Abstract
Introduction
People with Down syndrome (DS) are at high risk for Alzheimer's disease (AD). Defects in monoamine neurotransmitter systems are implicated in DS and AD but have not been comprehensively studied in DS.
Methods
Noradrenaline, adrenaline, and their metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG); dopamine and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid; and serotonin and its metabolite 5-hydroxyindoleacetic acid were quantified in 15 brain regions of DS without AD (DS, n = 4), DS with AD (DS+AD, n = 17), early-onset AD (EOAD, n = 11) patients, and healthy non-DS controls (n = 10) in the general population. Moreover, monoaminergic concentrations were determined in cerebrospinal fluid (CSF)/plasma samples of DS (n = 37/149), DS with prodromal AD (DS+pAD, n = 13/36), and DS+AD (n = 18/40).
Results
In brain, noradrenergic and serotonergic compounds were overall reduced in DS+AD versus EOAD, while the dopaminergic system showed a bidirectional change. For DS versus non-DS controls, significantly decreased MHPG levels were noted in various brain regions, though to a lesser extent than for DS+AD versus EOAD. Apart from DOPAC, CSF/plasma concentrations were not altered between groups.
Discussion
Monoamine neurotransmitters and metabolites were evidently impacted in DS, DS+AD, and EOAD. DS and DS+AD presented a remarkably similar monoaminergic profile, possibly related to early deposition of amyloid pathology in DS. To confirm whether monoaminergic alterations are indeed due to early amyloid β accumulation, future avenues include positron emission tomography studies of monoaminergic neurotransmission in relation to amyloid deposition, as well as relating monoaminergic concentrations to CSF/plasma levels of amyloid β and tau within individuals.
Keywords: Alzheimer's disease, Cerebrospinal fluid, Dementia, Dopamine, Down syndrome, Monoamines, MHPG, Neurotransmitter, Noradrenaline, Plasma, Serotonin, Trisomy 21
Highlights
-
•
Monoaminergic changes are found in Alzheimer's disease (AD) and Down syndrome (DS).
-
•
We characterized 15 brain regions of DS, DS+AD, early-onset AD, and non-DS controls.
-
•
Noradrenergic and serotonergic compounds were reduced in DS+AD versus early-onset AD postmortem brain tissue.
-
•
DS and DS+AD cases presented rather similar monoaminergic profiles in postmortem brain tissue.
-
•
Monoaminergic CSF/plasma levels were not related to clinical dementia status in DS.
1. Introduction
People with Down syndrome (DS), or trisomy 21, have an exceptionally high risk to develop Alzheimer's disease (AD): 68%–80% of people are diagnosed with dementia by the age of 65 years [1]. The additional copy of chromosome 21, encoding the amyloid precursor protein (APP), causes overproduction of amyloid β (Aβ) peptides. Very early in life, intracellular Aβ accumulation takes place in neurons, followed by extracellular Aβ aggregation and subsequent deposition in characteristic Aβ plaques [2], [3], [4], [5]. In DS brains, not only plaques but also neurofibrillary tangles are omnipresent from the age of 40 years [6]. The onset of clinical dementia symptoms, however, is subject to a marked variation in time [7], [8]. Because the dementia diagnosis in DS is complex, among others due to comorbidities, pre-existing intellectual disability, and behavior [9], sensitive and specific biomarkers for AD in DS would be very valuable. In the general non-DS population, the so-called “AD profile” (low Aβ42, high total-tau, and high phosphorylated-tau) in cerebrospinal fluid (CSF) has proven useful as a diagnostic aid [10]. However, the clinical utility in DS has not been demonstrated yet [11]. Therefore, the study of alternative biomarkers for AD in DS receives vast attention.
In this context, we previously analyzed monoamine neurotransmitters and metabolites in serum of 151 elderly DS individuals with AD (DS+AD) and without AD (DS), but also in a nondemented DS group at blood sampling that developed dementia over time (converters). Remarkably, serum levels of the primary noradrenergic metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG) were strongly decreased in DS+AD, but also in converted DS individuals. Individuals with MHPG levels below median had a more than 10-fold increased risk of developing dementia, suggesting that decreased serum MHPG levels may be predictive for conversion to AD [12].
Blood biomarkers, however, are subject to (confounding) peripheral effects. CSF biomarkers are generally regarded better indicators of biochemical changes in the central nervous system because of their direct contact with the extracellular space [13]. Very few studies have investigated CSF biomarkers in (moreover small) DS cohorts [11], including two on monoamines [14], [15]. Although a few postmortem studies were conducted several decades ago, a comprehensive profile of central monoaminergic changes in DS+AD is not established yet. Indeed, monoamines were quantified in a limited number of brain regions from a few DS cases with often long postmortem delays (PMDs). For instance, cell loss in the locus coeruleus (LC), major source of noradrenaline (NA), and reduced NA concentrations have been reported in elderly DS cases [16], [17], [18], [19], [20], [21], [22], [23], but an integrated study of regional changes in NA, dopamine (DA), serotonin (5-HT), and their primary metabolites is lacking. Vermeiren et al., for example, investigated monoaminergic profiles in a variety of postmortem brain regions in early-onset AD patients (EOAD) compared with age- and gender-matched control subjects. In EOAD patients, lower levels of serotonergic compounds were found in amygdala and hippocampus, complemented by lower NA levels in the prefrontal cortex and amygdala. No differences in MHPG levels could be observed [24].
To the best of our knowledge, this study is the first to comprehensively evaluate monoaminergic alterations in (1) postmortem brain tissues and (2) (paired) CSF/plasma samples from DS individuals with and without AD. Noradrenergic (NA; adrenaline; MHPG), dopaminergic (DA; 3,4-dihydroxyphenylacetic acid [DOPAC]; homovanillic acid [HVA]), and serotonergic (5-HT; 5-hydroxyindoleacetic acid [5-HIAA]) compounds were quantified using reversed phase high-performance liquid chromatography (RP-HPLC). In one of the largest collections of DS brain tissue (n = 21), 15 regions of DS cases without and with a neuropathologically confirmed diagnosis of AD (DS and DS+AD, respectively) were analyzed and compared with EOAD patients and healthy controls in the general population. Second, we report the monoaminergic results in (paired) CSF/plasma samples obtained from the largest DS cohort to have undergone lumbar punctures, comparing DS without dementia (DS), DS with prodromal AD (DS+pAD), and DS with clinically diagnosed AD (DS+AD).
2. Materials and methods
2.1. Postmortem samples
2.1.1. Study population
In total, postmortem samples from 21 elderly DS individuals were obtained from the Netherlands Brain Bank (NBB), Netherlands Institute for Neuroscience (Amsterdam, The Netherlands), the Neurological Tissue Bank—Biobanc, Hospital Clinic Barcelona—Institut d'Investigacions Biomediques August Pi i Sunyer (IDIBAPS; Barcelona, Spain), and the Institute Born-Bunge (IBB; Antwerp, Belgium). Specifically, brain samples from nine DS+AD individuals were obtained from the NBB (open access: www.brainbank.nl). All material has been collected from donors for or from whom written informed consent for a brain autopsy and the use of the material and clinical information for research purposes had been obtained by the NBB. Moreover, the IDIBAPS provided samples of two DS and five DS+AD donors for whom written informed consent was obtained from the next of kin. The study was approved by the Hospital Clinic de Barcelona Ethics Committee and in accordance with Spanish legislation. Finally, the IBB provided samples of DS (n = 2), DS+AD (n = 3), EOAD patients (n = 11), and healthy controls without neurological disease (n = 10). Since DS+AD presents early in life, we identified EOAD patients and controls <75 years of age as comparison groups. Ethical approval was granted by the medical ethics committee of the Hospital Network Antwerp (ZNA, approval numbers 2805 and 2806). The study was compliant with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects.
2.1.2. Assessment of AD neuropathologic changes
Neuropathological analysis was conducted according to the “ABC scoring” system [25]. Formalin-fixed paraffin-embedded samples were sectioned in concordance with the minimally recommended brain regions (if available). If possible, additional sections of the cingulate gyrus, amygdala, pons at the level of the LC, and medulla oblongata were included. Applied stains were hematoxylin-eosin, cresyl violet, Klüver-Barrera (myelin), and modified Bielschowsky silver staining. Moreover, antibodies against amyloid (4G8), phosphorylated-tau (AT8), ubiquitin, TDP-43, and p62 Lck ligands were used. All cases were diagnosed by experienced neuropathologists (E.G., A.S., and J.-J.M.) as not, low, intermediate, or high AD neuropathologic changes. Intermediate and high signify the diagnosis of AD [25].
2.1.3. Regional brain samples and dissection
Table 1 shows the selection of frozen samples for RP-HPLC analyses. Brains were included in the three biobanks between 1990 and 2011 and stored at −80°C. Postmortem delays: NBB (<10 hours), IDIBAPS (<12 hours), and IBB (DS: 20 and 36 hours; DS+AD: 15 hours, 20 hours, and one unknown; and EOAD/controls: <7 hours). Samples were dissected from the left hemispheres (undefined hemisphere for three IBB cases). Not all regions were available for all cases. Most samples from EOAD and controls have been published before [24]. For this study, Brodmann area (BA)7, substantia nigra (SN), caudate nucleus, globus pallidus, and putamen were additionally analyzed.
Table 1.
