcomes prior to LaserACE procedure.
| Patient | Prior LVC Type | Age LVC | Age LaserACE | Age Long-Term Exam | MRSE | Eye | Sphere | Cylinder | Axis | UDVA | UIVA | UNVA | CDVA | DCIVA | DCNVA | DoF (D) | Clinical Accommodation (D) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | PresbyLasik OU | 49 | 49 | 59 | 20/20 | OD | PL | −0.25 | 20 | 20/20 | 20/30 | 20/40 | 20/20 | 20/40 | 20/40 | 0.5 | 0.5 |
| OS | PL | −0.25 | 175 | 20/20 | 20/30 | 20/60 | 20/20 | 20/30 | 20/40 | 0.5 | 0.5 | ||||||
| 102 | Hyperopic Lasik OU hyperopic regression of RK | 46 | 48 | 59 | 20/20 | OD | PL | −0.50 | 155 | 20/15 | 20/100 | 20/100 | 20/15 | 20/100 | 20/60 | 1.07 | 1.7 |
| OS | −0.25 | −0.75 | 47 | 20/20 + 2 | 20/400 | 20/400 | 20/20 | 20/400 | 20/400 | 0.75 | 1.7 | ||||||
| 103 | Hyperopic/Astigmatism LASIK OU | 50 | 52 | 60 | 20/20 | OD | +0.50 | −0.75 | 156 | 20/20 | – | – | 20/20 | – | – | 0.5 | 0.5 |
| OS | +0.25 | −0.25 | 176 | 20/20 | – | – | 20/20 | – | – | 0.6 | 0.6 |
Abbreviations: LVC, laser vision correction; LaserACE, laser anterior ciliary excision; MRSE, manifest refraction spherical equivalent; UDVA, uncorrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; UNVA, uncorrected near visual acuity; CDVA, corrected distance visual acuity; DCIVA, distance corrected intermediate visual acuity; DCNVA, distance corrected near visual acuity; DoF, depth of focus.
Table 2.
Patient long-term visual outcomes after LaserACE procedure.
| Patient | Years After LaserACE | Post OP MRSE (1-month) | Post OP MRSE (Long-Term) | Eye | Sphere | Cylinder | Axis | IOP (mmHg) | UDVA | UIVA | UNVA | CDVA | DCIVA | DCNVA | Dif HOA | DoF (D) | True Accommodation (D) | Pseudo-accommodation (D) | Effective Range of Focus (D) | VSOTF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 10 | 20/20 | 20/25-3 | OD | +2.12 | −1.12 | 150 | 12 | 20/25-3 | 20/40-2 | 20/60 | 20/20 | 20/20 | 20/20 | 0.047 (2.1 mm pupil) | 1.23 | 0.31 | 1.14 | 1.45 | 0.555 |
| OS | +2.37 | −1.12 | 031 | 14 | 20/40-2 | 20/40 | 20/40 | 20/15 | 20/20 | 20/20 | 0.080 (2.0 mm pupil) | 2.82 | 0.06 | 2.10 | 2.16 | 0.281 | ||||
| 102 | 13 | 20/15 | 20/20-2 | OD | +1.75 | −0.75 | 178 | 14 | 20/20-2 | 20/20-2 | 20/20-1 | 20/15 | 20/20 | 20/20 | 0.066 (2.0 mm pupil) | 1.32 | 0.06 | 1.21 | 1.27 | 0.616 |
| OS | +2.00 | −0.25 | 166 | 13 | 20/25 | 20/20-2 | 20/20-2 | 20/15 | 20/20 | 20/20 | 0.038 (2.0 mm pupil) | 1.44 | 0.19 | 1.30 | 1.49 | 0.426 | ||||
| 103 | 8 | 20/20 | 20/15-3 | OD | +1.25 | −0.50 | 151 | 11 | 20/15-3 | 20/20 | 20/20 + 1 | 20/15 | 20/20 | 20/20 + 1 | 0.058 (2.0 mm pupil) | 1.03 | 0.68 | 1.09 | 1.77 | 0.609 |
| OS | +0.25 | −0.25 | 014 | 17 | 20/20-2 | 20/20 | 20/20 + 1 | 20/15 | 20/20 + 1 | 20/20 + 1 | 0.146 (2.5 mm pupil) | 1.12 | 0.06 | 1.14 | 1.20 | 0.405 |
Abbreviations: LaserACE, laser anterior ciliary excision; MRSE, manifest refraction spherical equivalent; IOP, intraocular pressure; UDVA, uncorrected distance visual acuity; UIVA, uncorrected intermediate visual acuity; UNVA, uncorrected near visual acuity; CDVA, corrected distance visual acuity; DCIVA, distance corrected intermediate visual acuity; DCNVA, distance corrected near visual acuity; Dif HOA, differential item functioning higher order aberrations; VSOTF, visual Strehl ratio based on the optical transfer function.
