Abstract
Introduction
Increased hospitalization is a major component of dementia impact on individuals and cost, but has rarely been studied in dementia with Lewy bodies (DLB). Our aim was to describe the risk and duration of hospital admissions in patients with DLB, and compare these to those in Alzheimer's disease (AD) and the general population.
Methods
A large database of mental health and dementia care in South London was used to assemble a cohort of patients diagnosed with DLB. These were 1:4 matched with patients diagnosed with AD on age, gender, and cognitive status.
Results
Rates of hospital admissions in the year after dementia diagnosis were significantly higher in 194 patients with DLB than in 776 patients with AD (crude incidence rate ratio 1.50; 95% confidence interval: 1.28–1.75) or the catchment population (indirectly standardized hospitalization rate 1.22; 95% confidence interval: 1.06–1.39). Patients with DLB had on average almost four additional hospital days per person-year than patients with AD. Multivariate Poisson regression models indicated poorer physical health early in the disease course as the main driver of this increased rate of hospitalization, whereby neuropsychiatric symptoms additionally explained the higher number of hospital days.
Discussion
Patients with DLB are more frequently admitted to general hospitals and utilize inpatient care to a substantially higher degree than patients with AD or the general elderly population. These data highlight an opportunity to reduce hospital days by identifying DLB earlier and providing more targeted care focused on the specific triggers for hospitalization and associations of prolonged stay.
Keywords: Dementia with Lewy bodies, Hospitalization, Health-care costs, Comorbidity, Health services
1. Introduction
Hospitalization is a major cost driver in dementia [1], [2], [3] with longer and more frequent, more potentially avoidable, and more unplanned hospital admissions in people with Alzheimer's disease (AD) than the general population [3], [4], [5], [6]. Dementia with Lewy bodies (DLB) is the second most common type of neurodegenerative dementia [7]. Given the complex clinical phenotype with motor, psychiatric, and autonomic symptoms in addition to dementia, people with DLB may have a higher risk of being hospitalized. Furthermore, the known increased risk of iatrogenic complications, functional decline, and delirium [8], [9], [10] when frail, elderly people are admitted to general hospitals is likely to be aggravated in patients with DLB. Neuropsychiatric symptoms, as well as fluctuations mimicking delirium, are frequent in DLB [11], [12] and may deteriorate in the unfamiliar general hospital setting, increasing the likelihood of neuroleptic use and sensitivity reactions [13]. Several studies have reported a relatively poor prognosis in DLB, including earlier mortality and nursing home admission [14], [15] and higher health-care costs [16], [17]. Yet, hospitalization has, to our knowledge, only been evaluated in the context of small scale analyses focusing on economic impact [16], [18], [19].
Our aim was to investigate the rate, number, duration, cost, and risk factors of hospitalization in patients with DLB and compare these with a matched AD cohort and the general elderly population. Such knowledge may inform health-planners to target care and interventions to lower hospitalization risk, thereby benefiting patients and caregivers as well as reducing costs.
2. Methods
2.1. Data source
Data for this study were obtained from the South London and Maudsley NHS Foundation Trust (SLaM) Biomedical Research Centre Clinical Record Interactive Search (CRIS) application. SLaM is one of the Europe's largest mental health and dementia care providers, serving a geographic catchment of four South London boroughs (Lambeth, Lewisham, Southwark, and Croydon) with a population of over 1.2 million residents. In 2007–2008, the CRIS application was developed with NIHR funding to provide researchers access to anonymized copies of SLaM's electronic health record within a robust governance framework [20], [21]. CRIS has received ethical approval as an anonymized data resource (Oxford Research Ethics Committee C, reference 08/H0606/71+5), and a number of data linkages have been set up, including to the national hospitalization data [22] used in this analysis. In addition to variables which can be extracted from compulsory structured fields in the source record, a range of bespoke natural language processing algorithms using General Architecture for Text Engineering software (https://gate.ac.uk) have allowed the extraction of entities recorded in text fields [21], [23]. Development (p.4–6), performance (p.8–11), and strengths and limitations (p.10–12) of using the General Architecture for Text Engineering software in CRIS have been described by Perera et al. [21].
2.2. Study cohort
We used CRIS to extract a sample of AD and DLB cases diagnosed in SLaM services between January 1, 2006 and March 31, 2013. Date of the first dementia diagnosis served as index date. Diagnosis of AD was classified according to the International Classification of Diseases, Tenth Revision (ICD-10) codes [24]. DLB cases were identified using the General Architecture for Text Engineering natural language processing software [21], which extracted any text strings associated with a diagnosis statement of Lewy body dementia or disease.
To assess the performance of the electronic case identification, a subset of 69 cases identified through the natural language processing software were examined by three clinicians with an experience of the diagnosis of DLB. In a consensus meeting with two senior experts, the raters ascertained the “research diagnoses” (applying McKeith criteria [25]) of probable DLB, possible DLB, dementia in Parkinson's disease, or no Lewy body disease (LBD). Of the 69 electronically identified cases, “research diagnoses” were probable DLB (59.5%, n = 41), possible DLB (18.8%, n = 13), and Parkinson's disease dementia (17.4%, n = 12). Only three case records (4.3%) contained false positives (no LBD).
We matched DLB and randomly selected AD cases on a 1:4 ratio according to gender, age, and Mini–Mental State Examination (MMSE) [26] score categories at dementia diagnosis. Age was categorized in 5-year bands from 55-59 to 95+ years. MMSE groups were 0–9, 10–14, 15–19, 20–24, and 25–30 score points at the time of first dementia diagnosis. DLB cases and AD controls were only included if data on all three matching variables were available.
