Abstract
Dermatophytes are amongst the most common causative agents of fungal infections worldwide and widespread in the developing countries. Various studies have found the significantly rising trend of this infection in India especially in last 4-5 years. The growing epidemic of recurrent/chronic dermatophytosis has led to the need for newer antifungal agents and/or preparations. Furthermore, resistance to commonly used topical and oral antifungals has increased alarmingly. Significantly increasing resistance has led to state of anxiety in physicians and significant distress to the patients socially, emotionally, and financially. Newer formulations or newer derivatives of existing drug classes and few newer drug classes are being developed to tackle this menace. Other forms of local therapies including lasers and photodynamic therapy are still in developmental phase and still need to be optimized in terms of dosing schedule, frequency of use and duration of therapy. Moreover, cost of these therapies remained most important obstacle in developing countries like India. We are hereby reviewing the newer formulations of topical therapies and drugs/interventions in experimental phase.
Keywords: Dermatophytosis, tinea, topical antifungal
Introduction
Superficial fungal infections of the skin, hair, and nails are common worldwide with a prevalence of 20–25%, of which dermatophytes are the most common causative agents.[1] Dermatophytosis is defined as an infection of the hair, nails, or skin by the dermatophytes which include three genera i.e., Trichophyton spp., Microsporum spp., and Epidermophyton. As dermatophytic infections of the hair mainly require systemic antifungal therapy, we will focus only on the topical therapy of dermatophytic infections of the skin and nails.
The most common clinical morphology is tinea corporis and cruris in most studies, and Trichophyton rubrum is the most commonly isolated species.[1,2] However, few studies have documented T. mentagrophytes as the most common isolate.[1,3,4] Recently, a study from a tertiary centre in New Delhi found T. interdigitale (56%) as the most common isolate followed by T. tonsurans (25.7%).[5]
Chronic and Recalcitrant Dermatophytosis
There is no standard definition of chronic dermatophytosis in literature. However, patients with disease duration of more than 6 months to 1 year, with or without recurrence, despite being treated with adequate course of antifungal drugs are considered as chronic dermatophytosis. Recurrent dermatophytosis is defined as reoccurrence of infection within few weeks of stopping the treatment.[6] Although there is sparse literature on the exact frequency of clinical resistance in the Indian subcontinent, it is being very commonly encountered in clinical practice. Adimi et al. assessed the efficacy of antifungal agents, i.e., griseofulvin, terbinafine, itraconazole, ketoconazole, fluconazole, voriconazole, clotrimazole, ciclopirox olamine, amorolfine, and naftifine over 15 different species of dermatophytes using Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (M38-A). Itraconazole and terbinafine showed the lowest and fluconazole had the greatest minimum inhibitory concentration (MIC).[7] A study conducted in a Mexican hospital assessed in-vitro resistance among 36 patients using the E-test method who did not respond to treatment for dermatophytosis. One strain of T. tonsurans and three strains each of T. rubrum and T. mentagrophytes showed resistance to azoles. One T. rubrum strain was resistant to all three azoles, i.e., itraconazole, ketoconazole, and fluconazole. All these seven dermatophyte strains were resistant to fluconazole.[8,9]
Various factors including host, agent, environmental, or topical corticosteroid abuse have been attributed for chronicity and recurrent dermatophytic infections in India.[6] Due to the increasing proportion of recalcitrant cases, there is an urgent need of newer topical and oral antifungal medications.
Treatment of dermatophyte infections
Besides pharmacotherapy, there are many important considerations while managing a case of dermatophytic infection. Improving hygiene of the skin, nails, and hair; avoidance of humidity and occlusive clothing; discontinuation of corticosteroid containing topical antifungal creams; and close examination of possible carriers, i.e., family members and pets, are important measures in the treatment.[10]
Role of Topical Antifungals
Treatment of dermatophytosis consists of oral or topical antifungal drugs or a combination of both, depending on the extent and severity, site of infection, and causative organism.[11] Topical antifungals are generally considered the first-line therapy for uncomplicated, superficial dermatomycoses owing to their high efficacy and low potential for systemic adverse effects. These drugs are compounded into various types of vehicles, i.e., creams, lotions, gels, or sprays to facilitate penetration and efficacy depending on the site of involvement. They readily penetrate the stratum corneum when applied to the skin surface, which leads to killing of the fungi or inhibition of their growth, achieving clinical and mycologic eradication. The main classes of topical antifungal drugs include the polyenes, the azoles, and the allylamine/benzylamines. Recently, various new antifungal drugs with increased efficacy and associated anti-inflammatory effect have been introduced in India and have broadened the armamentarium against this chronic dermatosis. A Cochrane review and meta-analysis found no significant differences among the topical antifungals in regard to clinical and mycological cure at the end of the treatment. However, terbinafine and butenafine led to sustained cure compared to clotrimazole.[1,12] Naftifine (1%) was found to be superior to placebo in terms of mycological cure rates, although the evidence was low. Combinations of azoles with corticosteroids were slightly more effective than azoles alone for clinical cure, but there was no statistically significant difference with regards to mycological cure.[13] This might be due to rapid improvement in inflammatory component and symptoms which leads to increased patient compliance also. However, inadvertent use of this combination therapy may be associated with treatment failure and adverse effects.[14] Recently, a specific cutaneous form, tinea pseudoimbricata, has been described after steroid abuse.[1]
The classification of important drugs is mentioned in Table 1. The development of new topical broad-spectrum antifungal agents has presented new treatment options for the management of superficial cutaneous mycoses, especially dermatophytosis, which has become a difficult to treat entity in this era. This article focuses on the newer topical antifungal drugs. Description of important drugs is presented below.
Table 1.