Fig. 5, Fig. 6 show the DoF for near (red) and distance (green) and the effective range of focus (EROF) measurements for each patient eye. Patient DoF increased by 0.84 ± 0.74 D on average compared to preoperative DoF. The largest EROF for a single patient eye was 2.16 D (Fig. 5A). Patient EROF averaged 1.56 ± 0.36 D for all patient eyes (n = 6). This was higher than preoperative clinical accommodation, which averaged 0.92 ± 0.61 D. True accommodation and pseudoaccommodation averaged 0.23 ± 0.24 D and 1.33 ± 0.38 D respectively.
Fig. 5.
Effective range of focus. Visual Strehl ratio based upon the optical transfer function is computed as a function of defocus using a through-focus curve. Patient eye (OD): A. 101, B. 102, C. 103.
Fig. 6.
Effective range of focus. Visual Strehl ratio based upon the optical transfer function is computed as a function of defocus using a through-focus curve. Patient eye (OS): A. 101, B. 102, C. 103.
Ray-tracing aberrometry results for each patient eye are shown in Fig. 7, Fig. 8. Averages of DoF, EROF, VSOTF, and HOA for OD, OS, and OU are summarized in Fig. 9.
Fig. 7.
Refractive and wavefront difference maps at distance and near using ray-tracing. Maps show accommodation and pseudoaccommodation components. Patient eye (OD): 1 A. 101, B. 102, C. 103.
Fig. 8.
Refractive and wavefront difference maps at distance and near using ray-tracing. Maps show accommodation and pseudoaccommodation components. Patient eye (OS): A. 101, B. 102, C. 103.
Fig. 9.
Patient averages of depth of focus, effective range of focus, visual Strehl ratio based on the optical transfer function, higher order aberrations: OD (black), OS (white), OU (gray).
4. Discussion
Presbyopia is the most common refractive error, but remains not entirely understood. Several factors have been shown to influence the loss of accommodation including lens inelasticity, ocular rigidity, the vitreous membrane, peripheral choroid, zonules, and ciliary muscles.2, 3, 4, 5, 6, 7 Age-related ocular rigidity can cause ocular biomechanical dysfunction of the accommodation apparatus.9,20,21
LaserACE aims to reverse this dysfunction by targeting the sclera overlying the ciliary body in three critical zones of anatomical and physiological significance.9,18 This may impact the age-related biomechanical and neuromuscular changes of the eye to reverse the loss of accommodation in presbyopes.22 In this brief report, the DCNVA for all patients remained at 20/20 or better up to 13 years postoperatively. This suggests that LaserACE can enhance the accommodative ability of presbyopes.
LaserACE limits treatment to the sclera, leaving the visual axis untouched. This permits the eyes in this study to receive further correction of their hyperopic regression, allowing them to utilize their residual accommodative ability. LaserACE also does not preclude these or other patients from future corneal or cataract procedures, such as receiving enhancements to their LVC or accommodative intraocular lenses (IOLs).
LaserACE patient CDVA, DCIVA, and DCNVA were 20/20 or better in each eye, up to 13 years postoperatively. These retained improvements in both DCIVA and DCNVA are encouraging compared to other treatments such as scleral bands, scleral implants, or accommodating IOLs. Scleral expansion bands can produce inconsistent and unpredictable results.23 A recent study found that 93% of eyes with scleral implants had DCNVA of 20/40 or better at 2 years postoperatively (Soloway B and Schanzlin DJ; ASCRS 2014 E-Abstract). Accommodating IOLs aim to change the IOL position to facilitate near focus, however to date results have been moderate (Ang RE; ASCRS 2014 E-Abstract). A recent study found that the mean DCNVA of patient eyes with the Crystalens accommodating IOL was 20/32 at 1 year postoperatively.24
Although each patient in this study had prior refractive surgical procedures prior to receiving the LaserACE procedure, they all met the preoperative inclusion criteria of being LVC corrected to within the parameter of ± 0.5 D of emmetropia. This allows for the procedural results to have the biggest effect on restoring near and intermediate visual acuity and depth of focus while keeping the distance visual acuity stable. The LaserACE procedure primarily affects lenticular aberrations, therefore to isolate the effects from the lens it is necessary to subtract the lenticular aberrations from the corneal aberrations. For this reason, the iTrace Aberrometer is the preferred diagnostic evaluation for the objective measurement of true physiological accommodation and pseudoaccommodation from the lens aberrations solely. Our results demonstrate that the response from the crystalline lens to the LaserACE procedure is both an increase in true accommodative power with spherical changes in the lens as well as changes in HOA, corresponding to improvements in DoF and quality of vision.
