Abstract
ApoA4 exerts anti-inflammatory effects, but the mechanism remains unclear. SERPINA3 is a member of the serine proteinase inhibitor gene family, and has been shown to be involved in anti-inflammation and associated with a number of human diseases. In this study, we revealed that ApoA4 stimulates the gene expression of SERPINA3 in mouse hepatocytes both in vivo and in vitro, in a dose- and time-dependent manner. The transcriptional response of SERPINA3 to ApoA4 is regulated through the binding of ApoA4 with nuclear receptors NR4A1 and NR1D1 on the SERPINA3 promoter, which was verified with ChIP, Luciferase activity assay and RNA interference-mediated NR4A1 or NR1D1 gene knockdown. These data suggests that ApoA4 transcriptionally induced SERPINA3 expression via NR1D1 and NR4A1. Our findings may throw light on the function of ApoA4 in inflammatory responses and acute-phase reactions, as well as the development of SERPINA3 relative diseases.
1. Introduction
ApoA4 is a lipoprotein primarily synthesized by enterocytes of the small intestine, and secreted into blood as a major component of high-density lipoprotein cholesterol and chylomicrons [1]. Numerous functions have been ascribed to ApoA4, which is involved in the metabolic procedure of lipid and glucose, and anti-inflammatory response [2]. ApoA4 knockout mice exhibited a significantly greater inflammatory response to DSS, which was reversed upon exogenous administration of ApoA4 to knockout mice [3]. The overexpression of ApoA4 or exogenous administration of ApoA4 in APOE0 mice reduces the susceptibility to atherogenesis and decreases the secretion of proinflammatory cytokines INF-γ and TNF-α after LPS administration [4, 5].
The serine protease inhibitor, alpha-1 antichymotrypsin (SERPINA3), acts as an inhibitor of several serine proteases involved in a wide range of biological processes [6]. SERPINA3 is a typical acute phase protein, wherein its amount of circulating protein dramatically increases in response to inflammation and its gene expression is stimulated in hepatic cells, macrophages, endothelial and epithelial cells, as well as activated astrocytes by various cytokines such as IL-6 and TNF-α [7]. Its major target is the cathepsin family, a pro-inflammatory enzyme released at sites of inflammation, which contributes to the activation of inflammatory cytokines, the degradation of pathogens, and the remodeling of tissues [8]. Therefore, by inhibiting cathepsin G, SERPINA3 should limit inflammation, coagulation, extracellular matrix remodeling and apoptosis [9]. SERPINA3 has been involved in the pathology of a number of devastating human diseases including Parkinson’s disease and Alzheimer’s disease. The disease can arise from gene expression disturbances that result in excess uncontrolled target protease activity and disruption of the downstream pathway [6, 10, 11]. It was reported that Nur77 (NR4A1) regulate SERPINA3 expression in astrocytes and HepG2 human liver cancer cells [12]. In the present study, SERPINA3 was first identified as an ApoA4-regulated gene transcriptionally through nuclear receptors NR4A1 and NR1D1 in hepatocytes.
2. Materials and Methods
2.1. Animal experiment
Age-matched 12 week-old male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). These mice were individually housed in a temperature-controlled vivarium with a 12-hour light/12-hour dark cycle (lights on at 05:00 hours). Laboratory chow (Teklad 7912, Madison, WI) and water were provided ad libitum until the time of sacrifice, except where noted. Mice were sacrificed at 13–16 weeks, intracardiac blood collection and multiple tissue extraction were performed, placed into RNALater (Ambion), and stored at −80°C. For the ApoA4 treatment group, 50 µg of recombinant mouse ApoA4 (r-m-ApoA4) was injected intraperitoneally for each mouse two hours before sacrifice. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and Xi’an Jiaotong University.
