Abstract
The key factors underlying the development of allergic diseases—the propensity for a minority of individuals to develop dysfunctional responses to harmless environmental molecules—remain undefined. We report a pathway of immune counter-regulation that suppresses the development of aeroallergy and shrimp-induced anaphylaxis. In mice, signaling through epithelially expressed dectin-1 suppresses the development of type 2 immune responses through inhibition of interleukin-33 (IL-33) secretion and the subsequent recruitment of IL-13–producing innate lymphoid cells. Although this homeostatic pathway is functional in respiratory epithelial cells from healthy humans, it is dramatically impaired in epithelial cells from asthmatic and chronic rhinosinusitis patients, resulting in elevated IL-33 production. Moreover, we identify an association between a single-nucleotide polymorphism (SNP) in the dectin-1 gene loci and reduced pulmonary function in two cohorts of asthmatics. This intronic SNP is a predicted eQTL (expression quantitative trait locus) that is associated with reduced dectin-1 expression in human tissue. We identify invertebrate tropomyosin, a ubiquitous arthropod-derived molecule, as an immunobiologically relevant dectin-1 ligand that normally serves to restrain IL-33 release and dampen type 2 immunity in healthy individuals. However, invertebrate tropomyosin presented in the context of impaired dectin-1 function, as observed in allergic individuals, leads to unrestrained IL-33 secretion and skewing of immune responses toward type 2 immunity. Collectively, we uncover a previously unrecognized mechanism of protection against allergy to a conserved recognition element omnipresent in our environment.
INTRODUCTION
Allergy is thought to result from maladaptive immune responses to ubiquitous, otherwise harmless environmental proteins, referred to as allergens. The immunological mechanisms underlying the propensity of specific proteins to behave as allergens in allergic individuals, but not in healthy individuals, are not well understood. Although much work has centered on the study of the allergen epitopes recognized by T and B cells, there is no compelling evidence for common structural characteristics among the diverse T and B cell epitopes recognized in allergic individuals (1–3). Thus, it appears doubtful that the presence of such B cell and T cell epitopes is sufficient to endow a protein with allergenic potential. Recent data suggest that the ability of such proteins to drive allergic responses in susceptible hosts may be associated with altered innate immune recognition and/or activation at mucosal surfaces.
Allergen recognition through pattern recognition receptors (PRRs) is thought to be the critical step that determines either mucosal homeostasis or the development of an inflammatory response to allergen exposure. PRRs recognize allergens through pathogen-associated molecular patterns (PAMPs), and the culmination of the signals [e.g., interleukin-33 (IL-33) and IL-25] emanating from such interactions, in a cell type–specific manner, dictates the development and magnitude of type 2 responses.
C-type lectin receptors (CLRs), a class of carbohydrate structure sensing PRRs, have recently been implicated in modulating T helper 2 (TH2) responses (4, 5). Although the CLR dectin-1 is critical for recognition of fungal β-glucans and mounting of TH17-mediated immune responses (6, 7), it has recently been shown to signal the presence of nonfungal ligands including the mammalian protein vimentin (8–10), suggesting that dectin-1 is a multipotent receptor whose function extends beyond fungal sensing. This highlights the complexity and diversity of PRR-ligand interactions. An expanded ligand repertoire for dectin-1 suggests the possibility of a role in the sensing of noninfectious allergens.
RESULTS
Dectin-1 protects against dust mite–induced allergic airway disease
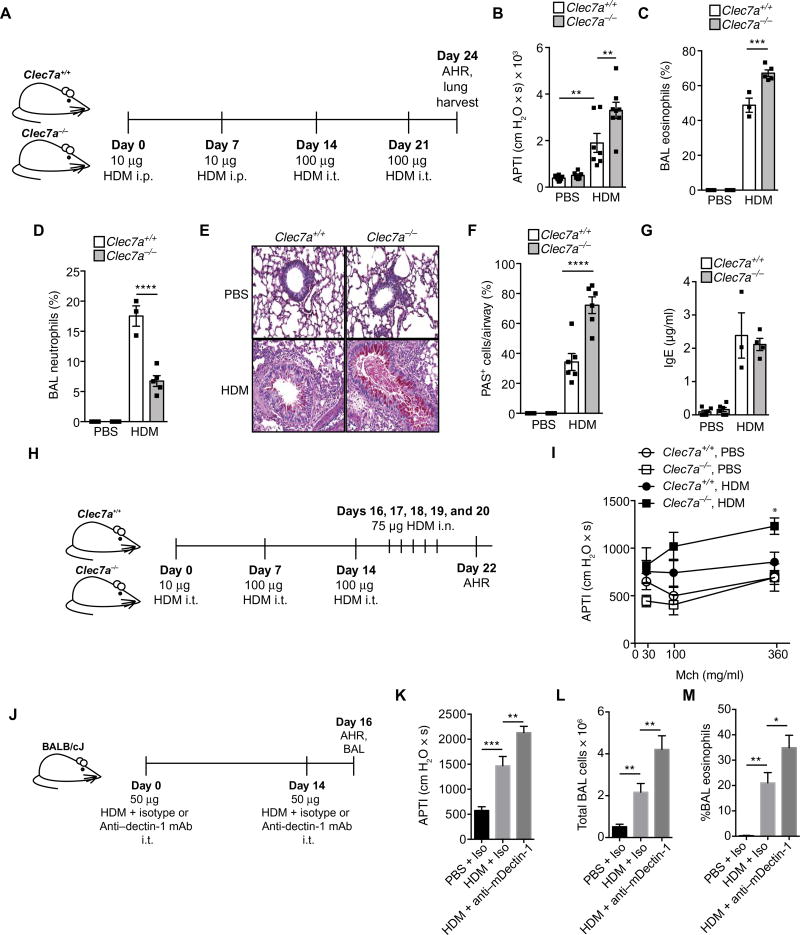
To determine the biological relevance of dectin-1, which is encoded by the Clec7a gene, in allergic airway inflammation, we sensitized Clec7a+/+ and Clec7a−/− littermate C57BL/6 mice with phosphate-buffered saline (PBS) or house dust mite (HDM) extract intraperitoneally followed by either PBS or HDM intratracheal challenges (Fig. 1A) and examined hallmarks of the allergic phenotype. Airway responses to cholinergic stimulation in PBS-exposed mice were similar in both genotypes. However, after HDM challenges, Clec7a−/− mice had significantly higher airway hyperresponsiveness (AHR) compared with Clec7a+/+ mice (Fig. 1B), suggesting an inherent protective role for airway-expressed dectin-1. Exacerbated AHR in Clec7a−/− mice was also accompanied by greater bronchoalveolar lavage (BAL) eosinophilia (Fig. 1C). Conversely, Clec7a deficiency was associated with diminished HDM-induced neutrophilia (Fig. 1D), supporting its previously reported role in neutrophil recruitment. Consistent with their exaggerated allergic phenotype, Clec7a-deficient mice had substantial mucus plugging of the airway lumen, and more mucus-positive epithelial cells, compared with Clec7a+/+ mice (Fig. 1, E and F). However, we find that loss of dectin-1 did not alter total HDM-induced IgE (Fig. 1G).
Fig. 1. Dectin-1 inhibits HDM-mediated allergic asthma.
Asthmatic phenotype in (A) Clec7a+/+ and Clec7a−/− mice sensitized with PBS or HDM [10 µg, intraperitoneally (i.p.); days 0 and 7] and challenged with PBS or HDM [100 µg, intratracheally (i.t.)] on days 14 and 21. Seventy-two hours after the last challenge, (B) AHR as measured by airway pressure time index (APTI), (C) BAL eosinophils, (D) BAL neutrophils, (E and F) mucus (PAS staining), and (G) serum total IgE were assessed. (H) Clec7a+/+ and Clec7a−/− mice were sensitized and challenged with PBS or HDM through the airways [intratracheally on days 0, 7, and 14 and intranasally (i.n.) on days 16 to 20]. (I) Forty-eight hours after the last challenge, AHR to nebulized methacholine (Mch) was determined. (J) Male BALB/c mice were sensitized and challenged with PBS or 50 µg of HDM in combination with either 30 µg of isotype control or anti–dectin-1–blocking mAbs (intratracheally on days 0 and 14). Forty-eight hours after the last challenge, (K) AHR was determined (nebulized methacholine, 10 mg/ml), and BAL was collected for evaluation of (L) total cells and (M) eosinophils. Data are representative of two to three independent experiments each containing n = 3 to 8 animals per group (B to G) or from n = 7 to 14 mice per group, pooled from two independent experiments (I), or from n = 6 to 10 mice per group, pooled from two independent experiments (K and L), or are representative of two independent experiments (M). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, as determined by one-way ANOVA followed by post hoc (Newman-Keuls) analysis. In (I), *P < 0.05 indicates comparison with Clec7a+/+ HDM (FDR).
Our data are in contrast to a report showing a proallergic role for dectin-1 in response to HDM (11). However, the study used vendor-bought, unrelated C57BL/6 as controls for comparison with Clec7a−/−mice, and not littermate controls, which may be a considerable confounder as previously reported (12–14). To further confirm the protective role of dectin-1 in allergen-mediated airway responses, we exposed Clec7a−/−, and Clec7a+/+, mice to HDM solely through the airways (Fig. 1H), and consistent with systemic HDM sensitization, these Clec7a−/− mice also developed exacerbated HDM-induced AHR (Fig. 1I). Further, we also gave BALB/c mice intratracheal administration (Fig. 1J) of a dectin-1–blocking antibody (30 µg) or isotype control antibody (30 µg) in combination with HDM (50 µg). Consistent with genetic dectin-1 deficiency, dectin-1 blockade resulted in exacerbated AHR (Fig. 1K), higher total BAL cells (Fig. 1L), and elevated BAL eosinophil numbers (Fig. 1M) as compared with mice receiving isotype control antibodies. Overall, we demonstrate that neutralizing dectin-1, via either genetic or antibody-mediated approaches, exacerbates dust mite–mediated allergic asthma. This demonstrates the generalizable phenomena that dectin-1 is a protective pathway against asthma pathogenesis.
