Abstract
Background
AR-V7 is an androgen receptor (AR) splice variant that lacks the ligand-binding domain and is isolated from prostate cancer cell lines. Increased expression of AR-V7 is associated with the transition from hormone-sensitive prostate cancer to more advanced castration-resistant prostate cancer (CRPC). Due to the loss of the ligand-binding domain, AR-V7 is not responsive to traditional AR-targeted therapies, and the mechanisms that regulate AR-V7 are still incompletely understood. Therefore, we aimed to explore existing classes of small molecules that may regulate AR-V7 expression and intracellular localization and their potential therapeutic role in CRPC.
Methods
We used AR high-content analysis (AR-HCA) to characterize the effects of a focused library of well-characterized clinical compounds on AR-V7 expression at the single-cell level in PC3 prostate cancer cells stably expressing green fluorescent protein (GFP)-AR-V7 (GFP-AR-V7:PC3). In parallel, an orthogonal AR-HCA screen of a small interfering (si)RNA library targeting 635 protein kinases was performed in GFP-AR-V7:PC3. The effect of the Src-Abl inhibitor PD 180970 was further characterized using cell-proliferation assays, quantitative PCR, and western blot analysis in multiple hormone-sensitive and CRPC cell lines.
Results
Compounds that tended to target Akt, Abl, and Src family kinases (SFKs) decreased overall AR-V7 expression, nuclear translocation, absolute nuclear level, and/or altered nuclear distribution. We identified 20 protein kinases that, when knocked down, either decreased nuclear GFP-AR-V7 levels or altered AR-V7 nuclear distribution, a set that included the SFKs Src and Fyn. The Src-Abl dual kinase inhibitor PD180970 decreased expression of AR-V7 by greater than 46% and decreased ligand-independent transcription of AR target genes in the 22RV1 human prostate carcinoma cell line. Further, PD180970 inhibited androgen-independent cell proliferation in endogenous–AR-V7–expressing prostate cancer cell lines and also overcame bicalutamide resistance observed in the 22RV1 cell line.
Conclusions
SFKs, especially Src and Fyn, may be important upstream regulators of AR-V7 expression and represent promising targets in a subset of CRPCs expressing high levels of AR-V7.
Keywords: Castration-resistant prostate cancer, Small Molecule Screen, Human Kinome siRNA Screen, High Content Analysis
INTRODUCTION
Castration-resistant prostate cancer (CRPC) is a leading cause of cancer-related deaths among males. An indicator of CRPC is the detection of increased serum prostate specific antigen (PSA), which signals the re-emergence of androgen receptor (AR) activity despite ongoing androgen deprivation therapy (ADT)1,2. Recently, the FDA has approved new second-generation ADT treatment options, including the CYP17A1 inhibitor abiraterone acetate and the AR antagonist enzalutamide, after these showed a clinical benefit3. Although these agents increase life expectancy for CRPC patients, the increase is limited to only a few months with many patients demonstrating resistance either initially or during ongoing treatment4–6.
One mechanism thought to contribute to resistance to ADT is increased expression of AR splice variants that lack the carboxy-terminal ligand-binding domain targeted by first and second generation ADT2,7, with AR-V7 being the most studied. This truncation results in a constitutively active receptor that accumulates in the nucleus8. Subsequent studies have demonstrated that expression of these variants is sufficient to induce androgen independence in cell culture models of prostate cancer7. In clinical studies, AR-V7 expression has been shown to increase in patients with CRPC, and expression is predictive of clinical outcome, including development of CRPC, time to development of CRPC, and cancer-specific survival4,6,7,9. Further, a recent prospective study showed that AR-V7 expression is predictive of resistance to the latest generation ADT, with 100% of patients with AR-V7–positive disease failing to respond to either enzalutamide or abiraterone5,6. These data suggest that AR-V7 is an important driver of CRPC, making the identification of mechanisms regulating its expression an urgent need in the medical community.
To define potential therapeutic classes of molecules capable of altering AR variant expression and/or intracellular distribution, we performed a focused, small-molecule screen of a panel of FDA-approved investigational drugs in a PC3 prostate cancer model stably expressing GFP-AR-V7. In parallel, potential pathway targets were identified through an orthogonal human kinome short interfering (si)RNA screen. This led to the identification of SFKs as potential regulators of AR-V7 expression and subcellular localization in prostate cancer cells. Inhibition of Src using the Src/Abl inhibitor PD180970 results in arrest of ligand-independent prostate cell growth in cell lines expressing increased levels of AR-V7. We also show that PD180970 treatment results in loss of AR-V7 expression and ligand-independent transcription from AR-dependent gene targets. Further, we demonstrate that PD180970 increases effectiveness of bicalutamide in inhibiting ligand-dependent growth in the 22RV1 cell line, which expresses high levels of endogenous AR-V7. These studies provide support for the role of Src inhibition in prostate cancers overexpressing truncated AR.
MATERIALS AND METHODS
Cell Culture
Human prostate (PC-3, 22RV1, LNCaP, LNCaP C4-2) and human breast (MCF7) cancer cell lines were obtained from the American Type Culture Collection (ATCC) and subsequently verified by genotyping. The GFP-AR-V7:PC3 and GFP-AR:HeLa cell lines were generated as previously described9,10. Cell culture methods are described in further detail in the Supporting Information.
High-Throughput Small-Molecule Screen
A set of 145 small-molecule compounds were utilized in a screen using the GFP-AR-V7:PC3 and GFP-AR:HeLa cell lines. In vitro screening was performed at concentrations ranging from 50 pM to 5 μM with a total exposure time of 48 hours. Samples were fixed and imaged using an IN Cell 6000 confocal image cytometer (GE Healthcare) equipped with either a 4× or 40× objective. Images were analyzed using the myImageAnalysis platform (Thermo Fisher Scientific) as previously described11. Further details about the compound library and methods used can be found in Table S1, Supplemental Figure 1A, and in the Supporting Information.