2.2. Northern blot analysis of SERPINA3 expression
Total RNA were obtained from mouse tissues using a TOTALLY RNA™ kit (Ambion), according to manufacturer’s instructions. Five µg of total tissue RNA were electrophoresed in 1.2% MOPS-Formaldehyde agarose gel and electro-blotted onto a positively charged nylon membrane for hybridization analysis with non-radioactive DIG labeled RNA probes in DIG Easy Hyb hybridization solution for 16 hours at 50°C. Then, the blots were washed and detected with DIG Wash and Block buffer, Anti-digoxigenin-AP, and CDP-Star (Roche Applied Science, Indianapolis, IN), according to manufacturer’s instructions. For the detection of mouse SERPINA3, DIG-labeled RNA probe specific for mouse SERPINA3 was generated using a plasmid containing mouse SERPINA3 cDNA (BC_013651, nt 1–2085) as the template and the DIG RNA labeling kit (Roche Applied Science). As an internal control, the blots were stripped and re-hybridized with a DIG-labeled probe for mouse GAPDH (NM_0080842, nt349-664).
2.3. Cells and cell culture
Primary mouse hepatocytes were isolated from three-month old male C57BL/6J mice, as previously described [13]; and cultured in a similar way as the HEK-293 and HepG2 cells (ATCC, Manassas, VA) in high glucose DMEM with 10% FBS and 1% pen/strep in a 5% CO2 atmosphere. The cell line cells were passaged 2–3 times a week. Cells in the exponential growth phase were used for experiments.
2.4. Gene expression by qRT-PCR and western blotting
Total RNA was isolated and quantitative real time PCR (qRT-PCR) was performed, as previously described [14]. All the primers were purchased from Integrated DNA Technologies (Coralville, IA). The sequences for mouse SERPINA3 are as follows: forward, 5’-AGGAACCAAGGATCTG-AGAGTG-3’; reverse, 5’-GCTGCTTCTGTGCCTGTCT-3’. Other primer pairs used in this study were the same, as previously described [13, 15]. Western blotting was performed as described in the western immunoblotting protocol provided by Cell Signaling Technology [16]. Blots were probed with anti-SERPINA3 (NOVUS, Littleton, CO) and anti-actin (Millipore, Billerica, MA), respectively.
2.5. Luciferase activity assay
HEK-293 cells were transient transfected, as previously described in luciferase activity assays [13, 15]. These cells were grown in 24-well plates, and 0.3 µg of SERPINA3-luciferase reporter with or without 0.3 µg of human Nr1d1 or human Nr4a1 expression vector, 20 pmol of siNr1d1 or siNr4a1 or si-control, and 5 ng of Renilla luciferase control Reporter vector were added into each well. Twenty-four hours after transfection, cells were treated with or without recombinant human ApoA4 protein (r-h-ApoA4) for 24 hours. Relative luciferase activities were measured using a Dual-luciferase Reporter Assay System kit (Promega, Madison, WI) and determined by dividing the light units generated by the firefly luciferase and those generated by the Renilla luciferase in the same reaction.
2.6. Gene overexpression and RNA interference
The SERPINA3 promoter region (−341 to +54) [12] was amplified from human genomic DNA. The resulting PCR product was inserted into pGL3-basic vector (Promega, Madison, WI). An AMAXA-based electroporation method (Nucleofector kit from Amaxa, Gaithersburg, MD) was used, according to manufacturer’s instructions, in order to deliver Nr1d1 and Nr4a1 plasmids or siRNA into HepG2 cells. The human Nr1d1 or Nr4a1 expression plasmas and double-strand RNAi oligos targeting either the negative control, human Nr1d1, or human Nr4a1 were the same, as previously described [13, 15].
2.7. Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed with anti-GFP (for ApoA4-GFP), anti-NR1D1 and NR4A1 antibodies, respectively, as previously described [13, 15]. The sequences of primers used to amplify the promoter region of human SerpinA3 or the promoter of the GAPDH gene as control by PCR were as follows: SerpinA3 forward: TCATTTCCAGTCCGAGAACAG, SerpinA3 reverse: GGATTTTCATGAATGCTGAGGC; GAPDH forward: TACTAGCGGTTTTACGGGCG, GAPDH reverse: TCGAACAGGAGGAGCAGAGAGCGA.
2.8. Statistical analysis
Data were presented as mean ± SE of at least three independent experiments performed using triplicate samples for cell culture experiments or 5–9 mice in each group for animal in vivo experiments. Intergroup differences were compared using two-tailed student t-tests or one-way ANOVA analysis, followed by Tukey’s multiple comparison test where appropriate; and the level of statistical significance was set at P<0.05.
