Abstract
Introduction
Overlapping and evolving symptoms lead to ambiguity in the diagnosis of dementia. Visuospatial function relies on parietal lobe function, which may be affected in the early stages of Alzheimer's disease (AD). This review evaluates visuospatial dysfunction in patients with AD, frontotemporal dementia, dementia with Lewy bodies, and vascular dementia to determine the diagnostic and prognostic potential of visuospatial tasks in AD.
Methods
A systematic search of studies (1960–2016) investigating visuospatial dysfunction in dementia was conducted.
Results
Tests measuring construction, specifically Block Design and Clock Drawing Test, and visual memory, specifically Rey-Osterrieth Complex Figure recall and topographical tasks, show the greatest diagnostic potential in dementia. The Benton visual retention, Doors and People, and topographical memory tests show potential as prognostic markers.
Discussion
Tests of visuospatial function demonstrate significant diagnostic and prognostic potential in dementia. Further studies with larger samples of pathologically confirmed cases are required to verify clinical utility.
Keywords: Alzheimer's disease, Frontotemporal dementia, Dementia with Lewy bodies, Vascular dementia, Visuospatial, Diagnosis, Prognosis, Clock Drawing Test, Visual object space perception battery, Rey-Osterrieth Complex Figure, Benton visual retention test
Highlights
-
•
Memory deficits have been demonstrated in Alzheimer's and non-Alzheimer's dementias.
-
•
Parietal lobes are uniquely affected in the early stages of Alzheimer's disease.
-
•
Visuospatial tasks demonstrate significant diagnostic and prognostic potential.
-
•
Computerized test protocols have been developed to test aspects of visuospatial function and memory.
-
•
Novel topographical memory tasks demonstrated the greatest prognostic potential.
1. Introduction
Overlapping and evolving symptoms make existing clinical diagnostic criteria for dementia [1] difficult to apply in a considerable proportion of patients [2]. In vivo markers of brain pathology (e.g., cerebrospinal fluid or amyloid PET imaging) [3] are still largely confined to research settings, so dementia is still primarily diagnosed on clinical grounds [4]. A final pathological diagnosis is restricted to the very few individuals who undergo postmortem examination or those in whom a genetic cause of dementia is identified. This diagnostic ambiguity is unacceptable because it hampers efforts to develop therapies by restricting clinical trial enrollment or necessitating large clinical trials to demonstrate efficacy [4].
It is challenging to make a diagnosis of AD in the earliest stages or in undifferentiated dementia presentations. Distinct atypical AD syndromes are recognized and characterized by prominent visual symptoms (e.g., posterior cortical atrophy), progressive aphasia (e.g., logopenic progressive aphasia), or motor symptoms (e.g., corticobasal syndrome). A proportion of AD patients will not meet the criteria for any single dementia syndrome, including the recognized atypical AD syndromes, but rather present with an undifferentiated mix of cognitive and behavioral symptoms and signs [2].
In vivo diagnostic markers of amyloid pathology have existed for more than 10 years but have not been deployed clinically due to issues with availability, cost, and specificity. For instance, Pittsburgh compound type B positron emission tomography presents logistical problems because of its cost and short half-life [5]. Cerebrospinal fluid analyses require an invasive procedure and demonstrate only modest specificity in undifferentiated dementia syndromes [6], [7]. Meanwhile, the diagnostic utility of structural neuroimaging in early AD is controversial as atrophy may be subtle or nonspecific [8].
Before the development of amyloid biomarkers, efforts to improve AD diagnosis emphasized improving neuropsychological tests of memory [9], [10]. Memory deficits are characteristic of typical AD, but similar deficits of immediate and delayed episodic memory [11], [12], [13] and autobiographical memory [14], [15] have been reported in frontotemporal dementia (FTD). Separately, early language impairment, which is characteristic of language forms of FTD, can be seen in AD [16], and individuals with nonfluent aphasia due to AD remain difficult to distinguish from patients with the progressive nonfluent aphasia phenotype of FTD [17], despite refinement of diagnostic criteria [18]. Limited specificity of memory and language deficits may not be surprising because their neuroanatomical substrates (i.e., the frontal and temporal lobes) can be affected by several underlying pathologies [19], [20], [21].
Unlike memory and language, visuospatial functioning is heavily reliant on parietal lobe integrity [22], [23]. Changes in medial and lateral parietal lobe function or structure occur early in AD [22], [24], [25], [26]. Consequently, tests of visuospatial abilities may prove to be more accurate in differentiating AD and non-AD dementias than other cognitive tests [8], [27], [28]. This review evaluates studies of visuospatial dysfunction in patients with AD, FTD, and other dementias often associated with AD pathology such as dementia with Lewy bodies (DLB) and vascular dementia (VaD). First, a critical overview of the various components of visuospatial function and their neural bases is presented. Then, the diagnostic and prognostic potential of visuospatial tasks in AD and non-AD dementias is considered.
2. Methods
Studies of visuospatial dysfunction in dementia were identified using a systematic search process. A combination of keywords, including “visuospatial function,” “Alzheimer's disease,” “frontotemporal dementia,” “dementia with Lewy bodies,” “vascular dementia,” and “neuropsychological test,” was searched in MEDLINE, EMBASE, and PubMed, generating a total of 297 abstracts from 1960 to 2016. Duplicates, non-English articles, and case studies/series were excluded. Articles were excluded primarily because of their focus on neurological disorders, neuropsychological tests, and cognitive domains beyond the scope of this review. Preference was given to studies of pathologically confirmed dementia cohorts. Seventy-two additional records were identified through bibliographic research. These were reviewed for relevance, and 100 papers comprising review articles and experimental studies regarding visuospatial function in dementia remained for full review. If peer-reviewed original studies or review articles were not available, textbooks were consulted.
3. What is visuospatial function?
Broadly defined, visuospatial function is the ability to specify the parts and overall configuration of a percept, appreciate its position in space, integrate a coherent spatial framework, and perform mental operations on spatial concepts [22], [23]. Visuospatial function is commonly conceptualized in three components: visual perception, construction, and visual memory [29].