Ray-tracing identifies the precise IQM to compute the mechanisms contributing to the improved EROF of the patients before and after the LaserACE procedure, as shown in Fig. 3. This allows for an understanding of how changes in lenticular aberrations contribute to the improved visual acuities for both intermediate and near without consideration of the pre-existing corneal aberrations, which remain unchanged after the LaserACE procedure. Comparing the distance corrected visual acuities to the uncorrected visual acuities reveal any influences from corneal treatments past or present.
Another study has shown that LaserACE alone can significantly improve the visual outcomes in presbyopes.17 However, it may also be the case that the accommodative improvements after LaserACE allow these patients to better utilize their previous LVC. In either case, the use of LaserACE improved the visual outcomes in these patients.
In conclusion, LaserACE provided improvement in near vision functionality for these LVC patients with long-term stability of up to 13 years without significant change to manifest refraction. The LaserACE procedure is not on the visual axis, therefore these patients could still receive correction to their hyperopic regression, which would allow them to utilize their restored accommodative ability.
Patient consent
Data were obtained from an IRB monitored and registered international clinical pilot study, which followed the tenets of the Declaration of Helsinki. The LaserACE patients provided written consent for imaging and publication of personal identifying information including medical record details.
Acknowledgements and disclosures
Funding
Financial support provided by Ace Vision Group, Inc.
Conflict of interest
AMH reports personal fees and non-financial support from Ace Vision Group Inc. during the conduct of the study. In addition, Dr. Hipsley has a patent 7871404 issued to Ace Vision Group Inc, a patent 8348932 issued to Ace Vision Group, Inc, a patent 20150157406 pending to Ace Vision Group, Inc, a patent 20140316388 pending to Ace Vision Group, Inc, a patent 20140163597 pending to Ace Vision Group, Inc, a patent 20120165849 pending to Ace Vision Group, Inc, a patent 20110190798 pending to Ace Vision Group, Inc, a patent 20080058779 pending to Ace Vision Group, Inc, and a patent 20070016175 pending to Ace Vision Group, Inc.
BH reports personal fees from Ace Vision Group Inc. during the conduct of the study. KMR is a Consultant to Ace Vision Group.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Holden B.A., Fricke T.R., Ho S.M. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126:1731–1739. doi: 10.1001/archopht.126.12.1731. [DOI] [PubMed] [Google Scholar]
- 2.von Helmholtz H. Mechanism of accommodation. In: Southall J.P.C., editor. Helmholtz's Treatise on Physiological Optics. vol. 1. Optical Society of America; Rochester, NY.: 1924. pp. 143–172. Trans. from the 3rd German Ed. [Google Scholar]
- 3.Detorakis E.T., Pallikaris I.G. Ocular rigidity: biomechanical role, in vivo measurements and clinical significance. Clin Exp Ophthalmol. 2013;41:73–81. doi: 10.1111/j.1442-9071.2012.02809.x. [DOI] [PubMed] [Google Scholar]
- 4.Croft M.A., McDonald J.P., Katz A., Lin T.-L., Lütjen-Drecoll E., Kaufman P.L. Extralenticular and lenticular aspects of accommodation and presbyopia in human versus monkey eyes. Investig Ophthalmol Vis Sci. 2013;54:5035–5048. doi: 10.1167/iovs.12-10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft M.A., Nork T.M., McDonald J.P., Katz A., Lütjen-Drecoll E., Kaufman P.L. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Invest Ophthalmol Vis Sci. 2013;54:5049–5058. doi: 10.1167/iovs.12-10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strenk S.A., Strenk L.M., Guo S. Magnetic resonance imaging of the anteroposterior position and thickness of the aging, accommodating, phakic, and pseudophakic ciliary muscle. J Cataract Refract Surg. 2010;36:235–241. doi: 10.1016/j.jcrs.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richdale K., Sinnott L.T., Bullimore M.A. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eyelens and ciliary muscle with age and accommodation. Invest Ophthalmol Vis Sci. 2013;54:1095–1105. doi: 10.1167/iovs.12-10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom W., Fawcett W. eleventh ed. Saunders; Philadelphia: 1986. A Textbook of Histology. [Google Scholar]