Population level data were acquired from the publicly available Admitted Inpatient Care data set of the Hospital Episode Statistics (HES) for England [22]. We were able to ascertain the number of hospitalizations in the source population from 2006–2013 in the available age bands of 60–74 and 75+ years. We calculated age-band specific standardized admission ratios for patients with DLB and AD within those age categories. Of note, these data sets represent the unselected general elderly population and thereby include people with dementia.
2.3. Outcomes
General hospital admissions (excluding admissions to psychiatric units) prior and after dementia diagnosis were extracted from the HES data [22]. The HES database records all admissions to all the National Health Service hospitals in England and includes diagnoses and procedures. Within the National Health Service, HES allows tracking of patients between different hospitals and across different years. These also allow distinction between elective (planned) and emergency (unplanned) admissions. We extracted the primary discharge diagnosis and determined days spent in hospital, and whether an admission was planned or unplanned. Two analytic approaches were adopted: (1) to reduce competing mortality effects, we ascertained and modeled number and days of hospitalization in a 1-year window around dementia diagnosis; (2) not limiting follow-up, we further calculated time to first hospitalization after the index date. HES data were available up to March 31, 2013 at the time of analysis, and either this date or the date of death served as cut-off date.
To determine hospitalization costs, we extracted healthcare resource groups (HRGs) from the HES database. Hospitals in England are funded through a prospective payment system, whereby payments are defined through HRGs which are allocated to each patient. HRG allocation is determined by age of the patient, primary diagnosis, procedures received (if any), and the level of complications [27]. Payments for each HRG are adjusted regularly, and we determined the cost incurred by each patient in the year according to the HRG version valid for the respective time period.
2.4. Covariates
Other variables extracted were demographic factors including ethnicity, marital status, a neighborhood-level index of socioeconomic deprivation [28], and data from the Health of the Nation Outcome Scales (HoNOS65+) instrument. This is a validated standard measure of patient well-being and functioning used in the UK mental health services [29], [30]. HoNOS65+ subscales are each rated 0 (no problem) to 4 (severe or very severe problem) and to ease interpretation, we dichotomized these subscale scores to “minor or no problem” (0–1) and “mild to severe problems” (2–4). Subscales included the “problems related to physical illness and disability scale”, used in this analysis as a general measure of physical well-being and multi-morbidity.
2.5. Statistical analysis
We used STATA 13 software (Stata Corp LP, College Station, TX). Baseline differences between the AD and DLB cohort were assessed using t-tests for continuous parametric data, the Wilcoxon rank sum test for nonparametric data, and chi-squared test for categorical variables. To compare the number of hospitalizations in the year after dementia diagnosis to the general population in the catchment area, we calculated age-specific indirectly standardized hospitalization ratios according to age bands (60–74 and 75+ years). We further assessed the differences in planned and unplanned admissions as well as hospital days between the two dementia cohorts using fixed-effect Poisson regression models (reporting results as incidence rate ratios comparing rates of admission or hospital days in the DLB with the AD group) and stratified Cox regression models. Both were adjusted for sociodemographic factors, mental and physical comorbidities, and functional status. Finally, we ascertained primary (ICD-10) discharge diagnoses grouped into disease categories, comparing incidence rates using the mid-P exact test [31] and corrected this analysis for multiple comparisons using the Benjamini–Hochberg false discovery rate procedure [32].
3. Results
Overall, we identified 10,159 cases with a dementia diagnosis during the observation period, 6300 of whom had a diagnosis of AD. We identified 200 patients diagnosed with DLB; of these, six were excluded as no MMSE score was recorded at the time of dementia diagnosis. The remaining 194 DLB cases were matched to 776 with an AD diagnosis according to age, gender, and MMSE score groups.
3.1. Baseline characteristics
Characteristics of the DLB and AD groups at the time of dementia diagnosis are presented in Table 1. The groups did not differ significantly on sociodemographic variables such as ethnicity, deprivation score, or marital status. In addition to the defining characteristics as hallucinations and/or delusions, the DLB cohort had an adverse neuropsychiatric and functional profile, with a significantly higher occurrence of problems due to aggression, self-injury, and depressed mood, as well as problems with activities of daily living, occupational and recreational activities, and social relationships. A significantly higher percentage of patients with DLB suffered from physical health problems on the HoNOS65+ “physical illness and disability scale” at the time of dementia diagnosis. As demonstrated in Table 1, patients with DLB had a higher number of hospital admissions, hospital days, and hospitalization costs in the year before dementia diagnosis.
Table 1.