Characteristics of postmortem study groups
| Characteristics | DS (n = 4) | DS+AD (n = 17) | EOAD (n = 11) | Controls (n = 10) | P value |
|---|---|---|---|---|---|
| Age at death in years (median; min.−max.) | 39.5 (35.0–44.0)a,b,c | 62.0 (44.0–80.0)a | 67.2 (57.6–73.0)b | 65.5 (57.2–73.3)c | .004 |
| Gender (N male and %) | 2 (50) | 5 (29.4) | 8 (72.7) | 6 (60) | n.s. |
| Psychoactive medication (yes/no/not reported) | 3/0/1 | 10/3/4 | 3/8/0 | 2/8/0 | .002 |
| Postmortem delay in hours (median; min.–max.) | 21.0 (11.5–36.0)a,b,c | 7.3 (3.8–20.0)a,b,e | 3.0 (2.8–7.0)b,d | 5.4 (2.3–7.0)c,e | <.001 |
| AD neuropathologic change | Low | High | Intermediate (1)/High (10) | Not (6)/Low (4) | |
| Available brain regions per study group | |||||
| Neocortex | |||||
| BA7: superior parietal lobule | 2 | 13 | 11 | 10 | |
| BA9/10/46: (pre)frontal cortex | 4 | 14 | 11 | 10 | |
| BA17: occipital pole (V1) | 3 | 7 | 11 | 10 | |
| BA22: superior temporal gyrus | 3 | 10 | 11 | 10 | |
| Limbic system | |||||
| Amygdala | 2 | 10 | 10 | 10 | |
| Hippocampus | 3 | 5 | 11 | 10 | |
| BA11/12: orbitofrontal cortex | 4 | 6 | 11 | 10 | |
| Cingulate gyrus | 3 | 8 | 11 | 10 | |
| Thalamus | 3 | 11 | 11 | 10 | |
| Basal ganglia | |||||
| Caudate nucleus | 3 | 16 | 11 | 10 | |
| Globus pallidus | 2 | 8 | 11 | 10 | |
| Putamen | 3 | 15 | 11 | 10 | |
| Substantia nigra | 2 | 14 | 11 | 10 | |
| Metencephalon | |||||
| Locus coeruleus (in pons) | – | 10 | 10 | 10 | |
| Cerebellar cortex | 2 | 9 | 10 | 10 | |
Abbreviations: AD, Alzheimer's disease; BA, Brodmann area; DS, Down syndrome without neuropathologic AD diagnosis; DS+AD, Down syndrome with neuropathologic AD diagnosis; EOAD, early-onset Alzheimer's disease; n.s., not significant.
NOTE. Gender and medication use were compared with Fisher's exact test. Kruskal-Wallis tests were performed to compare ages and postmortem delays between the groups. Post hoc Mann-Whitney U tests were performed to identify significant group differences (P < .015): (a) DS vs. DS+AD; (b) DS vs. EOAD; (c) DS vs. controls; (d) DS+AD vs. EOAD; (e) DS+AD vs. controls.
2.2. CSF/plasma samples
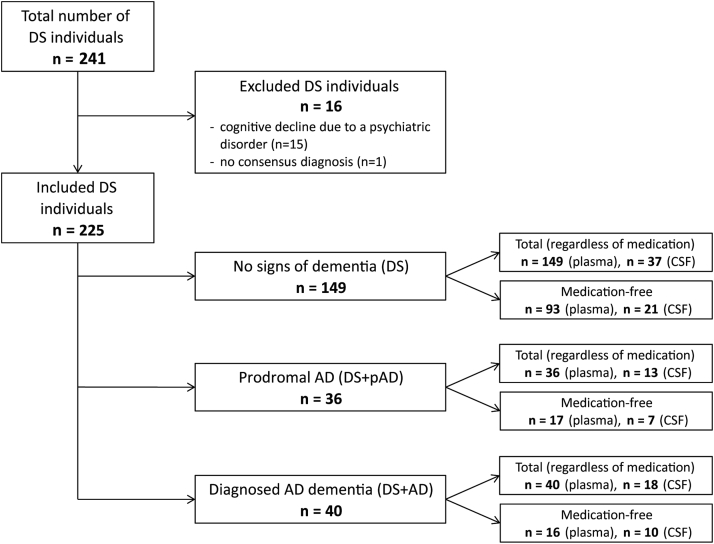
Samples of 241 DS adults were obtained from the Down Alzheimer Barcelona Neuroimaging Initiative study, a prospective biomarker study for AD in DS [26], [27], [28]. The person with DS and/or the legal representative provided written informed consent. The study was compliant with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and approved by the ethics committee of the Sant Pau hospital in Barcelona [27]. Neurologists and neuropsychologists established a consensus diagnosis of dementia, distinguishing between DS without dementia (DS), DS with prodromal AD (DS+pAD), and DS with diagnosed AD (DS+AD). Specifically, the DS group did not show evidence of cognitive decline. The DS+pAD group includes individuals who (1) presented cognitive/functional change but did not (yet) meet criteria for dementia or (2) showed significant cognitive decline in longitudinal assessment. The DS+AD group includes individuals with clear cognitive/functional change meeting the dementia criteria (IWG-2 [29]). In the diagnostic procedure, medical comorbidities and other possible causes of cognitive decline were assessed (differential diagnostics). DS cases with cognitive decline due to medical comorbidities or a psychiatric etiology were excluded (Fig. 1). Use of psychoactive medication around the moment of sampling was noted. Within the Down Alzheimer Barcelona Neuroimaging Initiative study, participants are offered a lumbar puncture, which was found to be feasible and safe [27]. For 68 individuals, paired CSF/plasma samples were obtained. The other 157 participants provided plasma-only. CSF and plasma samples were drawn on the same day. Lumbar punctures were performed between 9 and 12 am, directly followed by plasma collection. Samples were stored at −80°C.
Fig. 1.
Flow chart of CSF/plasma study groups. Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; DS, Down syndrome without (clinical) dementia; DS+AD, DS with diagnosed AD dementia; DS+pAD, Down syndrome with prodromal AD.
2.3. Reversed-phase HPLC
To quantify noradrenergic (NA; adrenaline; MHPG), dopaminergic (DA; DOPAC; HVA), and serotonergic (5-HT; 5-HIAA) compounds, a validated RP-HPLC setup with ion pairing (octane-1-sulfonic acid sodium salt) and amperometric electrochemical detection was used [30], previously applied to CSF and blood samples [12] and brain homogenates [24], [31], [32], [33]. Concentrations were calculated using Clarity Software (DataApex Ltd., 2008, Prague, Czech Republic).
2.4. Statistics
Histograms, normal quantile-quantile (Q-Q) plots, and Shapiro-Wilk tests (P < .05) demonstrated that the concentrations in the brain and CSF/plasma were (largely) not normally distributed. Consequently, nonparametric Kruskal-Wallis tests were applied to compare groups. If the P value was <.05, post hoc Mann-Whitney U tests were conducted. In brain, the three most relevant group comparisons were performed: DS versus DS+AD, DS+AD versus EOAD, and DS versus controls. The EOAD versus controls comparison has been largely published before [24]. Regarding CSF/plasma samples, we analyzed the total cohort (n = 225), that is, all individuals regardless of medication use, as well as the medication-free subpopulation because psychoactive medication may affect monoaminergic neurotransmission. Nonparametric Spearman's rank-order correlation tests established the relationship with age and between CSF and plasma concentrations. Cohort characteristics like gender and medication use were compared using Pearson's χ2 tests or Fisher's exact tests. To account for multiple comparisons, we applied the Benjamini-Hochberg procedure with a false discovery rate of 0.05 [34]. Original P values <.015 were regarded significant. Finally, we evaluated whether the results in the brain were possibly affected by psychoactive medication and PMDs. Within each group, we performed Mann-Whitney U tests to compare monoaminergic concentrations between those taking psychoactive medication and a subgroup that did not and performed Spearman's rank-order correlation tests to establish the association between PMDs and monoaminergic concentrations. IBM SPSS Statistics, version 23.0, was used.
3. Results
Based on the measured concentrations, five accompanying ratios were calculated: (1) MHPG:NA (noradrenergic turnover), (2) DOPAC:DA (dopaminergic turnover) and (3) HVA:DA (dopaminergic turnover), (4) 5-HIAA:5-HT (serotonergic turnover), and (5) HVA:5-HIAA (serotonergic inhibition on dopaminergic neurotransmission).
3.1. Monoaminergic characterization of postmortem brain tissue
Table 1 shows the general demographics, use of psychoactive medication, and PMDs for each of the four groups. Table 2 provides the monoaminergic concentrations (median and quartiles) that differed significantly between the groups. Specifically, DS versus DS+AD, DS+AD versus EOAD, and DS versus controls were compared. EOAD and controls were used as the reference group (compared in [24], thus not further described here). The supplementary material provides all concentrations and the accompanying ratios for noradrenergic (Supplementary Table 1), dopaminergic (Supplementary Table 2), and serotonergic (Supplementary Table 3) systems.
Table 2.