High-Throughput siRNA-Kinome Screen
A library of chemically synthesized and validated siRNA from the Stealth™ RNAI Human Kinase Collection (Invitrogen) were used to identify protein kinases involved in regulating AR-V7 expression and subcellular localization. GFP-AR-V7:PC3 cells were reverse transfected with the siRNA library using XtremeGene (Roche) and automated transfection methods. Assay plates were incubated at 37°C for 72 hours prior to fixation and imaging. Samples were imaged and analyzed as above. Further details about the siRNA library and methods used can be found in Table S2, Supplemental Figure 1B, and in the Supporting Information.
Data Analysis
Raw numerical data generated by myImageAnalysis were imported into Pipeline Pilot (Biovia) where normalization and hit selection methods adapted from Judson et al.12 were applied. Details are provided in the Supporting Information.
Cell Proliferation Assay
Cells were seeded into 384-well assay plates in DMEM/F12 medium containing 10% fetal bovine serum (FBS) and allowed to adhere overnight. Then, medium was replaced with fresh medium containing small-molecule compounds and the cells were further incubated for 96 hours. Cell proliferation and viability were quantified by fixing the cells, labeling the DNA with 4′,6-diamidino-2-phenylindole (DAPI), and imaging using the IN Cell Analyzer 6000 image cytometer equipped with a 4× objective. Cell number and viability were determined using myImageAnalysis software.
Quantitative Real-Time Polymerase Chain Reaction, Western Blot Analysis, and Immunofluorescence Analysis
These methods were performed as described previously. Details provided in Supporting Information.
RESULTS
Small-Molecule Inhibitors that Alter AR-V7 Expression and Intracellular Distribution
To expand upon our previous work in which we identified and characterized the PI3K inhibitor LY-290004 as a potent inhibitor of AR-V7, we used a custom collection of investigational clinical compounds (Table S1) and performed an image-based, AR-V7 high-content analysis (AR-HCA) screen to identify compounds that alter AR-V7 expression. The AR-HCA assay measures multiple aspects of AR expression simultaneously at the single-cell level, including expression level, nuclear translocation/accumulation, and nuclear localization pattern that captures the punctate or “hyperspeckling” associated with transcriptionally active AR signaling (Figure 1A). Compounds were tested at multiple concentrations using the GFP-AR-V7:PC3 cell line, as well as GFP-AR:HeLa cells to allow comparison to drug effects on full-length wildtype AR (WT-AR).
Figure 1.
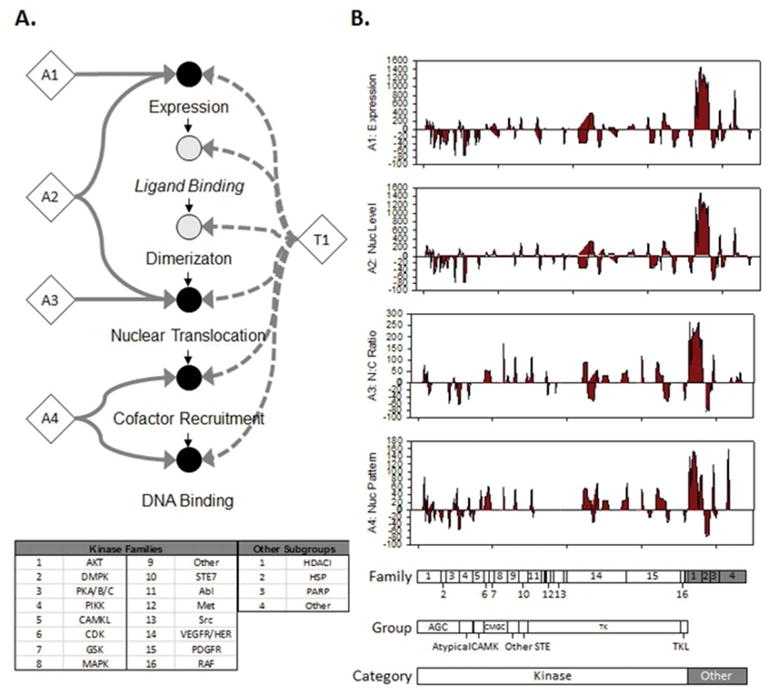
A high-content screen to identify small-molecule compounds capable of altering GFP-AR-V7 expression. A. A graphical representation of AR high-content analysis (AR-HCA) of the AR genomic signaling pathway. Filled circles represent components of the pathway directly measured by the AR-HCA assay. Diamonds represent AR-HCA assay endpoints (A) or toxicity assay (T). B. Plot showing maximal percent change in each assay response from controls after incubation with clinical compounds for 48 hours. Responses grouped by category, kinase group, and kinase family of characterized target of each compound are indicated below. Numbers used in diagram are explained in box at left. Nuc, nuclear; N:C, nuclear:cytoplasmic.
Using a previously established toxicity analysis11 that employs multiple metrics extracted from the DAPI-labeled nuclei present in the samples, we detected evidence of toxicity associated with 50 of the 145 compounds tested on GFP-AR-V7:PC3 cells, with similar results observed in GFP-AR:HeLa cells (data not shown). To mitigate the observed toxic effect, we incorporated filters in all subsequent analyses that removed unhealthy cells from AR-HCA analysis based on nuclear morphology and DNA-labeling intensity/pattern. A multivariate Cox analysis on this filtered population of cells demonstrated that toxicity was not predictive of an effect on either AR-V7 (1.2; 95% confidence interval (CI), 0.7 to 1.4) or WT-AR (1.0; 95% CI, 0.8 to 1.2) expression.