At its most basic level, visual perception involves light perception, contrast sensitivity, stimulus orientation, visual acuity, detection of color and motion, and processes mediated primarily by the occipital cortices. Progressive integration of this visual perception involves input from the parietal, temporal, and frontal cortices. Progressive integration of visual information occurs via two major visual processing streams: the ventral “what” stream and the dorsal “where” stream [27], [30], [31]. The ventral stream is responsible for (1) resolving visual interference; (2) the ability to identify an object masked by an overlapping picture; and (3) the ability to make sense of fragmented or ambiguously presented objects [29]. The dorsal “where” stream is responsible for spatial orientation and relies on posterior and inferior parietal regions [29].
Visual memory consists of two main components: recall (or recognition) of visual information and topographical memory. Topographical memory involves perception and encoding of spatial orientation to navigate surroundings. Topographical orientation is characterized as being either egocentric (relative to the self) or allocentric (relative to other objects).
3.1. Incidence of visuospatial deficits in dementia
Visuospatial dysfunction is among the earliest manifestations of AD [8], [32], eventually affecting 20%–43% of patients [27], [32], [33], [34]. One study showed disabling visuospatial disorientation in more than one-third of AD patients [35] while almost half of patients complained of visuospatial problems when questioned directly [34]. AD patients may describe impaired discrimination of form, colors and contrast, motion detection, as well as disturbances of higher order functions such as reading, visuospatial orientation, and visual search strategies [8], [27], [31], [32]. Recent work also indicates marked deficits in the ability to mentally envisage and provide accurate descriptions of visuospatial scenes in AD [36].
Deficits in processing of visuospatial information are present in some, but not all, dementias. For example, DLB is characterized by visual hallucinations, visual agnosia, and constructional impairments [37], [38]. Visuospatial deficits, more particularly impaired constructional praxis, results in poor size and form discrimination, misidentification of overlapping figures, and poor visual counting on neuropsychological testing [22], [37]. In this disease, these deficits have been associated with abnormalities of the primary visual and visual association cortices on brain imaging [22], [37], [38], [39]. In contrast, visuospatial abilities appear to be relatively preserved in the early stages of FTD, likely explained by the relative sparing of posterior brain structures by the disease [37], [39], [40], [41].
4. Neuropsychological tests assessing visuospatial function
4.1. A potential diagnostic marker?
A range of neuropsychological tests have been developed to assess visuospatial function, spanning from traditional paper-based tests to interactive computer simulations. Many visuospatial tasks rely on other cognitive abilities (e.g., memory, attention, and executive planning) and motor function, which often complicates interpretation of performance [8], [22], [38]. Several studies (Table 1) have demonstrated diagnostic potential for visuospatial tests in distinguishing AD from non-AD dementia syndromes [8] and in detecting dementias associated with AD copathology [38]. Studies investigating tests of visuospatial function as markers of AD pathology in undifferentiated or uncertain dementia presentations are sparse, and further research is required [8], [25], [39].
Table 1.
Summary of studies comparing visuospatial function in AD, DLB, FTD, and VaD
| Test | Studies | Subtest/scoring | Results |
|---|---|---|---|
| Visual perception | Calderon et al., 2001 [45] | Incomplete letters | DLB < AD |
| VOSP | Silhouettes | DLB = AD | |
| Cube analysis | DLB < AD | ||
| Siri et al., 2001 [44] | Cube analysis | AD < FTD | |
| Pengas et al., 2010 [73] | Cube analysis | AD = semantic dementia | |
| JLO | Ota et al., 2015 [65] | Overall score | DLB < AD |
| Simard et al., 2003 [47] | Overall score | DLB = AD | |
| Hodges et al., 1999 [49] | Overall score | AD = FTD | |
| Grossi et al., 2002 [48] | Overall score | AD = FTD | |
| Construction | Shimomura et al., 1998 [63] | WAIS-III | DLB < AD |
| Block design | Hansen et al., 1990 [61] | WAIS-III | DLB < AD |
| Galasko et al., 1996 [59] | WISC | DLB < AD | |
| Johnson et al., 2005 [62] | WISC | DLB/AD < DLB < AD | |
| Rascovsky et al., 2002 [56] | WISC | AD < FTD | |
| CDT | Ota et al., 2015 [65] | Copy | DLB = AD |
| Galasko et al., 1996 [59] | Copy | DLB < AD | |
| Gnanalingham et al., 1997 [60] | Copy | DLB < AD | |
| Cahn-Weiner et al., 2003 [64] | Command | DLB = AD | |
| Blair et al., 2006 [40] | Command | AD < FTD | |
| Rascovsky et al., 2002 [56] | Copy and command | AD < FTD | |
| Schmidtke and Olbrich, 2007 [54] | Circle predrawn | AD = FTD | |
| Moretti et al., 2002 [55] | Circle predrawn | AD < FTD = VaD | |
| Libon et al., 1993 [68] | Copy | VaD < AD | |
| Kitabayashi et al., 2001 [67] | Command | VaD < AD | |
| Heinik et al., 2002 [66] | Command | VaD < AD | |
| RCF copy | Grossi et al., 2002 [48] | Copy | AD = FTD |
| Kramer et al., 2003 [57] | Copy | AD = FTD | |
| Irish et al., 2014 [12] | Copy | AD = FTD | |
| Pachana et al., 1996 [41] | Copy | AD < FTD | |
| Irish et al., 2016 [88] | Copy | AD < semantic dementia | |
| Pengas et al., 2010 [73] | Copy | AD < semantic dementia | |
| Visual memory | Kramer et al., 2003 [57] | Recall | AD < FTD |
| RCF recall | Perry and Hodges, 2000 [89] | Recall | AD < FTD |
| Siri et al., 2001 [44] | Recall | AD < FTD | |
| Pachana et al., 1996 [41] | Recall | AD < FTD | |
| Irish et al., 2014 [12] | Recall | AD = FTD | |
| Galton et al., 2001 [90] | Recall | AD < semantic dementia | |
| Pengas et al., 2010 [73] | Recall | AD < semantic dementia | |
| Irish et al., 2016 [88] | Recall | AD < semantic dementia | |
| Benton visual retention test | Grossi et al., 2002 [48] | Copy | AD = FTD |
| Johnson et al., 2005 [62] | Copy and recall | DLB/AD < DLB < AD | |
| Doors | Irish et al., 2014 [12] | Recognition | AD = bvFTD |
| Graham et al., 2004 [71] | Recognition | AD = VaD | |
| Virtual supermarket test | Tu et al., 2015 [72] | AD < FTD | |
| Virtual route learning task | Pengas et al., 2010 [73] | AD < semantic dementia | |
| CANTAB PAL | Lee et al., 2003 [77] | AD < FTD | |
| CANTAB MTS | Lee et al., 2003 [77] | AD = FTD |
Abbreviations: AD, Alzheimer's disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; VOSP, visual object and space perception battery; JLO, judgment of line orientation; CDT, Clock Drawing Test; RCF, Rey-Osterrieth Complex Figure; CANTAB, Cambridge Neuropsychological Test Automated Battery; PAL, Paired Associates Learning Task; MTS, matching-to-sample task.
