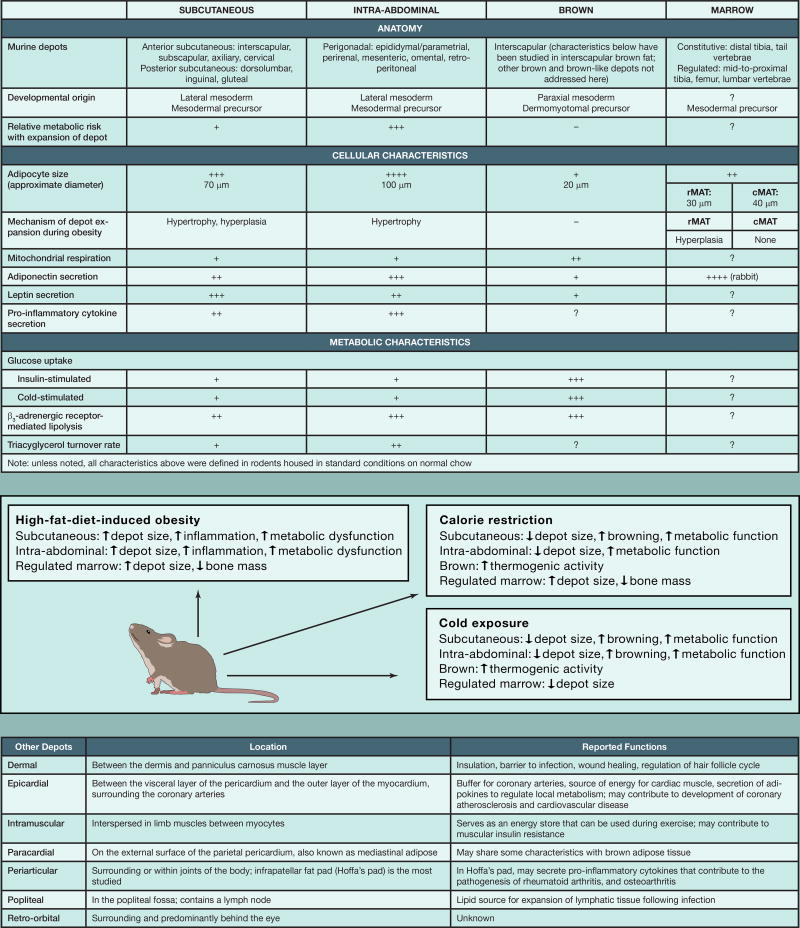
Adipocytes throughout the body have a wide array of functions: storage and release of energy in response to local and global needs, uncoupling of metabolism in brown(-like) adipocytes to generate heat, and secretion of adipokines to regulate whole-body metabolism and immune response (Cinti, 2012). These different properties of the depots are medically relevant since an increase in intra-abdominal, but not subcutaneous, adiposity is closely associated with development of metabolic syndrome. In Part I, we provided an atlas of rodent adipose anatomy. Here, we present an overview of the properties of the most well-characterized depots and known details of less-studied depots.
Subcutaneous adipocytes are contained within the anterior and posterior subcutaneous depots. The interscapular depot contains multilocular brown adipocytes, which are rich in mitochondria. Brown and brown-like adipocytes express uncoupling protein 1 (UCP1), which when activated can uncouple mitochondrial respiration, leading to non-shivering thermogenesis. This increase in mitochondrial respiration also promotes glucose and fatty acid uptake and oxidation in brown adipocytes (Kajimura et al., 2015). Some adipose depots are more prone to browning; although the posterior subcutaneous depot appears to be continuous, centrally located inguinal adipocytes become brown-like more readily than those in adjacent depots. However, at very low environmental temperatures, even epididymal adipose tissue is recruited to form brown-like thermogenic adipocytes (Yang et al., 2017).