Comparison of postmortem concentrations between the groups
| Brain region | Compound | N | DS (n = 4) | DS+AD (n = 17) | EOAD (n = 11) | Controls (n = 10) | P value |
|---|---|---|---|---|---|---|---|
| Neocortex | |||||||
| BA7 | MHPG | 2/13/11/10 | 71.0 (63.3–) | 72.3 (57.0–105.2)* | 119.4 (106.0–189.5)* | 259.7 (119.8–354.5) | <.001 |
| DA | 2/12/11/10 | 11.0 (6.5–) | 18.7 (12.5–28.7) | 10.7 (6.4–16.5) | 6.9 (4.5–9.9) | .004 | |
| 5-HIAA | 2/13/11/10 | 92.8 (70.4–) | 40.2 (30.4–71.1) | 55.3 (40.5–93.7) | 106.4 (75.3–158.9) | .003 | |
| BA9/10/46 | MHPG | 4/14/11/10 | 84.2 (77.7–86.9) | 107.4 (63.4–133.8)** | 471.2 (284.3–655.1)** | 265.7 (132.4–629.6) | <.001 |
| DA | 4/14/11/10 | 127.5 (11.4–497.4) | 373.9 (204.3–743.6)** | 7.2 (2.5–9.3)** | 7.5 (4.0–11.3) | <.001 | |
| 5-HT | 4/14/11/10 | 17.8 (13.2–30.6) | 28.7 (15.3–49.8)* | 11.1 (6.1–15.0)* | 11.7 (7.4–16.7) | .009 | |
| 5-HIAA | 4/14/11/10 | 81.1 (64.6–122.2) | 62.2 (33.9–87.4)* | 144.7 (110.5–186.6)* | 164.1 (128.2–215.6) | .001 | |
| BA17 | MHPG | 3/7/11/10 | 77.2 (61.3–)# | 89.2 (73.6–114.6) | 127.9 (101.6–182.0) | 285.3 (244.0–530.1)# | <.001 |
| 5-HIAA | 3/7/11/10 | 126.0 (93.6–) | 92.1 (46.9–144.1) | 95.6 (57.5–143.6) | 200.8 (154.3–263.9) | .008 | |
| BA22 | NA | 3/10/9/10 | 30.2 (18.3–) | 17.3 (12.5–27.4) | 10.0 (7.4–12.4) | 18.7 (13.6–28.5) | .014 |
| MHPG | 3/10/11/10 | 107.3 (80.4–)# | 87.0 (60.0–125.4)** | 520.7 (313.4–673.4)** | 360.5 (260.4–630.7)# | <.001 | |
| DA | 3/10/11/10 | 6.7 (6.5–) | 11.3 (8.1–25.2)* | 4.2 (3.2–6.8)* | 9.1 (5.4–18.4) | .041 | |
| 5-HIAA | 3/10/11/10 | 119.1 (98.7–) | 84.0 (60.4–131.7)* | 303.2 (119.6–450.2)* | 171.5 (121.7–320.6) | .004 | |
| Limbic system | |||||||
| Amygdala | NA | 2/10/10/10 | 21.8 (19.5–) | 25.3 (13.6–39.8)* | 59.0 (46.9–78.5)* | 84.5 (77.5–121.8) | <.001 |
| MHPG | 2/10/10/10 | 68.8 (58.2–) | 70.9 (62.5–94.9)** | 429.8 (180.0–964.3)** | 304.8 (193.5–759.3) | <.001 | |
| HVA | 2/10/10/10 | 265.8 (174.5–) | 376.2 (258.9–617.2) | 599.1 (398.7–866.2) | 1132.5 (751.0–1421.4) | .005 | |
| 5-HT | 2/10/10/10 | 59.3 (11.7–) | 33.0 (18.6–49.6)* | 121.1 (55.1–148.6)* | 244.9 (221.7–297.2) | <.001 | |
| 5-HIAA | 2/10/10/10 | 197.9 (162.9–) | 141.3 (102.8–227.1)** | 522.4 (334.9–795.2)** | 999.8 (754.5–1270.4) | <.001 | |
| Hippocampus | Adrenaline | 2/5/3/5 | 391.6 (236.5–) | 42.3 (20.6–139.0) | 6.4 (2.6–) | 10.1 (6.4–14.1) | .011 |
| MHPG | 3/5/11/10 | 75.9 (70.3–)# | 97.2 (76.9–124.8)** | 459.5 (193.2–1099.2)** | 416.3 (232.9–713.5)# | <.001 | |
| 5-HT | 3/5/11/10 | 46.6 (28.0–) | 13.5 (8.6–50.0) | 44.4 (20.7–65.3) | 87.8 (69.3–111.2) | .003 | |
| 5-HIAA | 3/5/11/10 | 141.3 (110.6–) | 47.7 (43.1–213.4)* | 336.1 (257.8–476.2)* | 383.9 (279.5–717.0) | .004 | |
| BA11/12 | MHPG | 4/6/11/10 | 91.8 (70.6–105.7)# | 103.1 (63.9–111.2)** | 437.1 (352.6–545.6)** | 361.7 (216.5–636.3)# | <.001 |
| 5-HIAA | 4/6/11/10 | 98.2 (85.4–189.7) | 56.7 (35.3–78.5)** | 238.5 (183.5–344.3)** | 229.9 (168.9–328.3) | <.001 | |
| Cingulate gyrus | MHPG | 3/8/11/10 | 128.0 (58.8–) | 126.0 (104.6–153.6)** | 567.8 (258.4–739.3)** | 336.9 (158.1–587.5) | <.001 |
| DA | 3/8/11/10 | 224.2 (7.9–) | 55.5 (40.6–211.2)** | 10.5 (3.8–13.0)** | 9.6 (3.0–17.4) | <.001 | |
| 5-HIAA | 3/8/11/10 | 66.0 (34.5–)# | 102.6 (58.5–191.8)** | 357.3 (281.2–379.3)** | 387.2 (313.7–475.7)# | <.001 | |
| Thalamus | MHPG | 3/11/11/10 | 159.2 (131.3–)# | 148.3 (110.2–177.8)** | 793.0 (628.6–1442.6)** | 441.9 (244.1–1345.4)# | <.001 |
| 5-HIAA | 3/11/11/10 | 673.7 (508.8–) | 689.3 (412.4–895.8)** | 1584.5 (1237.1–1952.8)** | 1525.8 (1168.1–1946.8) | <.001 | |
| Basal ganglia | |||||||
| Caudate nucleus | Adrenaline | 2/11/7/6 | 533.3 (355.2–) | 69.3 (42.7–151.0)* | 268.5 (176.5–587.7)* | 372.9 (185.7–743.0) | .006 |
| DA | 3/16/11/10 | 4297.2 (1845.8–) | 2395.2 (1178.5–2784.8)** | 4721.2 (3403.8–6905.9)** | 3965.1 (2987.2–4420.5) | .003 | |
| HVA | 3/16/11/10 | 2732.0 (2231.3–) | 3145.6 (1522.7–3756.0)* | 4681.2 (3714.5–5640.3)* | 4372.3 (3564.4–6818.8) | .014 | |
| 5-HT | 3/16/11/10 | 121.5 (33.1–) | 55.8 (35.6–97.0)** | 168.3 (139.5–230.2)** | 240.0 (188.0–285.2) | <.001 | |
| 5-HIAA | 3/16/11/10 | 170.9 (76.6–) | 217.6 (106.3–284.2)** | 537.2 (362.3–727.8)** | 579.6 (441.0–784.9) | <.001 | |
| Globus pallidus | DOPAC | 2/8/11/10 | 208.3 (28.0–) | 131.7 (61.8–141.5)* | 39.5 (19.5–85.8)* | 20.1 (10.5–34.7) | .004 |
| 5-HT | 2/8/11/10 | 149.7 (89.7–) | 97.4 (70.5–120.4)* | 171.9 (117.6–207.7)* | 161.8 (140.3–215.5) | .022 | |
| 5-HIAA | 2/8/11/10 | 1009.2 (384.6–) | 439.5 (329.9–978.8)* | 984.5 (864.7–1407.3)* | 1320.2 (1019.2–1614.4) | .015 | |
| Putamen | Adrenaline | 2/8/11/10 | 390.4 (325.6) | 135.0 (40.9–219.9)* | 581.9 (187.2–1523.4)* | 339.6 (100.7–797.8) | .038 |
| DOPAC | 3/15/11/10 | 207.5 (165.1–) | 611.6 (274.7–851.6) | 421.9 (235.7–625.7) | 202.2 (119.0–299.7) | .008 | |
| 5-HT | 3/15/11/10 | 189.5 (43.9–) | 77.8 (35.2–159.6)* | 189.2 (153.9–219.8)* | 219.7 (200.5–326.3) | <.001 | |
| 5-HIAA | 3/15/11/10 | 260.0 (231.7–) | 372.7 (203.7–669.5)** | 790.9 (638.7–1026.1)** | 998.4 (781.9–1400.3) | <.001 | |
| Substantia nigra | DA | 2/14/11/10 | 164 (126.8–) | 157.2 (89.0–292.4)** | 572.7 (284.9–623.7)** | 624.0 (295.7–936.8) | <.001 |
| DOPAC | 2/14/11/10 | 47.2 (47.1–) | 58.0 (27.1–87.4)* | 189.7 (80.3–211.0)* | 47.1 (29.2–101.0) | .005 | |
| HVA | 2/14/11/10 | 3061.7 (2010.5–) | 2421.8 (1995.7–3007.8)** | 3799.4 (3461.0–4385.5)** | 4331.0 (3241.6–5349.9) | <.001 | |
| 5-HT | 2/14/11/10 | 208.3 (167.6–) | 364.1 (243.1–420.4) | 487.2 (389.2–531.2) | 461.0 (404.1–588.8) | .009 | |
| Metencephalon | |||||||
| Locus coeruleus | NA | 0/10/10/10 | – | 88.6 (52.7–11.4)** | 255.1 (171.9–400.7)** | 347.3 (248.6–501.8) | <.001 |
| MHPG | 0/10/10/10 | – | 201.8 (151.8–259.5)* | 429.6 (304.6–530.7)* | 572.4 (158.9–836.2) | .032 | |
| DOPAC | 0/10/10/10 | – | 12.3 (8.1–19.2)** | 39.0 (23.6–71.3)** | 55.5 (33.1–98.6) | <.001 | |
| HVA | 0/10/10/10 | – | 730.2 (482.0–1003.5) | 1009.7 (905.7–1443.4) | 1351.0 (1195.0–1482.2) | <.001 | |
| Cerebellum | Adrenaline | 0/7/4/6 | – | 36.4 (34.7–44.5)* | 6.6 (3.6–12.7)* | 21.4 (10.3–50.2) | .017 |
| MHPG | 2/9/11/10 | 124.6 (100.3–) | 101.1 (85.2–148.6)* | 421.1 (232.5–708.2)* | 518.1 (386.8–713.4) | .001 | |
| DA | 2/9/11/10 | 32.1 (8.4–) | 15.5 (12.2–22.1)** | 3.6 (1.5–8.6)** | 3.6 (3.2–5.4) | <.001 | |
| DOPAC | 2/9/11/10 | 35.1 (6.4–) | 16.4 (11.3–26.8)* | 8.2 (5.8–11.1)* | 8.0 (6.0–8.3) | .005 | |
| 5-HT | 2/9/11/10 | 66.5 (22.2–) | 25.0 (14.5–34.9)** | 4.6 (2.1–9.1)** | 2.6 (2.3–6.8) | <.001 | |
| 5-HIAA | 2/9/11/10 | 30.8 (21.9–) | 41.7 (33.8–56.5)** | 206.0 (92.8–301.4)** | 98.5 (73.7–207.2) | <.001 | |
Abbreviations: AD, Alzheimer's disease; BA, Brodmann area; 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, serotonin; DA, dopamine; DS, Down syndrome without neuropathologic AD diagnosis; DS+AD, Down syndrome with neuropathologic AD diagnosis; DOPAC, 3,4-dihydroxyphenylacetic acid; EOAD, early-onset Alzheimer's disease; HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; NA, noradrenaline; n.s., not significant.