Classification and mechanism of action of antifungal antibiotics
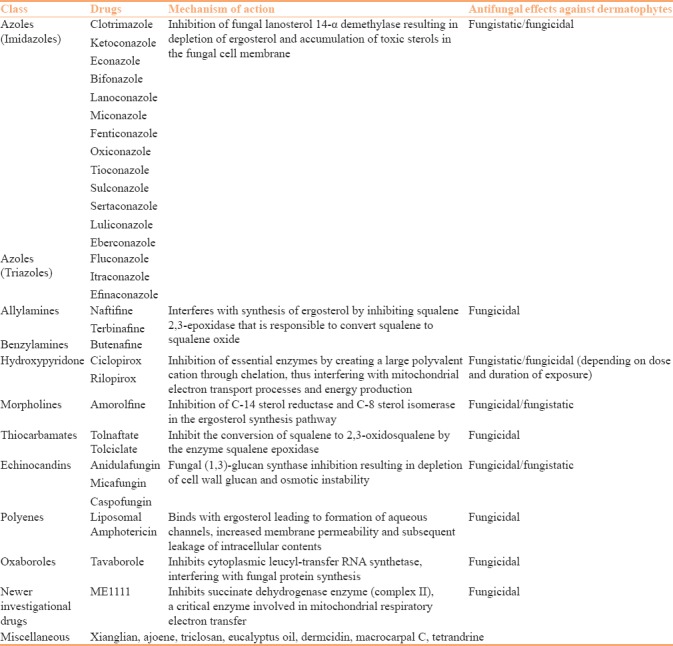
1. Azoles
Azole antifungals are composed of two chemically related groups, i.e., imidazoles and triazoles. Triazoles differ from imidazoles by having three nitrogen atoms in the azole ring whereas imidazoles has two. They act by blocking the lanosterol 14α-demethylase, an enzyme necessary for the biosynthesis of ergosterol. Ergosterol is the primary sterol derivative which is an essential component of the fungal cell membrane.[15] Decreased levels of ergosterol in cell membrane and accumulation of intracellular 14α-methylsterols lead to increased membrane rigidity, increased membrane permeability, disruption of membrane-bound enzymes, inhibition of growth, and ultimately cell death.[15] Various topical azoles, preparations, their uses, and frequency of application is described in Table 2.
Table 2.
Formulations and use of individual topical antifungal drugs

Sertaconazole
Sertaconazole, an imidazole antifungal agent, has both fungistatic and fungicidal activity depending on the concentration of the drug and the specific organisms involved in the infection. At higher concentration, it directly binds to nonsterol lipids in the fungal membrane leading to increased permeability and rapid leakage of key intracellular constituents (e.g., adenosine triphosphate) to such an extent that immediate cell death ensues.[16,17] It has broad-spectrum antifungal activity against all three genera of dermatophytes, Candida and Cryptococcus. In addition, it is also effective against Gram-positive cocci.[16] Notably, this sensitivity was maintained in isolates of dermatophytes and laboratory strains of Candida that exhibited reduced susceptibility to other azoles. In-vitro and animal studies have proven anti-inflammatory and antipruritic effects of sertaconazole which leads to increased compliance. Sertaconazole 2% cream was approved by the FDA in 2003 for the treatment of tinea pedis caused by T. rubrum, T. interdigitale, and Epidermophyton floccosum in individuals aged 12 years or more.[18] The recommended dosage of 2% sertaconazole cream is applied once or twice daily for a period of 4 weeks.[16] Various newer topical formulations of sertaconazole, i.e., anhydrous gel, microsponge, microemulsion, nanovesicles, loaded hydrogel, bioadhesive gel, transdermal patch, and nail patch are under development to enhance the dermal delivery and skin retention of the drug.
Luliconazole
Luliconazole, R-enantiomer of lanoconazole, is an imidazole antifungal agent.[19] It has been found that the MIC for luliconazole is 1–4 times lower than lanoconazole and terbinafine for T. rubrum and T. mentagrophytes strains.[19] Luliconazole 1% has also been found to successfully eradicate dermatophytic infection caused by T. mentagrophytes in experimental model in half the time or less required for 1% terbinafine cream and bifonazole 1% cream.[18] Besides dermatophytes, in-vitro studies have documented the effectiveness of luliconazole against Candida, Malassezia subspecies, and Aspergillus fumigatus.[19] Luliconazole was approved by the FDA in November 2013 for the treatment of tinea pedis, tinea cruris, and tinea corporis. The recommended dosage of 1% luliconazole is once daily for 1 week for tinea cruris and tinea corporis and 2 weeks for tinea pedis over affected area(s) and immediate surrounding area(s).[19] Recently, the role of luliconazole is being explored in the management of onychomycosis. It has excellent penetration capability through the nail layer.[19] A multicenter, double-blind, randomized study using once daily luliconazole 5% nail solution in cases with distal lateral subungual onychomycosis with 20–50% clinical involvement have found good efficacy and tolerability.[20] Jones et al. assessed safety and tolerability of 10% luliconazole solution in patients with moderate-to-severe distal subungual onychomycosis and found significant accumulation of the drug in the nail with minimal systemic exposure.[21] Another randomized controlled trial compared fractional carbon dioxide (CO2) laser combined with luliconazole 1% cream to fractional CO2 laser for the treatment of onychomycosis and found significantly higher efficacy in the former.[22]
Eberconazole
Eberconazole, an imidazole azole, has been found to have broad antimicrobial activity. It has shown good efficacy against dermatophytes, Candida, and Malassezia furfur. Similar to sertaconazole, it also possesses antibacterial property against Gram-positive bacteria.[23] It has anti-inflammatory activity similar to aspirin and ketoprofen, and this effect has been attributed to the inhibition of 5-lipooxygenase and to a lesser extent of cyclooxygenase-2.[23] A randomized controlled trial found the efficacy and safety of 1% eberconazole nitrate cream similar to that of 1% terbinafine hydrochloride cream in the treatment of localized (<20% involvement) tinea corporis and tinea cruris.[24] Recently, a study used ethyl cellulose microsponges as topical carriers of eberconazole nitrate and found four-fold higher retention of eberconazole nitrate in the stratum corneum layer compared with commercial cream,[25] which might lead to increased efficacy of the drug.