Dectin-1 regulates IL-13+ innate lymphoid cells
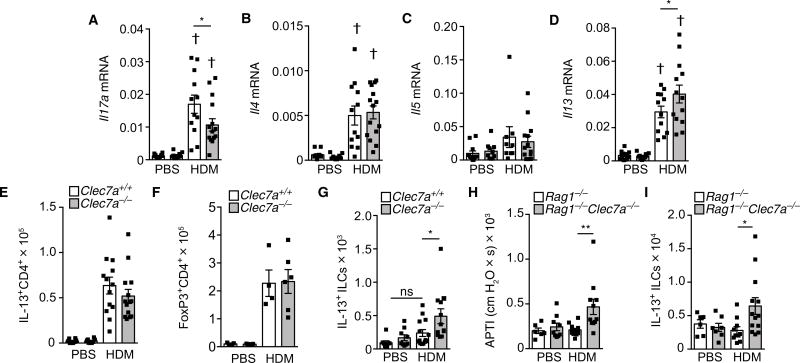
Consistent with its previously recognized role in driving TH17 responses, loss of dectin-1 resulted in decreased HDM-induced Il17a expression (Fig. 2A). However, because type 2 immune responses are the central drivers of allergic responses, we investigated their regulation by dectin-1. Allergen-driven type 2 cytokines (IL-4, IL-5, and IL-13) drive the hallmark features of allergic diseases, including AHR, eosinophilia, and mucus production. Among these, IL-13 has been shown to be a central mediator of allergic asthma (15). To determine whether the enhanced allergic phenotype of Clec7a-deficient mice resulted from increased type 2 cytokine expression, we examined lung type 2 cytokine levels in Clec7a+/+ and Clec7a−/− mice. We found that HDM-induced expression of Il4 and IL5 was not significantly regulated by dectin-1 (Fig. 2, B and C). However, we found that Clec7a-deficient mice expressed greater amounts of Il13 mRNA than controls (Fig. 2D). Despite greater Il13 expression, we observed equivalent numbers of IL-13+ T cells in both Clec7a+/+ and Clec7a−/− mice (Fig. 2E). Also, both genotypes had similar numbers of HDM-induced regulatory T cells (Tregs; Fig. 2F). These results suggest that the loss of dectin-1 does not modulate the development of CD4+ TH2 cells and that there is likely a non-CD4+ T cell source of IL-13 that is regulated by dectin-1.
Fig. 2. Dectin-1 regulates innate IL-13.
Clec7a+/+ and Clec7a−/− mice were sensitized and challenged with PBS or HDM. Seventy-two hours after the last challenge, lungs were harvested for determination of (A) levels of Il17A mRNA, (B) Il4 mRNA, (C) Il5 mRNA, (D) Il13 mRNA, (E) IL-13+CD4+ cells, and (F) FoxP3+CD4+ Tregs. (G) Numbers of IL-13+ ILCs in mice 24 hours after single PBS or HDM (100 µg) intratracheal inhalation. Rag1−/− or Rag1−/−Clec7a−/− mice were exposed to HDM as in Materials and Methods, and (H) AHR and (I) IL-13+ ILCs were determined. Data are means + SEM and are pooled from two independent experiments with n = 10 to 15 mice per group (A to E), representative of two experiments with n = 4 to 5 mice per group, or n = 6 to 13 animals per group (G to I). †P < 0.0001, as compared with PBS. *P < 0.05, **P < 0.01, as determined by one-way ANOVA followed by post hoc (Newman-Keuls) analysis.
Type 2 innate lymphoid cells (ILC2), which are a potent innate source of IL-13, participate in the maintenance of allergic diseases (16). Clec7a-deficient mice treated with a single inhalational exposure to HDM (100 µg) had significantly elevated numbers of IL-13+ ILC2 cells (lineage−CD45+ST2+IL-13+) in their lungs compared with littermate controls (Fig. 2G and fig. S1, A and B, for gating). To verify that dectin-1 regulates a non–T cell arm of type 2 immunity, we crossed Clec7a−/−mice onto the Rag1−/− background (devoid of B and T cells). Whereas Rag1−/− mice failed to mount allergen-induced AHR, Clec7a−/−Rag1−/−mice showed a significant increase in HDM-driven AHR (Fig. 2H) and higher levels of lung IL-13+ ILCs (Fig. 2I). These findings indicate that the increased asthmatic phenotype in Clec7a−/− mice is associated with aberrant IL-13 production from ILCs.
Dectin-1 regulates dust mite–induced epithelial IL-33 production
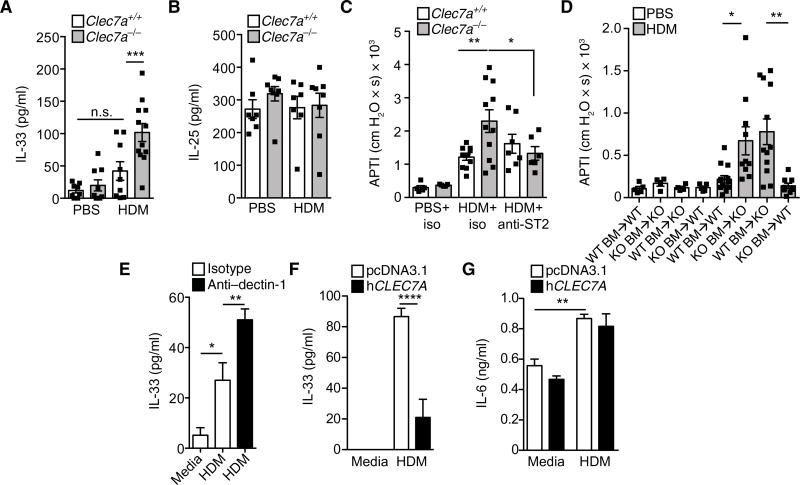
Given the central role of innate cytokines such as IL-25, IL-33, and TSLP (thymic stromal lymphopoietin) in regulating group 2 ILCs (17, 18), we investigated whether dectin-1 regulated their production. Genetic deletion of dectin-1 led to significantly increased levels of IL-33 in BAL after HDM exposure (Fig. 3A). This dysregulation was specific. HDM exposure failed to augment baseline BAL concentrations of IL-25 in Clec7a+/+ or Clec7a−/− mice (Fig. 3B), and TSLP was not detected in the BAL. To evaluate whether IL-33 drives the aberrant allergic phenotype of Clec7a−/− mice, we used antibody-mediated neutralization of IL-33 receptor (ST2) signaling (see protocol in Materials and Methods and fig. S1). Antibody-mediated blockade of ST2 reduced the aberrant AHR (Fig. 3C) and mucus production (fig. S2) seen in Clec7a-deficient mice while having no effect on Clec7a-sufficient mice, consistent with our data showing that littermate controls on the C57BL/6 background secrete little to no IL-33 in response to HDM. These data demonstrate that dectin-1 protects against asthma pathogenesis by regulating IL-33 production.
Fig. 3. Dectin-1 regulates epithelial IL-33.
BAL levels of (A) IL-33 and (B) IL-25 4 hours after a single PBS or HDM (100 µg) intratracheal exposure. n.s., not significant. (C) AHR in mice receiving HDM and blocking anti-ST2 or control antibodies. (D) AHR was determined in PBS- or HDM-treated Clec7a chimeric mice. WT, wild type; KO, knockout. (E) IL-33 levels in 16HBE cells treated with HDM in combination with isotype or neutralizing anti–hDectin-1 antibodies. IL-33 (F) and IL-6 (G) levels 4 hours after HDM treatment of 16HBE cells overexpressing human dectin-1 or empty vector. Data are means + SEM pooled from two to three independent experiments with n = 7 to 12 mice per group (A and B), n = 5 to 11 mice per group (C), or n = 4 to 13 mice per group (D) or are representative of two to three independent experiments with four replicate wells per condition (E to G). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, as determined by one-way ANOVA followed by post hoc (Newman-Keuls and Tukey’s) analysis.
Although dectin-1 expression has been primarily associated with antigen-presenting cells (APCs), recent studies have demonstrated that dectin-1 is also expressed by mucosal epithelial cells (19, 20). Moreover, because epithelial cells are a primary source of IL-33 in the lungs (21–25), we ascertained the contribution of Clec7a expression in hematopoietic and nonhematopoietic compartments to allergic airway disease. To this end, we generated Clec7a bone marrow (BM) chimeric mice. We observed about 97% chimerism 5 months after reconstitution (fig. S3, A and B), suggesting that host or donor Clec7a deficiency did not alter hematopoietic stem cell engraftment potential. Chimeric mice were then challenged with PBS or HDM (as shown in Fig. 1A). Next, we found, consistent with our previous data, that mice lacking Clec7a in both the hematopoietic and nonhematopoietic compartments (Clec7a−/− BM into Clec7a−/− host) had higher HDM-induced AHR (Fig. 3D), eosinophilia (fig. S3C), and mucus production (fig. S3, D and E) as compared with control mice, sufficient for Clec7a in both compartments (Clec7a+/+ BM into Clec7a+/+ host). However, we found, similar to whole-body Clec7a KO mice, that animals that lost dectin-1 on nonhematopoietic cells only (Clec7a+/+ BM into Clec7a−/− host) retained exacerbated allergen-induced AHR (Fig. 3D), BAL eosinophilia (fig. S3C), and mucus production (fig. S3, D and E). Conversely, mice that lost dectin-1 on hematopoietic cells, but retained it on nonhematopoietic cells (Clec7a−/− BM into Clec7a+/+ host), had a similar phenotype as control mice (Clec7a+/+ BM into Clec7a+/+ host; Fig. 3D and fig. S3, C to E).
These data suggest that sensing of dust mite by dectin-1 expressed on lung structural cells is both necessary and sufficient to protect against exacerbated experimental asthma. Consistent with its reported proinflammatory role on APCs (26, 27), mice deficient for dectin-1 in the hematopoietic compartment displayed lower HDM-induced mucus-positive epithelial cells (fig. S3, D and E). Our data demonstrate that dectin-1–mediated sensing of dust mite through structural cells of the lungs is protective and dominant because it recapitulates the total body Clec7a−/− phenotype.
Dectin-1 is robustly expressed by epithelial cells (CD45−EpCAM+) in human nasal tissues (fig. S4), consistent with previous studies demonstrating dectin-1 expression by human gut and airway epithelial cells (19, 20). Although nonepithelial structural cells (CD45−EpCAM−) in human nasal tissue also express dectin-1, such expression appears to be relatively minimal compared with epithelial cells (fig. S4). We next demonstrate that dectin-1 regulates IL-33 in human epithelia: (i) Antibody-mediated blockade of dectin-1 signaling led to enhancement of HDM-induced IL-33 release in a human bronchial epithelial cell line (Fig. 3E), and (ii) epithelial cell overexpression of dectin-1 secreted significantly less HDM-induced IL-33 than control cells (Fig. 3F), whereas the secretion of other cytokines, such as IL-6 (Fig. 3G), was not modulated. These data demonstrate that allergen recognition by epithelial expression of dectin-1 plays a critical role in regulating IL-33 specifically.