Demographics and baseline characteristics
| Risk factors | DLB cohort (n = 194) | AD cohort (n = 776) | P value∗ |
|---|---|---|---|
| Sociodemographic status and cognitive function† | |||
| Mean age at dementia diagnosis (95% CI)‡ | 79.9 (78.8–81.0) | 80.3 (79.7–80.8) | .560 |
| Female gender (%)‡ | 50.0 | 50.0 | 1.000 |
| Nonwhite ethnicity (%) | 22.2 | 20.9 | .694 |
| Married or cohabiting status (%) | 43.1 | 41.4 | .648 |
| Mean index of deprivation (95% CI) | 26.6 (25.0–28.1) | 26.2 (25.4–27.0) | .618 |
| Mean MMSE score at diagnosis (95% CI)‡ | 19.2 (18.3–20.1) | 18.7 (18.2–19.1) | .307 |
| HoNOS65+ symptoms/disorders (%)§ | |||
| Overactive, aggressive behavior | 38.0 | 17.7 | <.001 |
| Nonaccidental self-injury | 3.1 | 0.7 | .005 |
| Problem-drinking or drug taking | 2.1 | 4.2 | .167 |
| Hallucinations and/or delusions | 59.2 | 10.0 | <.001 |
| Depressed mood | 22.9 | 11.4 | <.001 |
| HoNOS65+ functional problems (%)§ | |||
| Activities of daily living | 63.4 | 52.3 | .001 |
| Living conditions | 10.9 | 10.9 | .973 |
| Occupational and recreational activities | 37.0 | 28.2 | .019 |
| Social relationships | 25.0 | 15.1 | <.001 |
| HoNOS65+ physical illness or disability (%)§ | 64.1 | 38.1 | <.001 |
| Hospitalization in the year before dementia diagnosis | |||
| Any hospitalization (%) | 59.3 | 40.3 | <.001 |
| Emergency hospitalization (%) | 51.6 | 31.7 | .009 |
| Mean number of hospitalizations (95% CI, n = total number) | 1.49 (1.32–1.67; n = 289) | 0.78 (0.72–0.85; n = 606) | <.001 |
| Mean number of emergency hospitalizations (95% CI, n = total number) | 1.17 (1.02–1.33; n = 226) | 0.58 (0.53–0.63; n = 448) | <.001 |
| Mean number of days spent in hospital (95% CI, n = total number) | 14.51 (13.98–15.06; n = 2815) | 6.58 (6.40–6.76; n = 5104) | <.001 |
| Mean number of £s/$s incurred due to hospitalization (95% CI, n = total number) | £3290 (£2366–£4213; n = £611,868)/$4195 ($3017–$5372; n = $780,217) | £1893 (£1564–£2222; n = 1,469,139)/$2345 ($1995–$2834; n = $1,873,357) | <.001 |
Abbreviations: DLB, dementia with Lewy bodies; AD, Alzheimer's disease; CI, confidence interval; MMSE, Mini–Mental State Examination; HoNOS65+, Health of the Nation Outcome Scales.
t-test (age), Wilcoxon rank sum (MMSE, index of deprivation), or χ2 test (categorical variables).
At the time of index dementia diagnosis.
Cohorts matched on categories of these variables.
Whether problem present at dementia diagnosis.
3.2. Hospitalization after dementia diagnosis
Mean follow-up time available for patients with DLB was 10.1 months and for patients with AD 11.3 months; 164 patients with DLB contributed 162.5 person-years follow-up time and 776 patients with AD 733.1 person-years. In this time period, 52.6% (102) of patients with DLB and 42.9% (333) of patients with AD had at least one admission to a general hospital. Mean numbers of planned and unplanned hospitalizations and hospital days within the first year after dementia diagnosis are presented in Table 2. Patients with DLB had significantly more hospitalizations, unplanned admissions, and days in hospital than their AD counterparts. No significant differences were detected in planned admissions.
Table 2.
Number of admissions and hospital days in the year after dementia diagnosis in DLB and AD cohorts
| Outcome | Mean number of admissions per person-year (95% CIs; n = total number of admissions) |
Mean number of hospital days per person-year (95% CIs; n = total number of hospital days) |
||
|---|---|---|---|---|
| DLB | AD | DLB | AD | |
| All hospital admissions/days | 1.31 (1.14–1.50; n = 213) | 0.86 (0.80–0.93; n = 634) | 10.80 (10.30–11.31; n = 1755) | 6.92 (6.73–7.11; n = 5073) |
| Planned admissions | 0.29 (0.22–0.38; n = 47) | 0.20 (0.17–0.23; n = 145) | ||
| Unplanned admissions | 1.02 (0.87–1.19; n = 166) | 0.67 (0.61–0.73; n = 489) | ||
Abbreviations: DLB, dementia with Lewy bodies; AD, Alzheimer's disease; CI, confidence interval.
Indirectly, standardized admission ratios showed differences between the general population and the two patient groups. The admission ratio for patients with DLB was higher than the general population (1.22 [95% confidence interval {CI}: 1.06–1.39]), whereas it was lower in the AD cohort (0.91 [95% CI: 0.84–0.99]).
3.3. Comparison of rates of hospital admissions and hospital days in the first year after dementia diagnosis
Unadjusted Poisson regression models for the year following dementia diagnosis confirmed that patients with DLB had significantly more hospital admissions, unplanned hospitalizations, and hospital days than patients with AD (see Table 3). However, differences in crude incidence rate ratios for hospital admissions were explained through adjusting for physical health problems at diagnosis and previous hospitalizations. A higher count for hospital days was detected for the AD group when regression models were adjusted for sociodemographic factors and HoNOS65+ mental health and functional problem scores in addition to accounting for physical illness. This reversed direction of association was only present when the HoNOS65+ “hallucinations and/or delusions” subscale was included in the model, and the strongest effect was detected in a Poisson regression model adjusting solely for physical illness score, previous hospitalization, and the HoNOS65+ “hallucinations and delusions” subscale (incidence rate ratio = 0.81; 95% CI 0.75–0.87).
Table 3.
Fixed-effects Poisson regression models comparing rates of admission/hospital days and stratified Cox regression models assessing risk of first hospitalization in DLB compared with AD
| Outcome |
Within the first year after dementia diagnosis |
Any time |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All hospital admissions |
Planned admissions |
Unplanned admissions |
Days in hospital |
First hospitalization |
||||||
| Model | IRR (95% CI) | P value | IRR (95% CI) | P value | IRR (95% CI) | P value | IRR (95% CI) | P value | HR (95% CI) | P value |
| Unadjusted | 1.50 (1.28–1.75) | <.001 | 1.43 (1.02–2.00) | .035 | 1.51 (1.27–1.81) | .001 | 1.57 (1.48–1.66) | <.001 | 1.76 (1.43–2.17) | <.001 |
| Adjusted for HoNOS65+ physical illness score and hospitalization in year before dementia diagnosis | 1.12 (0.94–1.33) | .218 | 1.18 (0.82–1.68) | .373 | 1.13 (0.93–1.38) | .224 | 1.03 (0.97–1.10) | .304 | 1.46 (1.17–1.83) | .001 |
| Adjusted for adjusted for the above and ethnicity, marital status, deprivation score, and HoNOS65+ mental health and functional problem scores | 1.07 (0.85–1.35) | .549 | 1.10 (0.70–1.72) | .676 | 1.06 (0.80–1.39) | .687 | 0.88 (0.81–0.96) | .006 | 1.46 (1.10–1.94) | .009 |
NOTE. Bold text indicates P < .05
Abbreviations: DLB, dementia with Lewy bodies; AD, Alzheimer's disease; CI, confidence interval; HoNOS65+, Health of the Nation Outcome Scales; IRR, incident rate ratio; HR, hazard ratio.