Lanoconazole
Lanoconazole, a racemic imidazole antifungal agent, has been widely used in Japan for management of tinea pedis, tinea corporis, and cutaneous candidiasis.[26] Uratsuji et al. documented the anti-inflammatory activity of lanoconazole by inhibition of IL-8.[27] In addition, it has been found to accelerate wound healing in animal models, however, the relevance of this feature in the management of dermatophytosis is yet to be addressed.[26] An animal model study showed lower efficacy of lanoconazole compared to terbinafine and luliconazole.[28] Once daily local application is currently recommended at affected sites. Occasionally, it can cause allergic contact dermatitis.[26]
Efinaconazole
Efinaconazole is a newer emerging triazole antifungal effective against dermatophytes, Candida species, and nondermatophyte molds. Efinaconazole 10% topical solution got FDA approval for the topical treatment of toenail onychomycosis caused by T. rubrum and T. mentagrophytes in June 2014.[29] It is to be applied once daily for 48 weeks with the help of a brush applicator to the affected toenail and its undersurface, nail folds, nail bed, and hyponychium.[29] The drug reaches the site of infection by both transungual delivery and spreads through the subungual space. It is deactivated by keratin to a lesser extent compared to other azoles.[19,29,30] Sugiura et al. compared the keratin affinity of efinaconazole to amorolfine and ciclopirox. Efinaconazole was found to penetrate the nail bed to a greater extent (14.3% efinaconazole vs 0.7% and 1.9% for ciclopirox and amorolfine in keratin suspensions, respectively). Moreover, it inhibited the growth of T. rubrum more than ciclopirox and amorolfine whereas its efficacy against T. mentagrophytes was similar to amorolfine and better than ciclopirox.[30] Notably, it has low surface tension which also leads to increased penetration and spreading. Debridement is not necessary for the action of efinaconazole.[29]
Pramiconazole
Pramiconazole is a newer triazole under development which has shown good in-vitro and clinical activity against dermatophytes, Candida and Malassezia. Both oral as well as topical preparations of pramiconazole have shown superior efficacy compared to itraconazole and terbinafine against Microsporum canis in guinea pigs.[31]
Other Newer 14α-Lanosterol Demethylase Inhibitors
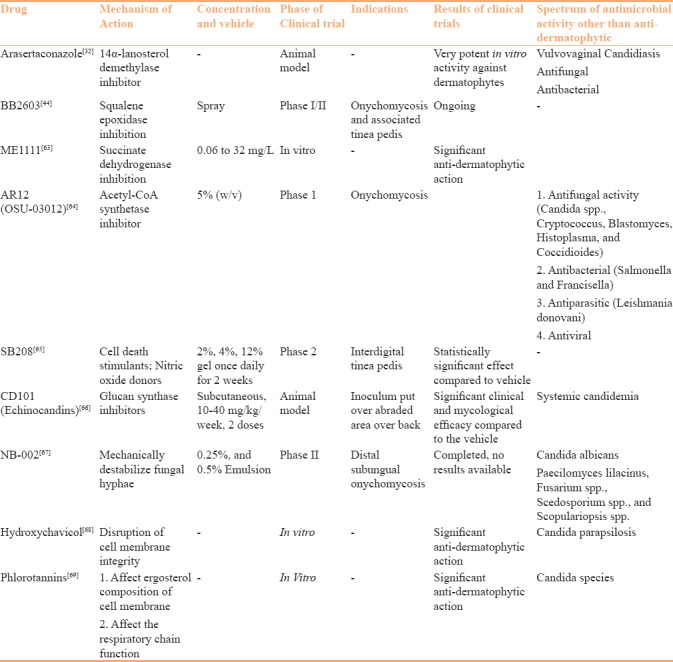
Novel 14α-lanosterol demethylase inhibitor arasertaconazole is under development and being studied in animal models [32] [Table 3].
Table 3.
2. Allylamines
Allylamines interfere with ergosterol synthesis similar to azoles but act at an earlier stage by inhibiting the formation of squalene epoxidase, which is a precursor of lanosterol and involved in the formation of cell membrane. Moreover, the inhibition of this pathway leads to the accumulation of high levels of squalene, which leads to increased membrane permeability and disruption of cellular organisation ensuring death of fungus. Terbinafine and naftifine are the two important drugs of this group.