β-Glucans do not modulate allergen-induced AHR or epithelial IL-33 production
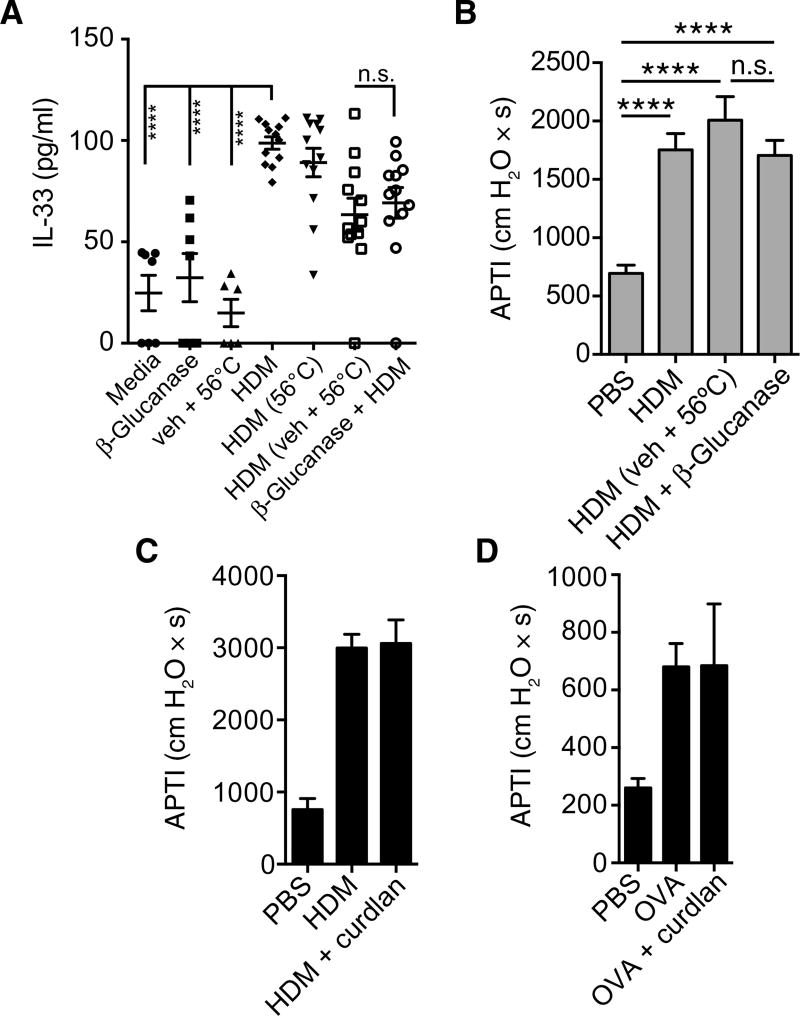
We show that, in a model of allergy involving a noninfectious source of allergens such as HDM, dectin-1 is protective. However, in mouse models of live fungal allergy, dectin-1 mediates proinflammatory responses to fungal β-glucans, which exacerbate asthma (6). These β-glucans, specifically, β-1,3/β-1,6-glucans, are the prototypical carbohydrate PAMP ligands for dectin-1. Dust mites are known to harbor fungi in their gastrointestinal tract (28), and β-glucans in dust mite extract have been shown to be necessary for HDM induction of CCL20 in the airway epithelium (29). However, β-glucan depletion of dust mite extracts via enzymatic (β-glucanase) digestion, when compared with vehicle control, had no effect on human epithelial IL-33 secretion (Fig. 4A). Supporting this observation, mice receiving either control-treated HDM or β-glucanase–digested HDM had equivalent airway responses (Fig. 4B). Conversely, exogenous administration of curdlan, a polysaccharide consisting of β-1-3–linked glucose (β-1-3-glucan), concomitant with HDM or ovalbumin (OVA) did not significantly alter allergen-induced AHR (Fig. 4, C and D). Together, these data suggest that β-glucan does not regulate IL-33 or airway responses and that dectin-1–mediated protection against HDM-driven asthma is independent of β-glucan signaling. This suggests that dectin-1 recognizes an alternative ligand in dust mite extract, the binding of which confers protection against type 2 immune responses.
Fig. 4. β-Glucans do not modulate epithelial IL-33 or AHR.
(A) Supernatant IL-33 levels measured from 16HBE cells exposed to media or HDM, which was preincu-bated with PBS, vehicle, or β-glucanase. (B) AHR in A/J mice receiving PBS, HDM, β-glucanase–treated HDM, or HDM exposed to control conditions (veh + 56°C; see Materials and Methods). AHR in C57BL/6 mice exposed to either (C) PBS, HDM, or HDM + curdlan or (D) PBS, OVA, or OVA + curdlan (details in Materials and Methods). Data are means + SEM pooled from two independent experiment (A) or are representative of two independent experiments (B to D) with n = 3 to 8 mice per group. ****P < 0.0001, as determined by one-way ANOVA followed by post hoc analysis.
Invertebrate tropomyosin is a ligand for dectin-1
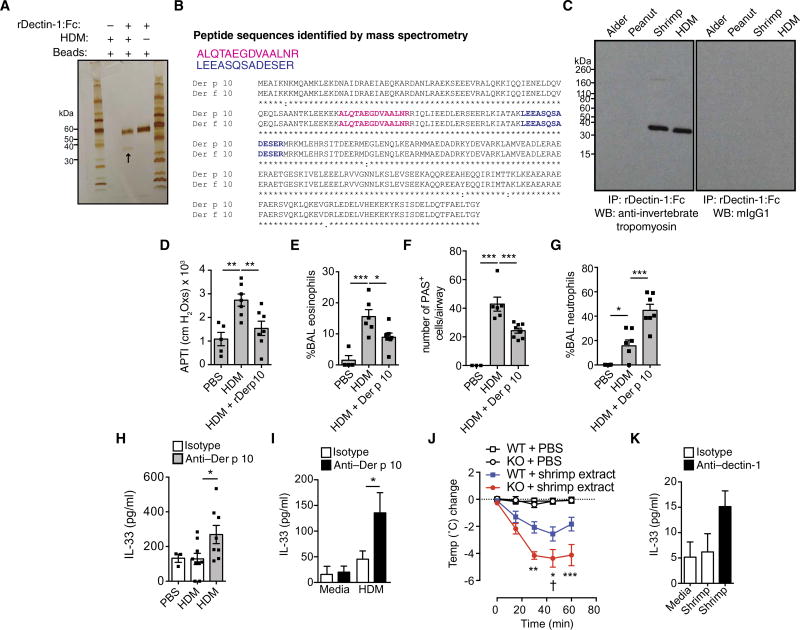
To identify this ligand, we incubated a recombinant extracellular domain human fusion dectin-1–Fc with PBS or HDM, followed by affinity purification and electrophoretic analysis. Our data reveal that dectin-1–Fc (57 kDa) binds a single 37-kDa ligand in HDM (Fig. 5A). This interaction was specific because the control Fc (53 kDa) did not bind any HDM protein ligand (fig. S5). We identified this ligand as mite invertebrate tropomyosin (Der p 10) by mass spectrometry (MS) analysis (Fig. 5B). Invertebrate tropomyosins from different allergenic sources are highly cross-reactive because they share in excess of 80% homology (30). Therefore, it is likely that antibodies generated against dust mite invertebrate tropomyosin (Der p 10) would cross-react with tropomyosin from other invertebrates such as its shrimp homolog Pen 1 a. Therefore, we examined the ability of dectin-1 to recognize invertebrate tropomyosins from other allergenic sources. We incubated allergen extracts (HDM, shrimp, alder, and peanut) with dectin-1–Fc, followed by immunoprecipitation and immunoblotting with anti–invertebrate tropomyosin (anti–Der p 10) or isotype control monoclonal antibodies (mAbs). We demonstrate that dectin-1 specifically binds to invertebrate tropomyosin in both mite and shrimp extracts, but not in tropomyosin-devoid plant allergen extracts (Fig. 5C).
Fig. 5. Identification of invertebrate tropomyosin as a ligand for dectin-1.
(A) Silver stain of a pull-down from rhDectin-1–Fc alone, protein G beads, or rhDectin-1–Fc incubated with HDM extract (see arrow). (B) Peptides identified by MS. (C) Coimmunoprecipitation (IP) of rDectin-1–Fc incubated with alder, peanut, shrimp, and HDM extracts and immunoblotted using anti–invertebrate tropomyosin (anti–Der p 10) or isotype control. WB, Western blot. (D) AHR, (E) BAL eosinophils, (F) lung PAS+ epithelial cells, and (G) BAL neutrophils from male BALB/c mice receiving PBS, HDM (50 µg), or HDM + 10 µg of recombinant Der p 10 intratracheally on days 0 and 5 and harvested on day 7 for analysis. (H) Four hours later, BAL IL-33 levels in male C57BL/6 mice were given PBS or HDM with isotype or anti–Der p 10 antibodies intratracheally. (I) Supernatant IL-33 from 16HBE cells treated for 2 hours with media or HDM in combination with isotype or anti–Der p 10 antibodies. (J) Change in body temperature in Clec7a+/+ (WT) and Clec7a−/− (KO) mice sensitized to shrimp extract. “†” represents death. (K) 16HBE human bronchial epithelial cells treated for 2 hours with PBS or shrimp extract with isotype control or neutralizing anti–hDectin-1 antibodies. Data are representative of two to three independent experiments (A and C) or are means + SEM of two to three independent experiments (I and K) with four replicate wells per condition or pooled from two to three independent experiments (D to G and H and J) and each containing n = 4 to 7 (D to G), n = 3 to 9 (H), or n = 9 to 11 (J) animals per group. *P < 0.05, **P < 0.01, ***P < 0.001, as determined by one- or two-way (I) ANOVA followed by post hoc (Newman-Keuls, Dunnett’s, and Bonferroni) analysis.
To confirm whether invertebrate tropomyosin–dectin-1 interactions can inhibit HDM-induced allergic inflammation, we treated wild-type mice with HDM alone or HDM supplemented with recombinant Der p 10 (dust mite tropomyosin). Hallmark features of type 2 inflammation including AHR (Fig. 5D), BAL eosinophilia (Fig. 5E), and mucus cell hyperplasia (Fig. 5F) were significantly abrogated by addition of rDer p 10 to dust mite extract. Consistent with published reports showing that dectin-1 ligation can drive neutrophilia, Der p 10 enhanced neutrophil influx (Fig. 5G). Next, we demonstrate that the protective effect of Der p 10–dectin-1 ligation is generalizable to other proallergic triggers. For this, we made use of a chitin-driven allergic airway inflammation model. Chitin, a β1-4 carbohydrate (31) that does not bind dectin-1 (32), is a well-established driver of type 2 inflammation, dominated by IL-5 and eosinophils (33, 34). We show that Der p 10 inhibits chitin-induced AHR (fig. S6A) and chitin-induced inflammation such as total BAL cell infiltration (fig. S6B) and BAL eosinophils (fig. S6C), but invertebrate tropomyosin (Der p 10)– dectin-1 interaction induces airway neutrophils (fig. S6D). These data establish a broad role for the sensing of invertebrate tropomyosin as a dampening signal for pro–type 2 stimuli.
Further, we show that blockade of Der p 10–dectin-1 interactions with anti–Der p 10 mAb treatment enhanced HDM-mediated IL-33 production in mice (Fig. 5H) and in cultured epithelial cells (Fig. 5I). These data demonstrate that the interaction of Der p 10 with dectin-1 inhibits type 2 immune responses by regulating IL-33 release, suggesting that pro–IL-33 signals such as HDM or chitin (33, 35) can be counter-regulated by the sensing of environmental invertebrate tropomyosin.