3.4. Assessment of time to first hospitalization after dementia diagnosis
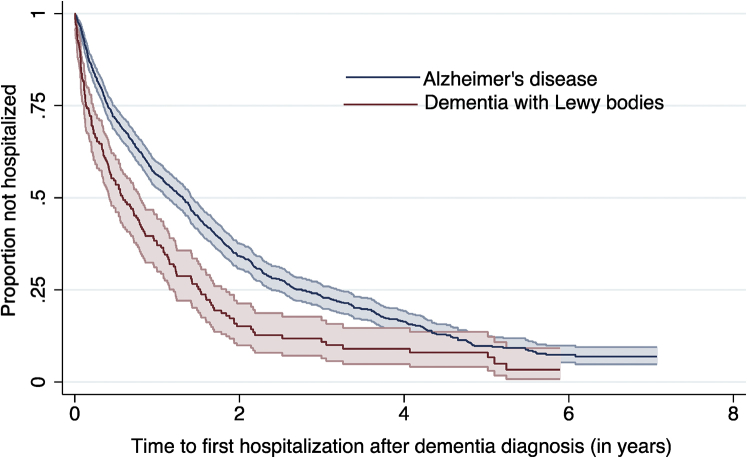
Mean time to first hospitalization after dementia diagnosis was 0.89 (standard deviation 1.15) years in the DLB group and 1.67 (standard deviation 1.62) years in the AD group. Kaplan–Meier curves illustrating time to first hospitalization after dementia diagnosis are presented in Figure 1. A higher risk of hospitalization in the DLB group was confirmed by the log-rank test (log-rank χ2 = 32.34, P < .001). An unadjusted fixed-effects Cox regression model showed a 76% increased hazard of hospitalization in DLB compared with AD. After adjusting for previous admissions and physical health problems, this association was attenuated to 46%, but remained significant. Further adjustments for sociodemographic factors, mental health, and functional problems did not substantially affect the strength of the association (see Table 3).
Fig. 1.
Kaplan–Meier curve illustrating time to first hospitalization after dementia diagnosis.
3.5. Discharge diagnoses coded following admissions
The most common primary discharge diagnoses were grouped into disease categories, and numbers of hospital admissions due to these categories are presented in Table 4. The two most common causes of admissions in both groups were infections and falls/fractures, followed by circulatory disease. Using the Mid-P exact test to compare incidence rates between groups, infections, dementia, anemia, and Parkinson's disease were significantly more often recorded as primary causes of admission in the DLB group. After adjusting for multiple comparisons, only infections (P = .012) and dementia (P = .012) remained significant. No differences were detected, for example, in falls/fractures, circulatory illness or ICD-10 codes indicating autonomic dysfunction.
Table 4.
Primary discharge diagnoses
| Primary cause of admission | ICD-10 codes used | DLB |
AD |
P value∗ | Corrected P value† |
|---|---|---|---|---|---|
| Percentage of total hospital admissions (total = 213) (n = number of admissions due to specific cause) | Percentage of total hospital admissions (total = 634) (n = number of admissions due to specific cause) | ||||
| Infections | A41, A48, J18, J22, J44, J69, L03, N30, N39 | 23.0% (n = 49) | 19.9% (n = 126) | .001 | .012 |
| Falls and fall-related injuries | R55, S00, S01, S02, S09, S52, S62, S72 | 7.0% (n = 15) | 10.1% (n = 64) | .825 | .900 |
| Circulatory illness | I20, I21, I25, I44, I47, I48, I49, I50, R00, R07 | 6.6% (n = 14) | 6.5% (n = 41) | .174 | .261 |
| Dementia | F01, F03, G30, G31, R41 | 6.1% (n = 13) | 2.7% (n = 17) | .002 | .012 |
| Senility and disorientation | R41, R54 | 5.2% (n = 11) | 4.7% (n = 30) | .166 | .261 |
| Stroke and TIA | G45, I61, I63, I64, I67 | 3.8% (n = 8) | 2.5% (n = 16) | .075 | .178 |
| Cancer | C18, C25, C34, C44, C50, C54, C61, C67, C78, C79, C92, D07 | 3.3% (n = 7) | 5.1% (n = 32) | 1.000 | 1.000 |
| Cataract | H25, H26 | 3.3% (n = 7) | 3.6% (n = 23) | .459 | .551 |
| Autonomic dysfunction | I95, K59, R33, R42 | 3.3% (n = 7) | 3.5% (n = 22) | .406 | .541 |
| Anemia | D50, D51, D52, D64 | 3.3% (n = 7) | 1.4% (n = 9) | .020 | .060 |
| Parkinson's Disease | G20 | 1.9% (n = 4) | 0.3% (n = 2) | .013 | .052 |
| Delirium | F05 | 1.4% (n = 3) | 0.5% (n = 3) | .089 | .178 |
NOTE. Bold text indicates P < .05
Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision; DLB, dementia with Lewy bodies; AD, Alzheimer's disease.
Comparison of incidence rates (number of hospitalizations per person-year) using the Mid-P exact test.