Terbinafine
Efficacy of both oral and topical terbinafine (TBF) is well-documented in the management of dermatophytosis and has been widely used since the development of this drug. A recently conducted Cochrane review concluded the efficacy of terbinafine superior to placebo and almost similar to azoles. However, in many studies, terbinafine 1% cream has been used once daily and for a shorter duration.[12] It is FDA approved for the treatment of interdigital-type tinea pedis (athlete's foot), tinea cruris (jock itch), and tinea corporis (ringworm). Recently, a randomized control trial found similar efficacy and tolerability of 1% terbinafine cream to 1% eberconazole nitrate cream in the management of localized tinea corporis and cruris.[24] Similarly, another RCT found an efficacy of 1% terbinafine cream similar to 2% sertaconazole cream.[33] Various newer formulations, i.e., TBF-loaded liposome film, TBF-loaded liposome and ethosome, TBF-loaded transferosome, liposome poloxamer gel, microemulsion-based gel, and ethosome chitosan gel are in the developmental phase to increased penetration leading to augmented efficacy. Tanrıverdi et al. found the efficacy of liposome film formulation to be better compared to TBF-loaded liposome and ethosome, liposome poloxamer gel, and ethosome chitosan gel in the treatment of onychomycosis.[34]
Naftifine
Naftifine is a topical fungicidal allylamine that is effective against dermatophytes, Candida and Aspergillus species. It is also effective against Gram-negative and Gram-positive bacteria. Furthermore, naftifine has anti-inflammatory activity by targeting prostaglandin pathway.[35] Similar to terbinafine, it's efficacy is similar to azoles.[12] Recently, good efficacy of 2% naftifine gel has been documented in moccasin type tinea pedis in many RCTs.[36,37,38] A newer formulation, colloidal nanocarriers containing this drug, leads to increased penetration into the stratum corneum compared to the marketed formulation.[39] Once daily application of naftifine 2% cream and gel for 2 weeks is FDA approved for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organism T. rubrum in adults over 18 years of age. Use of both the cream and gel formulations shows good rates of mycologic and clinical cure after 2–8 weeks of use.[35]
Butenafine
Butenafine is a benzylamine derivative which shows potent fungicidal activity particularly against dermatophytes, aspergillus, dimorphic, and dematiaceous fungi. In addition, it has anti-inflammatory activity.[40] Its mechanism of action is similar to allylamines. Butenafine 1% cream is FDA approved for the treatment of interdigital tinea pedis, tinea cruris, and corporis. Various RCTs have found significantly superior efficacy of topical butenafine in the management of tinea compared to placebo whereas almost similar efficacy to those of topical azoles.[12,40] However, it may have the advantage of once daily application compared to azoles which decreases cost of therapy and increases compliance of therapy.[40] Recently, Thaker et al. in an RCT found topical 1% butenafine to be more efficacious, cost-effective, and equally safe compared to 2% sertaconazole for the treatment of tinea infections.[41] However, Thaker et al. in another RCT comparing Whitfield's ointment with weekly oral fluconazole (150 mg) to topical 1% butenafine in the treatment of tinea and found the former to be more efficacious, safe, and cost-effective.[42] A study from India found superior efficacy of butenafine 1% cream compared to terbinafine 1% cream for the treatment of tinea cruris.[43]
Novel Agents
A newer squalene epoxidase inhibitor BB2603 in a spray formulation is currently ongoing phase I/II trials for the treatment of onychomycosis and its associated tinea pedis [44] [Table 3].
3. Oxaboroles
Oxaboroles represent a new class of drug that acts primarily by blocking protein synthesis via the inhibition of leucylaminoacyl transfer RNA (t-RNA) synthetase.[45] Premature termination of protein synthesis leads to growth inhibition and death of the fungus. It has broad-spectrum antifungal activity including dermatophytes, such as T. rubrum and T. mentagrophytes, C. albicans, and nondermatophytic moulds.[19,45,46]
Tavaborole
Tavaborole, the first oxaborole, was approved by the FDA in July 2014 as a topical treatment of toenail onychomycosis caused by T. rubrum and T. mentagrophytes.[47] It has 1000 times greater selectivity for fungal aminoacyl transfer RNA synthetase (AARS) compared to mammalian AARS.[19] Tavaborole has a low-molecular-weight (152 kDa) leading to increased penetration through full-thickness human nail plates.[47] An in-vitro study on cadaveric nail demonstrated that 5% tavaborole solution has superior penetration capability compared to 8% ciclopirox solution after 14 days of daily application.[48] Once daily application is recommended for 48 weeks and it should be applied over the entire surface of the toenail and underneath the tip of the toenail.[19,48] Mycologic cure rate of tavaborole is lower than oral antifungal agents (30–36% vs 50–76%, respectively), however, it might provide an important alternative or adjuvant to available antifungal therapies.[48]
4. Hydroxypyridones
Hydroxypyridones are weak acids and show broad-spectrum antimicrobial activity. They act by chelating trivalent metal cations causing inhibition of metal-dependent enzymes leading to less degradation of cytoplasmic peroxides, increased sensitivity of cells to oxidative stress, and decreased levels of iron permeases or transporters. This unique and multilevel mechanism of action is responsible for very low incidence of resistance.[49] Ciclopirox is a prototype drug of this group. Rilopirox and octopirox (piroctone olamine) are other recently described drugs of this group. Various newer formulations, i.e., ciclopirox, dissolved in lipid diffusion enhancers, hydrosoluble preparations, formulation containing isopropyl alcohol, potassium hydroxide, and urea have led to excellent efficacy and permeation properties of ciclopirox while managing onychomycosis.[49,50,51] An RCT comparing P-3051 formulation (water-soluble ciclopirox 8% formulation in hydroxypropyl chitosan) to amorolfine 5% lacquer in the treatment of mild-to-moderate toenail onychomycosis found statistical superiority of P-3051 over amorolfine after 48 weeks in terms of treatment success while fungal eradication by P-3051 was statistically superior at week 24.[52]
5. Morpholines
Morpholines inhibit two enzymes, i.e., C-14 sterol reductase and C-8 sterol isomerase, in the ergosterol synthesis pathway. Amorolfine is the most commonly used drug of this group. It has been primarily used for the management of onychomycosis in lacquer formulation. Efficacy and safety of amorolfine cream have been found to be comparable to various azoles.[12,53]
6. Photodynamic therapy
Photodynamic therapy (PDT) involves the systemic or topical application of a photosensitizing agent followed by the selective illumination of the target lesion with light of an appropriate wavelength which leads to generation of free radicals and subsequent cell death. Aminolevulinic acid and methylene blue are the most commonly used photosensitizing agents for PDT. Various in-vitro as well as in-vivo studies have shown promising results.[54] In a recently conducted RCT, PDT using methylene blue as photosensitizing agent was found to have significantly superior efficacy over weekly 300 mg of oral fluconazole. PDT may offer a good prospect as alternative antifungal therapy in the current era of rapidly growing antifungal resistance.