NOTE. Monoamines and metabolites (ng/g tissue) are expressed as median (50%) with the interquartile range (25%–75%) between brackets. Kruskal-Wallis tests were used to compare the four groups. If P < .05, post hoc Mann-Whitney U tests were performed. After all analyses had been conducted, we accounted for multiple comparisons by performing the Benjamini-Hochberg procedure. Here, we provide P values (Kruskal-Wallis) that remained significant after correction (P < .015). Those in italics are no longer regarded significant but are nevertheless provided since the post hoc Mann-Whitney U tests remained significant in the Benjamini-Hochberg procedure. Post hoc comparisons were performed for DS vs. controls (#P < .015) and DS+AD vs. EOAD (∗P < .015; ∗∗P <.001). The accompanying ratios and the nonsignificant comparisons are provided in the supplementary material (Supplementary Tables 1-3).
Since psychoactive medication may affect monoaminergic concentrations, we assessed the donors' clinical documentation (Table 1). Comparing individuals who did and did not use psychoactive medication within each group yielded no significant monoaminergic differences in DS+AD and control groups, whereas only a single significant difference was found in the EOAD group: NA levels in the caudate nucleus were lower in individuals using medication (P = .014). In the DS group, the effect of medication could not be established: three in four used medication, and for the last person, it was unknown. Nevertheless, the use of psychoactive medication did not appear to have evidently impacted monoaminergic concentrations in DS+AD, EOAD, and control groups.
Given the very limited availability of postmortem DS tissue, it was impossible to select for short PMDs, particularly in the DS group. Apart from three cases, PMD was <12 hours in the DS+AD group. Because PMDs differed between groups (Table 1), we subsequently examined whether PMD was associated with monoaminergic concentrations. Spearman's rank-order correlation tests within each group revealed few significant associations with PMDs: HVA (cingulate gyrus, r = −0.90, P = .002) in the DS+AD group; MHPG (caudate nucleus, r = 0.85, P = .001 and SN, r = 0.91, P < .001), DA (BA11/12, r = 0.74, P = .01), HVA (BA9/10/46, r = 0.80, P = .003; BA22, r = 0.88, P < .001; BA11/12, r = 0.91, P < .001; hippocampus, r = 0.86, P = .001; thalamus, r = 0.75, P = .007; and cerebellum, r = 0.78, P = .004) in the EOAD group; and 5-HT in the globus pallidus (r = −0.85, P = .002) in the control group. Although the PMD range was the largest in the DS+AD group, most significant correlations were observed in the EOAD group with overall lower PMDs (range: 2.8–7 hours). Although an effect of PMDs cannot be ruled out, our main findings do not appear to be evidently affected by PMDs.
3.1.1. Noradrenergic system
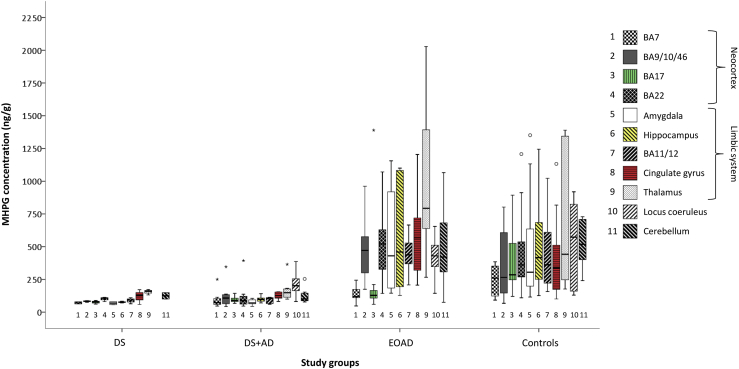
Between groups, NA differed significantly in the BA22, amygdala, and LC. Specifically, NA levels were not altered between DS and DS+AD, but lower in DS+AD compared with EOAD (amygdala and LC, Table 2). This pattern was more pronounced for MHPG (Fig. 2). Compared with EOAD, DS+AD presented significantly lower MHPG levels in the LC, cortical (BA7, BA9/10/46, and BA22) and limbic projection areas (amygdala, hippocampus, BA11/12, cingulate gyrus, and thalamus), and cerebellum. Consequently, the MHPG:NA ratio was consistently lower in DS+AD (BA9/10/46, BA22, hippocampus, BA11/12, cingulate gyrus, thalamus, and cerebellum), indicating a reduced noradrenergic turnover. Moreover, MHPG was significantly lower in DS compared with controls (Fig. 2) for BA17, BA22, hippocampus, BA11/12, and thalamus. Neither NA nor MHPG levels differed significantly in the basal ganglia, whereas adrenaline levels were lower in the caudate nucleus and putamen and higher in the cerebellum in DS+AD (vs. EOAD). Taken together, the noradrenergic system—particularly MHPG—was strongly impaired in DS (vs. controls) and DS+AD (vs. EOAD).
Fig. 2.
MHPG concentrations (ng/g tissue) for each study group in neocortical areas, the limbic system, locus coeruleus, and cerebellum. The boxes represent the IQR (25%–75%) with the black horizontal line indicating the median. The whiskers indicate values within 1.5 IQR. Mild outliers (1.5–3 IQR) are indicated with an open circle, whereas extreme outliers (>3 IQR) with an asterisk. One extreme value (EOAD, MHPG concentration in hippocampus of 3806 ng/g tissue) is not shown with respect to scaling. Evidently, MHPG levels were consistently lower in DS (vs. controls) and DS+AD (vs. EOAD). DS vs. DS+AD did not differ significantly. Individual comparison statistics are provided in Table 2. Abbreviations: BA, Brodmann area; DS, Down syndrome without neuropathologic AD diagnosis; DS+AD, Down syndrome with neuropathologic AD diagnosis; EOAD, early-onset Alzheimer's disease; IQR, interquartile range; MHPG, 3-methoxy-4-hydroxyphenylglycol.
3.1.2. Dopaminergic system
No significant differences were observed for DS versus DS+AD and DS versus controls. Compared with EOAD, bidirectional dopaminergic changes became evident in DS+AD: DA levels were significantly higher in the BA9/10/46, BA22, cingulate gyrus, and cerebellum and lower in the basal ganglia (caudate nucleus and SN). Similarly, in DS+AD (vs. EOAD), HVA was reduced in the caudate nucleus and SN. Consequently, the HVA:DA ratio, indicative of dopaminergic turnover, was consistently lower in DS+AD (vs. EOAD) in cortical areas (BA7, BA9/10/46, and BA22), limbic regions (BA11/12, cingulate gyrus, and thalamus), and the cerebellum. In contrast, the HVA:DA ratio was increased in the SN. The pattern for DOPAC was bidirectional as well: values were decreased in the SN and LC and increased in the globus pallidus and cerebellum. The DOPAC:DA ratio was significantly lower in the BA9/10/46, BA22, cingulate gyrus, and LC for DS+AD versus EOAD. In short, the dopaminergic system was evidently affected in DS+AD with higher DA levels (and thus lower HVA:DA and DOPAC:DA ratios) in cortical areas, limbic regions, and the cerebellum and lower DA and HVA levels in the basal ganglia.