In susceptible individuals, invertebrate tropomyosin is a major contributor to shellfish allergies (35). Thus, we investigated the role of dectin-1 in a model of shrimp extract–driven systemic anaphylaxis. Clec7a+/+ and Clec7a−/− mice that were orally sensitized to shrimp, and systemically challenged with shrimp extract, displayed a drop in body temperature, consistent with anaphylactic shock (Fig. 5J). However, in line with our data with the respiratory mite allergen, Clec7a−/− mice developed significantly greater hypothermia than littermate controls (Fig. 5J). Moreover, consistent with the fact that IL-33 signaling has been shown to be central in driving experimental food anaphylaxis (36, 37), especially during sensitization through the gut (36), we found that dectin-1 blockade drove more IL-33 release from epithelial cells in response to shrimp extract (Fig. 5K). Our data indicate that the shrimp invertebrate tropomyosin–dectin-1 interaction normally serves to dampen IL-33 production and the development of anaphylaxis. This provides evidence of a broader context of dectin-1– invertebrate tropomyosin interactions in regulating IL-33–mediated allergic diseases.
Dectin-1 is repressed in allergic individuals
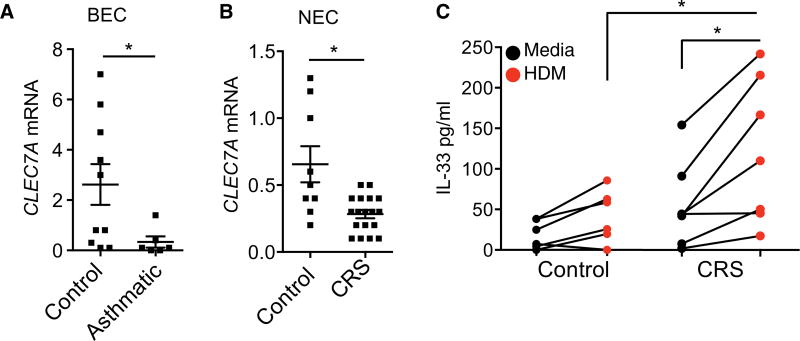
To explore whether this protective pathway is disrupted in humans with allergic disorders, we examined the expression and regulation of dectin-1 in epithelial cells from control and allergic individuals, which had positive skin prick test for dust mites. We found that CLEC7A expression was significantly repressed in primary bronchial epithelial cells isolated from asthmatics as compared with controls (Fig. 6A). Extending our observations to patients with chronic rhinosinusitis (CRS), who are commonly HDM-sensitized (38) and display a dysregulated IL-33–ILC2 axis (39, 40), we also found repressed CLEC7A levels in the sino-nasal epithelial cells (NECs) from these patients as compared with healthy controls (Fig. 6B). The reduced dectin-1 expression in allergic patients is associated with a significantly greater secretion of IL-33 as compared with epithelial cells from healthy individuals after HDM exposure (Fig. 6C). These studies support a link between deficient epithelial cell dectin-1 signaling, IL-33, and allergic disease in humans.
Fig. 6. Dectin-1 is repressed in allergic individuals.
CLEC7A expression in (A) bronchial epithelial cells (BEC) from controls and asthmatics and (B) NECs from control and CRS patients. (C) IL-33 levels in control and CRS NECs stimulated with media or HDM for 2 hours. (A to C) Data are means + SEM from n = 6 to 19 patients per group (A and B) or from a minimum of 7 patients (C). *P < 0.05, as determined by Student’s t test with Welch’s correction or one-way ANOVA followed by post hoc (Newman-Keuls) analysis.
A CLEC7A polymorphism is associated with decreased lung function in asthma
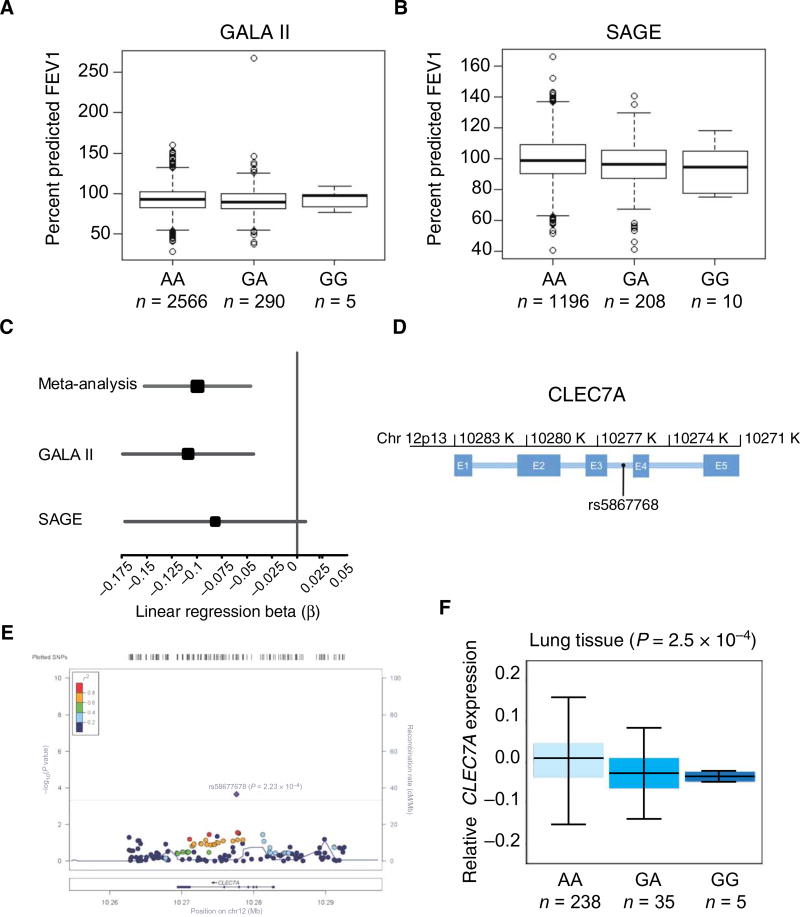
Next, we wanted to determine whether there is a genetic susceptibility associated with a CLEC7A polymorphism and asthma. To this end, we evaluated 143 single-nucleotide polymorphisms (SNPs) in CLEC7A in GALA II (Genes-environments and Admixture in Latino Americans) (n = 2861; Latino American cohort) and SAGE (Study of African Americans, Asthma, Genes and Environments) (n = 1414; African American cohort), two separate studies of childhood asthma (Fig. 7, A and B). To increase the power of our analyses and capture effects shared between the two populations, we performed a meta-analysis across the two studies (Fig. 7C). We identified a single significant association: The G allele of rs58677678 (Fig. 7D) was associated with reduced lung function [forced expiratory volume in 1 s (FEV1)], a cardinal feature of asthma pathogenesis (P = 2.23 × 10−4; Fig. 7E and table S1). In the context of the 143 SNPs that were evaluated in this study, there is strong evidence that this association is not due to random chance (Bonferroni threshold: 0.05/143 = 3.5 × 10−4). Moreover, we have determined the frequency of the CLEC7A polymorphism in the 1000 Genomes population, and confirm its presence in other populations (fig. S7). This intronic SNP is a predicted expression quantitative trait locus (eQTL) for CLEC7A (41, 42) in multiple tissues; specifically, in the lung, genotype at rs58677678 is significantly correlated with CLEC7A gene expression (Fig. 7F and table S2). In all assayed tissues, individuals with increased copies of the rs58677678 G allele have decreased expression of CLEC7A (Fig. 7F and table S2). Together, these findings suggest that genetic variants in CLEC7A may contribute to asthma severity and lung function.
Fig. 7. rs58677678 genomic location, allelic variation, and association with lung function.
(A and B) Baseline FEV1 by rs58677678 genotype for GALA II and SAGE. FEV1 is strongly influenced by age, sex, height, and ethnicity; therefore, baseline FEV1 is shown as the percentage of the predicted normal value achieved for each participant (y axis). Predicted normal values were derived using the Hankinson reference equation. (C) Forest plot of association analysis results. The size of the square represents the magnitude of the effect size, and the lines indicate the 95% confidence intervals in each study. β is the change in FEV1 per addition of one G allele (risk allele) of rs58677678. (D) Schematic of CLEC7A indicating the genomic position of rs5867768. K, kilobases; E, exon. Intronic regions are indicated as blue horizontal lines between exons. (E) LocusZoom plot of meta-analysis for association between CLEC7A variants and FEV1 across two independent studies (GALA II and SAGE). The top asthma-associated variant is highlighted in purple (rs58677678; P = 2.23 × 10−4, β = 0.100). The solid red line indicates the P value threshold for Bonferroni adjustment for 143 SNPs (3.50 × 10−4). Measurements of linkage disequilibrium with rs58677678 (r2) are from the 1000 Genome AMR (Ad Mixed American) population. Values of pairwise r2 between rs58677678 and each of the other 142 SNPs in the region shown above are displayed using the color scheme shown in the top left corner of the figure. (F) Expression of CLEC7A in human lung tissue by rs58677678 genotype. The x axis indicates genotype of rs58677678 (n is the number of individuals with the associated genotype). The y axis represents rank-normalized CLEC7A expression in the lungs. Data used to calculate the correlation between rs58677678 genotype and expression of CLEC7A were ascertained from the GTEx public database portal. P values indicate the significance of the correlation between rs58677678 genotype and CLEC7A expression for the specified tissue.
DISCUSSION
In susceptible individuals, exposure to common allergens drives aberrant type 2 responses and the development of allergic diseases. Conversely, these exposures do not lead to pathology in healthy individuals, suggesting that protective pathways are critical in maintaining tissue homeostasis. Innate sensing of allergens through PRRs is thought to be a critical step that determines either mucosal homeostasis or an inflammatory response to allergens. It has been recently demonstrated that some environmental exposures, such as endotoxin, can protect against the development of allergy (43, 44), suggesting that we have evolved protective pathways to sense our environment. Despite this, little is known about the role of innate sensors in preventing overzealous immune activation in the context of allergic diseases. Here, we report that dectin-1 controls type 2 immunity in the lungs in response to the dust mite aeroallergen. Specifically, we demonstrate that the mechanism responsible for the dectin-1–mediated protection of asthma is through the regulation of IL-33–driven IL-13 production from ILCs. Our data are consistent with previous findings showing that airway exposure of Clec7a−/− mice to Aspergillus versicolor spores leads to exacerbated IL-13 production (45).
PRRs, including dectin-1, are classically associated with APCs; however, we and others show that mucosal epithelial cells express these sensors (20, 46, 47). Moreover, it appears that cell type–specific expression of PRRs can lead to differential immune activation. This has recently been shown in the context of epithelial Toll-like receptor 4 (TLR4) that drives TH2 responses, whereas TLR4 on APC drives TH17 immunity (48, 49). Our data using chimeric mice establish that dectin-1 expressed by lung structural cells is responsible for the protective phenotype. Whereas APC-expressed dectin-1 is conventionally associated with a proinflammatory function, we show that engagement of epithelial dectin-1 is protective. Our data support the concept that PRR-mediated environmental sensing by barrier epithelial cells is critical in regulating type 2 immunity (50). Control of dust mite–induced epithelial IL-33 is central to the protective role of dectin-1. However, although little is known about the regulation of IL-33 release from epithelial cells, recent data suggest that it is released from preformed stores in a redox-dependent manner (51). Because dectin-1 has been previously reported to modulate pathways associated with cellular redox (52), this raises the interesting possibility that dectin-1 may prevent overzealous secretion of IL-33 by controlling cellular redox.