8. Lasers
Many studies have investigated the efficacy of lasers for the management of onychomycosis. The exact mechanism of action of lasers in treating onychomycosis is not known but proposed mechanism includes heat disintegration of fungal elements. Temperature exceeding 50°C leads to direct thermal killing. Various studies have used lasers with wavelengths varying from 780 to 3000 nm, but Nd: YAG 1064 nm laser is the most commonly used.[55] Although it provides an alternative treatment option with less procedural duration, current literature suggests limited success rate.[55,56]
New wine in old bottles: Older antifungal drugs in newer formulations
Due to widely increasing resistance, older drugs in newer formulations have also become the focus of attention. More recently, topical amphotericin B in lipid-based gel formulation has been found to effective in the treatment of various mucocutaneous fungal infections including dermatophytosis with a good safety profile.[57] Amphotericin B incorporated in microemulsion and nanoemulsion formulation shows an increased skin retention of the drug leading to better in-vitro antifungal activity.[1,58] Aqueous micellar solutions of econazole has 13-fold higher deposition of drug compared to conventional preparation.[59] Similarly, solid lipid nanoparticles and nanostructured lipid carriers have been found to increase occlusion, better penetration, less degradation of active drug, and sustained release over a longer duration leading to increased efficacy. These two drug delivery systems have been used for various drugs including clotrimazole, miconazole, ketoconazole, fluconazole, terbinafine, and griseofulvin, and are currently being considered among the most promising way of enhancing drug cutaneous penetration and efficacy for topical antifungal therapy. Transferosomes, also known as ultradeformable or flexible liposomes, have been used as carriers for griseofulvin and amphotericin B in the treatment of dermatophytosis with better efficacy.[59]
7. Future prospects
In-vitro studies have confirmed stronger in-vitro activity of echinocandins (anidulafungin, caspofungin, micafungin) against dermatophytes.[60,61,62] A recently developed novel antifungal drug, ME1111, fulfils the characteristics of an ideal antifungal drug for onychomycosis as it has potent antidermatophyte activity, low molecular weight leading to increased penetration, and maintaining good inhibitory activity even in the presence of keratin. It primarily acts by the inhibition of succinate dehydrogenase (complex II), a critical enzyme involved in electron transport chain in mitochondria.[63] AR12 (OSU-03012) is an acetyl CoA synthase inhibitor which is in phase 1 trials for malignancy but has also been found to have action on onychomycosis.[64] Other clinically relevant drugs have been briefly mentioned in Table 3.
Conclusion
The growing epidemic of recurrent/chronic dermatophytosis has led to the need for newer antifungal agents and/or preparations. Newer formulations or newer derivatives of existing drug classes and few newer drug classes have been described in the last 10 years, which provide hope to tackle this menace. Many newer agents are still undergoing experimental trials and require further study before they become commercially available. Newer topical agents, PDT, and lasers have all been tried, especially for onychomycosis which offers special challenges in successful management. Topical therapy has added advantage because of low risk of systemic side effects. It becomes difficult to choose one topical agent over the other given the efficacy is not much different between these. Similarly, all agents are quite safe for long-term use with no significant difference in the safety profile. However, long-term unsupervised use may lead to development of potential resistance. Many newer agents have an additional benefit of anti-inflammatory nature, thereby having relative preference over others, especially in inflamed and highly symptomatic lesions. Additional factors i.e., cost, easy availability, frequency, and ease of application may tilt the balance towards any one agent.
Judicious use of newer antifungals, emphasizing patient compliance, and prescribing without combining with oral/topical corticosteroids are all adjunctive steps necessary to offer cure to patients presenting with difficult to treat dermatophytosis.69
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J. 2016;7:77–86. doi: 10.4103/2229-5178.178099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakshmanan A, Ganeshkumar P, Mohan SR, Hemamalini M, Madhavan R. Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015;33(Suppl):134–6. doi: 10.4103/0255-0857.150922. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia VK, Sharma PC. Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134. doi: 10.1186/2193-1801-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahai S, Mishra D. Change in spectrum of dermatophytes isolated from superficial mycoses cases:First report from Central India. Indian J Dermatol Venereol Leprol. 2011;77:335. doi: 10.4103/0378-6323.79718. [DOI] [PubMed] [Google Scholar]
- 5.Dabas Y, Xess I, Singh G, Pandey M, Meena S. Molecular Identification and Antifungal Susceptibility Patterns of Clinical Dermatophytes Following CLSI and EUCAST Guidelines. J Fungi. 2017;3:17. doi: 10.3390/jof3020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogra S, Uprety S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J. 2016;7:73. doi: 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adimi P, Hashemi SJ, Mahmoudi M, Mirhendi H, Shidfar MR, Emmami M, et al. In-vitro Activity of 10 Antifungal Agents against 320 Dermatophyte Strains Using Microdilution Method in Tehran. Iran J Pharm Res. 2013;12:537–45. [PMC free article] [PubMed] [Google Scholar]
- 8.Ghannoum M. Azole Resistance in Dermatophytes: Prevalence and Mechanism of Action. J Am Podiatr Med Assoc. 2016;106:79–86. doi: 10.7547/14-109. [DOI] [PubMed] [Google Scholar]