3. Results
3.1. ApoA4 stimulates endogenous SERPINA3 expression in mouse in vivo
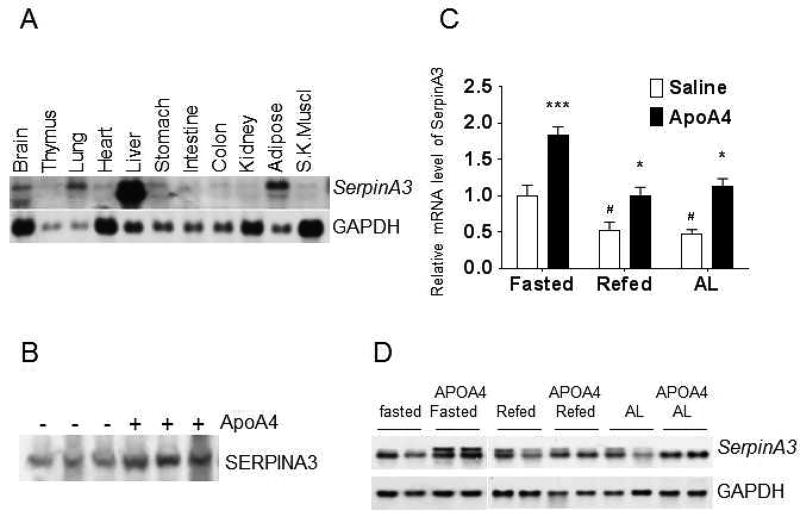
SERPINA3 is highly expressed in human liver, but microarray expression profiles show a wide range of expression in human multiple tissue types [6, 17]. In the present study, Northern blotting results on mouse multiple tissues reveled that SERPINA3 was highly expressed in mouse liver, and was mainly expressed in the adipose, lung and brain (Fig. 1A). NR4A1 enhances endogenous SERPINA3 in human tumor cancer HepG2 cells [12], and we have previously reported that ApoA4 binds to NR4A1 and induces its expression in mouse primary hepatocytes, HepG2 cells [15]. All previous studies above suggest that ApoA4 might be the upstream stimuli of NR4A1 action on SERPINA3 expression. In order to test this hypothesis, plasma SERPINA3 protein level was detected from ad libitum (AL) mice two hours after the administration r-m-ApoA4 by western blotting. Data revealed that SERPINA3 protein level in mouse plasma was elevated compared with that from saline negative control mice (Fig. 1B). In order to determine whether this elevation of SERPINA3 come from mouse hepatocytes and is in the transcriptional level, mice were either 24-hour fasted (Fasting), 24-hour fasted and re-fed for 24-hours (Refed), or fed ad libitum. Then, the mRNA level of SERPINA3 was measured in mouse liver isolated two hours after mice were treated with ApoA4 (i.p., r-m-ApoA4) both by qRT-PCR and Northern blotting. Notably, SERPINA3 mRNA in the liver of mice in the 24-hour Fasting group was significantly higher than Refed and AL mice (Fig. 1C). Furthermore, ApoA4 significantly induced SERPINA3 gene expression in mice in the Refed and AL groups, compared with saline control mice (Figs. 1C and 1D). Interestingly, two bands of SERPINA3 appeared in the Northern blotting, which might be splicing isoforms induced by ApoA4.
Figure 1. SERPINA3 expression in mouse multiple-tissues and ApoA4 induces SERPINA3 expression in mouse liver.
A: Multiple tissues were isolated from male C57BL/6J mice. Five µg of total RNA from tissues were analyzed by Northern blotting and probed with a non-radioactive DIG labeled RNA probe specific for mouse SERPINA3 with GAPDH as a control. B, C and D: Two hours after the administration of recombinant mouse ApoA4 (r-m-A4, 2 µg/g of body weight) by i.p., blood and liver samples were collected, and total RNA was isolated from the livers of mice (n=8–9 per group) that were fed ad libitum, fasted for 24 hours, or fasted for 24 hours and re-fed for 24 hours. Plasma SERPINA3 proteins were detected by western blotting from 5 µl of plasma obtained from mice fed ad libitum (B). SERPINA3 mRNA levels (C) were quantitated by real-time PCR and normalized to those of GAPDH RNA. Five µg of total RNA from the livers were analyzed by Northern blotting with a DIG labeled RNA probe specific for mouse SERPINA3 (D). Data are presented as mean ± SE. *P<0.05, ***P<0.001 vs. saline control by t-test; #P<0.05 vs. Fasted saline control by t-test.