β-1-3/1-6-Glucans are the prototypical ligands for dectin-1, but their role in allergic disease is controversial in mice and humans. Some groups have shown that β-1-3-glucans have a protective role (53, 54), and others show that environmental exposure to β-1-3-glucans is associated with greater asthma burden in children (55) and can exacerbate allergic asthma in mice (56). Mice exposed to a mixture of β-1-3-glucan and HDM extract develop exacerbated eosinophilia, which was independent of dectin-1 (56). In addition to dectin-1, β-glucans can bind several other receptors (TLR2, CR3, scavenger receptors, and lactosyl ceramide) (57–60). In the context of HDM exposure, it is possible that β-glucans in the dust mite extract may have greater affinity for these other receptors or that different β-glucan moieties drive different cellular processes. Nevertheless, our data demonstrate that β-1-3-glucans have no significant effects on epithelial IL-33 secretion or the development of allergen-induced AHR. On the basis of this, we set out to find an alternative ligand for dectin-1. We identified invertebrate tropomyosin as a ligand for dectin-1. Although innocuous in most individuals, invertebrate tropomyosin has been identified as a major arthropod allergen in susceptible individuals (61). Invertebrate tropomyosins are present in several subphyla and classes of arthropods (including crustaceans, arachnids, and mites/insects) as well as in helminths (62). Invertebrate tropomyosins from various sources are highly homologous, explaining why antibodies against dust mite tropomyosin (Der p 10/Der f 10) can cross-react with shrimp tropomyosin (Pen 1 a). Although fungi contain tropomyosins (63), they are intracellular and not normally accessible to the host’s immune cells. However, β-glucan moieties, which become exposed during fungal cell replication (64), engage dectin-1 to drive TH17 antifungal responses. On the other hand, exposure to arthropods (mites, cockroaches, and crustacea) usually arises from nonreplicating, noninfectious, cellular fragments/debris/feces, inhaled or ingested, and thus containing exposed tropomyosin molecules that, sensed through dectin-1, can dampen overzealous activation of type 2 responses. Moreover, similar to our data showing a dampening of type 2 immune responses by invertebrate tropomyosin, other invertebrate products such as helminth-derived proteins can prevent aberrant type 2 inflammation. Recently, Heligmosomoides polygyrus excretory/secreted products have been shown to suppress IL-33 production from epithelial cells (65, 66). Although preventing anti-parasitic type 2 responses is advantageous to worms, it suggests the broader concept that some environmental proteins can drive homeostatic responses.
Our studies in mice translate into humans in that we demonstrate that this homeostatic pathway is impaired in allergic airway epithelial cells from asthmatics and patients with CRS. This impairment in the dectin-1–mediated protective response is associated with a genetic variant in a gene for dectin-1, CLEC7A (rs58677678A/G), associated with lower lung function (FEV1), the clinical hallmark of asthma, across two independent cohorts of children (GALA II and SAGE). This intronic SNP predicts an eQTL resulting in reduced CLEC7A expression in the lungs and other human tissues.
Although our report highlights an important regulatory role for dectin-1 in mice and humans, our mouse studies did not show a role of dectin-1 in the regulation of immunoglobulin E (IgE). A limitation of using mice to model human allergic diseases is that, in mice, only IL-4 is essential for B cell–mediated IgE production, whereas in humans both IL-4 and IL-13 can drive IgE. Therefore, it is plausible that dectin-1 may regulate IgE in humans through its control of IL-13. Moreover, although we provide initial insight into a genetic role for CLEC7A and asthma, it would be of importance to further explore our genetic findings in other populations of allergic individuals and to conduct a larger study of CLEC7A/dectin-1 expression and the G allele of rs58677678 in asthmatic individuals.
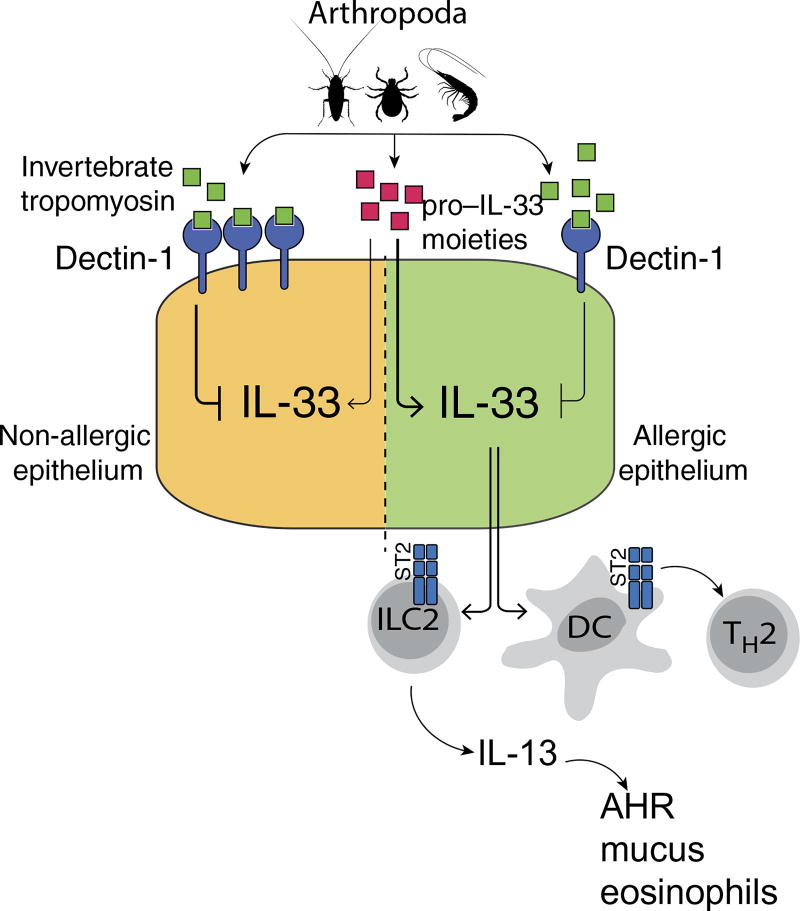
We are repeatedly exposed not only to environmentally ubiquitous sources of allergens that contain pro–IL-33 moieties such as endotoxin and chitin (33–35, 67–70) but also to other abundant proteins such as invertebrate tropomyosin. We have found that mammals have evolved a protective response to continuous exposure to invertebrate tropomyosin. In normal individuals, invertebrate tropomyosin binding of dectin-1 at the airway surface results in prevention/suppression of IL-33–driven inflammation and sensitization to invertebrate tropomyosin. In contrast, in allergic/asthmatic individuals, decrements in epithelial dectin-1 expression, likely as a result of a mutation in CLEC7A, result in aberrant secretion of IL-33 at mucosal surfaces. IL-33, in turn, drives IL-13 production from ILC2s and conditions dendritic cells (DCs) to promote type 2 immune responses. Thus, exposure to inhaled or ingested invertebrate tropomyosin in the presence of IL-33 from local epithelial cells may promote DC activation and antigen presentation to T cells, leading to TH2 cell skewing (Fig. 8). Our results provide critical new insights into the mechanisms underlying susceptibility to a wide range of allergic disorders, including asthma, rhinosinusitis, and food allergy.
Fig. 8. Invertebrate tropomyosin–dectin-1 interaction in mediating protection against allergic diseases.
In healthy individuals, invertebrate tropomyosin binding of dectin-1 at mucosal surfaces results in suppression of IL-33–driven inflammation. In contrast, in allergic individuals, decreased levels of epithelial dectin-1 result in aberrant secretion of IL-33, which in turn drives IL-13 production from ILC2 and conditions local DCs to promote type 2 immune responses.
MATERIALS AND METHODS
Study design
The objective of this study was to investigate the role of dectin-1 in HDM-induced experimental asthma. Generally, in vivo experiments consisted of enumerating inflammatory cells by differential counting, or flow cytometry, evaluation of mucus production and determination of AHR in response to HDM. All mouse experiments used littermate controls for comparison. In vitro experiments were used to examine the effect of dectin-1 and dectin-1 ligand on epithelial cell IL-33 release. The number of independent experiments is outlined in the figure legends, where appropriate. Experiments were not randomized, and the investigators were not blinded during experiments and endpoint analyses.
Allergen sensitization and challenge
Seven- to 10-week-old Clec7a+/+ and Clec7a−/− mice were sensitized to HDM by intraperitoneal injection with PBS (100 µl) or HDM (10 µg/100 µl in PBS; Greer Laboratories) on days 0 and 7. Mice were then challenged intratracheally with PBS (40 µl) or HDM (100 µg/40 µl) on days 14 and 21. Seventy-two hours after the last challenge, the allergic phenotype was assessed. Alternatively, Clec7a+/+ and Clec7a−/−mice were exposed to HDM only via the airways; for this, animals were sensitized to 10 µg of HDM intratracheally on day 0 and then 100 µg of HDM intratracheally on days 7 and 14, followed by five intranasal HDM challenges (75 µg on days 16 to 20). Forty-eight hours after the last challenge, airway responses were determined. For experiments involving ST2 blockade, a day before each sensitization (i.e., days −1 and 6) and challenge (i.e., days 13 and 20), mice were given 250 µg of either isotype control antibody (rat IgG2a; Bio X Cell) or anti-mouse ST2 antibody (Amgen) intraperitoneally. For experiments involving Clec7a−/−Rag1−/−, mice were sensitized intraperitoneally with PBS or HDM (10 µg; days 0 and 7) followed by intranasal administration of PBS or HDM (62.5 µg/25 µl) every other day for 8 days. Forty-eight hours after the last challenge, the allergic phenotype was assessed. For rDer p 10 administration, 7- to 9-week-old male BALB/c mice were given PBS, 50 µg of HDM, or 10 µg of rDer p 10 plus HDM intratracheally in a total volume of 40 to 50 µl on days 0 and 5 and measured on day 7. For anti–mDectin-1, male BALB/c mice were given 50 µg of HDM combined with either 30 µg of isotype control (rat IgG2a) or a neutralizing dectin-1 mAb intratracheally in a total volume of 50 µl on days 0 and 14. On day 16, the allergic phenotype was assessed. For curdlan (a source of β1-3-glucans) in vivo experiments, C57BL/6 mice were sensitized intraperitoneally with either PBS, OVA (20 µg), or HDM (10 µg) intraperitoneally on days 0 and 7, followed by challenges with either PBS, OVA (750 µg), OVA plus curdlan (100 µg), HDM (100 µg), or HDM plus curdlan (100 µg) intratracheally on days 14 and 21. AHR was determined on day 24.
Lung mucus stain
Lungs were excised and fixed in 10% neutral buffered formalin, processed, paraffin-embedded, and sectioned. Sections were stained with periodic acid–Schiff (PAS). To quantify mucus-producing airway epithelial cells, the percentage of PAS+ cells were determined using a light microscope.
Experiments using β-glucanase–treated HDM
β-Glucanase treatment of HDM was performed as previously published. Briefly, HDM extracts were incubated with vehicle or β-glucanase for 1 hour at 56°C (29). A/J mice were challenged with PBS, HDM, HDM plus vehicle, or β-glucanase–treated HDM on days 0 (100 µg, intratracheally) and 14 (100 µg, intratracheally), followed by AHR measurement on day 17.