The intra-abdominal depots, such as the mesenteric, perirenal, retroperitoneal, and gonadal fat pads, are contained within the peritoneal cavity. Relative to adipocytes in subcutaneous depots, intra-abdominal adipocytes are typically larger and secrete less leptin. In addition, expansion of the intra-abdominal depots is highly associated with development of systemic insulin resistance. With positive energy balance, subcutaneous adipose tissue expands by adipocyte hypertrophy and hyperplasia (Jeffery et al., 2016). In contrast, intra-abdominal depots expand during obesity predominantly by adipocyte hypertrophy (Jeffery et al., 2016), which is associated with metabolic dysfunction because larger adipocytes have increased lipolysis, attract inflammatory macrophages, and are insulin resistant.
Marrow adipose tissue accounts for ~10% of total fat mass in healthy humans, and in rodents can be classified as constitutive or regulated. Studies have demonstrated region-specific differences in marrow adipocyte properties, including development, regulation, gene expression, and lipid composition (Scheller et al., 2015). In addition to serving as a lipid reservoir, marrow adipose tissue is a disproportionate source of circulating adiponectin, and because of its enclosure within bone, is integral to our understanding of osteoblasts, osteoclasts, and hematopoietic cells.
In addition to the adipose depots discussed above, there are several other understudied depots that are likely to have important local roles yet to be discovered. These include intramuscular periarticular, paracardial, epicardial, and retro-orbital adipocytes. The popliteal depot provides lipids for expansion of lymphatic tissue following infection (Pond, 2005). The dermal depot has roles in thermal insulation, wound healing, and hair follicle growth, and serves as a barrier to infection (Alexander et al., 2015).
The transcriptional programming of adipogenesis has been studied extensively in vitro. Studies have shown that induction of differentiation results in rapid activation of transcription factors, leading to dramatic changes in the epigenome. These events culminate in the induction of the master regulator of adipogenesis, peroxisome proliferator-activated receptor γ (PPARγ), and other key adipogenic transcription factors, including CCAAT-enhancer binding protein α (C/EBPα) and C/EBPβ, which drive the adipocyte gene program (Lefterova et al., 2014). Whereas PPARγ is uniformly required for adipogenesis, the importance of C/EBP family members appears to vary among different depots. Similarly, the importance of several other adipogenic transcriptional regulators has also been shown to be highly depot specific. However, there is currently limited knowledge about the transcriptional regulators that orchestrate adipogenesis in vivo. Development of novel animal models like the nuTRAP mouse (Roh et al., 2017), along with detailed genome-wide studies (Loft et al., 2017), will allow a more detailed molecular definition of differences in development, gene expression, and function between the diverse adipose depots described here. We fully anticipate additional surprises as our understanding of these depot-specific differences deepens.
Figure 1.
Acknowledgments
O.A.M. is supported by NIH grants DK062876 and DK092759; D.P.B. is supported by the University of Michigan Medical Scientist Training Program (T32GM007863), University of Michigan Training Program in Organogenesis (T32HD007605), and the Tylenol Future Care Fellowship; S.M. is supported by the Danish Council for Independent Research | Natural Science, the Lundbeck Foundation, and the Novo Nordisk Foundation; and I.F. is supported by the Danish Diabetes Academy, which is supported by the Novo Nordisk Foundation.
References
- Alexander CM, Kasza I, Yen CL, Reeder SB, Hernando D, Gallo RL, Jahoda CA, Horsley V, MacDougald OA. J. Lipid Res. 2015;56:2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. Dis. Model. Mech. 2012;5:588–594. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, Church CD, Colman L, Berry R, Rodeheffer MS. Cell Metab. 2016;24:142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. Trends Endocrinol. Metab. 2014;25:293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft A, Forss I, Mandrup S. Trends Endocrinol. Metab. 2017;28:104–120. doi: 10.1016/j.tem.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Pond CM. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:17–30. doi: 10.1016/j.plefa.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Roh HC, Tsai LT, Lyubetskaya A, Tenen D, Kumari M, Rosen ED. Cell Rep. 2017;18:1048–1061. doi: 10.1016/j.celrep.2016.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, et al. Nat. Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Sui W, Zhang M, Dong M, Lim S, Seki T, Guo Z, Fischer C, Lu H, Zhang C, et al. JCI Insight. 2017;2:e89044. doi: 10.1172/jci.insight.89044. [DOI] [PMC free article] [PubMed] [Google Scholar]