P value corrected for multiple comparisons using the Benjamini–Hochberg procedure.
3.6. Hospitalization costs in the first year after dementia diagnosis
Based on HRGs assigned to patients during their hospital stays, patients with DLB incurred hospitalization costs of £2896 (95% CI £2887–£2904)/$3692 (95% CI $3681–$3703) per person-year and patients with AD £2453 (95% CI £2450–£2457)/$3128 (95% CI $3124–$3133).
4. Discussion
Patients with DLB were more frequently admitted to acute hospitals than the general population. During the year before and after dementia diagnosis, patients with DLB had more frequent and longer hospitalizations than patients with AD, which seemed to be driven by poor physical health and neuropsychiatric symptoms. While patients with DLB had almost four hospital days more per person-year than patients with AD, this difference was hypothetically reversed when the presence/severity of hallucinations and/or delusions was included in the regression model.
Main discharge diagnoses were infections and falls/fractures in both groups. On average, patients with DLB incurred more than £400/$550 in hospitalization costs compared to patients with AD in the year after dementia diagnosis.
Despite the relatively high prevalence [7] and poor prognosis of DLB, the condition has only attracted a fraction of dementia research funding [12]. DLB is underdiagnosed in the UK, US, and other high-income countries, and there is a lack of specialist services for this high-risk patient group [12], [13], [33]. Our study suggests that the estimated 80,000 people with DLB in the UK will have an excess of more than 27,000 hospitalizations, spend over 300,000 days more in hospital, and incur more than £35 million/$45 million additional hospitalization costs in a single year than the same number of patients with AD. It is possible that improved diagnosis, more careful monitoring, better information for patients, caregivers, and the primary care health system, combined with assertive management of the cognitive, psychiatric, and physical symptoms according to recently updated guidelines [34] may substantially reduce the risk and duration of hospitalizations in these patients. This would benefit patients, their families and could lead to substantial cost savings but needs to be demonstrated in carefully designed studies using complex interventions. Some of these are already on-going, as the DIAMOND-Lewy research programme, which aims to improve recognition and proactive management of Lewy body dementias through development and implementation of assessment and management toolkits [35], [36], and the Study of HAllucinations in Parkinson's disease, Eye disease, and Dementia, which specifically targets visual hallucinations, their longitudinal effects on patients and caregivers, and aims pilot management strategies for these symptoms [37]. However, it is likely that improved diagnosis and management will come with added costs. For example, in patients with possible DLB, who are presenting with only one of the four core features, establishing a correct subtype diagnosis relies on costly biomarkers as scans measuring dopamine uptake, myocardial scintigraphy, or polysomnography to detect rapid eye movement (REM) sleep behavior disorder [34]. It nevertheless appears reasonable to believe that complex interventions, as providing a more holistic support for patients with DLB, can reduce hospitalization and thus offset the added costs related to these diagnostic procedures. One likely benefit of having an established diagnosis that can be concluded directly from this analysis is that a more DLB-focused approach to hallucinations can reduce the length of stay. Although there are yet no robust evidence for how hallucinations in DLB can be treated, there is some indication that cholinesterase inhibitors may be beneficial [34], [38]. Clozapine [39] or pimavanserin [40], which have proven efficacy for psychotic symptoms and tolerability in Parkinson's disease, may be useful in patients with DLB. Of note, it is important to avoid antipsychotics due to the risk of a severe hypersensitivity syndrome [41] or at least restrict the use to only severely ill patients, with low dosage and in short periods. In addition, more assertive input from liaison psychiatry services for general hospitals may potentially reduce the duration of stay. Again, these hypotheses need to be tested in systematic trials.
Previous studies examining rates of hospitalization after diagnosis of DLB are scarce, with small sample sizes [16], [18], and hospital admission has only been one of several cofactors in economic analyses. A US economic evaluation found that patients with DLB had an average 10.3 days of hospital care per year, which was significantly higher than 1.6 days of hospital care per year in the AD control group [18]. In contrast, a Scandinavian study found that patients with AD used more inpatient care (6.1 vs. 4.1 hospital days per year) [16]. In a 5-year follow-up study, patients with DLB discontinued to visit a Japanese memory clinic significantly more often due to hospitalization or death than patients with AD (30% vs. 14%) [19]. Our study is able to provide more detail, and to our knowledge, we assembled one of the largest samples of patients with DLB drawn from a single-dementia assessment and treatment provider. Furthermore, the use of national hospitalization statistics guarantees near-complete outcome data, and in regression models, we were able to adjust for a range of potential clinical, social, and demographic confounders.
Although general patterns of increased hospitalization in dementia have consistently been reported from several countries with varying systems of health-care organization and financing, the exact numbers of bed days vary widely between studies, from 22 to 25 days per person-year in AD in Finland [2] to 13.8 in the US [42] or 10.7 in Taiwan [43]. In our AD cohort, admission rates and bed days (6.9 days per person-year) were lower than in US and Finnish data [6], [44], and the indirectly standardized admission ratios were slightly lower than the general elderly population in our catchment area. One explanation is that the unselected general population sample used in our analysis included people suffering from dementia, including DLB, while the aforementioned studies had compared patients with AD with cognitively healthy controls. It might further reflect a higher threshold for hospital admission in the UK or a greater focus on outpatient care in the UK health-care system. The higher number of bed days in the DLB cohort further highlights the increased use of scarce resource in this group.