We observed small molecule inhibitors targeting 98 of a possible 219 protein targets significantly altered overall AR-V7 expression level (Figure 1B, Table S3). Of these, 71 were associated with increased AR-V7 expression with the highest frequency of hits seen with inhibitors targeting HDACs (71%) and PDGFR family kinases (51%). Inhibition of 27 targets resulted in decreased AR-V7 expression with SFKs (85%) and the Abl kinase (50%) having the highest frequency of hits. In the GFP-AR:HeLa cell line, a similar number of targets was associated with altered WT-AR expression, with inhibition of 50 targets increasing and of 49 targets decreasing WT-AR expression (Supplemental Figure 2B, Table S4). Again, HDAC inhibitors consistently (100%) increased WT-AR expression; however, inhibitors of SFKs (0%) and Abl (12%) did not commonly decrease WT-AR expression (Supplemental Figure 2) suggesting an isoform- and/or cell–line-specific effect of these compounds.
A similar pattern emerged when level of nuclear AR-V7 was specifically examined (Figure 1B). Inhibition of 54 targets was associated with increased nuclear level of AR-V7, with 88% of HDAC inhibitors exhibiting this effect. A decreased level of nuclear AR-V7 was observed with inhibition of 40 targets, with 71% of SFK and 50% of Abl inhibitors showing this effect. In comparison, inhibition of 48 targets was associated with increased nuclear level of WT-AR, with 100% of the HDAC inhibitors exhibiting this effect (Supplemental Figure 2B). Although inhibition of 45 targets resulted in decreased nuclear levels of WT-AR, the SFK and Abl inhibitors were less active in this regard, with only 12% and 25%, respectively, of the compounds being scored as hits.
We next examined the effects of the drugs on the measured nuclear to cytoplasmic (NC) ratio of AR-V7 (Figure 1B). Targeting of only 53 proteins was associated with altered AR-V7 intracellular distribution, and only HDAC inhibitors (88%) showed a large and consistent increase in the NC ratio indicating increased nuclear translocation and/or accumulation of AR-V7. No single class of targets was able to consistently decrease the measured NC ratio of AR-V7. The intracellular distribution of WT-AR was more sensitive than AR-V7 to drug treatment, with 100% of HDAC inhibitors, as well as 100% of CDK inhibitors in the library, resulted in an increase in the NC ratio of WT-AR (Supplemental Figure 2B). Only inhibitors of heat shock proteins (60%) and PKC family kinases (60%) demonstrated a consistent ability to decrease the NC ratio of WT-AR.
Finally, we examined the effect of small-molecule–compound treatment on the nuclear localization pattern of AR-V7. Inhibition of 76 targets resulted in altered nuclear patterning of AR-V7 (Figure 1B). Forty targets were associated with increased nuclear speckling, and these were targeted by inhibitors of HDACs and GSK kinases. In contrast, inhibition of 36 targets was associated with a decreased nuclear speckle pattern, and active compounds included inhibitors of VEGFR kinases (36%), Raf kinases (66%), and Akt-related kinases (33%). Similar responses were observed for WT-AR in the GFP-AR:HeLa cell line (Supplemental Figure 2B), with additional increased activity of CDK and heat shock protein inhibitors. Taken together, HDAC inhibitors appeared to consistently increase all four metrics (expression level, nuclear level, NC ratio, and nuclear speckling) across both AR-V7 and WT-AR in the cell lines used for screening, whereas SFK and Abl inhibitors appeared to specifically inhibit overall expression and nuclear levels of AR-V7, but not of WT-AR.
Human Kinome siRNA Screen Supports Role of MAPK and Src Kinases in AR-V7 Regulation
To confirm the role of kinases in regulating AR-V7 and to identify additional kinases not targeted by the compounds tested, we modified our existing AR-HCA strategy to allow for the screening of the Invitrogen Stealth RNAi Human Kinome library (Supplemental Figure 1B) in the GFP-AR-V7:PC3 cell line. Toxicity analysis identified 101 siRNAs (18.3%) with significantly elevated toxicity index as determined by criteria described in Methods. These samples were subsequently removed from further analysis.
To identify potential therapeutic targets that might alter AR-V7 activity, we focused on those kinases that had the greatest effect on nuclear accumulation or nuclear localization pattern of AR-V7. A total of 251 (49%) siRNAs altered AR-V7 nuclear accumulation and 111 (20%) altered AR-V7 nuclear patterning (Figure 2A and B, Table S5). The large number of active siRNAs is consistent with the high level of kinase activity observed in cancer cells and with the results of previous studies using a kinome-wide siRNA library13,14. Due to the highly active nature of the kinome siRNA library, we restricted analysis to the 40 most active siRNAs for each metric.
Figure 2.