3.2. Induction of endogenous SERPINA3 expression by ApoA4 in primary mouse hepatocytes and HepG2 cells
In order to confirm that the effect of ApoA4 on SERPINA3 is the result of the directly stimulation of ApoA4 in liver cells and to exclude potential secondary factors induced by ApoA4 treatment on SERPINA3 expression, mouse primary hepatocytes were isolated and cultured; and the mRNA level of SERPINA3 from hepatocytes with or without ApoA4 treatment was determined. This result was consistent with the effects observed in mouse livers in vivo (Figs. 2C and 2D), and the expression of SERPINA3 induced by ApoA4 revealed a U-shaped with an elevated dose of ApoA4 in a time depended manner. At four hours after ApoA4 treatment, 20 µg/ml of ApoA4 had a maximum effect on SERPINA3 expression (Figs. 2A and 2B). In addition, SERPINA3 protein levels increased at two and four hours after treatment with 20 µg/ml of ApoA4 (Figs. 2C and 2D). In order to detect the effect of ApoA4 on SERPINA3 expression in human hepatic HepG2 cells, immunoblotting was performed with the cells treated with r-h-ApoA4. The data revealed that SERPINA3 protein was elevated by ApoA4 stimulation (Supplementary Fig. 1). All these data suggests that ApoA4 acts directly on hepatocytes to stimulate endogenous SERPINA3 gene expression.
Figure 2. ApoA4 induces SERPINA3 expression from primary mouse hepatocytes in vitro.
Primary mouse hepatocytes were prepared from C57BL6 mice, cultured in DMEM with 10% FBS medium, and treated with an elevated dose of r-m-ApoA4 for four hours (A) or 20 µg/ml of r-m-ApoA4 for the indicated number of hours (B). The amount of SERPINA3 mRNA was determined by real-time PCR (A and B). The protein levels of SERPINA3 were detected by western blotting with anti-SERPINA3 antibodies and loading control β-Actin (C and D). The mean ± SE of more than triplicate samples is shown. *P<0.05 **P<0.01 vs. 0 min or vehicle control by t-test.
3.3. ApoA4 increases SERPINA3 promoter activity
It has been reported that NR4A1 protein increases SERPINA3 activity by binding to the NGFI/Nur77 binding responsive element (NBRE) site in the SERPINA3 promoter [12]. We have previously reported the interaction of ApoA4 with NR4A1 and the increase in NR4A1 expression induced by ApoA4 [15]. In order to determine the expression of SERPINA3 induced by ApoA4 mimics NR4A1 on SERPINA3, luciferase activity driven by the human SERPINA3 promoter was analyzed in cells incubated with ApoA4. Similar to the effect of NR4A1, our data indicated that ApoA4 increases SERPINA3 promoter activity, and that this activity was elevated in ApoA4 in a dose dependent manner (Supplementary Fig. 2A). Furthermore, 20 µg/ml of ApoA4 started to exhibit this effect, which increased with the elevated dose of ApoA4. ApoA4 and APOA-I are closely related and share a number of functions [18]. In order to ensure that the activity increased by ApoA4 is specific from ApoA4, the same procedure were also performed with same amount of recombinant human ApoA-I protein (h-m-ApoA-I). The change in SERPINA3 promoter activity cannot be detected in cells treated with ApoA-I (Supplementary Fig. 2B). Thus, it appears that ApoA4 specifically and transcriptionally induces SERPINA3 gene expression both in mouse and human hepatic cells.