4.1.1. Visual perception
Higher visual processing is commonly assessed using the visual object and space perception battery (VOSP) [42]. For example, the “incomplete letters” subtest is an efficient test of visual interference [27], [43] while spatial skills are assessed via the “cube analysis” and “dot counting” subtests. The VOSP is simple, paper-based, and independent of language and motor function [27], [29], [43]. Administration of the full suite of tasks, however, is time consuming and performance requires intact attention which is often compromised in AD [29], [43]. Although evidence is limited, the “cube analysis” subtest may be helpful in discriminating AD from FTD [44]. Meanwhile, DLB patients appear to be impaired on the VOSP, with some studies reporting equivalent or worse performance than in AD patients depending on the subtest [45].
Spatial orientation may also be assessed by Benton's judgment of line orientation (JLO) test [46]. The JLO test requires minimal motor skills and comprehension abilities, is free of practice effects, and addresses a relatively low-level visuospatial skill [43]. Disadvantages include administration time (30 items), dependence on intact visual attention [29], and an oversimplified multiple-choice format [47]. Like the VOSP, the total score of the JLO test may show little difference between dementia syndromes [47], [48], [49], but detailed error analysis might be more useful [47].
4.1.2. Construction
Object movement tasks, such as the Block Design subtest from the Wechsler adult intelligence scales IV [50] and 3D block construction [51], are often used to assess constructional ability [27]. Both tests involve secondary functions including spatial orientation, motor skills, and executive planning, so separating the contributions of each function is difficult [27], [29].
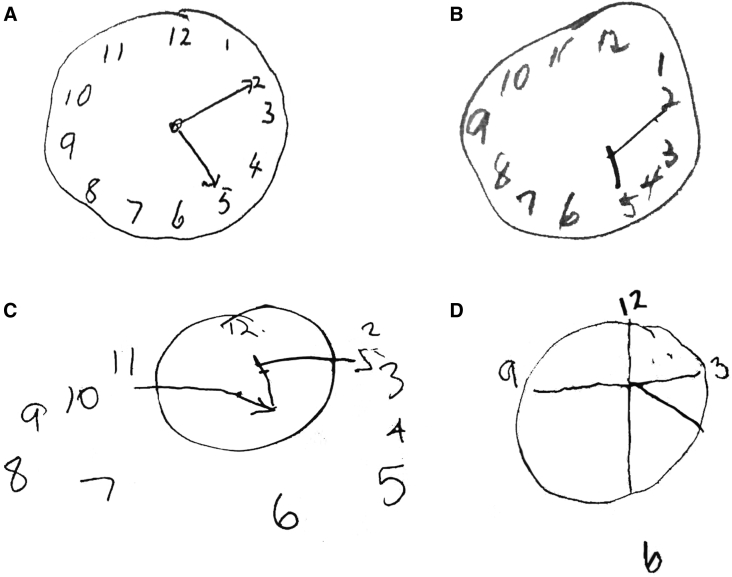
Drawing tasks such as the Clock Drawing Test (CDT) and the Rey-Osterrieth Complex Figure (RCF) [52] test are commonly used to assess construction [27]. The CDT copy (where the picture of a clock is shown) and command (where the clock is drawn from memory) components are quick and easy to administer, irrespective of culture and language (Fig. 1) [27], [43], [53]. The test requires executive processing, semantic and linguistic competence, numerical knowledge, motor skills, memory concentration, and attention [40], [53]. Although this complex integration of functions makes the CDT an excellent screening tool [22], [40], [53], its ability to differentiate focal impairments without error analysis is modest [40], [53]. For this reason, over a dozen scoring criteria have been proposed to categorize errors; however, none have achieved universal acceptance [40].
Fig. 1.
Clock Drawing Test (A–D). Four examples of increasingly impaired clock faces from different patients with dementia.
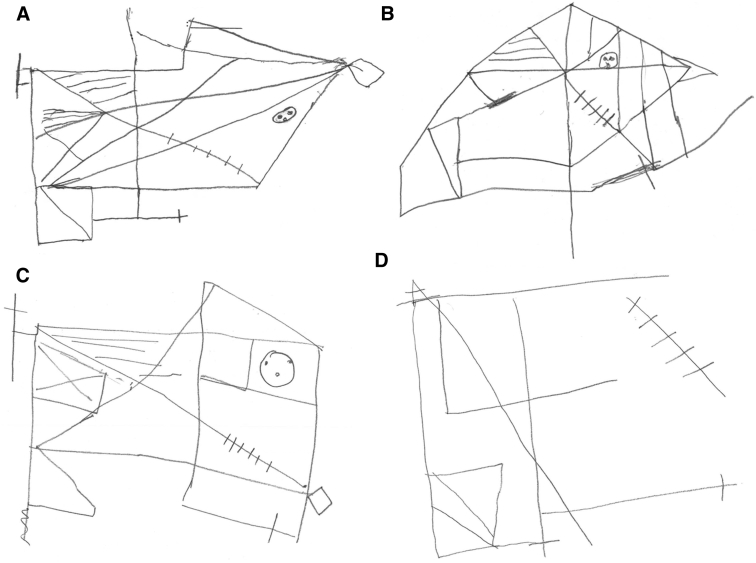
The RCF test (Fig. 2) requires executive planning, organization, motor, spatial orientation, attention, and concentration, all of which contribute to intact performance [22], [27], [29], [43]. Some aspects of the RCF test scoring system are subject to ambiguity (e.g., the distinction of distortion compared to misplacement). Furthermore, the scoring system neglects qualitative aspects of performance such as strategy, delays, and organization [43].