- 9.Manzano-Gayosso P, Méndez-Tovar LJ, Hernández-Hernández F, López-Martínez R. Antifungal resistance: An emerging problem in Mexico. Gac Med Mex. 2008;144:23–6. [PubMed] [Google Scholar]
- 10.Durdu M, Ilkit M, Tamadon Y, Tolooe A, Rafati H, Seyedmousavi S. Topical and systemic antifungals in dermatology practice. Expert Rev Clin Pharmacol. 2017;10:225–37. doi: 10.1080/17512433.2017.1263564. [DOI] [PubMed] [Google Scholar]
- 11.Rotta I, Sanchez A, Gonçalves PR, Otuki MF, Correr CJ. Efficacy and safety of topical antifungals in the treatment of dermatomycosis: A systematic review. Br J Dermatol. 2012;166:927–33. doi: 10.1111/j.1365-2133.2012.10815.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Gohary M, van Zuuren EJ, Fedorowicz Z, Burgess H, Doney L, Stuart B, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;8:CD009992. doi: 10.1002/14651858.CD009992.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zuuren EJ, Fedorowicz Z, El-Gohary M. Evidence-based topical treatments for tinea cruris and tinea corporis: A summary of a Cochrane systematic review. Br J Dermatol. 2015;172:616–41. doi: 10.1111/bjd.13441. [DOI] [PubMed] [Google Scholar]
- 14.Schaller M, Friedrich M, Papini M, Pujol RM, Veraldi S. Topical antifungal-corticosteroid combination therapy for the treatment of superficial mycoses: Conclusions of an expert panel meeting. Mycoses. 2016;59:365–73. doi: 10.1111/myc.12481. [DOI] [PubMed] [Google Scholar]
- 15.Zhang AY, Camp WL, Elewski BE. Advances in topical and systemic antifungals. Dermatol Clin. 2007;25:165–83. doi: 10.1016/j.det.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Croxtall JD, Plosker GL. Sertaconazole: A review of its use in the management of superficial mycoses in dermatology and gynaecology. Drugs. 2009;69:339–59. doi: 10.2165/00003495-200969030-00009. [DOI] [PubMed] [Google Scholar]
- 17.Carrillo-Muñoz AJ, Giusiano G, Ezkurra PA, Quindós G. Sertaconazole: Updated review of a topical antifungal agent. Expert Rev Anti Infect Ther. 2005;3:333–42. doi: 10.1586/14787210.3.3.333. [DOI] [PubMed] [Google Scholar]
- 18.Gupta AK, Foley KA, Versteeg SG. New Antifungal Agents and New Formulations Against Dermatophytes. Mycopathologia. 2017;182:127–41. doi: 10.1007/s11046-016-0045-0. [DOI] [PubMed] [Google Scholar]
- 19.Saunders J, Maki K, Koski R, Nybo SE. Tavaborole, Efinaconazole, and Luliconazole: Three New Antimycotic Agents for the Treatment of Dermatophytic Fungi. J Pharm Pract. 2016 doi: 10.1177/0897190016660487. 0897190016660487. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe S, Kishida H, Okubo A. Efficacy and safety of luliconazole 5% nail solution for the treatment of onychomycosis: A multicenter, double-blind, randomized phase III study. J Dermatol. 2017;44:753–9. doi: 10.1111/1346-8138.13816. [DOI] [PubMed] [Google Scholar]
- 21.Jones T, Tavakkol A. Safety and tolerability of luliconazole solution 10-percent in patients with moderate to severe distal subungual onychomycosis. Antimicrob Agents Chemother. 2013;57:2684–9. doi: 10.1128/AAC.02370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou BR, Lu Y, Permatasari F, Huang H, Li J, Liu J, et al. The efficacy of fractional carbon dioxide (CO2) laser combined with luliconazole 1% cream for the treatment of onychomycosis: A randomized, controlled trial. Medicine (Baltimore) 2016;95:e5141. doi: 10.1097/MD.0000000000005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moodahadu-Bangera LS, Martis J, Mittal R, Krishnankutty B, Kumar N, Bellary S, et al. Eberconazole--pharmacological and clinical review. Indian J Dermatol Venereol Leprol. 2012;78:217–22. doi: 10.4103/0378-6323.93651. [DOI] [PubMed] [Google Scholar]
- 24.Choudhary SV, Aghi T, Bisati S. Efficacy and safety of terbinafine hydrochloride 1% cream vs eberconazole nitrate 1% cream in localised tinea corporis and tinea cruris. Indian Dermatol Online J. 2014;5:128. doi: 10.4103/2229-5178.131079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bothiraja C, Gholap AD, Shaikh KS, Pawar AP. Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv. 2014;5:781–94. doi: 10.4155/tde.14.43. [DOI] [PubMed] [Google Scholar]
- 26.Rubin AI, Bagheri B, Scher RK. Six novel antimycotics. Am J Clin Dermatol. 2002;3:71–81. doi: 10.2165/00128071-200203020-00001. [DOI] [PubMed] [Google Scholar]
- 27.Uratsuji H, Nakamura A, Yamada Y, Hashimoto K, Matsumoto T, Ikeda F, et al. Anti-inflammatory activity of lanoconazole, a topical antifungal agent. Mycoses. 2015;58:197–202. doi: 10.1111/myc.12297. [DOI] [PubMed] [Google Scholar]
- 28.Ghannoum MA, Long L, Kim HG, Cirino AJ, Miller AR, Mallefet P. Efficacy of terbinafine compared to lanoconazole and luliconazole in the topical treatment of dermatophytosis in a guinea pig model. Med Mycol. 2010;48:491–7. doi: 10.3109/13693780903373811. [DOI] [PubMed] [Google Scholar]
- 29.Lipner SR, Scher RK. Efinaconazole in the treatment of onychomycosis. Infect Drug Resist. 2015;8:163–72. doi: 10.2147/IDR.S69596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiura K, Sugimoto N, Hosaka S, Katafuchi-Nagashima M, Arakawa Y, Tatsumi Y, et al. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob Agents Chemother. 2014;58:3837–42. doi: 10.1128/AAC.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Wit K, Paulussen C, Matheeussen A, van Rossem K, Cos P, Maes L. In Vitro Profiling of Pramiconazole and In Vivo Evaluation in Microsporum canis Dermatitis and Candida albicans Vaginitis Laboratory Models. Antimicrob Agents Chemother. 2010;54:4927–9. doi: 10.1128/AAC.00730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biological Characterization of the Antimicrobial Activity of Arasertaconazole Nitrate | Aspergillus & Aspergillosis Website [Internet] [Last accessed on 2017 Sep 17]. Available from: http://www.aspergillus.org.uk/content/biological-characterization-antimicrobial-activity-arasertaconazole-nitrate .