3.1.3. Serotonergic system
5-HT and 5-HIAA did not differ significantly between DS and DS+AD. 5-HIAA levels in the cingulate gyrus and the 5-HIAA:5-HT ratio in the (pre)frontal cortex were significantly lower in DS than in controls. Compared with EOAD, 5-HT levels in DS+AD were significantly lower in the amygdala and basal ganglia (caudate nucleus, globus pallidus, and putamen) and higher in the (pre)frontal cortex and cerebellum. In comparison with EOAD, 5-HIAA was consistently lower in DS+AD, namely in cortical areas (BA9/10/46, BA22), limbic system (amygdala, hippocampus, BA11/12, cingulate gyrus, and thalamus), the basal ganglia (caudate nucleus, globus pallidus, and putamen), and the cerebellum. Similarly, the 5-HIAA:5-HT ratio was reduced in the BA9/10/46, BA22, BA11/12, and cerebellum in DS+AD versus EOAD, thus indicating an overall decreased serotonergic turnover in DS+AD. In summary, a serotonergic deficit became apparent in DS+AD, with a pronounced overall reduction in 5-HIAA levels (and thus a reduced 5-HIAA:5-HT ratio) as compared with EOAD.
Finally, the HVA:5-HIAA ratio, indicating serotonergic inhibition on dopaminergic neurotransmission, clearly differed between groups. Apart from a significantly higher HVA:5-HIAA ratio in the cingulate gyrus in DS (vs. controls), significance was, again, observed for the DS+AD versus EOAD comparison. The ratio was invariably higher in DS+AD for cortical regions (BA9/10/46 and BA22), limbic system (amygdala, hippocampus, BA11/12, and cingulate gyrus), and the cerebellum, suggestive of a reduced serotonergic inhibition on the dopaminergic system.
3.2. Monoaminergic characterization of (paired) CSF/plasma samples
Samples of 225 DS individuals were included in analysis (Fig. 1). Paired samples were available for 68 individuals and plasma-only samples for 157 cases. Table 3, Table 4, respectively, show the study group characteristics and monoaminergic concentrations. The accompanying ratios are provided in the supplementary material, Supplementary Table 4. Remarkably, CSF/plasma concentrations did not differ between the three groups apart from DOPAC levels in CSF (medication-free subpopulation) and plasma (total population and medication-free subpopulation). DOPAC levels were consistently higher in DS+AD compared with DS (CSF medication-free, P = .001; plasma total, P < .001; and plasma medication-free, 0.002) but did not differ for the DS versus DS+pAD and DS+pAD versus DS+AD comparisons. Similarly, plasma HVA (total), plasma 5-HIAA (total), and CSF DOPAC:DA (medication-free) were higher in DS+AD versus DS. In contrast, CSF HVA:5-HIAA (total) was decreased in DS+AD. Moreover, DA (r = −0.31, P = .012), DOPAC (r = +0.71, P < .001), MHPG (r = +0.70, P < .001), and adrenaline (r = +0.49, P < .001) correlated significantly in CSF and plasma (paired samples, total population). Groups differed in age with DS+AD logically being the oldest. DOPAC (CSF, r = +0.362, P = .002; plasma, r = +0.386, P < .001), HVA (plasma, r = +0.169, P = .011), and 5-HIAA (CSF, r = +0.365, P = .002; plasma, r = +0.345, P < .001) correlated significantly with age. After exclusion of individuals younger than 45 years, that is, resembling the elderly DS cohort in our previously published serum study [12], comparison between DS (CSF/plasma, n = 8; plasma-only, n = 34), DS+pAD (CSF/plasma, n = 11; plasma-only, n = 31), and DS+AD (CSF/plasma, n = 16; plasma-only, n = 38) yielded no significant monoaminergic differences, again suggesting that DOPAC changes most likely relate to aging rather than dementia status.
Table 3.
Characteristics of CSF/plasma study groups
| Characteristics | No dementia (DS, n = 149) | Prodromal AD (DS+pAD, n = 36) | Diagnosed AD dementia (DS+AD, n = 40) | P value |
|---|---|---|---|---|
| Age (median; min.–max.) | 36.9 (19.2–61.2)a,b | 49.8 (37.7–59.4)a,c | 55.1 (42.1–69.2)b,c | <.001 |
| Gender (N male and %) | 82 (55.0) | 16 (44.4) | 25 (62.5) | n.s. |
| APOE status | 1 (ε2/2), 15 (ε2/3), 2 (ε2/4), 106 (ε3/3), 23 (ε3/4), 2 (ε4/4) | 1 (ε2/2), 6 (ε2/3), 21 (ε3/3), 8 (ε3/4) | 1 (ε2/3), 1 (ε2/4), 30 (ε3/3), 8 (ε3/4) | n.s. |
| Levothyroxine (%) | 61 (40.9) | 12 (33.3) | 14 (35.0) | n.s. |
| Any psychoactive drugs (%) | 56 (37.6) | 19 (52.8) | 24 (60.0) | n.s. |
| Antidepressants | 36 (24.2) | 11 (30.6) | 9 (22.5) | n.s. |
| Antiepileptics | 13 (8.7) | 7 (19.4) | 10 (25.0) | .014 |
| Antipsychotics | 20 (13.4) | 8 (22.2) | 13 (32.5) | n.s. |
| Anxiolytics | 14 (9.4) | 3 (8.3) | 8 (20.0) | n.s. |
| Antidementia | 1 (0.7) | 1 (2.8) | 5 (12.5) | .002 |
Abbreviations: APOE, apolipoprotein E gene; DS, Down syndrome without (clinical) dementia; DS+AD, Down syndrome with diagnosed AD dementia; DS+pAD, Down syndrome with prodromal AD; n.s., not significant.
NOTE. Age at the moment of sampling is provided as median with range (minimum–maximum). The Kruskal-Wallis test was performed to compare ages between groups. Post hoc Mann Whitney U tests were performed to identify significant group differences (P < .015): (a) DS vs. DS+pAD; (b) DS vs. DS+AD; and (c) DS+pAD vs. DS+AD. Gender, APOE status, and medication use were compared using Pearson's χ2 test or Fisher's exact test.
Table 4.
Comparison of CSF/plasma concentrations between the groups
| Compound | CSF or plasma | Total or medication free | N | DS | DS+pAD | DS+AD | P value |
|---|---|---|---|---|---|---|---|
| NA | CSF | Total | 37/13/18 | 2.8 (0.9–5.5) | 1.4 (0.6–4.9) | 1.8 (0.8–5.6) | n.s. |
| Medication free | 21/7/10 | 2.8 (1.0–6.9) | 1.4 (0.6–4.7) | 1.4 (0.8–5.5) | n.s. | ||
| Plasma | Total | 145/35/37 | 0.4 (0.2–0.9) | 0.6 (0.2–0.9) | 0.6 (0.4–1.1) | n.s. | |
| Medication free | 90/16/15 | 0.5 (0.2–1.0) | 0.4 (0.1–0.7) | 0.4 (0.2–1.0) | n.s. | ||
| Adrenaline | CSF | Total | 35/13/16 | 0.5 (0.3–0.9) | 0.7 (0.3–1.3) | 0.4 (0.3–1.2) | n.s. |
| Medication free | 20/7/10 | 0.7 (0.3–1.0) | 0.8 (0.2–2.1) | 0.4 (0.4–1.2) | n.s. | ||
| Plasma | Total | 147/35/39 | 2.9 (1.9–4.2) | 2.6 (1.9–4.0) | 3.3 (1.4–4.3) | n.s. | |
| Medication free | 92/17/16 | 2.9 (2.0–4.0) | 3.0 (2.0–4.2) | 2.7 (1.2–3.9) | n.s. | ||
| MHPG | CSF | Total | 37/13/18 | 30.2 (21.5–44.6) | 24.0 (18.8–40.0) | 22.4 (19.6–46.0) | n.s. |
| Medication free | 21/7/10 | 31.1 (21.8–45.0) | 24.0 (15.9–41.1) | 28.5 (20.7–43.4) | n.s. | ||
| Plasma | Total | 149/36/40 | 76.2 (51.2–102.4) | 70.9 (50.4–111.6) | 75.6 (55.3–114.4) | n.s. | |
| Medication free | 93/17/16 | 77.3 (51.5–100.6) | 70.4 (45.5–119.3) | 63.0 (47.9–107.5) | n.s. | ||
| DA | CSF | Total | 37/13/18 | 0.6 (0.4–0.9) | 0.5 (0.2–0.7) | 0.6 (0.3–1.2) | n.s. |
| Medication free | 21/7/10 | 0.6 (0.4–0.9) | 0.6 (0.3–1.0) | 0.8 (0.4–1.3) | n.s. | ||
| Plasma | Total | 149/35/40 | 0.5 (0.3–1.0) | 0.5 (0.3–1.1) | 0.7 (0.3–1.3) | n.s. | |
| Medication free | 93/16/16 | 0.5 (0.3–1.0) | 0.6 (0.4–1.1) | 0.7 (0.4–1.9) | n.s. | ||
| DOPAC | CSF | Total | 37/13/18 | 0.6 (0.4–1.3) | 1.2 (0.6–2.7) | 1.7 (0.7–3.3) | .033 |
| Medication free | 21/7/10 | 0.5 (0.4–1.0)§§ | 0.9 (0.5–1.5) | 2.8 (1.1–5.6)§§ | .003 | ||
| Plasma | Total | 149/36/40 | 2.7 (1.9–4.1)§§ | 3.1 (2.6–4.3) | 4.3 (3.0–6.2)§§ | <.001 | |
| Medication free | 93/17/16 | 2.5 (1.9–3.6)§ | 3.1 (2.7–3.6) | 5.3 (2.9–6.2)§ | .004 | ||
| HVA | CSF | Total | 37/13/18 | 54.6 (40.5–67.7) | 55.1 (43.3–74.8) | 55.3 (33.3–67.3) | n.s. |
| Medication free | 21/7/10 | 56.3 (41.5–64.1) | 46.5 (35.2–79.5) | 56.4 (33.1–67.2) | n.s. | ||
| Plasma | Total | 149/36/40 | 9.4 (7.5–11.6)§ | 10.3 (7.4–12.7) | 10.6 (8.7–15.0)§ | .039 | |
| Medication free | 93/17/16 | 9.5 (7.4–12.2) | 10.5 (7.6–13.0) | 9.9 (8.5–14.2) | n.s. | ||
| 5-HT | CSF | Total | 11/4/6 | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | n.s. |
| Medication free | 6/2/2 | 0.1 (0.1–0.2) | 0.1 (0.1–) | 0.1 (0.0–) | n.s. | ||
| Plasma | Total | 149/36/40 | 9.5 (3.9–20.3) | 11.1 (2.4–20.8) | 9.7 (5.2–17.2) | n.s. | |
| Medication free | 93/17/16 | 12.4 (7.1–25.0) | 17.3 (8.0–28.6) | 10.6 (8.4–20.7) | n.s. | ||
| 5-HIAA | CSF | Total | 37/13/18 | 24.9 (18.1–28.9) | 26.5 (21.5–35.4) | 28.9 (23.2–34.3) | n.s. |
| Medication free | 21/7/10 | 26.6 (22.6–29.7) | 26.5 (22.0–36.1) | 33.2 (25.2–38.4) | n.s. | ||
| Plasma | Total | 149/36/40 | 4.5 (3.7–5.4)§ | 4.7 (4.2–5.7) | 5.0 (4.1–6.6)§ | .020 | |
| Medication free | 93/17/16 | 4.5 (3.6–5.4) | 5.3 (4.2–6.5) | 4.9 (3.6–6.7) | n.s. |
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, serotonin; AD, Alzheimer's disease; CSF, cerebrospinal fluid; DA, dopamine; DS, Down syndrome without (clinical) dementia; DS+pAD, Down syndrome with prodromal AD; DS+AD, DS with diagnosed AD dementia; DOPAC, 3-4-dihydroxyphenylacetic acid; HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; NA, noradrenaline; n.s., not significant.