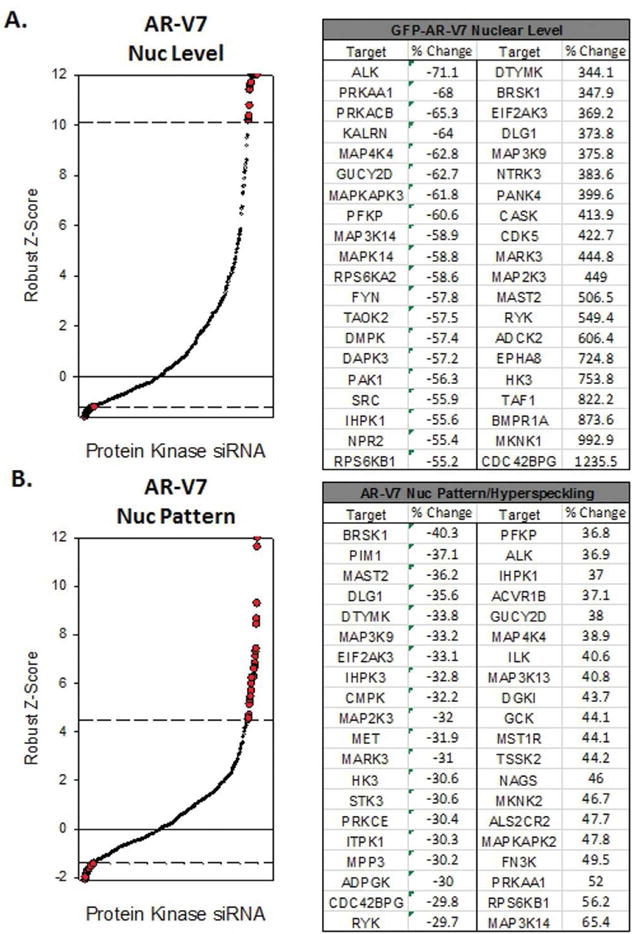
A high-content short interfering (si)RNA screen to identify protein kinases capable of altering AR-V7 nuclear accumulation and/or nuclear localization pattern. A and B. Waterfall plots of nuclear GFP-AR-V7 levels (A) and nuclear GFP-AR-V7 patterning (B) in siRNA screening samples. Dashed lines indicate thresholds of decreased or increased response compared to scrambled siRNA controls. The siRNAs exceeding the thresholds are shown in red and listed in the corresponding tables. Nuc, nuclear.
Twelve siRNAs decreased nuclear accumulation of AR-V7. Of these, six siRNAs targeted kinases involved in MAPK signaling pathways (ALK, MAP4K4, MAPKAPK3, MAPK14, RPS6KA2, TAOK2), whereas two (FYN, SRC) targeted SFKs. Treatment with five siRNAs resulted in decreased AR-V7 nuclear speckling and targeted a diverse set of kinases involved in multiple pathways, including cell cycle regulation and NF-κB signaling.
SRC/ABL Inhibitors Result in the Dose-Dependent Loss of GFP-AR-V7 Expression
Due to the observation that multiple small-molecule inhibitors of SRC/ABL and two siRNAs targeting SFKs were identified by the AR-HCA screens as decreasing GFP-AR-V7 nuclear accumulation, we chose to further validate the activity of this class of compounds. To determine if the effects were dose- and target-dependent, we treated GFP-AR-V7:PC3 cells with four structurally diverse SRC/ABL inhibitors at concentrations ranging from 50 pM to 5 μM for 48 hours (Figure 3).
Figure 3.
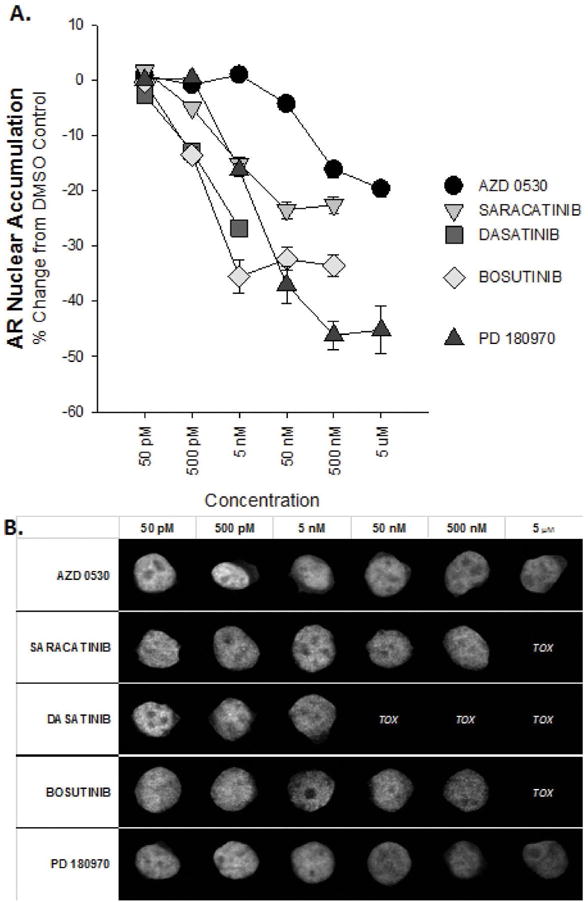
Multiple classes of Src-Abl inhibitors reduce nuclear AR-V7 accumulation in a dose- dependent manner. A. Single doses of the indicated Src-Abl inhibitor preparations reduced nuclear accumulation of AR-V7 in the PC3-GFP-AR-V7 cell line after 48 hours of treatment. Effect on nuclear accumulation was measured using an image-based approach with an N > 500 cells per sample. B. Representative images of cells showing percent change in (A) within 1% of the mean value are shown. Intensities are normalized to those of dimethyl sulfoxide (DMSO)-treated control samples.
When two preparations of the same inhibitor, AZD0530 and saracatinib, were tested, nuclear GFP-AR-V7 decreased by a similar level (20.28±0.25% and 24.79±39%, respectively).
Treatment with a substituted pyrazolopyrimidine, PD180970, resulted in the greatest decrease in nuclear GFP-AR-V7 levels (46.08±0.49%). Consistent with the known potency of these compounds, the observed effects occurred with EC50 values between 0.5 and 134 nM. Importantly, despite their structural diversity, all four compounds resulted in some degree of loss of nuclear GFP-AR-V7, suggesting that the observed effect was due to targeted inhibition of SFKs and unlikely to be a nonspecific effect.