- 33.Chatterjee D, Ghosh SK, Sen S, Sarkar S, Hazra A, De R. Efficacy and tolerability of topical sertaconazole versus topical terbinafine in localized dermatophytosis: A randomized, observer-blind, parallel group study. Indian J Pharmacol. 2016;48:659–64. doi: 10.4103/0253-7613.194850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanrıverdi ST, Hilmioǧlu Polat S, Yeşim Metin D, Kandiloǧlu G, Özer Ö. Terbinafine hydrochloride loaded liposome film formulation for treatment of onychomycosis: In vitro and in vivo evaluation. J Liposome Res. 2016;26:163–73. doi: 10.3109/08982104.2015.1067892. [DOI] [PubMed] [Google Scholar]
- 35.Gupta AK, Ryder JE, Cooper EA. Naftifine: A review. J Cutan Med Surg. 2008;12:51–8. doi: 10.2310/7750.2008.06009. [DOI] [PubMed] [Google Scholar]
- 36.Stein Gold LF, Parish LC, Vlahovic T, Plaum S, Kircik L, Fleischer AB, et al. Efficacy and safety of naftifine HCl Gel 2% in the treatment of interdigital and moccasin type tinea pedis: Pooled results from two multicenter, randomized, double-blind, vehicle-controlled trials. J Drugs Dermatol. 2013;12:911–8. [PubMed] [Google Scholar]
- 37.Stein Gold LF, Vlahovic T, Verma A, Olayinka B, Fleischer AB. Naftifine Hydrochloride Gel 2%: An Effective Topical Treatment for Moccasin-Type Tinea Pedis. J Drugs Dermatol. 2015;14:1138–44. [PubMed] [Google Scholar]
- 38.Vlahovic TC. The Role of Naftifine HCl 2% Gel and Cream in Treating Moccasin Tinea Pedis. J Drugs Dermatol. 2016;15(2 Suppl):s56–9. [PubMed] [Google Scholar]
- 39.Erdal MS, Özhan G, Mat MC, Özsoy Y, Güngör S. Colloidal nanocarriers for the enhanced cutaneous delivery of naftifine: Characterization studies and in vitro and in vivo evaluations. Int J Nanomed. 2016;11:1027–37. doi: 10.2147/IJN.S96243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singal A. Butenafine and superficial mycoses: Current status. Expert Opin Drug Metab Toxicol. 2008;4:999–1005. doi: 10.1517/17425255.4.7.999. [DOI] [PubMed] [Google Scholar]
- 41.Thaker SJ, Mehta DS, Shah HA, Dave JN, Mundhava SG. A comparative randomized open label study to evaluate efficacy, safety and cost effectiveness between topical 2% sertaconazole and topical 1% butenafine in tinea infections of skin. Indian J Dermatol. 2013;58:451–6. doi: 10.4103/0019-5154.119955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaker SJ, Mehta DS, Shah HA, Dave JN, Kikani KM. A comparative study to evaluate efficacy, safety and cost-effectiveness between Whitfield's ointment+oral fluconazole versus topical 1% butenafine in tinea infections of skin. Indian J Pharmacol. 2013;45:622–4. doi: 10.4103/0253-7613.121378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das S, Barbhuniya JN, Biswas I, Bhattacharya S, Kundu PK. Studies on comparison of the efficacy of terbinafine 1% cream and butenafine 1% cream for the treatment of Tinea cruris. Indian Dermatol Online J. 2010;1:8–9. doi: 10.4103/2229-5178.73249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yau S. Blueberry Therapeutics commence Phase I/II clinical trial of lead product BB2603 [Internet] Blueberry Therapeutics. 2017. [Last accessed on 2017 Sep 17]. Available from: http://www.blueberrytherapeutics.com/blueberry-therapeutics-commence-phase-iii-clinical-trial-lead-product-bb2603/.