Fig. 2.
Rey-Osterrieth Complex Figure (A–D). Four examples of increasingly impaired copy drawings of the original Rey-Osterrieth Complex Figure across different dementia subtypes.
Variability in test paradigms and the impact of other cognitive or motor impairments [43], [54] limit the reliability of construction tasks as markers of AD. For example, FTD patients perform better than AD patients on the block design and CDT [40], [55], [56], but performance on the RCF copy is less consistent [12], [41], [48], [57], perhaps reflecting executive planning impairment and impulsivity in FTD [8], [58], rather than visuospatial deficits per se. The pattern in DLB is unclear; some studies demonstrate worse performance on construction tasks than in AD [59], [60], [61], [62], [63], while others report the opposite pattern [64], [65]. Inattention, impaired motor skills, and executive dysfunction in DLB have all been proposed as potential confounds on construction tasks [64], [65]. VaD patients have been shown to perform worse on the CDT than AD patients [66], [67], [68]. In a study by Moretti, these findings were contradicted [55]; however, their predrawn circle may measure numerical and spatial ability rather than construction.
4.1.3. Visual memory
The delayed recall component of the RCF (i.e., reproduction from memory after a delay of 3–45 minutes) is a common test used to assess visual recall and recognition. This test, however, is subject to the same confounds as the copy component [43]. Visual recognition memory can be tested using tasks such as the Benton visual retention test [69], which incorporates visual recognition, recall, and spatial orientation [69], as well as additional motor, construction, and visual perception abilities [29]. Impulsivity, however, may impair performance, and correct responses may be determined strategically [43]. Nevertheless, the Benton visual retention test has been rarely administered across dementia syndromes, and thus, its diagnostic utility in this context remains unclear.
The doors subtest of the “Doors and People” test is a forced visual recognition multiple-choice test involving pictures of doors. The test primarily detects a learning deficit, particularly in the early stages of AD [70]. Again, this test has not been extensively tested across the range of dementia syndromes; however, the available evidence demonstrates little value in discriminating AD from FTD or VaD [12], [71].
4.2. New directions in the assessment of visuospatial dysfunction
Computerized testing paradigms to assess topographical memory demonstrate promise as diagnostic tools for AD. Topographical memory tests represent a novel and ecologically valid approach to test functions purported to rely on the integrity of posterior parietal brain regions. Computerized tests of topographical memory are increasingly being used to simulate virtual environments and engage egocentric spatial processes, while reducing motor and language demands. For example, the virtual supermarket [72] and the virtual route learning tests [73] guide the participant through a supermarket and town, respectively, and require the participant to repeat routes, choose a route to a specific landmark, or indicate the direction of the starting point [72], [73]. Early studies have demonstrated that patients with FTD tend to outperform those with AD on such computerized topographical memory tasks, even after accounting for episodic memory differences [72]. A systematic review confirms the clinical utility of topographical memory tasks in distinguishing AD from FTD [74]. The use of computerized testing protocols and automated scoring may reduce administration and interpretation time, making these approaches particularly appealing for use in dementia.
Computerized tests of visuospatial memory also show promise as AD diagnostic tools. For example, performance on the Groton Maze Learning Test [75], which incorporates testing of visuospatial memory and executive function, appears sensitive to cognitive changes in healthy aging. The Cambridge Neuropsychological Test Automated Battery is a battery of computerized tests, including the Paired Associates Learning (PAL) task and the simultaneous and delayed matching-to-sample (MTS) tasks [76]. The PAL task primarily tests visuospatial associative learning. In this task, a visual stimulus is briefly displayed in one of up to eight boxes. The stimulus later reappears, and the subject must choose which box it was originally displayed in. The MTS task is a purer task of visual recognition memory, where a rectangular pattern is shown and must be matched to 1 of 4 options displayed below; the options are presented simultaneously to the original stimulus or after a delay [77]. One small study demonstrated significantly poorer performance on the PAL task in AD than in FTD. Although performance on the MTS task did not differ between groups overall, the pattern of errors differed slightly between diagnostic groups [77]. Similar studies have demonstrated impaired performance in AD compared to controls [77], [78], [79], [80], and relatively intact performance in FTD compared to controls [81], on both PAL and MTS tasks. A further study reported even greater impairment in DLB than in AD patients on the delayed MTS task [82]. The CogState battery includes tests of visual recognition (One Card Learning), visual recall (Continuous Paired Associate Learning), and visual attention (Identification task) [83], [84]. A number of small studies comparing performance on these tasks demonstrated little discriminative ability between AD, DLB, and FTD [85], [86], [87]. Studies of computerized testing protocols in larger patient cohorts are required to establish their clinical utility.
4.3. Prognostic marker
Visuospatial function shows potential as a cognitive marker for the detection of preclinical AD [10], [27]. Specifically, visuospatial deficits may be detected up to 5 years before the onset of AD symptoms in a nondemented elderly population [91] often before the development of frank memory impairment [25], [92]. Separately, one study of 1425 participants demonstrated that six errors or more on the Benton visual retention test doubled the risk of developing AD, even up to 15 years before diagnosis [93]. The Doors and People test has demonstrated value in predicting those who convert from mild cognitive impairment (MCI) to AD [94], [95], as have tests of topographical memory [10], [27], [93], [96], the Groton Maze Learning Test [97], and the PAL task of the Cambridge Neuropsychological Test Automated Battery [78], [94], [98].