Airway measurements
Briefly, mice were anesthetized by intraperitoneal administration of ketamine/xylazine and tracheotomized before insertion of an 18-gauge cannula into the trachea. Mice were paralyzed with suxamethonium chloride (3 mg/kg), intubated, and respirated at a rate of 120 breaths per minute with a constant tidal volume (0.2 ml). After a stable baseline was achieved, mice were exposed to nebulized methacholine (10 to 30 mg/ml; Sigma). After 10 s, dynamic airway pressure (cm H2O × s) was recorded for 5 min. After airway reactivity measurements, serum, BAL fluid, and lungs were collected and processed as previously described (71).
Food anaphylaxis
Female Clec7a−/− and Clec7a+/+ littermate controls were sensitized by oral gavage of 0.5 mg of shrimp extract containing 10 µg of cholera toxin in 200 µl on days 0, 7, and 14. Mice were then challenged systemically with 0.5 mg of shrimp extract intraperitoneally. Body temperature was monitored every 15 min for 1 hour, using a dual laser infrared thermometer as previously described (72).
Cytokine and IgE ELISAs
IL-25, IL-6, and IL-13 levels were measured using ELISA DuoSets (R&D Systems). IL-33 was measured using antibody pairs (R&D Systems): capture antibody (AF3625) and biotinylated detection antibody (BAF3625). Serum total IgE levels were determined by enzyme-linked immunosorbent assay (ELISA; BD OptEIA, BD Biosciences).
Real-time PCR
RNA was extracted from snap-frozen lungs or cultured cells using TRIzol (Invitrogen). Complementary DNA (cDNA) was generated by reverse transcribing 0.25 to 1 µg of RNA using SuperScript III (Invitrogen) and random primers (Invitrogen). cDNA was diluted sixfold using double-distilled water before polymerase chain reaction (PCR). Real-time PCR was performed using SYBR Green (Bio-Rad), and gene expression was measured using specific primer pairs, which span at least one intron to avoid coamplification of genomic DNA. For mouse samples, target gene expression was normalized to expression of the Rps14 gene. For human samples, target gene expression was normalized to RPS13. The following primer sequences were used: Il4, 5′-TGAACGAGGTCACAGGAGAA-3′ (sense) and 5′-CGAGCT-CACTCTCTGTGGTG-3′ (antisense); Il5, 5′-GCAATGAGACGAT-GAGGCTT-3′ (sense) and 5′-CCCACGGACAGTTTGATTCT-3′ (antisense); Il13, 5′-CACACTCAACCATGCTGC-3′ (sense) and 5′-TGTGTCTCTCCCTCTGACCC-3′ (antisense); Il17a, 5′-ACTACCT-CAACCGTTCCACG-3′ (sense) and 5′-AGAATTCATGTGGTG-GTCCAG-3′ (antisense); Rps14, 5′-TGGTGTCTGCCACATCTTTG-CATC-3′ (sense) and 5′-AGTCACTCGGCAGATGGTTTCCTT-3′ (antisense); human CLEC7A, 5′-TGGGAGGATGGATCAACATT-3′ (sense) and 5′-TGGGTTTTCTTGGGTAGCTG-3′ (antisense); human RPS13, 5′-ACTTGTGCAACACCATGTGAA-3′ (sense) and 5′-ACGACGTGAAGGAGCGATT-3′ (antisense).
Flow cytometry analysis
Mouse lung cells were obtained by digestion of lung tissue with Liberase TL (0.05 mg/ml; Roche) and deoxyribonuclease I (0.5 mg/ml; Sigma) for 45 min at 37°C in 5% CO2. Digested tissue was filtered through a 70- µm nylon mesh (BD Biosciences) and centrifuged. The pellet was resuspended in red blood cell lysis buffer (ACK lysis buffer). Recovered cells were counted (trypan blue exclusion), plated at 4 × 106 to 5 × 106 cells/ml, and stimulated with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 µg/ml) for 16 hours, and then brefeldin A and monensin (eBioscience) were added for the last 3 to 4 hours. All cells were filtered using a 40-µm nylon mesh (BD Biosciences), washed with PBS and labeled with live/dead dye (Zombie Aqua, BioLegend) for 10 min at room temperature (RT), and blocked with anti-CD16/32 (BioLegend) for an additional 20 min at RT. For CD4+IL-13+ staining, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 10 min at RT and permeabilized in 0.1% saponin (Sigma) for 20 min at RT before staining using phycoerythrin (PE)–Cy7– conjugated anti-CD4 (RM4-5, eBioscience) and eFluor 660–conjugated anti–IL-13 (eBio13A, eBioscience). For IL-13+ ILCs, cells were surface-stained using peridinin chlorophyll protein (PerCP)–Cy5.5–conjugated lineage antibodies [CD8 (53-6.7, BioLegend), CD4 (GK1.5, BioLegend), CD3ε (145-2C11, BD Biosciences), T cell receptor β (TCRβ) (H57-597, BD Biosciences), CD11b (M1/70, BD Biosciences), CD11c (N418, eBioscience), Gr1 (RB6-8C5, eBioscience), B220 (RA3-6B2, BD Biosciences), and NK1.1 (PK136, BD Biosciences)], Alexa Fluor 700 anti-CD45 (30-F11, BD Biosciences), and fluorescein isothiocyanate (FITC)–anti-ST2 (T1/ST2, MD Bioproducts), then fixed and permeabilized (as above), and stained with PE–anti–IL-13 (ebio13A). Data were acquired on an LSR II flow cytometer (BD Biosciences) and gated to exclude debris and to select single cells (SSC-W/SSC-A). Data were analyzed using FACSDiva (BD Biosciences). Positive gates were based on fluorescence minus one (FMO) controls.
Cell culture
16HBE cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) [10% fetal bovine serum (FBS)], and the immortalized human bronchial epithelial cell line 16HBE was grown as previously described (29). Cells were seeded at 15,000 cells per well in a 96-well flat-bottomed dish in complete DMEM (10% FBS) overnight. Cells were then serum-starved (0.1% FBS) for 24 hours and then stimulated with media or HDM (100 µg/ml) or shrimp extract (100 µg/ml), and supernatants were harvested 2 to 4 hours after for determination of cytokine concentration (IL-6 and IL-33). In some experiments, cells were pretreated with isotype (5.0 to 7.5 µg/ml) or neutralizing antibodies to Der p 10 or dectin-1 for 30 min before stimulation. β-Glucanase treatment of HDM was performed as previously published (29). For transfections, 16HBE cells were seeded at 10,000 cells per well of a 96-well flat-bottomed dish and transfected using FuGENE HD (Promega). Transfected cells were then serum-starved (0.1% FBS) overnight and then stimulated with media or HDM (100 µg/ml), and supernatants were harvested 2 to 4 hours after for determination of cytokine concentration.
Human primary nasal tissue epithelial cell culture
Human NECs and sinonasal mucosa were obtained from nasal brushings, and tissue was removed during endoscopic sinus surgery from control and patients with nasal polyps as previously described (39). The research protocol was approved through the Johns Hopkins Institutional Review process, and all patients gave signed informed consent. Inclusion criteria included continuous symptoms of rhinosinusitis for greater than 12 weeks as defined by the American Academy of Otolaryngology—Head and Neck Surgery Chronic Rhinosinusitis Task Force, computed tomography of the sinuses revealing isolated or diffuse sinus mucosal thickening or air-fluid levels, and nasal polyps visible on diagnostic endoscopy. None of the patients had a history of tobacco use, cystic fibrosis, ciliary dyskinesia, systemic inflammatory or autoimmune disease, or immunodeficiency. Isolated sino-NECs were grown in Bronchial Epithelial Cell Growth Media (BEGM BulletKit, Lonza). At 80% confluency, cells were lifted using trypsin-EDTA (0.05%; Gibco) and seeded into a 96-well culture plate pre-coated with fibronectin-collagen. At 80 to 90% confluency, cells were starved overnight [BEGM media without the bovine pituitary extract (BEGMnoBP)]. Cells were treated with HDM (200 µg/ml) for 2 hours, and the supernatants were collected for IL-33 ELISA. The characteristics of the patients are summarized in table S3.
Human bronchial epithelial cells from asthmatics
Fifteen volunteers were recruited from the Asthma Clinic at Institut Universitaire de Cardiologie et de Pneumologie (IUCPQ; Québec, Canada), and epithelial cells were isolated and cultured as previously described (73). RNA isolated (TRIzol) from nonstimulated cells was harvested and reverse-transcribed to obtain cDNA, followed by real-time PCR. The study was approved by the IUCPQ (Québec, Canada) ethics committee, and all patients signed an informed consent form. The asthmatic patients were diagnosed according to the American Thoracic Society criteria, and the characteristics of the patients are summarized in table S4.
BM chimera
Seven- to 8-week-old Clec7a+/+ and Clec7a−/− mice were irradiated at a dose of 10 Gy. Fresh BM isolated from nonirradiated Clec7a+/+ and Clec7a−/− mice was injected intravenously (2 × 106 cells/100 µl) to irradiated mice. To avoid infections, irradiated mice were then kept on drinking water supplemented with neomycin (1.1 mg/ml) for 2 weeks after radiation. Afterward, mice were switched back to neomycin-free water. The degree of chimerism was evaluated based on the differential expression of CD45 isoforms. Because our experimental mice (Clec7a+/+ and Clec7a−/−) express the CD45.2 isoform, we used the CD45.1-expressing B6.SJL-Ptprca Pepcb/BoyJ strain. B6.SJL-Ptprca Pepcb/BoyJ mice are dectin-1–sufficient and hence were used as wild-type controls. We transferred either CD45.1 BM (BoyJ) into irradiated Clec7a−/− host (CD45.2). Another group of irradiated wild-type (BoyJ, CD45.1) mice received Clec7a−/− CD45.2 BM. At the end of 5 months, the expression of CD45.1 and CD45.2 isoforms in the lungs was evaluated. Chimeric mice were then exposed to HDM as described above.
Dectin-1–Der p 10 coimmunoprecipitation
HDM (250 µg), shrimp (250 µg), peanut (250 µg), and alder (250 µg) extracts were preincubated with 25 µl of prewashed (PBS) protein G magnetic beads for 4 hours at 4°C on a rocker to remove nonspecific binding. Beads were removed using a magnet, and cleared allergen extracts were incubated with dectin-1–Fc (3.5 µg) overnight at 4°C on a rocker. Twenty-four hours later, 25 µl of protein G magnetic beads (prewashed) was added and incubated for 4 hours at 4°C on a rocker. Beads were magnetically purified and washed three times with PBS to remove any nonspecific binding. The pull-down for each extract was split in two equal parts and loaded onto an SDS gel (4 to 15% Tris-Glycine eXtended, Bio-Rad) and separated by electrophoresis. Proteins were transferred onto polyvinylidene difluoride membranes (Bio-Rad) and incubated with anti–Der p 10 (2 µg/ml) or isotype control antibody (2 µg/ml; BioLegend, clone MG1-45) overnight at 4°C. The blots were developed the following day after incubation with horseradish peroxidase–conjugated secondary antibody (anti-mouse IgG; Santa Cruz Biotechnology).