3.4. Transcriptional regulation of the SERPINA3 promoter by ApoA4 through NR1D1 and NR4A1
NR4A1 protein increases the activity of SERPINA3 through binding to the NBRE site in the SERPINA3 promoter [12], and our previous publication demonstrated that APOA4 activates NR4A1 for regulating the target gene expression through interacting with NR4A1 and inducing NR4A1 expression [15]. Consistent with previously reported observations [12], in this study, the same effect of NR4A1 on SERPINA3 promoter activity was observed. However, SERPINA3 promoter activity unexpectedly started to increase with 37.5 ng (which is only 1/8 of the common dose) of NR4A1, and decreased with increase in dosage (Fig. 3A). NR4A1-specific small interfering RNA (siRNA) totally inhibited SERPINA3 promoter activity, because luciferase activity almost could not be detected in cells transfected with the siRNA of NR4A1. Furthermore, ApoA4 enhanced the effect of NR4A1 on SERPINA3 promoter activity by further increasing luciferase expression (Fig. 3B). In cells overexpressing NR4A1, as well as those treated with ApoA4, luciferase activity was 35.9% and 74.1% more than that in cells transfected only with NR4A1 and in cells only treated with ApoA4, respectively. These data indicate that ApoA4 works on SERPINA3 through NR4A1.
Figure 3. ApoA4 regulates the activation of the SERPINA3 promoter through NR4A1 and NR1D1 detected by luciferase activity assay.
HEK-293 cells were co-transfected with 300 ng of SERPINA3 luciferase reporter plasmid with the indicated amount (A and C) or 100 ng (B and D) of pcDNA3.1-Nr4a1 (A and B) or pcDNA3.1-Nr1d1, or pcDNA3.1-Nr1d1-602H (C and D) expression plasmid or siRNA of Nr4a1 (B), or the siRNA of Nr1d1 (D), or the empty vector for 24 hours. Then, these were incubated with or without 50 µg of r-h-ApoA4 for 24 hours. Cells were lysed with lysis buffer, and the lysates were subjected to luciferase activity assay. A and B: SERPINA3 luciferase activity in NR4A1 overexpression or NR4A1 knockdown cells with or without r-h-ApoA4, respectively. C and D: NR1D1 overexpression or NR1D1 knockdown cells with or without r-h-ApoA4, respectively. The mean ± SE of more than triplicate samples is shown. a, b, c, d and e: P<0.05 compared to each other by one-way ANOVA, followed by Tukey’s multiple comparison test.
We previously reported the interaction between ApoA4 and nuclear receptor NR1D1, the increase of NR1D1 expression induced by ApoA4, and the regulation of each between NR4A1 and NR1D1 [13, 15]. In order to confirm the involvement of NR1D1 in the modulation of SERPINA3 gene expression, we detected luciferase activity by using the same experimental processor transfected with NR1D1 or NR1D1 602HF mutant plasmids [19] with or without ApoA4 treatment. The same result was obtained as that observed with NR4A1, and the effect of NR1D1 on SERPINA3 promoter activity in NR1D1 was dose dependent (Fig. 3C). The overexpression of NR1D1 added the effect of ApoA4 on the SERPINA3 promoter and NR1D1-specific small interfering RNA (siRNA), and NR1D1 602HF mutant plasma attenuated SERPINA3 promoter activity (Fig. 3D). These results indicate that ApoA4 stimulates SERPINA3 expression in the transcriptional level mediated by nuclear receptor NR4A1, as well as NR1D1. The knockdown of NR4A1 that resulted from the total loss of effect of ApoA4 on SERPINA3 promoter activity suggests that NR4A1 is required for ApoA4 to induce SERPINA3 expression.
3.5. The direct binding of ApoA4 and NR4A1 or NR1D1 with the human SERPINA3 promoter
The direct association of exogenous ApoA4 and endogenous NR4A1 or NR1D1 with the proximal region of the human SerpinA3 promoter was verified by ChIP. It was observed that ApoA4, along with NR4A1 and NR1D1, have the ability to directly bind to the human SERPINA3 promoter region (Fig. 4), in which NR4A1 has a higher ability to binding compared with NR1D1. All these data indicate that both NR4A1 and NR1D1 mediate the transcriptional increase of SERPINA3 expression stimulated by ApoA4 through ApoA4 binding with NR4A1 or NR1D1 on the promoter of SERPINA3.
Figure 4. The direct binding of exogenous ApoA4 and endogenous NR4A1 or NR1D1 with the proximal region of the human SERPINA3 promoter detected by ChIP.