The rates of decline in performance on computerized visual memory tasks have also been associated with amyloid deposition on PiB-PET in healthy controls and patients with MCI or conversion from MCI to AD. Specifically, declines in performance on the Cogstate's One Card Learning, One Back Learning, and Continuous Paired Associate tasks over 36 months were reported to be significantly greater in controls and MCI patients with high amyloid deposition than those with low amyloid deposition [99]. Furthermore, intraindividual decline in the One Card Learning task was predictive of cerebral amyloid deposition in healthy elderly adults [100].
5. Summary
The diagnosis and management of dementia is challenging, in part because of diagnostic uncertainty and variability in prognosis. Visuospatial function is a relatively underreported symptom that relies on parietal lobes structures that are damaged in early-stage AD. Neuropsychological measures of visuospatial function may offer a practical and noninvasive approach to the diagnosis of AD or AD copathology. Current neuropsychological assessments demonstrate significant diagnostic and prognostic potential, but existing studies are limited by small numbers, clinically defined (not autopsy-confirmed) cohorts [38], and confounds by nonvisuospatial cognitive abilities and/or motor deficits. Tests of topographical memory demonstrate the greatest promise, but further studies with larger sample sizes of pathologically confirmed cases are required, as a prelude to implementation in diagnostically undifferentiated dementia cohorts.
Research in Context.
-
1.
Systematic review: A systematic search of studies (1960–2016) investigating visuospatial dysfunction in dementia was conducted. The resulting articles were evaluated, and 100 papers remained for full review.
-
2.
Interpretation: Our study has demonstrated that neuropsychological measures of visuospatial function may prove sensitive to parietal lobe damage, offering a practical and noninvasive approach to the diagnosis of AD or AD copathology. Tests of construction, specifically Block Design and Clock Drawing Test, and visual memory, specifically Rey Complex Figure recall and topographical tasks, show the greatest diagnostic potential. The Benton visual retention, Doors and People, and topographical memory tests show potential as prognostic markers.
-
3.
Future direction: Existing studies are limited by small numbers, non–autopsy-confirmed cohorts, and are potentially confounded by nonvisuospatial cognitive abilities and/or motor deficits. Further studies with larger sample sizes of pathologically confirmed cases are required.
Acknowledgments
This work was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council of Australia (NHMRC) program grant (#1037746) and the Australian Research Council (ARC) Centre of Excellence in Cognition and its Disorders Memory Node (#CE110001021). M.I. is supported by an ARC Future Fellowship (FT160100096). O.P. is supported by an NHMRC Senior Research Fellowship (APP1103258). J.R.B. is supported by an NHMRC Early Career Fellowship (#1072451).
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.American Psychiatric Association . American Psychiatric Pub; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- 2.Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the Clinical Diagnosis of Alzheimer Disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie C.W., Terrera G.M., Quinn T.J. Dementia trials and dementia tribulations: methodological and analytical challenges in dementia research. Alzheimers Res Ther. 2015;7:31. doi: 10.1186/s13195-015-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabinovici G.D., Rosen H.J., Alkalay A., Kornak J., Furst A.J., Agarwal N. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77:2034–2042. doi: 10.1212/WNL.0b013e31823b9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida O.P. Review: most laboratory tests do not add to the diagnostic accuracy of clinical criteria for dementia. Evid Based Ment Health. 2002;5:26. doi: 10.1136/ebmh.5.1.26. [DOI] [PubMed] [Google Scholar]
- 7.De Leon M.J., DeSanti S., Zinkowski R., Mehta P.D., Pratico D., Segal S. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Intern Med. 2004;256:205–223. doi: 10.1111/j.1365-2796.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 8.Harciarek M., Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer's disease: a review. Neuropsychol Rev. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- 9.Burrell J.R., Hodges J.R. Oxford University Press, Incorporated; New York, NY: 2015. Dementia. Landmark Pap. Neurol., 2015; pp. 289–324. [Google Scholar]
- 10.Chapman R.M., Mapstone M., McCrary J.W., Gardner M.N., Porsteinsson A., Sandoval T.C. Predicting conversion from mild cognitive impairment to Alzheimer's disease using neuropsychological tests and multivariate methods. J Clin Exp Neuropsychol. 2011;33:187–199. doi: 10.1080/13803395.2010.499356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisch S., Dukart J., Vogt B., Horstmann A., Becker G., Villringer A. Dissociating memory networks in early Alzheimer's disease and frontotemporal lobar degeneration - a combined study of hypometabolism and atrophy. PLoS One. 2013;8:e55251. doi: 10.1371/journal.pone.0055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irish M., Piguet O., Hodges J.R., Hornberger M. Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer's disease. Hum Brain Mapp. 2014;35:1422–1435. doi: 10.1002/hbm.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennington C., Hodges J.R., Hornberger M. Neural correlates of episodic memory in behavioral variant frontotemporal dementia. J Alzheimers Dis. 2011;24:261–268. doi: 10.3233/JAD-2011-101668. [DOI] [PubMed] [Google Scholar]