PD180970 Inhibits Androgen-independent Prostate Cancer Cell Growth
Src and other SFKs are known to be involved in prostate cancer cell proliferation, and AR-V7 has been implicated in androgen-independent prostate cancer growth. Therefore, we examined the effect of PD180970 on cell growth in androgen-dependent and androgen-independent cancer cell lines. Using an image-based approach, we observed that a single treatment with PD180970 resulted in a dose-dependent decrease in cell number in all cell lines examined after 72 hours, regardless of the presence of androgens (Figure 4). In cell lines that either lack AR expression (PC3), or do not demonstrate a proliferative response to androgens (MCF7), the inhibitory effect of PD180970 on cell proliferation was not affected by dihydrotestosterone (DHT). Similarly, in the androgen-responsive LNCAP cell line that expresses minimal levels of AR-V7, although DHT-induced proliferation was observed, the concentration at which an effect of PD180970 treatment was observed was not significantly altered by DHT treatment.
Figure 4.
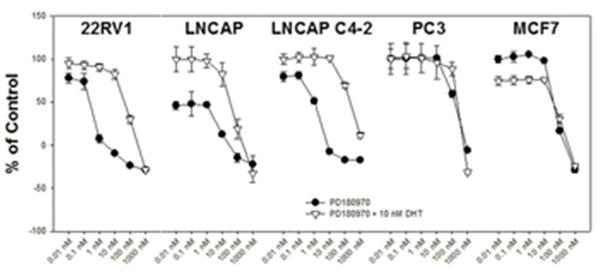
The Src-Abl inhibitor PD180970 inhibits androgen-independent in vitro prostate cancer cell proliferation. Four PCa cell lines (22RV1, LNCAP, LNCAP C4-2, and PC3) as well as the breast cancer cell line MCF7 were exposed to a single treatment with PD180970 at the indicated concentrations ranging from 0.01 nM to 1000 nM in the presence or absence of 10 nM dihydrotestosterone (DHT). After 72 hours, cell proliferation was measured using an image-based assay in which nuclei were counted. Data were normalized to that from either DHT-(PCa cell lines) or DMSO-(MCF7) treated control wells.
In contrast, in prostate cancer cells known to express either low (LNCAP C4-2) or high (22RV1) levels of AR-V7, the growth inhibitory effect of PD180970 was observed at lower concentrations in the absence of DHT. In the presence of DHT, the potency of PD180970 is similar to that observed in the other cell lines tested. This suggests that PD180970 inhibits growth in a manner that is masked by WT-AR signaling of androgen-responsive growth in cell lines that express both WT-AR and AR-V7.
PD180970 Decreases AR-V7 Protein Stability
Due to the observed effects on nuclear accumulation of AR-V7 in the GFP-AR-V7:PC3 cell line and effects on androgen-independent proliferation in cell lines with elevated AR-V7 expression, we sought to understand the effects of PD180970 on endogenous AR-V7 expression in the 22RV1 cell line. In untreated cells, a prominent band representing full-length AR and two weaker bands representing truncated AR isoforms were observed, with the large isoform previously characterized as AR-V7 (Figure 5A). With DHT treatment for 24 hours, we observed stabilization of full-length AR, whereas AR-V7 expression was decreased by 47%. With PD180970 treatment alone, expression of both full-length AR and AR-V7 decreased by 23% and 76%, respectively. Combined DHT and PD180970 treatment resulted in attenuated stabilization of full-length AR and 86% decrease in AR-V7 expression. These observations support the idea that PD180970 may decrease androgen-independent growth in 22RV1 cells by downregulating AR-V7.
Figure 5.
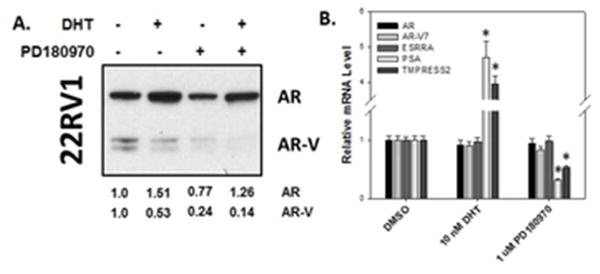
PD180970-mediated downregulation of AR-V expression reduces expression of endogenous androgen-regulated genes. 22RV1 cells were treated with PD180970 prior to 24-hour incubation with either 10 nM DHT or 0.5% DMSO. A. Androgen receptor (AR) levels, detected by the N20 antibody that recognizes both full-length AR and AR-Vs, were assessed by western blotting. Protein levels assessed by densitometry and normalized to that of DMSO-treated controls (set as 1.0) are shown below blot. B. Levels of full-length AR, AR-V, ESRRA, PSA, and TMPRSS2 mRNAs were measured by quantitative real-time PCR and normalized to that of GAPDH mRNA. Values represent the mean ± standard error of the mean (SEM).
To determine if the altered AR-V7 expression level was due to changes in protein stability or decreased transcription, we quantified full-length AR and AR-V7 mRNA levels in 22RV1 cells after treatment with either DHT or PD180970 (Figure 4B). Neither DHT or PD180970 treatment significantly altered levels of full-length AR or AR-V7 mRNA, suggesting that differences in AR-V7 protein levels were due to altered stability.