HepG2 cells were treated by r-h-ApoA4-GFP for the indicated time, and cell lysates were performed for immune-precipitation using anti-GFP, anti-NR4A1 (A) and anti-NR1D1 (B) antibodies, respectively. The promoter region of SERPIA3 was amplified from the precipitation products by quantitative real-time PCR. The mean ± SE of three samples is shown. *P<0.05, **P<0.01 vs. 0 min control.
4. Discussion
The identification of ApoA4-target genes is a vital step towards understanding the regulatory mechanism of ApoA4 action. In a previous publication, it was shown that ApoA4 suppresses G6Pase and PEPCK through NR1D1 and NR4A1 to down-regulate hepatic gluconeogenesis [13, 15]. SERPINA3 is a target of nuclear receptor NR4A1 [12]. Therefore, we investigated whether SERPINA3 is an ApoA4 target gene, and whether it is regulated via NR4A1 and NR1D1. After we determined that SERPINA3 was dominantly express in mouse liver by Northern Blotting in multiple tissues, we detected acute responses of SERPINA3 from mouse liver, as well as the secreted plasma protein levels to recombinant ApoA4 administration, by Quantitative PCR and Northern Blotting for mRNA, and in turn, by western blot for protein. Both SERPINA3 mRNA and its plasma protein increased after ApoA4 treatment. The response of SERPINA3 to ApoA4 in primary mouse hepatocytes and human hepatocytes HepG2 in vitro demonstrates that the effect of ApoA4 on SERPINA3 is a direct effect, which occurred on both mouse and human hepatocytes. However, the decrease of SERPINA3 expression with the dose increase of ApoA4 (50 and 100 µg/ml, Fig. 2A) suggests that other suppressing factors were possibly activated by ApoA4. The increase in SERPINA3 transcripts by ApoA4 via NR1D1 or NR4A1 was verified by luciferase reporter assays. However, unexpectedly, with the exogenous diminishing dose of their plasmids compared with the common dose of NR4A1 or NR1D1, SERPINA3 promotor activity was elevated. Although the combination of some amount ApoA4 and NR4A1 or NR1D1 have a co-effect, and the knockdown of endogenous NR4A1 or NR1D1 abolished the effect of these two nuclear receptors, as well as the NRs with ApoA4 on SERPINA3 promoter activity, the transfection with the overdose of NR4A1 or NR1D1 could not increase SERPINA3 transcription. This was consistent with over-dose of ApoA4 that resulted in the loss of its effect on SERPINA3 expression. In summary, ApoA4 elevates SERPINA3 through NR4A1 or NR4A1 in ApoA4, and with NR4A1 or NR1D1 in a dose-dependent manner. NR4A1 and NR1D1 are not the only factors that modulate SERPINA3 expression. Hence, the involvement of other suppressing factors should not be excluded.
Previous studies have shown that SERPINA3 is associated with a wide range of physiological activities such as hematopoiesis, apoptosis, wound healing, and embryonic development. The reduced expression of SERPINA3 raises a number of atrophy human diseases, including chronic obstructive pulmonary disease, Parkinson’s disease, Alzheimer’s disease, Stroke, Cystic Fibrosis and Cerebral Hemorrhage [6, 9, 10, 20, 21]. ApoA4, an endogenous protein secreted from the intestine as a novel ligand of NR4A1, increases SERPINA3; which implies that ApoA4 may be involved in regulating the above diseases, and can be utilized as a therapy target. Obviously, the dose-depended effects of ApoA4 on SERPINA3 need to be elucidated, and whether or how other co-factors are involved in this regulating network needs to be further explored.
In comparing with NR1D1, higher fold changes of SERPINA3 promoter activity with NR4A1 overexpression, the total lost activity with NR4A1 knockdown and the higher binding DNA of the SERPINA3 promoter region with NR4A1 indicate that keeping SERPINA3 transcriptional activity requires NR4A1 and that NR4A1 plays an essential role in the effect of ApoA4 on SERPINA3. NR4A1 is widely expressed in different types of tissues as early immediate-response genes, which are induced by a large number of stimuli including prostaglandins, growth factors and fatty acids [22]. The cellular outcome is the stimulus- and cell context-dependent differential activation of NR4A1 target genes that regulate cell cycle, apoptosis, inflammation, atherogenesis, glucose metabolism, or DNA repair [23, 24]. Our findings show that ApoA4 stimulates SERPINA3 via NR4A1, and that this may throw light on the function of ApoA4 in inflammatory acute-phase reactions, as well as the development of SERPINA3 and NR4A1 relative diseases.