- 14.Irish M., Hornberger M., Lah S., Miller L., Pengas G., Nestor P.J. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer's disease. Neuropsychologia. 2011;49:2694–2702. doi: 10.1016/j.neuropsychologia.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Irish M., Hornberger M., Wahsh S.E., Lam B.Y.K., Lah S., Miller L. Grey and white matter correlates of recent and remote autobiographical memory retrieval—insights from the dementias. PLoS One. 2014;9:e113081. doi: 10.1371/journal.pone.0113081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorno-Tempini M.L., Dronkers N.F., Rankin K.P., Ogar J.M., Phengrasamy L., Rosen H.J. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chare L., Hodges J.R., Leyton C.E., McGinley C., Tan R.H., Kril J.J. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014;85:865–870. doi: 10.1136/jnnp-2013-306948. [DOI] [PubMed] [Google Scholar]
- 18.Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castiglioni S., Pelati O., Zuffi M., Somalvico F., Marino L., Tentorio T. The frontal assessment battery does not differentiate frontotemporal dementia from Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22:125–131. doi: 10.1159/000093665. [DOI] [PubMed] [Google Scholar]
- 20.Hornberger M., Wong S., Tan R., Irish M., Piguet O., Kril J. In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer's disease. Brain. 2012;135:3015–3025. doi: 10.1093/brain/aws239. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs H.I.L., Van Boxtel M.P.J., Jolles J., Verhey F.R.J., Uylings H.B.M. Parietal cortex matters in Alzheimer's disease: an overview of structural, functional and metabolic findings. Neurosci Biobehav Rev. 2012;36:297–309. doi: 10.1016/j.neubiorev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Geldmacher D.S. Visuospatial dysfunction in the neurodegenerative diseases. Front Biosci J Virtual Libr. 2003;8:e428–e436. doi: 10.2741/1143. [DOI] [PubMed] [Google Scholar]
- 23.Stiles J., Akshoomoff N., Haist F. Academic Press; San Diego, CA: 2013. Neural circuit development and function in the brain: comprehensive developmental neuroscience. [Google Scholar]
- 24.Buckner R.L., Snyder A.Z., Shannon B.J., LaRossa G., Sachs R., Fotenos A.F. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal P.K., Joshi J., Saharan S. Visuospatial perception: an emerging biomarker for Alzheimer's disease. J Alzheimers Dis. 2012;31:S117–S135. doi: 10.3233/JAD-2012-120901. [DOI] [PubMed] [Google Scholar]
- 26.Possin K.L., Laluz V.R., Alcantar O.Z., Miller B.L., Kramer J.H. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer's disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49:43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iachini T., Iavarone A., Senese V.P., Ruotolo F., Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2:43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- 28.Tiraboschi P., Salmon D.P., Hansen L.A., Hofstetter R.C., Thal L.J., Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129:729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 29.Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. 5th ed. Oxford University Press; New York, NY: 2012. Neuropsychological assessment. [Google Scholar]
- 30.Barton J. LWW; 2014. Higher cortical visual deficits: CONTINUUM: lifelong learning in neurology.http://journals.lww.com/continuum/Fulltext/2014/08000/Higher_Cortical_Visual_Deficits.14.aspx Available at: Accessed March 26, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quental N.B.M., Brucki S.M.D., Bueno O.F.A. Visuospatial function in early Alzheimer's disease—the use of the visual object and space perception (VOSP) battery. PLoS One. 2013;8:e68398. doi: 10.1371/journal.pone.0068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quental N.B.M., Brucki S.M.D., Bueno O.F.A. Visuospatial function in early Alzheimer's disease. Dement Neuropsychol. 2009;3:234–240. doi: 10.1590/S1980-57642009DN30300010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronin-Golomb A., Corkin S., Rizzo J.F., Cohen J., Growdon J.H., Banks K.S. Visual dysfunction in Alzheimer's disease: relation to normal aging. Ann Neurol. 1991;29:41–52. doi: 10.1002/ana.410290110. [DOI] [PubMed] [Google Scholar]
- 34.Mendez M.F., Mendez M.A., Martin R., Smyth K.A., Whitehouse P.J. Complex visual disturbances in Alzheimer's disease. Neurology. 1990;40:439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- 35.Monacelli A.M., Cushman L.A., Kavcic V., Duffy C.J. Spatial disorientation in Alzheimer's disease: the remembrance of things passed. Neurology. 2003;61:1491–1497. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- 36.Irish M., Halena S., Kamminga J., Tu S., Hornberger M., Hodges J.R. Scene construction impairments in Alzheimer's disease—a unique role for the posterior cingulate cortex. Cortex. 2015;73:10–23. doi: 10.1016/j.cortex.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Cronin-Golomb A. Wiley-Blackwell; West Sussex, UK: 2011. Visuospatial function in Alzheimer's disease and related disorders. Handb. Alzheimers Dis. Dement; p. 457. [Google Scholar]
- 38.Salmon D.P., Bondi M.W. Neuropsychological assessment of dementia. Annu Rev Psychol. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weintraub S., Wicklund A.H., Salmon D.P. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair M., Kertesz A., Mcmonagle P., Davidson W., Bodi N. Quantitative and qualitative analyses of clock drawing in frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2006;12:159–165. doi: 10.1017/S1355617706060255. [DOI] [PubMed] [Google Scholar]
- 41.Pachana N.A., Boone K.B., Miller B.L., Cummings J.L., Berman N. Comparison of neuropsychological functioning in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc. 1996;2:505–510. doi: 10.1017/s1355617700001673. [DOI] [PubMed] [Google Scholar]
- 42.Warrington E.K., James M. 1991. The visual object and space perception battery. [Google Scholar]
- 43.Strauss E., Sherman E.M.S., Spreen O. Oxford University Press; 2006. A compendium of neuropsychological tests: administration, norms, and commentary. [Google Scholar]
- 44.Siri S., Benaglio I., Frigerio A., Binetti G., Cappa S.F. A brief neuropsychological assessment for the differential diagnosis between frontotemporal dementia and Alzheimer's disease. Eur J Neurol. 2001;8:125–132. doi: 10.1046/j.1468-1331.2001.00179.x. [DOI] [PubMed] [Google Scholar]