PD180970 Inhibits Androgen-independent Transcription of AR Target Genes
Androgen-induced transcription of TMPR2SS and PSA in 22RV1 is dependent on full-length AR, whereas both full-length AR and AR-V7 have been shown to play a role in androgen-independent transcription. To test whether PD180970 had an effect on endogenous gene expression, the levels of transcripts for these well-characterized androgen-regulated genes were measured. PD180970 decreased androgen-independent induction of endogenous PSA and TMPRSS2 mRNA (Figure 5B). PD180970 did not alter the level of transcripts of ESSR1, a PPAR-γ regulated gene. This result, in combination with a lack of an effect on full-length AR and AR-V7 mRNA levels, supports the idea that PD180970 has a targeted effect on AR-regulated gene expression, likely due to downregulation of AR-V7.
Inhibition of Full-length AR Signaling Sensitizes 22RV1 cells to PD180970-induced Growth Inhibition
Given that PD180970 treatment has a predominant effect on AR-V7 with minimal effects on full-length AR, we sought to determine if blockade of androgen-mediated full-length AR signaling would alter the effect seen with PD180970 treatment in the presence of androgens after 72 hours of treatment (Figure 5). Consistent with previous growth assays, PD180970 treatment in the presence of DHT significantly inhibited cell proliferation at concentrations at and above 100 nM, with an EC50 value of 184.2 nM. We observed inhibition of 22RV1 proliferation by the AR ligand-binding domain inhibitor bicalutamide at 1 uM with a calculated EC50 value of 1.2 uM. When combined with 1 uM bicalutamide, PD180970 inhibited 22RV1 proliferation in the presence of DHT at the 10 nM dose and had a reduced EC50 value of 12.6 nM. This result suggests that blocking of androgen-mediated stimulation by bicalutamide makes the cells more sensitive to inhibition of proliferation by PD180970.
DISCUSSION
Recent studies continue to link increased expression of a naturally occurring AR splice variant, AR-V7, to advanced CRPC and treatment resistance4–7; in parallel, there is growing interest in the medical and research community in understanding factors that regulate AR isoform expression and function. However, there is a paucity of data from studies that more thoroughly examine the landscape of potential drug targets to regulate AR-V7 expression. Previous studies have used screening approaches that examined either phosphorylation sites on kinases or peptide kinase substrates to identify changes linked to CaP or CRPC cell models, with effects on AR-V7 expression identified in follow-up experiments13. More recent studies have used high content screening methods to screen large small molecule libraries to identify molecules capable of altering the sub-cellular distribution of a GFT WT-AR fusion protein in a CRPC cell model15. In the present study, we used image-based, high content screening to quantify the levels and localization of GFP-AR-V7 as a primary endpoint in a stable PC-3 prostate cancer cell line treated with a panel of 145 highly active and clinically relevant compounds. Further, we paired the small-molecule inhibitors with a screen of a human kinome siRNA library targeting more than 500 protein kinases. Importantly, our image-based method allowed us to discriminate between generalized toxic effects and specific effects on AR-V7 biology. Whereas the methodologies used in this and other recent studies are markedly different, all support the conclusion that kinase signaling is highly active in CaP cells, and that disruption of signaling in these pathways can alter AR-V7 expression and function.
The mechanisms involved in the regulation of AR-V7 mRNA expression and protein stability are still being explored. In the current study, HDAC inhibitors proved to be highly active in increasing the expression, nuclear level, and nuclear speckling pattern of both AR-V7 and WT-AR. Previous work has shown that treatment of CaP cells with HDAC inhibitors results in DNA damage and apoptosis, an effect enhanced in the presence of AR expression16.
Surprisingly, the loss of WT-AR transcriptional activity with HDAC inhibitor treatment is associated with an increase in WT-AR protein level17,18. Our results suggest that a similar phenomenon likely occurs with AR-V7 and, due to the fact that AR-V7 expression is driven by the CMV promoter, the changes in protein level likely reflect altered degradation pathways as opposed to loss of AR-mediated feedback inhibition of its own expression. Recently, based upon follow-on data from our original screens described in this report, we published a study that identifies CUDC-101, an inhibitor of Her2/Neu/EGFR/HDAC, as an effective in vitro and in vivo inhibitor of AR-V7 transcriptional activity and cell proliferation. This effect of CUDC-101 was found to be primarily driven by its inhibitory effect on HDAC activity19. Our findings and those of others indicate that further study of HDAC inhibitors in patients with AR-V7–positive CaP may yield promising results.
In addition to the ubiquitous effect of HDACs on both AR-V7 and WT-AR, AR-V7 nuclear levels and localization were consistently decreased by dual-specificity compounds targeting Src and/or Abl kinases. Due to the fact that these inhibitors tend to exhibit activity against multiple members within a protein family20,21, it remained unclear whether one or both was mediating the effect on AR-V7 expression. To overcome this limitation and to further examine the kinase landscape regulating AR-V7 expression, we performed an unbiased screen of the human kinome in which kinases relating to the MAPK signaling pathways (the SFKs Src and Fyn, but not Abl) were identified as capable of altering AR-V7 expression. Further evidence suggesting a role for Src, but not Abl, is found in previous studies that have shown that Src, Fyn, and Abl are overexpressed in various CaP cell lines, including PC3, but that the Abl kinase is not activated22.
There is extensive evidence of the important role that the Src kinases plays in CRPC (reviewed in2), with recent studies showing increased levels of Src and phosphorylated (p)-Src in tumor tissue isolated from CRPC patients23. Further, in vitro studies show that increased expression of p-Src results in altered AR phosphorylation, which results in resistance to AR antagonists and increased CaP proliferation in hormone-depleted conditions23, features shared with increased AR-V7 expression. Fyn, a kinase associated with neuronal development, has recently been shown to be elevated in metastatic CaP tissue24. Interestingly, Src and Fyn have not been previously shown to directly affect WT-AR or AR-V7 protein stability. However, the PI3K/Akt/mTOR pathway, through the direct phosphorylation of WT-AR/AR-V7 and the Mdm2/proteasome pathway, plays an important role in AR turnover25. Therefore, further work is needed to determine if the Src and Fyn kinases are directly regulating AR-V7 expression through phosphorylation events, or are acting as upstream regulators of other pathways, including the PI3K/Akt/mTOR pathway.