NOTE. Concentrations of monoamines and metabolites (ng/ml) are expressed as median (50%) with the interquartile range (25%–75%) between brackets. The number (N) of samples is provided as certain compounds were not detectable in all samples. Kruskal-Wallis tests were performed to compare the three groups. If P < .05, post hoc Mann-Whitney U tests were performed. After all analyses had been conducted, we accounted for multiple comparisons by performing the Benjamini-Hochberg procedure. The P values in italics are no longer regarded significant but are nevertheless provided since the post hoc Mann-Whitney U tests remained significant in the Benjamini-Hochberg procedure. Post hoc comparisons were performed for DS vs. DS+pAD, DS vs. DS+AD (§P <.015 and §§P <.001) and DS+pAD vs. DS+AD. The accompanying ratios are provided in the supplementary material (Supplementary Table 4).
4. Discussion
Monoaminergic profiles were evaluated in 15 postmortem brain regions and (paired) CSF/plasma samples. In brain, pronounced noradrenergic, dopaminergic, and serotonergic differences were found for DS+AD versus EOAD and to a lesser extent for DS versus controls (primarily decreased MHPG levels), but not for DS versus DS+AD. Similarly, CSF/plasma concentrations were virtually unaltered between the diagnostic DS groups.
In AD, studies have demonstrated LC neuronal loss and reduced NA levels [21], [35], [36], [37], [38], [39]. Noradrenergic abnormalities have been implicated in DS too [40]. Here, we demonstrate that the noradrenergic system was more severely impacted in DS+AD versus EOAD and to a lesser extent in DS versus non-DS controls. NA, MHPG, and the MHPG:NA ratio were significantly reduced in most brain areas, but not in the basal ganglia, which is in accordance with the modest noradrenergic innervation of the basal ganglia [35]. These results are also in agreement with earlier studies reporting AD-related loss of LC neurons [21], [22], [23], [36] and reduced NA levels in various brain regions in DS+AD compared with controls [16], [17], [18], [19], [20]. Our results demonstrate that MHPG concentrations were most severely impacted in DS+AD (even more than in EOAD), but also in DS, thus already before the neuropathological criteria for AD were met.
DA is produced in the SN and ventral tegmental area (VTA). In AD, a variable SN neuronal loss and diminished DA levels have been described [41], [42]. Whereas previous studies did not report evident dopaminergic alterations in DS [16], [18], we found significantly increased DA levels (and thus decreased HVA:DA and DOPAC:DA ratios) in cortical areas, limbic regions and cerebellum, and a general decrease in DA and HVA levels in the basal ganglia. Indeed, lower DA levels in the caudate nucleus have been reported in DS+AD versus EOAD and age-matched controls [20]. Ascending dopaminergic projections are subdivided into the nigrostriatal (from SN to striatum), mesolimbic (from VTA to limbic system), and mesocortical (from VTA to cortex) pathways [35]. Previously, a mild cell loss (though often not significant) in the SN, but also in the VTA, was found in DS+AD compared with controls or younger counterparts [23], [36], [43], [44]. Our results may suggest a more severe impairment of the nigrostriatal pathway (reduced DA levels in caudate and SN), whereas the mesolimbic and mesocortical pathways seem to be somewhat overactive, possibly as a compensatory mechanism.
Concerning the serotonergic system, neuronal loss in the dorsal raphe nuclei (5-HT production site) and reduced levels of 5-HT and 5-HIAA in various brain regions have been reported in AD and DS [16], [17], [18], [35], [36], [37], [39], [45]. Compared with EOAD, we observed an even more severe serotonergic impairment in DS+AD, presenting decreased 5-HIAA levels in 11 brain regions, while 5-HT was reduced in the amygdala and basal ganglia but increased in the (pre)frontal cortex and cerebellum.
Interestingly, the DS and DS+AD groups showed remarkably similar monoaminergic profiles, although both groups had different AD neuropathologic changes (low vs. high). Importantly, the four DS cases with low AD neuropathologic change already presented high amyloid burden (“ABC scoring system” [25]: A2,B1,C2; A3,B1,C1; A3,B0,C0, and A3,B0,C0, respectively). The third copy of the APP gene in DS causes very early Aβ overproduction and accumulation. Deposition of Aβ plaques occurs at ages as early as 12 years and precedes tau pathology by many years [4]. Previously, noradrenergic and serotonergic depletion was found to be more severe in EOAD (mutations in APP or PSEN1/2, promoting the amyloidogenic pathway) than in late-onset AD [38]. Inverse relations between Aβ accumulation and, respectively, NA, DA, and 5-HT signaling have been described [35], [46]. This may suggest that the monoaminergic system is particularly affected by (early) Aβ pathology, being altered long before full-blown AD pathology is present. For a comprehensive summary about the pathophysiological link between monoaminergic alterations and AD pathology, see the review by Trillo et al. [35].
In the context of abnormal brain development, monoamines were quantified in the frontal cortex of fetal DS tissue (20 weeks) compared with controls. DA, 5-HT, and 5-HIAA levels were significantly reduced in DS [47]. This suggests that monoamines are already impacted by trisomy 21 itself, which may be further impaired by progressive Aβ pathology during life. Compared with age-matched controls, smaller brain volumes were found in DS, among others of (pre)frontal cortex, hippocampus, brainstem and cerebellum [48], [49], [50]. Fewer neurons (cortical dysgenesis), altered neuronal distribution, and reduced synaptic density were described in DS as well [49]. Consequently, the compensatory reserve is likely to be lower, which could result in a particularly early vulnerability (functional impact) to additional neuropathology. To differentiate between the alterations caused by trisomy 21 and AD pathology, respectively, future monoaminergic studies should include DS samples without early Aβ plaque load. In the present study, we were unable to collect more than four such cases (limiting the generalizability of the findings in this group) because inclusion of DS cases in brain banks, those without pathology in particular, is very limited. In fact, the 21 cases analyzed here were obtained by three large brain banks in a timeframe of 25 years. Contemporary standardized (multicenter) brain banking efforts for DS are thus imperative [51], [52], focusing, among others, on the collection of tissues with short(er) PMDs and good clinical documentation. Although our main findings did not appear to be evidently impacted by PMDs or psychoactive medication use, such effects cannot be fully ruled out because of the unavailability of DS tissue with low PMDs and no psychoactive medication use.
The apparent lack of monoaminergic changes between DS and DS+AD in the brain was also reflected in CSF/plasma. The CSF/plasma groups were distinguished based on a clinical dementia diagnosis, whereas from a neuropathological perspective, the (amyloid) pathology is likely to be quite comparable. In future studies, it would be useful to relate monoaminergic values in DS to (in vivo) pathologic staging, such as the CSF “AD profile” [11] or positron emission tomography of Aβ/tau [5]. Furthermore, mounting evidence indicates an important role of neuroinflammation in the pathogenesis of AD (in DS) [53], and it would thus be valuable to look further than Aβ and tau pathology and examine the role of neuroinflammatory processes in monoaminergic alterations as well.