- 45.Calderon J., Perry R.J., Erzinclioglu S.W., Berrios G.E., Dening T.R., Hodges J.R. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benton A., Hamsher K., Varney N., Spreen O. Oxford University Press; New York, NY: 1983. Judgement of line orientation, contributions to neuropsychological assessment. [Google Scholar]
- 47.Simard M., van Reekum R., Myran D. Visuospatial impairment in dementia with Lewy bodies and Alzheimer's disease: a process analysis approach. Int J Geriatr Psychiatry. 2003;18:387–391. doi: 10.1002/gps.839. [DOI] [PubMed] [Google Scholar]
- 48.Grossi D., Fragassi N.A., Chiacchio L., Valoroso L., Tuccillo R., Perrotta C. Do visuospatial and constructional disturbances differentiate frontal variant of frontotemporal dementia and Alzheimer's disease? An experimental study of a clinical belief. Wiley Intersci. 2002;17:641–648. doi: 10.1002/gps.654. [DOI] [PubMed] [Google Scholar]
- 49.Hodges J.R., Patterson K., Ward R., Garrard P., Bak T., Perry R. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer's disease: a comparative neuropsychological study. Neuropsychology. 1999;13:31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- 50.Wechsler D. 2014. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) [Google Scholar]
- 51.Benton A.L., Fogel M.L. Three-dimensional constructional praxis: a clinical test. Arch Neurol. 1962;7:347–354. doi: 10.1001/archneur.1962.04210040099011. [DOI] [PubMed] [Google Scholar]
- 52.Osterrieth P. Le test de copie d'une figure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- 53.Shulman K.I. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.Schmidtke K., Olbrich S. The Clock Reading Test: validation of an instrument for the diagnosis of dementia and disorders of visuo-spatial cognition. Int Psychogeriatr. 2007;19:307–321. doi: 10.1017/S104161020600456X. [DOI] [PubMed] [Google Scholar]
- 55.Moretti R., Torre P., Antonello R.M., Cazzato G., Bava A. Ten-point clock test: a correlation analysis with other neuropsychological tests in dementia. Int J Geriatr Psychiatry. 2002;17:347–353. doi: 10.1002/gps.600. [DOI] [PubMed] [Google Scholar]
- 56.Rascovsky K., Salmon D.P., Ho G.J., Galasko D., Peavy G.M., Hansen L.A. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–1808. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- 57.Kramer J.H., Jurik J., Sha S.J., Rankin K.P., Rosen H.J., Johnson J.K. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Hutchinson A.D., Mathias J.L. Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: a meta-analytic review. J Neurol Neurosurg Psychiatry. 2007;78:917–928. doi: 10.1136/jnnp.2006.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galasko D., Katzman R., Salmon D.P., Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain Cogn. 1996;31:166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- 60.Gnanalingham K.K., Byrne E.J., Thornton A., Sambrook M.A., Bannister P. Motor and cognitive function in Lewy body dementia: comparison with Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry. 1997;62:243–252. doi: 10.1136/jnnp.62.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen L., Salmon D., Galasko D., Masliah E., Katzman R., DeTeresa R. The Lewy body variant of Alzheimer's disease: A clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- 62.Johnson D.K., Morris J.C., Galvin J.E. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- 63.Shimomura T., Mori E., Yamashita H., Imamura T., Hirono N., Hashimoto M. Cognitive loss in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 1998;55:1547–1552. doi: 10.1001/archneur.55.12.1547. [DOI] [PubMed] [Google Scholar]
- 64.Cahn-Weiner D.A., Williams K., Grace J., Tremont G., Westervelt H., Stern R.A. Discrimination of dementia with Lewy bodies from Alzheimer disease and Parkinson disease using the Clock Drawing Test. Cogn Behav Neurol. 2003;16:85–92. doi: 10.1097/00146965-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Ota K., Murayama N., Kasanuki K., Kondo D., Fujishiro H., Arai H. Visuoperceptual assessments for differentiating dementia with Lewy bodies and Alzheimer's disease: illusory contours and other neuropsychological examinations. Arch Clin Neuropsychol. 2015;30:256–263. doi: 10.1093/arclin/acv016. [DOI] [PubMed] [Google Scholar]
- 66.Heinik J., Solomesh I., Raikher B., Lin R. Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study. Int J Geriatr Psychiatry. 2002;17:699–703. doi: 10.1002/gps.678. [DOI] [PubMed] [Google Scholar]
- 67.Kitabayashi Y., Ueda H., Narumoto J., Nakamura K., Kita H., Fukui K. Qualitative analyses of clock drawings in Alzheimer's disease and vascular dementia. Psychiatry Clin Neurosci. 2001;55:485–491. doi: 10.1046/j.1440-1819.2001.00894.x. [DOI] [PubMed] [Google Scholar]
- 68.Libon D.J., Swenson R.A., Barnoski E.J., Sands L.P. Clock drawing as an assessment tool for dementia. Arch Clin Neuropsychol. 1993;8:405–415. [PubMed] [Google Scholar]
- 69.Benton A., Sivan A. Psychological Corporation; San Antonio, TX: 1992. Benton Visual Retention Test: Manual. [Google Scholar]
- 70.Greene J.D.W., Baddeley A.D., Hodges J.R. Analysis of the episodic memory deficit in early Alzheimer's disease: evidence from the doors and people test. Neuropsychologia. 1996;34:537–551. doi: 10.1016/0028-3932(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 71.Graham N.L., Emery T., Hodges J.R. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- 72.Tu S., Wong S., Hodges J.R., Irish M., Piguet O., Hornberger M. Lost in spatial translation—a novel tool to objectively assess spatial disorientation in Alzheimer's disease and frontotemporal dementia. Cortex. 2015;67:83–94. doi: 10.1016/j.cortex.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Pengas G., Patterson K., Arnold R.J., Bird C.M., Burgess N., Nestor P.J. Lost and found: bespoke memory testing for Alzheimer's disease and semantic dementia. J Alzheimers Dis. 2010;21:1347–1365. doi: 10.3233/jad-2010-100654. [DOI] [PubMed] [Google Scholar]