Further evaluation of a set of structurally diverse Src inhibitors demonstrated a consistent inhibitory effect on AR-V7 expression in the GFP-AR-V7:PC3 model, with the greatest effect observed with the pyrazolopyrimidine PD180970. As androgen depletion induces AR-V7 expression in a variety of cell lines, we demonstrated a growth inhibitory effect of PD180970 treatment in multiple androgen-dependent and androgen-independent cell lines. This effect was greatest in the AR-V7–expressing 22RV1 and LNCaP C4-2 CaP cell lines, suggesting that the SFK inhibitor effects are associated with AR-V7 expression. Interestingly, the enhanced effect of PD180970 treatment in AR-V7–expressing cells was lost in the presence of DHT, suggesting that WT-AR signaling confers resistance to PD180970 inhibition. Conversely, inhibition of WT-AR by use of the AR antagonist bicalutamide (Casodex) resensitizes 22RV1 cells to the effect of PD180970 treatment in the presence of DHT. This is in line with studies in which suppression of AR-V7 expression makes cancer cells more sensitive to hormonal therapies6. Given that the majority of CRPCs continues to express WT-AR, we suggest that inhibition of SFKs may be more effective when combined with therapies targeting WT-AR, including enzalutamide and abiraterone acetate, in patients with AR-V7–positive disease.
Src and the SFKs are known to play an important role in many intracellular signaling pathways and are therefore a popular target in cancer therapy. Early clinical studies with SFK inhibitors in CRPC were promising, with evidence of patient response in a majority of patients, with minimal drug-related adverse events26. However, in later clinical trials for patients with metastatic CRPC in which SRK inhibitors were either combined with docetaxel or used with patients who had failed docetaxel therapy, there was no evidence of improved survival27–29. However, very few drugs have been shown to improve upon the effect of taxanes when given in combination or after a taxane based therapy27,28. Further, there is tremendous molecular heterogeneity in this patient group, and it is likely that the studies included patients with varying degrees of dependence on AR-V7 and SFKs. Thus, a focus on a more optimized clinical patient subgroup may lead to a clearer benefit, similar to the manner in which molecular characterization of breast cancer now drives treatment options.
CONCLUSIONS
Advances in drug development have resulted in exciting changes to the therapeutic landscape for advanced prostate cancer. For several years, the primary treatment option for CRPC has been limited to docetaxel chemotherapy, which extends survival, but only for a very limited time [39]. Whereas the treatment of advanced prostate cancer has been extremely challenging, novel therapeutic options have gained FDA approval, including the androgen-synthesis inhibitor abiraterone acetate and the AR antagonist enzalutamide. Although these new therapies have expanded the treatment options for patients, sustainable suppression of prostate cancer growth still remains a primary challenge, especially in patients with AR-V7–positive disease. Understanding the pathways that might regulate AR-V7 expression may lead to new therapeutic strategies in this challenging patient population. In the current study, we attempted to characterize the effect of a diverse panel of clinically relevant small-molecule inhibitors and identify kinase pathways that regulate AR-V7 expression, leading to the identification of the SFKs Src and Fyn as potentially important protein kinases.
Supplementary Material
Figure 6.
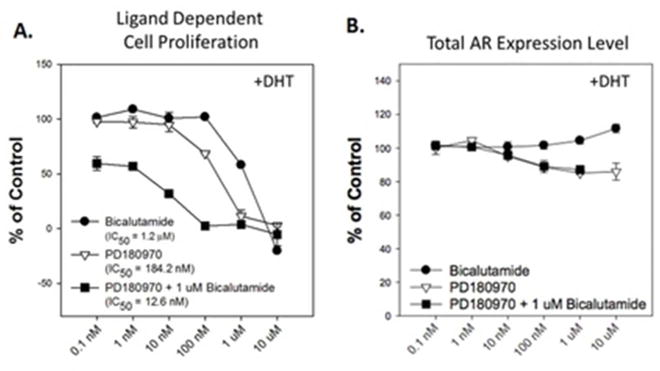
Bicalutamide treatment sensitizes DHT-treated prostate cancer cells to PD180970- mediated growth inhibition without altering total AR expression. 22RV1 cells were pretreated with the indicated concentrations of Bicalutamide, PD180970, or PD180970 + 1 μM Bicalutamide prior to 72-hour incubation with 10 nM DHT. Samples were fixed and labeled for immunofluorescence using anti-AR (N20) antibody. Nuclei were counterstained with DAPI and imaged. A. Effects on cell proliferation determined by counting total number of per sample and normalizing to DHT-only treated samples (100%) and day 0 fixed samples (0%). B. Effects on AR expression determining total anti-AR label intensity per cell and normalizing to DHT-only treated samples (100%).
Acknowledgments
Support was provided by the Integrated Microscopy Core at Baylor College of Medicine with funding from the John S. Dunn Gulf Coast Consortium for Chemical Genomics, the Dan L. Duncan Cancer Center (NIH P30CA125123), and the NIH (HD007495, and DK56338). Research conducted in joint participation with Diana Helis Henry Medical Research Foundation (M.A.M., M.M.). ATS is a K12 Scholar supported by NIH grant K12DK0083014, the multidisciplinary K12 Urologic Research (KURe) Career Development Program awarded to Dr. Dolores J. Lamb.