- 45.Poulakos M, Grace Y, Machin JD, Dorval E. Efinaconazole and Tavaborole. J Pharm Pract. 2017;30:245–55. doi: 10.1177/0897190016630904. [DOI] [PubMed] [Google Scholar]
- 46.Evans JM, Wang AL, Elewski BE. Successful Treatment of Paecilomyces lilacinus Onychomycosis with Efinaconazole and Tavaborole. Skin Appendage Disord. 2016;1:169–71. doi: 10.1159/000443773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zane LT, Chanda S, Coronado D, Del Rosso J. Antifungal agents for onychomycosis: New treatment strategies to improve safety. Dermatol Online J. 2016;22 [PubMed] [Google Scholar]
- 48.Jinna S, Finch J. Spotlight on tavaborole for the treatment of onychomycosis. Drug Des Devel Ther. 2015;9:6185–90. doi: 10.2147/DDDT.S81944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subissi A, Monti D, Togni G, Mailland F. Ciclopirox: Recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70:2133–52. doi: 10.2165/11538110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 50.Hafeez F, Hui X, Selner M, Rosenthal B, Maibach H. Ciclopirox delivery into the human nail plate using novel lipid diffusion enhancers. Drug Dev Ind Pharm. 2014;40:838–44. doi: 10.3109/03639045.2013.788016. [DOI] [PubMed] [Google Scholar]
- 51.Thapa RK, Choi JY, Go TG, Kang MH, Han SD, Jun JH, et al. Development of ciclopirox nail lacquer with enhanced permeation and retention. Arch Pharm Res. 2016;39:953–9. doi: 10.1007/s12272-016-0774-0. [DOI] [PubMed] [Google Scholar]
- 52.Iorizzo M, Hartmane I, Derveniece A, Mikazans I. Ciclopirox 8% HPCH Nail Lacquer in the Treatment of Mild-to-Moderate Onychomycosis: A Randomized, Double-Blind Amorolfine Controlled Study Using a Blinded Evaluator. Skin Appendage Disord. 2016;1:134–40. doi: 10.1159/000441569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee M, Ghosh AK, Basak S, Das KD, Gangopadhyay DN. Comparative evaluation of effectivity and safety of topical amorolfine and clotrimazole in the treatment of tinea corporis. Indian J Dermatol. 2011;56:657–62. doi: 10.4103/0019-5154.91823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smijs TGM, Pavel S. The susceptibility of dermatophytes to photodynamic treatment with special focus on Trichophyton rubrum. Photochem Photobiol. 2011;87:2–13. doi: 10.1111/j.1751-1097.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 55.Dembskey N, Abrahamse H. Laser Therapy for the Treatment of Onychomycosis: Best Evidence Based Practice or Not? Clin Res Foot Ankle. 2016;4:2. [Google Scholar]
- 56.Bristow IR. The effectiveness of lasers in the treatment of onychomycosis: A systematic review. J Foot Ankle Res. 2014;7:34. doi: 10.1186/1757-1146-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheikh S, Ahmad A, Ali SM, Paithankar M, Barkate H, Raval RC, et al. Topical Delivery of Lipid Based Amphotericin B Gel in the Treatment of Fungal Infection: A Clinical Efficacy, Safety and Tolerability Study in Patients. J Clin Exp Dermatol Res. 2014;5:248. [Google Scholar]
- 58.Hussain A, Singh VK, Singh OP, Shafaat K, Kumar S, Ahmad FJ. Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated topical delivery of Amphotericin B. Drug Deliv. 2016;23:3101–10. doi: 10.3109/10717544.2016.1153747. [DOI] [PubMed] [Google Scholar]
- 59.Bseiso EA, Nasr M, Sammour O, Gawad NAAE. Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol. 2015;81:457. doi: 10.4103/0378-6323.162328. [DOI] [PubMed] [Google Scholar]
- 60.Bao Y, Wan Z, Li R. In vitro antifungal activity of micafungin and caspofungin against dermatophytes isolated from China. Mycopathologia. 2013;175:141–5. doi: 10.1007/s11046-012-9571-6. [DOI] [PubMed] [Google Scholar]
- 61.Badali H, Mohammadi R, Mashedi O, de Hoog GS, Meis JF. In vitro susceptibility patterns of clinically important Trichophyton and Epidermophyton species against nine antifungal drugs. Mycoses. 2015;58:303–7. doi: 10.1111/myc.12315. [DOI] [PubMed] [Google Scholar]
- 62.Baghi N, Shokohi T, Badali H, Makimura K, Rezaei-Matehkolaei A, Abdollahi M, et al. In vitro activity of new azoles luliconazole and lanoconazole compared with ten other antifungal drugs against clinical dermatophyte isolates. Med Mycol. 2016;54:757–63. doi: 10.1093/mmy/myw016. [DOI] [PubMed] [Google Scholar]
- 63.Tabata Y, Takei-Masuda N, Kubota N, Takahata S, Ohyama M, Kaneda K, et al. Characterization of Antifungal Activity and Nail Penetration of ME1111, a New Antifungal Agent for Topical Treatment of Onychomycosis. Antimicrob Agents Chemother. 2016;60:1035–9. doi: 10.1128/AAC.01739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kushwaha AS, Sharma P, Shivakumar HN, Rappleye C, Zukiwski A, Proniuk S, et al. Trans-ungual Delivery of AR-12, a Novel Antifungal Drug. AAPS PharmSciTec. 2017:1–4. doi: 10.1208/s12249-017-0752-y. [DOI] [PubMed] [Google Scholar]
- 65.Novan - SB208 [Internet] Novan Therapeutics. [Last accessed on 2017 Sep 17]. Available from: http://www.novan.com/pipeline/sb208/.
- 66.Hager CL, Long L, Ghannoum M. 689 Efficacy of CD101, a novel echinocandin, in the treatment of dermatophytosis using a Guinea Pig (GP) Model. J Invest Dermatol. 2017;137:S118. [Google Scholar]
- 67.Pannu J, McCarthy A, Martin A, Hamouda T, Ciotti S, Fothergill A, et al. NB-002, a novel nanoemulsion with broad antifungal activity against dermatophytes, other filamentous fungi, and Candida albicans. Antimicrob Agents Chemother. 2009;53:3273–9. doi: 10.1128/AAC.00218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali I, Satti NK, Dutt P, Prasad R, Khan IA. Hydroxychavicol: A phytochemical targeting cutaneous fungal infections. Sci Rep. 2016;6:37867. doi: 10.1038/srep37867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopes G, Pinto E, Andrade PB, Valentão P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PloS One. 2013;8:e7220. doi: 10.1371/journal.pone.0072203. [DOI] [PMC free article] [PubMed] [Google Scholar]