- 74.Serino S., Cipresso P., Morganti F., Riva G. The role of egocentric and allocentric abilities in Alzheimer's disease: a systematic review. Ageing Res Rev. 2014;16:32–44. doi: 10.1016/j.arr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Pietrzak R.H., Cohen H., Snyder P.J. Spatial learning efficiency and error monitoring in normal aging: an investigation using a novel hidden maze learning test. Arch Clin Neuropsychol. 2007;22:235–245. doi: 10.1016/j.acn.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Robbins T.W. IET Conference Publications; 1994. Cambridge Neuropsychological Test Automated Battery (CANTAB): utility and validation. IEE Colloquium on ‘Computer-Aided Tests of Drug Effectiveness’. p. 3/1-3/3. [Google Scholar]
- 77.Lee A.C.H., Rahman S., Hodges J.R., Sahakian B.J., Graham K.S. Associative and recognition memory for novel objects in dementia: implications for diagnosis. Eur J Neurosci. 2003;18:1660–1670. doi: 10.1046/j.1460-9568.2003.02883.x. [DOI] [PubMed] [Google Scholar]
- 78.Égerházi A., Berecz R., Bartók E., Degrell I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:746–751. doi: 10.1016/j.pnpbp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 79.Sahakian B.J., Owen A.M. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 80.O'Connell H., Coen R., Kidd N., Warsi M., Chin A.-V., Lawlor B.A. Early detection of Alzheimer's disease (AD) using the CANTAB paired Associates Learning Test. Int J Geriatr Psychiatry. 2004;19:1207–1208. doi: 10.1002/gps.1180. [DOI] [PubMed] [Google Scholar]
- 81.Rahman S., Robbins T.W., Sahakian B.J. Comparative cognitive neuropsychological studies of frontal lobe function: implications for therapeutic strategies in frontal variant frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10:15–28. doi: 10.1159/000051207. [DOI] [PubMed] [Google Scholar]
- 82.Sahgal A., Galloway P.H., McKeith I.G., Edwardson J.A., Lloyd S. A comparative study of attentional deficits in senile dementias of Alzheimer and Lewy body types. Dement Geriatr Cogn Disord. 1992;3:350–354. [Google Scholar]
- 83.Mielke M.M., Weigand S.D., Wiste H.J., Vemuri P., Machulda M.M., Knopman D.S. Independent comparison of CogState computerized testing and a standard cognitive battery with neuroimaging. Alzheimers Dement J Alzheimers Assoc. 2014;10:779–789. doi: 10.1016/j.jalz.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wild K., Howieson D., Webbe F., Seelye A., Kaye J. The status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement J Alzheimers Assoc. 2008;4:428–437. doi: 10.1016/j.jalz.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hammers D., Spurgeon E., Ryan K., Persad C., Heidebrink J., Barbas N. Reliability of repeated cognitive assessment of dementia using a brief computerized battery. Am J Alzheimers Dis Dementiasr. 2011;26:326–333. doi: 10.1177/1533317511411907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammers D., Spurgeon E., Ryan K., Persad C., Barbas N., Heidebrink J. Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol. 2012;25:89–99. doi: 10.1177/0891988712447894. [DOI] [PubMed] [Google Scholar]
- 87.Hammers D., Spurgeon E., Ryan K., Persad C., Talton K., Coulas T. Diagnostic discrimination of mild cognitive impairment, Alzheimer's disease, and other dementia groups using the CogState clinic battery. Alzheimers Dement J Alzheimers Assoc. 2010;6:e51–e52. [Google Scholar]
- 88.Irish M., Bunk S., Tu S., Kamminga J., Hodges J.R., Hornberger M. Preservation of episodic memory in semantic dementia: the importance of regions beyond the medial temporal lobes. Neuropsychologia. 2016;81:50–60. doi: 10.1016/j.neuropsychologia.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Perry R.J., Hodges J.R. Relationship between functional and neuropsychological performance in early Alzheimer disease. Alzheimer's Dis Associated Disord. 2000;14:1–10. doi: 10.1097/00002093-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Galton C.J., Patterson K., Graham K., Lambon-Ralph M.A., Williams G., Antoun N. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57:216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- 91.Wilson R.S., Leurgans S.E., Boyle P.A., Bennett D.A. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood J.S., Firbank M.J., Mosimann U.P., Watson R., Barber R., Blamire A.M. Testing visual perception in dementia with Lewy bodies and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21:501–508. doi: 10.1016/j.jagp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 93.Kawas C.H., Corrada M.M., Brookmeyer R., Morrison A., Resnick S.M., Zonderman A.B. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 94.Blackwell A.D., Sahakian B.J., Vesey R., Semple J.M., Robbins T.W., Hodges J.R. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer's disease. Dement Geriatr Cogn Disord. 2003;17:42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- 95.Ivanoiu A., Adam S., Van der Linden M., Salmon E., Juillerat A.C., Mulligan R. Memory evaluation with a new cued recall test in patients with mild cognitive impairment and Alzheimer's disease. J Neurol. 2005;252:47–55. doi: 10.1007/s00415-005-0597-2. [DOI] [PubMed] [Google Scholar]
- 96.Morganti F., Stefanini S., Riva G. From allo- to egocentric spatial ability in early Alzheimer's disease: a study with virtual reality spatial tasks. Cogn Neurosci. 2013;4:171–180. doi: 10.1080/17588928.2013.854762. [DOI] [PubMed] [Google Scholar]
- 97.Thomas E., Snyder P.J., Pietrzak R.H., Jackson C.E., Bednar M., Maruff P. Specific impairments in visuospatial working and short-term memory following low-dose scopolamine challenge in healthy older adults. Neuropsychologia. 2008;46:2476–2484. doi: 10.1016/j.neuropsychologia.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 98.Fowler K.S., Saling M.M., Conway E.L., Semple J.M., Louis W.J. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- 99.Lim Y.Y., Maruff P., Pietrzak R.H., Ellis K.A., Darby D., Ames D. Aβ and cognitive change: examining the preclinical and prodromal stages of Alzheimer's disease. Alzheimers Dement. 2014;10:743–751.e1. doi: 10.1016/j.jalz.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Darby D.G., Brodtmann A., Pietrzak R.H., Fredrickson J., Woodward M., Villemagne V.L. Episodic memory decline predicts cortical amyloid status in community-dwelling older adults. J Alzheimers Dis. 2011;27:627–637. doi: 10.3233/JAD-2011-110818. [DOI] [PubMed] [Google Scholar]