Surprisingly, the CSF/plasma results did not reflect earlier results obtained in serum [12]. Whereas MHPG, for instance, was evidently decreased in DS+AD serum, MHPG levels were virtually unaltered in CSF/plasma. This raises the question what causes this apparent discrepancy. Our methodology has been validated [30], and the reported values have orders of magnitude comparable to earlier studies [14], [15], [54]. The—likely multifactorial—answer remains to be elucidated, including the effect of (alterations in) peripheral determinants, such as non-brain sources of catecholamines (e.g., the sympathetic nervous system is the main source of peripheral NA) and enzymes involved in catecholaminergic turnover [55], as well as (pre)analytical variables. O'Bryant et al. (2015) addressed variables that can impact findings in blood, including controllable variables (e.g., fasting status, tube type, centrifugation parameters, time from collection to freezing, and freezing temperature) and uncontrollable variables (e.g., diet, activity level, comorbidities, and medication). In particular, serum versus plasma, type of needle, additive in the collection tubes, and presence of hemolysis may influence the stability and detectability of biomarkers [56]. In CSF, similar variables may impact biomarker levels [57], [58]. Indeed, a few variables differ identifiably between our serum and plasma studies, such as fasting status, storage temperature, and storage time. Retrospectively identifying the cause of the discrepancy is virtually impossible. New initiatives should, therefore, systematically study the effect of these variables on monoaminergic concentrations.
In conclusion, this study is the first to comprehensively examine monoaminergic alterations in a unique collection of postmortem brain regions and (paired) CSF/plasma samples of DS individuals. Despite various limitations described previously, brain samples of DS+AD (vs. EOAD) revealed generalized impairments in the noradrenergic and serotonergic systems (overall decrease) and a bidirectional dopaminergic change. For DS (vs. controls), significantly decreased MHPG levels were noted, though to a lesser extent than for DS+AD (vs. EOAD). DS and DS+AD groups showed remarkably similar monoaminergic profiles in the brain. CSF/plasma concentrations did not differ between the diagnostic DS groups either. The underlying cause for the discrepancy with earlier serum findings remains unclear and requires further study. To confirm whether the more profound monoaminergic alterations in DS (vs. non-DS) are indeed due to early Aβ accumulation, (longitudinal) studies using positron emission tomography imaging of monoamines might provide a new avenue. For instance, neuroimaging of NA transporters in LC and key projection areas using [11C]methylreboxetine [59] in relation to amyloid deposition (e.g. [11C]Pittsburgh compound B) may be of utmost importance in this respect. Moreover, to further investigate disease progression, it would be valuable to relate monoaminergic concentrations to CSF/plasma levels of Aβ and tau within individuals, for instance to the CSF AD profile (low Aβ42, high total-tau, and high phosphorylated-tau) [11].
Research in Context.
-
1
Systematic review: Alterations in monoamine neurotransmitters and metabolites have been implicated in Alzheimer's disease (AD) and Down syndrome (DS). However, monoaminergic profiles have not been extensively studied in cerebrospinal fluid (CSF) and postmortem brain samples of DS with/without AD.
-
2
Interpretation: This is the first study to comprehensively characterize DS samples with regard to AD diagnosis. In CSF/plasma, monoaminergic levels were not related to the clinical status of dementia in DS. In brain, evident noradrenergic and serotonergic deficits were found in DS+AD versus early-onset AD patients, and to a lesser extent in DS versus non-DS healthy controls. Our results reveal a rather similar monoaminergic profile in both DS and DS+AD, possibly caused by early trisomy 21–related accumulation of amyloid β (Aβ).
-
3
Future directions: Positron emission tomography studies of monoaminergic neurotransmission may reveal whether monoaminergic impairment in DS relates to early Aβ accumulation. Longitudinal studies in relation to Aβ imaging would be of utmost importance.
Acknowledgments
This study was financially supported by the Gratama-Stichting/Stichting Groninger Universiteitsfonds (2015–04), the Research School for Behavioural and Cognitive Neurosciences of the University of Groningen (RUG), Interuniversity Poles of Attraction of the Belgian Federal Science Policy Office (IAP network P7/16), the Belgian Foundation for Alzheimer Research (SAO-FRA 15002), and the Carlos III National Institute of Health of Spain (CM14/00029, PI13/01532, PI11/02425 and PI14/01126) jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, ‘Una manera de hacer Europa’, Fundació Bancaria La Caixa, and Marató de TV3 (20141210). This work has also been partially supported by a grant from the Grifols Foundation, the Generalitat de Catalunya (2014SGR-0235), and the Fundació Catalana de Síndrome de Down (FCSD). Further general support was received from the Alzheimer Research Center of the University Medical Center Groningen (UMCG), Neurosearch Antwerp, and IBB. These public sponsors were not involved in designing the study, collecting and interpreting data, and writing the manuscript. The authors express their gratitude to all individuals with Down syndrome and their caregivers for their participation. We also thank the staff members involved at the different centers, specifically Michiel Kooreman (NBB) for his assistance with the postmortem material, Tinne Koninckx and Karen Sterck (IBB) for their immunohistochemical work, and Laia Muñoz and Raúl Núñez (Sant Pau) for the laboratory and sample handling.
Footnotes
The authors have declared no conflict of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2017.11.001.
Supplementary data
References
- 1.Wiseman F.K., Al-Janabi T., Hardy J., Karmiloff-Smith A., Nizetic D., Tybulewicz V.L.J. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16:564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori C., Spooner E.T., Wisniewski K.E., Wisniewski T.M., Yamaguchi H., Saido T.C. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- 3.Gyure K.A., Durham R., Stewart W.F., Smialek J.E., Troncoso J.C. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- 4.Lemere C.A., Blusztajn J.K., Yamaguchi H., Wisniewski T., Saido T.C., Selkoe D.J. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 5.Head E., Helman A.M., Powell D., Schmitt F.A. Down syndrome, beta-amyloid and neuroimaging. Free Radic Biol Med. 2017 doi: 10.1016/j.freeradbiomed.2017.09.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann D.M. Alzheimer's disease and Down's syndrome. Histopathology. 1988;13:125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 7.Zigman W.B., Lott I.T. Alzheimer's disease in Down syndrome: neurobiology and risk. Ment Retard Dev Disabil Res Rev. 2007;13:237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]
- 8.Krinsky-McHale S.J., Devenny D.A., Gu H., Jenkins E.C., Kittler P., Murty V.V. Successful aging in a 70-year-old man with down syndrome: a case study. Intellect Dev Disabil. 2008;46:215–228. doi: 10.1352/2008.46:215-228. [DOI] [PubMed] [Google Scholar]
- 9.Dekker A.D., Strydom A., Coppus A.M.W., Nizetic D., Vermeiren Y., Naudé P.J.W. Behavioural and psychological symptoms of dementia in Down syndrome: early indicators of clinical Alzheimer’s disease? Cortex. 2015;73:36–61. doi: 10.1016/j.cortex.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Blennow K., Dubois B., Fagan A.M., Lewczuk P., de Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekker A.D., Fortea J., Blesa R., De Deyn P.P. Cerebrospinal fluid biomarkers for Alzheimer's disease in Down syndrome. Alzheimers Dement (Amst) 2017;8:1–10. doi: 10.1016/j.dadm.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekker A.D., Coppus A.M., Vermeiren Y., Aerts T., van Duijn C.M., Kremer B.P. Serum MHPG strongly predicts conversion to Alzheimer's disease in behaviorally characterized subjects with Down syndrome. J Alzheimers Dis. 2015;43:871–891. doi: 10.3233/JAD-140783. [DOI] [PubMed] [Google Scholar]
- 13.Hampel H., Lista S., Khachaturian Z.S. Development of biomarkers to chart all Alzheimer's disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 14.Kay A.D., Schapiro M.B., Riker A.K., Haxby J.V., Rapoport S.I., Cutler N.R. Cerebrospinal fluid monoaminergic metabolites are elevated in adults with Down's syndrome. Ann Neurol. 1987;21:408–411. doi: 10.1002/ana.410210416. [DOI] [PubMed] [Google Scholar]
- 15.Schapiro M.B., Kay A.D., May C., Ryker A.K., Haxby J.V., Kaufman S. Cerebrospinal fluid monoamines in Down's syndrome adults at different ages. J Ment Defic Res. 1987;31:259–269. doi: 10.1111/j.1365-2788.1987.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 16.Godridge H., Reynolds G.P., Czudek C., Calcutt N.A., Benton M. Alzheimer-like neurotransmitter deficits in adult Down's syndrome brain tissue. J Neurol Neurosurg Psychiatry. 1987;50:775–778. doi: 10.1136/jnnp.50.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds G.P., Godridge H. Alzheimer-like brain monoamine deficits in adults with Down's syndrome. Lancet. 1985;2:1368–1369. doi: 10.1016/s0140-6736(85)92666-2. [DOI] [PubMed] [Google Scholar]