Recombinant Der p 10
Escherichia coli–expressed rDer p 10 was generated as previously described (74) and treated with endotoxin removal beads (Miltenyi Biotec).
Human dectin-1 expression vector
The human CLEC7A open reading frame was PCR-amplified from human peripheral blood mononuclear cells and cloned into pcDNA3.1 (Thermo Fisher Scientific).
Mass spectrometry
For identification of the protein ligand of dectin-1 in dust mite extract, we performed a pull-down using 5 µg of control-Fc or dectin-1–Fc and 200 µg of HDM (as described above). To visualize the ligand, we ran the pull-downs on an SDS gel followed by MS-grade silver stain (Thermo Fisher Scientific). The 37-kDa band was excised for MS analysis. Der p 10 was identified as the sole ligand with two peptides at a 1% false discovery rate (FDR).
Statistical analysis
Data were analyzed in GraphPad Prism 6. Comparisons between more than two groups were done using one-way analysis of variance (ANOVA) followed by post hoc test (Figs. 1 to 4) or using two-way ANOVA followed by Bonferroni correction for repeated measures (Fig. 5J). Comparisons between two groups were done using Student’s t test with Welch’s correction (Fig. 6, A and B) or one-way ANOVA followed by post hoc test (Fig. 5C). P values of less than 0.05 were considered significant.
Study populations
One hundred forty-three SNPs for CLEC7A were tested for an association with FEV1 in 4275 children who participated in SAGE and GALA II. The genotype and phenotype information analyzed in this article is publicly available via dbGaP (accession numbers phs001274. v1.p1, phs001180.v1.p1, and phs00921.v1.p1).
SAGE study
The SAGE study includes 1989 African American asthma cases and controls recruited from clinics in the San Francisco Bay Area (1176 cases, 813 controls). The analysis included 1414 individuals with baseline FEV1 measurement. Baseline FEV1 measurement is reported as the percentage of the predicted normal value of FEV1 achieved by the participant (FEV1 percent predicted). The predicted normal value of FEV1 for each participant was derived using the Hankinson reference equation (75). Asthma was defined as having a history of physician-diagnosed asthma and two or more asthma symptoms (wheezing, coughing, and/or shortness of breath) in the preceding 2 years. Atopy was not an inclusion criterion for the study. Controls had no reported history of asthma, allergies, lung disease, chronic illness or medication use, coughing, wheezing, or shortness of breath in the preceding 2 years; had <10 pack-years of smoking history; and had not smoked in the year preceding enrollment. Genotypes were called on Affymetrix Axiom LAT 1 (World Array 4) and LAT plus HLA genome-wide arrays using Affymetrix Power Tools software. Quality control was performed by removing SNPs with call rates of <95% and/or deviated from the Hardy-Weinberg equilibrium (P < 10−6). Samples with call rates of <95%, discrepancy between genetic sex and reported sex, or cryptic relatedness (proportion of identity by descent > 0.3) were removed.
GALA II study
The GALA II study includes 4436 Latino asthma cases and controls recruited from community clinics and hospitals from New York, Chicago, San Francisco, Houston, and Puerto Rico (2275 cases, 2161 controls). The analysis included 2861 individuals with baseline FEV1 measurement. Asthma was defined as having a history of physician-diagnosed asthma and two or more asthma symptoms (wheezing, coughing, and/or shortness of breath) in the preceding 2 years. Atopy was not an inclusion criterion for the study. Controls had no reported history of asthma, allergies, lung disease, chronic illness or medication use, coughing, wheezing, or shortness of breath in the preceding 2 years; had <10 pack-years of smoking history; and had not smoked in the year preceding enrollment. Genotyping and quality control (QC) process were performed as described in SAGE.
Genetic association testing
Genotype data of all samples were submitted to the Michigan Imputation Server (https://imputationserver.sph.umich.edu/) for imputation to the Haplotype Reference Consortium r1 2015 reference panel (www.haplotype-reference-consortium.org/) using the Minimac3 algorithm (http://genome.sph.umich.edu/wiki/Minimac3) and SHAPEIT (www.shapeit.fr/) as the phasing method. Imputed markers were excluded from the analysis if they were monomorphic or their R2 statistics were below 0.3. The analysis included 153 genotyped and imputed SNPs in GALA II and 197 SNPs in SAGE. We excluded markers with minor allele frequency lower than 0.01, resulting in a final list of 143 SNPs that overlapped between GALA II and SAGE. Global ancestry was estimated using ADMIXTURE (76) for each individual. For African Americans, we assumed a two-population model of admixture (European and African ancestry), and for Latinos, we assumed a three-population model (European, Native American, and African ancestry). Reference haplotypes were from the HapMap phase II CEU (European) and YRI (African), and 71 Native American individuals were genotyped on the Axiom LAT1 array as described previously (77). The association of each SNP with FEV1 was performed using linear regression in PLINK 1.9 (78, 79). Regression models were adjusted for asthma status, age, sex, and global ancestry. In GALA II, an additional variable of ethnicity was added as a covariate. P values presented are from two-sided testing, with an a of 0.05. The Bonferroni correction was applied to create a significance threshold corrected for multiple testing (Bonferroni threshold: 0.05/143 = 3.5 × 10−4). The results of GALA II and SAGE were combined with meta-analysis using METAL (80).
Supplementary Material
Materials and Methods
Fig. S1. Flow-gating scheme for lung ILC2.
Fig. S2. ST2 blockade in Clec7a−/− mice reduces HDM-induced airway mucus.
Fig. S3. Dectin-1 on lung structural cells protects against manifestations of allergic asthma.
Fig. S4. Dectin-1 expression on APCs and epithelial cells from human nasal tissue.
Fig. S5. Control Fc protein does not bind to invertebrate tropomyosin.
Fig. S6. Invertebrate tropomyosin abrogates chitin-induced airway inflammation.
Fig. S7. Allelic variation in rs58677678 in 1000 Genomes populations.
Table S1. Location, frequency, and effect size of rs58677678.
Table S2. Association between CLEC7A SNP (rs58677678) and expression of CLEC7A gene in multiple tissues.
Table S3. Patient information for CRS.
Table S4. Patient information for asthmatics.
Acknowledgments
We thank A. Scott and C. Karp for helpful discussions and review of the manuscript. We acknowledge R. Cole and T. Boronina at the Johns Hopkins Mass Spectrometry and Proteomics Facility for assistance with MS analyses. We thank Amgen for providing neutralizing anti-mouse ST2 antibodies.
Funding: This work was funded by the National Institute of Allergy and Infectious Diseases (grants U19AI070235 and R01 AI083315 to M.W.-K.) and the NIH (grants R56AI118791 and R01AI127644 to S.L. and R01AI072502 to A.P.L.). S.L. was also supported by a Parker B. Francis Fellowship, and N.G. was supported by the National Institute of Environmental Health Sciences (grant 5T32ES007141). E.G.B., M.W., S.O., S.H., C.E., and D.H. were supported in part by the Sandler Family Foundation, the American Asthma Foundation, the NIH (grants R01 HL117004, R21ES24844 R01 ES015794, R01 HL088133, R01 HL078885, R01 HL104608, R01MD010443, and R01 HL135156), and the Tobacco-Related Disease Research Program (grant 24RT-0025). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
immunology.sciencemag.org/cgi/content/full/3/20/eaam9841/DC1 Materials and Methods
Author contributions: N.G., S.L., and M.W.-K. designed the study. N.G. and S.L. performed the experiments and analyzed the data with help from U.S., A. Sharma, A. Singh, and X.X. J.-H.K. was instrumental in carrying out BM chimera studies. N.Y. provided technical assistance with the allergic phenotype. S.V. and Y.R. made recombinant dust mite tropomyosin. S.P., I.H.S., and J.C. isolated and prepared airway epithelial cells from controls and asthmatics. M.W., D.H., P.G., S.H., C.E., A.M., S.O., and E.G.B. performed genetic analyses. A.P.L. provided samples from control and CRS patients. N.G., S.L., and M.W.-K. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
References
- 1.Thomas WR, Hales BJ, Smith W-A. Structural biology of allergens. Curr. Allergy Asthma Rep. 2005;5:388–393. doi: 10.1007/s11882-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 2.Aalberse RC. Structural biology of allergens. J. Allergy Clin. Immunol. 2000;106:228–238. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 3.Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. J. Allergy Clin. Immunol. 2009;123:558–566. doi: 10.1016/j.jaci.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates TH2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Kawasaki H, Hsu S-C, Lee RT, Yao X, Plunkett B, Fu J, Yang K, Lee YC, Huang S-K. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat. Med. 2010;16:1128–1133. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. The β-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 2012;189:3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiagarajan PS, Yakubenko VP, Elsori DH, Yadav SP, Willard B, Tan CD, Rodriguez ER, Febbraio M, Cathcart MK. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc. Res. 2013;99:494–504. doi: 10.1093/cvr/cvt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, Nishio J, Negishi H, Tamura T, Saijo S, Iwakura Y, Taniguchi T. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. eLife. 2014;3:e04177. doi: 10.7554/eLife.04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Hirose K, Norimoto A, Tamachi T, Yokota M, Saku A, Takatori H, Saijo S, Iwakura Y, Nakajima H. Dectin-1 plays an important role in house dust mite-induced allergic airway inflammation through the activation of CD11b+ dendritic cells. J. Immunol. 2017;198:61–70. doi: 10.4049/jimmunol.1502393. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbarth SC, Williams A, Colegio OR, Meng H, Strowig T, Rongvaux A, Henao-Mejia J, Thaiss CA, Joly S, Gonzalez DG, Xu L, Zenewicz LA, Haberman AM, Elinav E, Kleinstein SH, Sutterwala FS, Flavell RA. Corrigendum: NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature. 2016;530:504. doi: 10.1038/nature16074. [DOI] [PubMed] [Google Scholar]
- 13.Eisenbarth SC, Williams A, Colegio OR, Meng H, Strowig T, Rongvaux A, Henao-Mejia J, Thaiss CA, Joly S, Gonzalez DG, Xu L, Zenewicz LA, Haberman AM, Elinav E, Kleinstein SH, Sutterwala FS, Flavell RA. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature. 2012;484:510–513. doi: 10.1038/nature11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamantopoulos M, Ronchi F, Van Hauwermeiren F, Vieira-Silva S, Yilmaz B, Martens L, Saeys Y, Drexler SK, Yazdi AS, Raes J, Lamkanfi M, McCoy KD, Wullaert A. Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity. 2017;47:339–348. doi: 10.1016/j.immuni.2017.07.011. e4. [DOI] [PubMed] [Google Scholar]