As reported in previous studies [5], [19], [44], infections were the most frequently reported causes of admission, both in the AD and DLB cohort. Similar to our study, one Japanese study [19] found that infections, particularly bronchopneumonia, were more common causes for hospitalization in patients with DLB than AD. This might be related to the lower level of functioning and higher support needs in patients with DLB earlier in the disease course [17]. Surprisingly, the occurrence of falls and fall-related injuries didn't differ between the groups, given that these are a supportive clinical feature of DLB [25]. We were not able to determine the nature of the falls, although a Swedish study [45] also showed no difference in hospitalization due to syncope between the two dementia subtypes. Behavioral/neuropsychiatric symptoms have been reported as leading causes for hospitalization in AD [46] and as increased in patients with DLB [11], [12] potentially underlying our observed increased frequency of dementia recorded as the primary discharge diagnosis in DLB; furthermore, while the presence of hallucinations and/or delusions did not significantly affect the risk of hospitalization, it explained the higher number of days spent in hospital in patients with DLB.
In our study, the limitations of using routine electronic health record data need to be considered. Diagnoses of dementia are clinical and not autopsy confirmed, and a considerable proportion of DLB cases are likely to be undetected or classified as AD. Furthermore, patients with AD might suffer from other or additional pathologies. Conversely, 19% of our test cases with a clinician diagnosis of DLB were classified as “possible DLB” by expert raters. Although this is partly due to incomplete recording of symptomatology in the electronic records, some of these patients might not suffer from LBD at autopsy. Most “possible DLB” cases without DLB will have AD, and thus the inclusion of such cases is likely to reduce the difference between AD and DLB. Overall, although exact estimates need to be treated with caution, we are more likely to underestimate rather than to overestimate the burden of DLB. Furthermore, a clear distinction between DLB and dementia in Parkinson's disease was not always possible; however, the two conditions share the same underlying pathology, and once dementia develops, there are no clinical or biological markers to reliably differentiate the two Lewy body dementias [25]. Importantly, ours was an observational study, and although following matching no significant differences in demographics persisted in the two cohorts, residual confounding can never be fully excluded.
Physical health was captured through the HoNOS65+ physical illness and disability subscale. Although the scale is widely used as a routine measure of clinical outcome in mental health and dementia services in the UK [30] and has been shown to be a reliable predictor of mortality [47], it is relatively brief without details on the specific conditions determining its score. Furthermore, hospitalization data, captured from HES [22], describes the reason for hospitalization through an ICD-10 code recorded at discharge and cannot always accurately explain why the individual admission occurred. For example, a diagnosis of dementia might refer to an episode of worsening neuropsychiatric symptoms but could also be related to a general deterioration in health or social circumstances.
Although it is well established that the underlying neurodegenerative process begins years before a formal dementia diagnosis is made [48], data on hospitalization before dementia diagnosis needs to be evaluated with caution. Only patients surviving until a dementia diagnosis will be able to enter the cohort and this immortal time bias limits the generalizability of predementia diagnosis estimates presented in the baseline demographics section.
Data were collected from the UK mental health and dementia services as well as general hospitals over a time-span of 7 years from 2006 to 2013. Although the diagnostic criteria for DLB [25] have not altered over this period, changes have taken place to the way dementia and inpatient care is delivered in the UK, which might affect our results. We have excluded psychiatric hospital admissions because inpatient psychiatric for the elderly care varies substantially between different regions in the UK and has undergone considerable structural changes in the studied period. A sizeable reduction of psychiatric inpatient beds has led to inpatient care only being provided to those with highest complexity [49]. Although our results should be a representative of the source population in South East London, generalizations to other systems of health-care delivery, particularly beyond the UK context, cannot readily be made.
To overcome some of these limitations, a prospective study design could be used. Improved diagnostic criteria [34] and increased awareness of DLB could improve recruitment into prospective cohorts. Furthermore, recently, several consortia, as the pan-European consortium on DLB [50] or the Dementia with Lewy Bodies Consortium in the US [51], have formed to collect data on larger scale multi-center samples. There is also an increasing literature on the prodromal stages in DLB [52] with delirium being proposed as a presenting feature of DLB [53]. Trials could evaluate if more proactive identification of DLB in patients admitted to general hospitals with delirium can lead to improved long-term outcomes.
To conclude, we report increased and prolonged admissions to general hospitals in DLB and suggest that trials with complex interventions, involving specialized services with input from differing medical specialties (neurology, geriatrics, and psychiatry) and specific case management, should be conducted to test whether hospitalizations in DLB can be reduced.
Research in Context.
-
1.
Systematic review: We reviewed the literature using PubMed and further examined references from the identified studies. Several studies have investigated rates, duration, and cost implications of hospitalization in patients with Alzheimer's disease. Despite dementia with Lewy bodies being the second most common form of neurodegenerative dementia, hospitalization has only been evaluated as one of several outcomes in small economic studies.
-
2.
Interpretation: We found that patients with dementia with Lewy bodies are more frequently admitted to general hospitals, have a longer length of stay, and generate higher costs than patients with Alzheimer's disease. Main trigger for hospitalization appears to be poor physical health, while length of stay seems to be determined by the presence of neuropsychiatric symptoms.
-
3.
Future research: Further investigation is needed to identify interventions to address triggers for hospitalization early and to develop strategies to reduce stay.
Acknowledgments
C.M., G.P., and R.S. receive salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Conflict of interest: J.T.O'B. has acted as a consultant for GE Healthcare, Cytox, TauRx, Axona, Piramal, and Lilly and has received grants from Avid (Lilly). C.B. has received honoraria and grant funding from Acadia pharmaceuticals, Lundbeck, Takeda, Axovant, Lilly, Otsuka, and Orion pharmaceutical companies. R.S. has received research funding from Roche, Pfizer, Janssen, Lundbeck, and In-Silico-Bioscience. D.A. has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, and GE Health, and serves as paid consultant for H. Lundbeck and Axovant.