Supplementary Material
Highlights.
ApoA4 stimulates the expression of SERPINA3 in hepatocytes.
The mechanism works through ApoA4 on the transcription of SERPINA3 via NR4A1 and NR1D1.
ApoA4 may act as an anti-inflammatory response in SERPINA3 relative diseases.
Acknowledgments
Funding
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NO. DK 92138 and DK 76928 awarded to Patrick Tso) and the Cincinnati MMPC (NO. U24 DK059630). This work was also supported by the Shaanxi Provincial Key Scientific and Technological Project (NO. 2016JZ032) and The Key Scientific Research Fund from the Hospital (NO. YJ [ZD] 201410) to Xiaoming Li.
Abbreviations
- ApoA4
Apolipoprotein A-IV
- SERPINA3
Serpin peptidase inhibitor clade A member 3
- ChIP
Chromatin immunoprecipitation
- DSS
Dextran sulfate sodium
- LPS
Lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liang Y, Jiang XC, Liu R, Liang G, Beyer TP, Gao H, Ryan TP, Dan Li S, Eacho PI, Cao G. Liver X receptors (LXRs) regulate apolipoprotein AIV-implications of the antiatherosclerotic effect of LXR agonists. Molecular endocrinology. 2004;18:2000–2010. doi: 10.1210/me.2003-0477. [DOI] [PubMed] [Google Scholar]
- 2.Fei Wang ABK, Lo Chun-Min, Liu Min, Howles Philip, Tso Patrick. Apolipoprotein AIV: a protein intimately involved in metabolism. Journal of lipid research. 2015 Feb. doi: 10.1194/jlr.R052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vowinkel T, Mori M, Krieglstein CF, Russell J, Saijo F, Bharwani S, Turnage RH, Davidson WS, Tso P, Granger DN, Kalogeris TJ. Apolipoprotein A-IV inhibits experimental colitis. Journal of Clinical Investigation. 2004;114:260–269. doi: 10.1172/JCI21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recalde D, Ostos MA, Badell E, Garcia-Otin AL, Pidoux J, Castro G, Zakin MM, Scott-Algara D. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:756–761. doi: 10.1161/01.ATV.0000119353.03690.22. [DOI] [PubMed] [Google Scholar]
- 5.Geronimo FR, Barter PJ, Rye KA, Heather AK, Shearston KD, Rodgers KJ. Plaque stabilizing effects of apolipoprotein A-IV. Atherosclerosis. 2016;251:39–46. doi: 10.1016/j.atherosclerosis.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Crystal Baker OB, Kalsheker Noor, Morgan Kevin. SERPINA3 (aka alpha-1-antichymotrypsin) Frontiers in Bioscience. 2007;12:15. doi: 10.2741/2275. [DOI] [PubMed] [Google Scholar]
- 7.Lannan EA, Galliher-Beckley AJ, Scoltock AB, Cidlowski JA. Proinflammatory Actions of Glucocorticoids: Glucocorticoids and TNFα Coregulate Gene ExpressionIn VitroandIn Vivo. Endocrinology. 2012;153:3701–3712. doi: 10.1210/en.2012-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath AJ, Irving JA, Rossjohn J, Law RH, Bottomley SP, Quinsey NS, Pike RN, Coughlin PB, Whisstock JC. The murine orthologue of human antichymotrypsin: a structural paradigm for clade A3 serpins. The Journal of biological chemistry. 2005;280:43168–43178. doi: 10.1074/jbc.M505598200. [DOI] [PubMed] [Google Scholar]
- 9.Chelbi ST, Wilson ML, Veillard AC, Ingles SA, Zhang J, Mondon F, Gascoin-Lachambre G, Doridot L, Mignot TM, Rebourcet R, Carbonne B, Concordet JP, Barbaux S, Vaiman D. Genetic and epigenetic mechanisms collaborate to control SERPINA3 expression and its association with placental diseases. Hum Mol Genet. 2012;21:1968–1978. doi: 10.1093/hmg/dds006. [DOI] [PubMed] [Google Scholar]