- 9.Hipsley A., Dementiev D. VisioDynamics theory: a biomechanical model for the aging ocular organ. In: Ashok G., Urzua G., Dementiev D., Pinelli R., editors. Step by Step Innovations in Presbyopia Management. Jaypee Brothers Medical Publishers; New Delhi: 2006. pp. 269–315. [Google Scholar]
- 10.Ylmaz ÖF Alagöz N., Pekel G., Azman E. Intracorneal inlay to correct presbyopia: long-term results. J Cataract Refract Surg. 2011;37:1275–1281. doi: 10.1016/j.jcrs.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Rocha K.M., Soriano E.S., Chalita M.R. Wavefront analysis and contrast sensitivity of aspheric and spherical intraocular lenses: a randomized prospective study. Am J Ophthalmol. 2006;142:750–756. doi: 10.1016/j.ajo.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Williams D., Yoon G.Y., Porter J., Guirao A., Hofer H., Cox I. Visual benefit of correcting higher order aberrations of the eye. J Refract Surg. 2000;16:S554–S559. doi: 10.3928/1081-597X-20000901-12. [DOI] [PubMed] [Google Scholar]
- 13.Cline D., Hofstetter H.W., Griffin J.R. Butterworth-Heinemann; 1997. Dictionary of Visual Science. [Google Scholar]
- 14.Yi F., Iskander D.R., Collins M.J. Estimation of the depth of focus from wavefront measurements. J Vis. 2010;10:1–9. doi: 10.1167/10.4.3. [DOI] [PubMed] [Google Scholar]
- 15.Thibos L.N., Hong X., Bradley A., Applegate R.A. Accuracy and precision of objective refraction from wavefront aberrations. J Vis. 2004;4:329–351. doi: 10.1167/4.4.9. [DOI] [PubMed] [Google Scholar]
- 16.Iskander D.R. Computational aspects of the visual Strehl ratio. Optom Vis Sci. 2006;83:57–59. doi: 10.1097/01.opx.0000195563.82891.3b. [DOI] [PubMed] [Google Scholar]
- 17.Hipsley A., Ma D.H.-K., Sun C.-C., Jackson M.A., Goldberg D., Hall B. Visual outcomes 24 months after LaserACE. Eye Vis. 2017;4:15. doi: 10.1186/s40662-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hipsley A., McDonald M. Laser scleral matrix microexcisions (LaserACE/erbium YAG laser) In: Pallikaris I.G., Plainis S., Charman W.N., editors. Presbyopia: Origins, Effects, and Treatment. 2012. pp. 219–225. Slack Incorporated New Jersey. [Google Scholar]
- 19.Lütjen-Drecoll E., Kaufman P.L., Wasielewski R., Ting-Li L., Croft M.A. Morphology and accommodative function of the vitreous zonule in human and monkey eyes. Investig Ophthalmol Vis Sci. 2010;51:1554–1564. doi: 10.1167/iovs.09-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battaglioli J.L., Kamm R.D. Measurements of the compressive properties of scleral tissue. Invest Ophthalmol Vis Sci. 1984;25:59–65. [PubMed] [Google Scholar]
- 21.Strenk S.A., Semmlow J.L., Strenk L.M., Munoz P., Gronlund-Jacob J., DeMarco J.K. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 22.Goldberg D.B. Computer-animated model of accommodation and presbyopia. J Cataract Refract Surg. 2015;41:437–445. doi: 10.1016/j.jcrs.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Malecaze F.J., Gazagne C.S., Tarroux M.C., Gorrand J.-M. Scleral expansion bands for presbyopia. Ophthalmology. 2001;108:2165–2171. doi: 10.1016/s0161-6420(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 24.Szigeti A., Kránitz K., Takacs A.I., Miháltz K., Knorz M.C., Nagy Z.Z. Comparison of long-term visual outcome and IOL position with a single-optic accommodating IOL after 5.5-or 6.0-mm Femtosecond laser capsulotomy. J Refract Surg. 2012;28:609–613. doi: 10.3928/1081597X-20120815-04. [DOI] [PubMed] [Google Scholar]