References
- 1.Zhao Y., Kuo T.C., Weir S., Kramer M.S., Ash A.S. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer's. BMC Health Serv Res. 2008;8:108. doi: 10.1186/1472-6963-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taipale H., Purhonen M., Tolppanen A.M., Tanskanen A., Tiihonen J., Hartikainen S. Hospital care and drug costs from five years before until two years after the diagnosis of Alzheimer's disease in a Finnish nationwide cohort. Scand J Public Health. 2016;44:150–158. doi: 10.1177/1403494815614705. [DOI] [PubMed] [Google Scholar]
- 3.Phelan E.A., Borson S., Grothaus L., Balch S., Larson E.B. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin P.J., Fillit H.M., Cohen J.T., Neumann P.J. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer's disease and related disorders. Alzheimers Dement. 2013;9:30–38. doi: 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Natalwala A., Potluri R., Uppal H., Heun R. Reasons for hospital admissions in dementia patients in Birmingham, UK, during 2002-2007. Dement Geriatr Cogn Disord. 2008;26:499–505. doi: 10.1159/000171044. [DOI] [PubMed] [Google Scholar]
- 6.Lyketsos C.G., Sheppard J.M., Rabins P.V. Dementia in elderly persons in a general hospital. Am J Psychiatry. 2000;157:704–707. doi: 10.1176/appi.ajp.157.5.704. [DOI] [PubMed] [Google Scholar]
- 7.Vann Jones S.A., O'Brien J.T. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673–683. doi: 10.1017/S0033291713000494. [DOI] [PubMed] [Google Scholar]
- 8.Lefevre F., Feinglass J., Potts S., Soglin L., Yarnold P., Martin G.J. Iatrogenic complications in high-risk, elderly patients. Arch Intern Med. 1992;152:2074–2080. [PubMed] [Google Scholar]
- 9.Covinsky K.E., Palmer R.M., Fortinsky R.H., Counsell S.R., Stewart A.L., Kresevic D. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 10.Inouye S.K. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 11.Bjoerke-Bertheussen J., Ehrt U., Rongve A., Ballard C., Aarsland D. Neuropsychiatric symptoms in mild dementia with lewy bodies and Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;34:1–6. doi: 10.1159/000339590. [DOI] [PubMed] [Google Scholar]
- 12.Mueller C., Ballard C., Corbett A., Aarsland D. The prognosis of dementia with Lewy bodies. Lancet Neurol. 2017;16:390–398. doi: 10.1016/S1474-4422(17)30074-1. [DOI] [PubMed] [Google Scholar]
- 13.Zweig Y.R., Galvin J.E. Lewy body dementia: the impact on patients and caregivers. Alzheimers Res Ther. 2014;6:21. doi: 10.1186/alzrt251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oesterhus R., Soennesyn H., Rongve A., Ballard C., Aarsland D., Vossius C. Long-term mortality in a cohort of home-dwelling elderly with mild Alzheimer's disease and Lewy body dementia. Dement Geriatr Cogn Disord. 2014;38:161–169. doi: 10.1159/000358051. [DOI] [PubMed] [Google Scholar]
- 15.Rongve A., Vossius C., Nore S., Testad I., Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer's dementia. Int J Geriatr Psychiatry. 2014;29:392–398. doi: 10.1002/gps.4015. [DOI] [PubMed] [Google Scholar]
- 16.Bostrom F., Jonsson L., Minthon L., Londos E. Patients with Lewy body dementia use more resources than those with Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22:713–719. doi: 10.1002/gps.1738. [DOI] [PubMed] [Google Scholar]
- 17.Vossius C., Rongve A., Testad I., Wimo A., Aarsland D. The use and costs of formal care in newly diagnosed dementia: a three-year prospective follow-up study. Am J Geriatr Psychiatry. 2014;22:381–388. doi: 10.1016/j.jagp.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Murman D.L., Kuo S.B., Powell M.C., Colenda C.C. The impact of parkinsonism on costs of care in patients with AD and dementia with Lewy bodies. Neurology. 2003;61:944–949. doi: 10.1212/wnl.61.7.944. [DOI] [PubMed] [Google Scholar]
- 19.Hanyu H., Sato T., Hirao K., Kanetaka H., Sakurai H., Iwamoto T. Differences in clinical course between dementia with Lewy bodies and Alzheimer's disease. Eur J Neurol. 2009;16:212–217. doi: 10.1111/j.1468-1331.2008.02388.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart R., Soremekun M., Perera G., Broadbent M., Callard F., Denis M. The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: development and descriptive data. BMC Psychiatry. 2009;9:51. doi: 10.1186/1471-244X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera G., Broadbent M., Callard F., Chang C.K., Downs J., Dutta R. Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open. 2016;6:e008721. doi: 10.1136/bmjopen-2015-008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hospital Episode Statistics (HES). Available at: http://content.digital.nhs.uk/hes. Accessed March 3, 2017.