- 18.Risser D., Lubec G., Cairns N., Herrera-Marschitz M. Excitatory amino acids and monoamines in parahippocampal gyrus and frontal cortical pole of adults with Down syndrome. Life Sci. 1997;60:1231–1237. doi: 10.1016/s0024-3205(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 19.Yates C.M., Ritchie I.M., Simpson J., Maloney A.F., Gordon A. Noradrenaline in Alzheimer-type dementia and Down syndrome. Lancet. 1981;2:39–40. doi: 10.1016/s0140-6736(81)90269-5. [DOI] [PubMed] [Google Scholar]
- 20.Yates C.M., Simpson J., Gordon A., Maloney A.F., Allison Y., Ritchie I.M. Catecholamines and cholinergic enzymes in pre-senile and senile Alzheimer-type dementia and Down's syndrome. Brain Res. 1983;280:119–126. doi: 10.1016/0006-8993(83)91179-4. [DOI] [PubMed] [Google Scholar]
- 21.German D.C., Manaye K.F., White C.L., Woodward D.J., McIntire D.D., Smith W.K. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- 22.Marcyniuk B., Mann D.M., Yates P.O., Ravindra C.R. Topography of nerve cell loss from the locus coeruleus in middle aged persons with Down's syndrome. J Neurol Sci. 1988;83:15–24. doi: 10.1016/0022-510x(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 23.Mann D.M., Yates P.O., Marcyniuk B., Ravindra C.R. Loss of neurons from cortical and subcortical areas in Down's syndrome patients at middle age. Quantitative comparisons with younger Down's patients and patients with Alzheimer's disease. J Neurol Sci. 1987;80:79–89. doi: 10.1016/0022-510x(87)90223-1. [DOI] [PubMed] [Google Scholar]
- 24.Vermeiren Y., Janssens J., Aerts T., Martin J.J., Sieben A., Van Dam D. Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer's disease. J Alzheimers Dis. 2016;53:1079–1096. doi: 10.3233/JAD-160320. [DOI] [PubMed] [Google Scholar]
- 25.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortea J., Carmona-Iragui M., Fernandez S., Benejam B., Videla L., Alcolea D.A. Down Alzheimer Barcelona Neuroimaging Initiative (DABNI): A prospective longitudinal biomarker cohort to study Alzheimer's disease in Down syndrome. Alzheimers Dement. 2016;12:P380–P381. [Google Scholar]
- 27.Carmona-Iragui M., Santos T., Videla S., Fernandez S., Benejam B., Videla L. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer's disease in subjects with Down syndrome. J Alzheimers Dis. 2017;55:1489–1496. doi: 10.3233/JAD-160827. [DOI] [PubMed] [Google Scholar]
- 28.Carmona-Iragui M., Balasa M., Benejam B., Alcolea D.A., Fernandez S., Videla L. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer's disease. Alzheimers Dement. 2017;13:1251–1260. doi: 10.1016/j.jalz.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois B., Feldman H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 30.Van Dam D., Vermeiren Y., Aerts T., De Deyn P.P. Novel and sensitive reversed-phase high-pressure liquid chromatography method with electrochemical detection for the simultaneous and fast determination of eight biogenic amines and metabolites in human brain tissue. J Chromatogr. 2014;1353:28–39. doi: 10.1016/j.chroma.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Vermeiren Y., Van Dam D., Aerts T., Engelborghs S., De Deyn P.P. Monoaminergic neurotransmitter alterations in postmortem brain regions of depressed and aggressive patients with Alzheimer's disease. Neurobiol Aging. 2014;35:2691–2700. doi: 10.1016/j.neurobiolaging.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Vermeiren Y., Van Dam D., Aerts T., Engelborghs S., Martin J.J., De Deyn P.P. The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Res Ther. 2015;7:1–18. doi: 10.1186/s13195-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeiren Y., Van Dam D., Aerts T., Engelborghs S., De Deyn P.P. Brain region-specific monoaminergic correlates of neuropsychiatric symptoms in Alzheimer's disease. J Alzheimers Dis. 2014;41:819–833. doi: 10.3233/JAD-140309. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 35.Trillo L., Das D., Hsieh W., Medina B., Moghadam S., Lin B. Ascending monoaminergic systems alterations in Alzheimer's disease. Translating basic science into clinical care. Neurosci Biobehav Rev. 2013;37:1363–1379. doi: 10.1016/j.neubiorev.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Mann D.M., Yates P.O., Marcyniuk B., Ravindra C.R. Pathological evidence for neurotransmitter deficits in Down's syndrome of middle age. J Ment Defic Res. 1985;29:125–135. doi: 10.1111/j.1365-2788.1985.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 37.Lyness S.A., Zarow C., Chui H.C. Neuron loss in key cholinergic and aminergic nuclei in Alzheimer disease: A meta-analysis. Neurobiol Aging. 2003;24:1–23. doi: 10.1016/s0197-4580(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 38.Arai H., Ichimiya Y., Kosaka K., Moroji T., Iizuka R. Neurotransmitter changes in early- and late-onset Alzheimer-type dementia. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:883–890. doi: 10.1016/0278-5846(92)90106-o. [DOI] [PubMed] [Google Scholar]
- 39.Simic G., Babić Leko M., Wray S., Harrington C.R., Delalle I., Jovanov-Milošević N. Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol. 2017;151:101–138. doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips C., Fahimi A., Das D., Mojabi F.S., Ponnusamy R., Salehi A. Noradrenergic system in Down syndrome and Alzheimer's disease: A target for therapy. Curr Alzheimer Res. 2016;13:68–83. doi: 10.2174/1567205012666150921095924. [DOI] [PubMed] [Google Scholar]
- 41.Zarow C., Lyness S.A., Mortimer J.A., Chui H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 42.Storga D., Vrecko K., Birkmayer J.G., Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203:29–32. doi: 10.1016/0304-3940(95)12256-7. [DOI] [PubMed] [Google Scholar]
- 43.Mann D.M., Yates P.O., Marcyniuk B. Dopaminergic neurotransmitter systems in Alzheimer's disease and in Down's syndrome at middle age. J Neurol Neurosurg Psychiatry. 1987;50:341–344. doi: 10.1136/jnnp.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibb W.R., Mountjoy C.Q., Mann D.M., Lees A.J. The substantia nigra and ventral tegmental area in Alzheimer's disease and Down's syndrome. J Neurol Neurosurg Psychiatry. 1989;52:193–200. doi: 10.1136/jnnp.52.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seidl R., Kaehler S.T., Prast H., Singewald N., Cairns N., Gratzer M. Serotonin (5-HT) in brains of adult patients with Down syndrome. J Neural Transm. 1999;57:221–232. doi: 10.1007/978-3-7091-6380-1_14. [DOI] [PubMed] [Google Scholar]
- 46.Cirrito J.R., Disabato B.M., Restivo J.L., Verges D.K., Goebel W.D., Sathyan A. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci U S A. 2011;108:14968–14973. doi: 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittle N., Sartori S.B., Dierssen M., Lubec G., Singewald N. Fetal Down syndrome brains exhibit aberrant levels of neurotransmitters critical for normal brain development. Pediatrics. 2007;120:e1465–e1471. doi: 10.1542/peds.2006-3448. [DOI] [PubMed] [Google Scholar]
- 48.Beacher F., Daly E., Simmons A., Prasher V.P., Morris R., Robinson C. Brain anatomy and ageing in non-demented adults with Down's syndrome: an in vivo MRI study. Psychol Med. 2010;40:611–619. doi: 10.1017/S0033291709990985. [DOI] [PubMed] [Google Scholar]
- 49.Wisniewski K.E. Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. Am J Med Genet Suppl. 1990;7:274–281. doi: 10.1002/ajmg.1320370755. [DOI] [PubMed] [Google Scholar]
- 50.Teipel S.J., Hampel H. Neuroanatomy of Down syndrome in vivo: A model of preclinical Alzheimer's disease. Behav Genet. 2006;36:405–415. doi: 10.1007/s10519-006-9047-x. [DOI] [PubMed] [Google Scholar]
- 51.Hartley D., Blumenthal T., Carrillo M.C., DiPaolo G., Esralew L., Gardiner K.J. Down syndrome and Alzheimer's disease: Common pathways, common goals. Alzheimers Dement. 2015;11:700–709. doi: 10.1016/j.jalz.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Head E., Lott I.T., Wilcock D.M., Lemere C.A. Aging in Down syndrome and the development of Alzheimer's disease neuropathology. Curr Alzheimer Res. 2016;13:18–29. doi: 10.2174/1567205012666151020114607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilcock D.M., Griffin W.S. Down's syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation. 2013;10:84. doi: 10.1186/1742-2094-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppus A.M., Fekkes D., Verhoeven W.M., Tuinier S., Egger J.I., van Duijn C.M. Plasma amino acids and neopterin in healthy persons with Down's syndrome. J Neural Transm. 2007;114:1041–1045. doi: 10.1007/s00702-007-0656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstein D.S., Eisenhofer G., Kopin I.J. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 56.O'Bryant S.E., Gupta V., Henriksen K., Edwards M., Jeromin A., Lista S. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Bastard N., De Deyn P.P., Engelborghs S. Importance and impact of preanalytical variables on Alzheimer disease biomarker concentrations in cerebrospinal fluid. Clin Chem. 2015;61:734–743. doi: 10.1373/clinchem.2014.236679. [DOI] [PubMed] [Google Scholar]
- 58.Mattsson N., Andreasson U., Persson S., Arai H., Batish S.D., Bernardini S. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395.e6. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietrzak R.H., Gallezot J.D., Ding Y.S., Henry S., Potenza M.N., Southwick S.M. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry. 2013;70:1199–1205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.