- 10.Horvath S, Mirnics K. Immune system disturbances in schizophrenia. Biol Psychiatry. 2014;75:316–323. doi: 10.1016/j.biopsych.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21:1009–1026. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yongjuan Zhao YLaDZ. Alpha 1-antichymotrypsin/SerpinA3 is a novel target of orphan nuclear receptor Nur77. FEBS Journal. 2008;275:14. doi: 10.1111/j.1742-4658.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Xu M, Wang F, Kohan AB, Haas MK, Yang Q, Lou D, Obici S, Davidson WS, Tso P. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1D1. The Journal of biological chemistry. 2014;289:2396–2404. doi: 10.1074/jbc.M113.511766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Xu M, Liu M, Ji Y, Li Z. TNF-alpha and IL-6 inhibit apolipoprotein A-IV production induced by linoleic acid in human intestinal Caco2 cells. J Inflamm (Lond) 2015;12:22. doi: 10.1186/s12950-015-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Xu M, Wang F, Ji Y, Davidso NW, Li Z, Tso P. Interaction of ApoA-IV with NR4A1 and NR1D1 Represses G6Pase and PEPCK Transcription: Nuclear Receptor-Mediated Downregulation of Hepatic Gluconeogenesis in Mice and a Human Hepatocyte Cell Line. PloS one. 2015;10 doi: 10.1371/journal.pone.0142098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Wang F, Xu M, Howles P, Tso P. ApoA-IV improves insulin sensitivity and glucose uptake in mouse adipocytes via PI3K-Akt Signaling. Sci Rep. 2017;7:41289. doi: 10.1038/srep41289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E, Lancet D, Shmueli O. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21:650–659. doi: 10.1093/bioinformatics/bti042. [DOI] [PubMed] [Google Scholar]
- 18.Issam A Haddad JMO, Fitzpatrick Tracy, Karathanasis Sotirios K. Linkage, Evolution, and Expression of the Rat Apolipoprotein A-I, C-111, and A-IV Genes. The Journal of biological chemistry. 1986;261:10. [PubMed] [Google Scholar]
- 19.Lei Yin NW, Curtin Joshua C, Qatanani Mohammed, Szwergold Nava R, Reid Robert A, Waitt Gregory M, Parks Derek J, Pearce Kenneth H, Wisely G Bruce, Lazar Mitchell A. Reverba, a Heme Sensor That Coordinates Metabolic and Circadian Pathways. Science. 2007;318:4. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 20.Winkler IG, Hendy J, Coughlin P, Horvath A, Levesque JP. Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. The Journal of experimental medicine. 2005;201:1077–1088. doi: 10.1084/jem.20042299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann DC, Textoris C, Oehme F, Klaassen T, Goppelt A, Romer A, Fugmann B, Davidson JM, Werner S, Krieg T, Eming SA. Pivotal Role for 1-Antichymotrypsin in Skin Repair. Journal of Biological Chemistry. 2011;286:28889–28901. doi: 10.1074/jbc.M111.249979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerstin Wenzl KT, Neumeister Peter, Deutsch Alexander JA. The Nuclear Orphan Receptor NR4A1 and NR4A3 as Tumor Suppressors in Hematologic Neoplasms. Current Drug Targets. 2015;16:9. doi: 10.2174/1389450115666141120112818. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Jiang R, Cheng X, Zhang Q, Hu Y, Zhang H, Cao Y, Zhang M, Wang J, Ding L, Diao Z, Sun H, Yan G. Decreased expression of NR4A nuclear receptors in adenomyosis impairs endometrial decidualization. Mol Hum Reprod. 2016;22:655–668. doi: 10.1093/molehr/gaw042. [DOI] [PubMed] [Google Scholar]
- 24.Yng-Tay Chen J-WL, Tsai Ya-Ching, Tsa Fuu-Jen. Inhibition of DNA methyltransferase 1 increases nuclear receptor subfamily 4 group A member 1 expression and decreases blood glucose in type 2 diabetes. Oncotarget. 2016;7:11. doi: 10.18632/oncotarget.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.