Footnotes
Disclosure Statement: Authors do not have any disclosures relating to any affiliations that they consider to be relevant and important nor has a direct interest in the work presented.
References
- 1.Sadar MD. Small molecule inhibitors targeting the ‘achilles’ heel’ of androgen receptor activity. Cancer Res. 2011;71:1208–13. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelman IH. Androgen receptor activation in castration-recurrent prostate cancer: the role of Src-family and Ack1 tyrosine kinases. Int J Biol Sci. 2014;10:620–6. doi: 10.7150/ijbs.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.Qu Y, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep. 2015;5:7654. doi: 10.1038/srep07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonarakis ES, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Z, et al. A Novel Androgen Receptor Splice Variant is Upregulated during Prostate Cancer Progression and Promotes Androgen-depletion-resistant Growth. Cancer. 2010;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu R, et al. Distinct Transcriptional Programs Mediated by the Ligand-Dependent Full-Length Androgen Receptor and Its Splice Variants in Castration-Resistant Prostate Cancer. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mediwala SN, et al. The activity of the androgen receptor variant AR-V7 is regulated by FOXO1 in a PTEN-PI3K-AKT-dependent way. Prostate. 2012 doi: 10.1002/pros.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcelli M, et al. Quantifying effects of ligands on androgen receptor nuclear translocation, intranuclear dynamics, and solubility. J Cell Biochem. 2006;98:770–88. doi: 10.1002/jcb.20593. [DOI] [PubMed] [Google Scholar]
- 11.Szafran AT, Mancini MA. The myImageAnalysis project: a web-based application for high-content screening. Assay Drug Dev Technol. 2014;12:87–99. doi: 10.1089/adt.2013.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judson RS, et al. Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High Throughput Screening Assays for the Estrogen Receptor. Toxicol Sci. 2015;148:kfv168. doi: 10.1093/toxsci/kfv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiota M, et al. Potential Role for YB-1 in Castration-Resistant Prostate Cancer and Resistance to Enzalutamide Through the Androgen Receptor V7. J Natl Cancer Inst. 2016;108:djw005. doi: 10.1093/jnci/djw005. [DOI] [PubMed] [Google Scholar]
- 14.Azorsa DO, et al. High-content siRNA screening of the kinome identifies kinases involved in Alzheimer ’ s disease-related tau hyperphosphorylation. 2010 doi: 10.1186/1471-2164-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston PA, et al. Development and Implementation of a High-Throughput High-Content Screening Assay to Identify Inhibitors of Androgen Receptor Nuclear Localization in Castration-Resistant Prostate Cancer Cells. Assay Drug Dev Technol. 2016;14:226–39. doi: 10.1089/adt.2016.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis L, et al. Combinatorial antitumor effect of HDAC and the PI3K-Akt-mTOR pathway inhibition in a Pten defecient model of prostate cancer. Oncotarget. 2013;4:2225–36. doi: 10.18632/oncotarget.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis L, et al. Concurrent HDAC and mTORC1 inhibition attenuate androgen receptor and hypoxia signaling associated with alterations in MicroRNA expression. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacopino F, et al. Valproic acid activity in androgen-sensitive and -insensitive human prostate cancer cells. Int J Oncol. 2008;32:1293–1303. doi: 10.3892/ijo_32_6_1293. [DOI] [PubMed] [Google Scholar]
- 19.Sun H, Mediwala SN, Szafran AT, Mancini MA, Marcelli M. CUDC-101, a Novel Inhibitor of Full-Length Androgen Receptor (flAR) and Androgen Receptor Variant 7 (AR-V7) Activity: Mechanism of Action and In Vivo Efficacy. Horm Cancer. 2016 doi: 10.1007/s12672-016-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraker aJ, et al. Biochemical and cellular effects of c-Src kinase-selective pyrido[2, 3-d]pyrimidine tyrosine kinase inhibitors. Biochem Pharmacol. 2000;60:885–898. doi: 10.1016/s0006-2952(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 21.Rosée P La, et al. Activity of the Bcr-Abl Kinase Inhibitor PD180970 against Clinically Relevant Bcr-Abl Isoforms That Cause Resistance to Advances in Brief Activity of the Bcr-Abl Kinase Inhibitor PD180970 against Clinically Relevant Bcr-Abl Isoforms That Cause Resistance. Cancer Res. 2002;62:7149–7153. [PubMed] [Google Scholar]
- 22.Chang YM, et al. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008;27:6365–6375. doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Z, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Posadas EM, et al. FYN is overexpressed in human prostate cancer. BJU Int. 2010;103:171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Xie N, Gleave ME, Rennie PS, Dong X. AR-v7 protein expression is regulated by protein kinase and phosphatase. Oncotarget. 2015;6:33743–54. doi: 10.18632/oncotarget.5608. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yu EY, et al. Once-daily dasatinib: Expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77:1166–1171. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonarakis ES, Eisenberger Ma. Phase III Trials With Docetaxel-Based Combinations for Metastatic Castration-Resistant Prostate Cancer: Time to Learn From Past Experiences. J Clin Oncol. 2013;31:8–11. doi: 10.1200/JCO.2013.48.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fizazi KS, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–1747. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 29.Posadas EM, et al. Saracatinib as a metastasis inhibitor in metastatic castration-resistant prostate cancer: A University of Chicago Phase 2 Consortium and DOD/PCF Prostate Cancer Clinical Trials Consortium Study. Prostate. 2016;76:286–93. doi: 10.1002/pros.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