- 23.Cunningham H., Tablan V., Roberts A., Bontcheva K. Getting more out of biomedical documents with GATE's full lifecycle open source text analytics. Plos Comput Biol. 2013;9:e1002854. doi: 10.1371/journal.pcbi.1002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . 10th ed. Health Organization; (ICD-10). Geneva: 2009. International statistical classification of diseases and related health problems. [Google Scholar]
- 25.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 26.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Mason A., Ward P., Street A.D. Diagnosis-related groups in Europe: moving towards transparency, efficiency and quality in hospitals. In: Busse R., editor. England: the Healthcare Resource Group system. Open University Press; Maidenhead: 2011. pp. 197–220. [Google Scholar]
- 28.Noble M.M.D., Wilkinson K., Whitworth A., Barnes H., Dibben C. Communities and Local Government; London: 2008. The English indices of deprivation 2007. [Google Scholar]
- 29.Burns A., Beevor A., Lelliott P., Wing J., Blakey A., Orrell M. Health of the Nation Outcome Scales for elderly people (HoNOS 65+) Br J Psychiatry. 1999;174:424–427. doi: 10.1192/bjp.174.5.424. [DOI] [PubMed] [Google Scholar]
- 30.Pirkis J.E., Burgess P.M., Kirk P.K., Dodson S., Coombs T.J., Williamson M.K. A review of the psychometric properties of the Health of the Nation Outcome Scales (HoNOS) family of measures. Health Qual Life Outcomes. 2005;3:76. doi: 10.1186/1477-7525-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miettinen O., Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Ser B. 1995;57:289–300. [Google Scholar]
- 33.Palmqvist S., Hansson O., Minthon L., Londos E. Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer's disease with common cognitive tests. Int J Geriatr Psychiatry. 2009;24:1405–1412. doi: 10.1002/gps.2277. [DOI] [PubMed] [Google Scholar]
- 34.McKeith I.G., Boeve B.F., Dickson D.W., Halliday G., Taylor J.P., Weintraub D. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas A.J., Taylor J.P., McKeith I., Bamford C., Burn D., Allan L. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry. 2017;32:1280–1304. doi: 10.1002/gps.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newcastle University. DIAMOND-Lewy research programme. Available at: http://research.ncl.ac.uk/diamondlewy/. Accessed August 17, 2017.
- 37.King's College London. Study of HAllucinations in Parkinson's disease, Eye disease, and Dementia (SHAPED). Available at: https://www.kcl.ac.uk/ioppn/depts/oldage/research/Non-medication-studies/Other-Clinical-Disorders/Study-of-HAllucinations-in-Parkinsons-disease,-Eye-disease,-and-Dementia-(SHAPED.aspx. Accessed August 17, 2017.
- 38.Ikeda M., Mori E., Kosaka K., Iseki E., Hashimoto M., Matsukawa N. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: results from a 52-week, open-label, multicenter extension study. Dement Geriatr Cogn Disord. 2013;36:229–241. doi: 10.1159/000351672. [DOI] [PubMed] [Google Scholar]
- 39.Morgante L., Epifanio A., Spina E., Zappia M., Di Rosa A.E., Marconi R. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27:153–156. doi: 10.1097/01.wnf.0000136891.17006.ec. [DOI] [PubMed] [Google Scholar]
- 40.Cummings J., Isaacson S., Mills R., Williams H., Chi-Burris K., Corbett A. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 41.Walker Z., Possin K.L., Boeve B.F., Aarsland D. Lewy body dementias. Lancet. 2015;386:1683–1697. doi: 10.1016/S0140-6736(15)00462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu C.W., Cosentino S., Ornstein K., Gu Y., Andrews H., Stern Y. Use and cost of hospitalization in dementia: longitudinal results from a community-based study. Int J Geriatr Psychiatry. 2015;30:833–841. doi: 10.1002/gps.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung S.D., Liu S.P., Sheu J.J., Lin C.C., Lin H.C., Chen C.H. Increased healthcare service utilizations for patients with dementia: a population-based study. PLoS One. 2014;9:e105789. doi: 10.1371/journal.pone.0105789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolppanen A.M., Taipale H., Purmonen T., Koponen M., Soininen H., Hartikainen S. Hospital admissions, outpatient visits and healthcare costs of community-dwellers with Alzheimer's disease. Alzheimers Dement. 2015;11:955–963. doi: 10.1016/j.jalz.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Fereshtehnejad S.M., Damangir S., Cermakova P., Aarsland D., Eriksdotter M., Religa D. Comorbidity profile in dementia with Lewy bodies versus Alzheimer's disease: a linkage study between the Swedish Dementia Registry and the Swedish National Patient Registry. Alzheimers Res Ther. 2014;6:65. doi: 10.1186/s13195-014-0065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nourhashemi F., Andrieu S., Sastres N., Ducasse J.L., Lauque D., Sinclair A.J. Descriptive analysis of emergency hospital admissions of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15:21–25. doi: 10.1097/00002093-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Mueller C., Perera G., Hayes R.D., Shetty H., Stewart R. Associations of acetylcholinesterase inhibitor treatment with reduced mortality in Alzheimer's disease: a retrospective survival analysis. Age Ageing. 2018;47:88–94. doi: 10.1093/ageing/afx098. [DOI] [PubMed] [Google Scholar]
- 48.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinner G., Hillam J., Branton T., Ramakrishnan A. Royal College of Psychiatrists, Faculty of Old Age Psychiatry; London: 2011. Inpatient care for older people within adult mental health services. Faculty Report FR/OA/1. [Google Scholar]
- 50.Kramberger M.G., Auestad B., Garcia-Ptacek S., Abdelnour C., Olmo J.G., Walker Z. Long-Term Cognitive Decline in Dementia with Lewy Bodies in a Large Multicenter, International Cohort. J Alzheimers Dis. 2017;57:787–795. doi: 10.3233/JAD-161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkinson's Disease Biomarkers Programme. Dementia with Lewy Bodies Consortium (U01). Available at: https://pdbp.ninds.nih.gov/Dementia_with_Lewy_Bodies_Consortium_U01. Accessed August 17, 2017.
- 52.Sadiq D., Whitfield T., Lee L., Stevens T., Costafreda S., Walker Z. Prodromal dementia with Lewy bodies and prodromal Alzheimer's disease: a comparison of the cognitive and clinical profiles. J Alzheimers Dis. 2017;58:463–470. doi: 10.3233/JAD-161089. [DOI] [PubMed] [Google Scholar]
- 53.McKeith I., Taylor J.P., Thomas A., Donaghy P., Kane J. Revisiting DLB diagnosis: a consideration of prodromal DLB and of the diagnostic overlap with Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29:249–253. doi: 10.1177/0891988716656083. [DOI] [PubMed] [Google Scholar]