- 15.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie ANJ. Type-2 innate lymphoid cells in asthma and allergy. Ann. Am. Thorac. Soc. 2014;11(suppl. 5):S263–S270. doi: 10.1513/AnnalsATS.201403-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliphant CJ, Barlow JL, McKenzie ANJ. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–385. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H-M, Yuk J-M, Shin D-M, Jo E-K. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J. Clin. Immunol. 2009;29:795–805. doi: 10.1007/s10875-009-9319-3. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Kedar S, Baram L, Elad H, Brazowski E, Guzner-Gur H, Dotan I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur. J. Immunol. 2014;44:3729–3740. doi: 10.1002/eji.201444876. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Hardman CS, Panova V, McKenzie ANJ. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur. J. Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate TH2-type responses. J. Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard J-P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 25.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard J-P, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, Holtzman MJ. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodridge HS, Shimada T, Wolf AJ, Hsu Y-MS, Becker CA, Lin X, Underhill DM. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J. Immunol. 2009;182:1146–1154. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 2006;176:3717–3724. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- 28.Van Asselt L. Interactions between domestic mites and fungi. Indoor Built Environ. 1999;8:216–220. [Google Scholar]
- 29.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through β-glucan-dependent pathways. J. Allergy Clin. Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reese G, Ayuso R, Lehrer SB. Tropomyosin: An invertebrate pan-allergen. Int. Arch. Allergy Immunol. 1999;119:247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 31.Blackwell J. Structure of β-chitin or parallel chain systems of poly-β-(1→4)-N-acetyl-d-glucosamine. Biopolymers. 1969;7:281–298. doi: 10.1002/bip.1969.360070302. [DOI] [PubMed] [Google Scholar]
- 32.Brown GD, Gordon S. Immune recognition: A new receptor for β-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 33.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie ANJ, Krummel MF, Liang H-E, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity. 2014;40:414–424. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reese TA, Liang H-E, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, Taki Y, Futatsugi-Yumikura S, Tsutsui H, Ishii KJ, Yoshimoto T, Akira S, Nakanishi K. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl. Acad. Sci. U.S.A. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013;131:187–200. doi: 10.1016/j.jaci.2012.08.002. e8. [DOI] [PubMed] [Google Scholar]
- 37.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, Yoshimoto T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int. Immunol. 2014;26:539–549. doi: 10.1093/intimm/dxu058. [DOI] [PubMed] [Google Scholar]
- 38.Armenaka MC, Grizzanti JN, Oriel B, Rosenstreich DL. Increased immune reactivity to house dust mites in adults with chronic rhinosinusitis. Clin. Exp. Allergy. 1993;23:669–677. doi: 10.1111/j.1365-2222.1993.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 39.Paris G, Pozharskaya T, Asempa T, Lane AP. Damage-associated molecular patterns stimulate interleukin-33 expression in nasal polyp epithelial cells. Int. Forum Allergy Rhinol. 2014;4:15–21. doi: 10.1002/alr.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, Liu Y-J, Luong A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GTEx Consortium, Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GTEx Consortium, The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Branco Madeira F, Beyaert R, van Loo G, Bracher F, von Mutius E, Chanez P, Lambrecht BN, Hammad H. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 44.Stein MM, Hrusch J, Gozdz CL, Igartua C, Pivniouk V, Murray SE, Ledford JG, Marques dos Santos M, Anderson RL, Metwali N, Neilson JW, Maier RM, Gilbert JA, Holbreich M, Thorne PS, Martinez FD, von Mutius E, Vercelli D, Ober C, Sperling AI. Innate immunity and asthma risk in Amish and Hutterite farm children. N. Engl. J. Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mintz-Cole RA, Gibson AM, Bass SA, Budelsky AL, Reponen T, Hershey GKK. Dectin-1 and IL-17A suppress murine asthma induced by Aspergillus versicolor but not Cladosporium cladosporioides due to differences in β-glucan surface exposure. J. Immunol. 2012;189:3609–3617. doi: 10.4049/jimmunol.1200589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Zhao G-Q, Che C-Y, Li N, Lin J, Xu Q, Wang Q, Liu Y, Qiu S. Expression of dectin-1 during fungus infection in human corneal epithelial cells. Int. J. Ophthalmol. 2014;7:34–37. doi: 10.3980/j.issn.2222-3959.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heyl KA, Klassert TE, Heinrich A, Müller MM, Klaile E, Dienemann H, Grünewald C, Bals R, Singer BB, Slevogt H. Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. MBio. 2014;5:e01492–e14. doi: 10.1128/mBio.01492-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAlees JW, Whitehead GS, Harley ITW, Cappelletti M, Rewerts CL, Holdcroft AM, Divanovic S, Wills-Karp M, Finkelman FD, Karp CL, Cook DN. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 2015;8:863–873. doi: 10.1038/mi.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Hristova M, Habibovic A, Veith C, Janssen-Heininger YMW, Dixon AE, Geiszt M, van der Vliet A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 2016;137:1545–1556. doi: 10.1016/j.jaci.2015.10.003. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, van der Veer BMJW, Deen PMT, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JWM, Ng A, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HS, Kwon JY, Joo C-K. Topical administration of β-13-glucan to modulate allergic conjunctivitis in a murine model. Invest. Ophthalmol. Vis. Sci. 2016;57:1352–1360. doi: 10.1167/iovs.15-17914. [DOI] [PubMed] [Google Scholar]
- 54.Kawashima S, Hirose K, Iwata A, Takahashi K, Ohkubo A, Tamachi T, Ikeda K, Kagami S-i, Nakajima H. β-glucan curdlan induces IL-10-producing CD4+ T cells and inhibits allergic airway inflammation. J. Immunol. 2012;189:5713–5721. doi: 10.4049/jimmunol.1201521. [DOI] [PubMed] [Google Scholar]
- 55.Maheswaran D, Zeng Y, Chan-Yeung M, Scott J, Osornio-Vargas A, Becker AB, Kozyrskyj AL. Exposure to beta-(1,3)-D-glucan in house dust at age 7–10 is associated with airway hyperresponsiveness and atopic asthma by age 11–14. PLOS ONE. 2014;9:e98878. doi: 10.1371/journal.pone.0098878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadebe S, Kirstein F, Fierens K, Redelinghuys P, Murray GI, Williams DL, Lambrecht BN, Brombacher F, Brown GD. β-Glucan exacerbates allergic airway responses to house dust mite allergen. Respir. Res. 2016;17:35. doi: 10.1186/s12931-016-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia Y, Větvička V, Yan J, Hanikýřová M, Mayadas T, Ross GD. The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b–opsonized target cells. J. Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 58.Zimmerman JW, Lindermuth J, Fish PA, Palace GP, Stevenson TT, DeMong DE. A novel carbohydrate-glycosphingolipid interaction between a β-(1–3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998;273:22014–22020. doi: 10.1074/jbc.273.34.22014. [DOI] [PubMed] [Google Scholar]
- 59.Kikkert R, Bulder I, de Groot ER, Aarden LA, Finkelman MA. Potentiation of Toll-like receptor-induced cytokine production by (1→3)-β-d-glucans: Implications for the monocyte activation test. J. Endotoxin Res. 2007;13:140–149. doi: 10.1177/0968051907080024. [DOI] [PubMed] [Google Scholar]
- 60.Rice PJ, Kelley JL, Kogan G, Ensley HE, Kalbfleisch JH, Browder IW, Williams DL. Human monocyte scavenger receptors are pattern recognition receptors for (1→3)-β-D-glucans. J. Leukoc. Biol. 2002;72:140–146. [PubMed] [Google Scholar]
- 61.Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J. Immunol. 1993;151:5354–5363. [PubMed] [Google Scholar]
- 62.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: Implications for the hygiene hypothesis. J. Allergy Clin. Immunol. 2011;127:479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pruyne D. Tropomyosin function in yeast. Adv. Exp. Med. Biol. 2008;644:168–186. doi: 10.1007/978-0-387-85766-4_14. [DOI] [PubMed] [Google Scholar]
- 64.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osbourn M, Soares DC, Vacca F, Cohen ES, Scott IC, Gregory WF, Smyth DJ, Toivakka M, Kemter AM, le Bihan T, Wear M, Hoving D, Filbey KJ, Hewitson JP, Henderson H, Gonzàlez-Cìscar A, Errington C, Vermeren S, Astier AL, Wallace WA, Schwarze J, Ivens AC, Maizels RM, McSorley HJ. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity. 2017;47:739–751. doi: 10.1016/j.immuni.2017.09.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McSorley HJ, Blair NF, Smith KA, McKenzie ANJ, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–1078. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim LK, Morita R, Kobayashi Y, Eisenbarth SC, Lee CG, Elias J, Eynon EE, Flavell RA. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E2891–E2899. doi: 10.1073/pnas.1507393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Dea EM, Amarsaikhan N, Li H, Downey J, Steele E, Van Dyken SJ, Locksley RM, Templeton SP. Eosinophils are recruited in response to chitin exposure and enhance TH2-mediated immune pathology in Aspergillus fumigatus infection. Infect. Immun. 2014;82:3199–3205. doi: 10.1128/IAI.01990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, Decker DC, de Araujo CA, Bryce PJ, Sperling AI. IL-33-dependent induction of allergic lung inflammation by FcγRIII signaling. J. Clin. Invest. 2013;123:2287–2297. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, Vogel SN. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J. Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lajoie S, Lewkowich I, Herman NS, Sproles A, Pesce JT, Wynn TA, Grusby MJ, Hamid Q, Wills-Karp M. IL-21 receptor signalling partially mediates TH2-mediated allergic airway responses. Clin. Exp. Allergy. 2014;44:976–985. doi: 10.1111/cea.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J. Clin. Invest. 2014;124:5442–5452. doi: 10.1172/JCI77798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haj-Salem I, Fakhfakh R, Bérubé J-C, Jacques E, Plante S, Simard MJ, Bossé Y, Chakir J. MicroRNA-19a enhances proliferation of bronchial epithelial cells by targeting TGFβR2 gene in severe asthma. Allergy. 2015;70:212–219. doi: 10.1111/all.12551. [DOI] [PubMed] [Google Scholar]
- 74.Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, Swoboda I, Thomas WR, Thalhamer J, Valenta R, Vrtala S. Molecular characterization of Der p 10: A diagnostic marker for broad sensitization in house dust mite allergy. Clin. Exp. Allergy. 2011;41:1468–1477. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 76.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torgerson DG, Gignoux CR, Galanter JM, Drake KA, Roth LA, Eng C, Huntsman S, Torres R, Avila PC, Chapela R, Ford JG, Rodríguez-Santana JR, Rodríguez-Cintrón W, Hernandez RD, Burchard EG. Case-control admixture mapping in Latino populations enriches for known asthma-associated genes. J. Allergy Clin. Immunol. 2012;130:76–82. doi: 10.1016/j.jaci.2012.02.040. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Purcell SM, Chang CC. https://www.cog-genomics.org/plink2.
- 80.Willer CJ, Li Y, Abecasis GR. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Fig. S1. Flow-gating scheme for lung ILC2.
Fig. S2. ST2 blockade in Clec7a−/− mice reduces HDM-induced airway mucus.
Fig. S3. Dectin-1 on lung structural cells protects against manifestations of allergic asthma.
Fig. S4. Dectin-1 expression on APCs and epithelial cells from human nasal tissue.
Fig. S5. Control Fc protein does not bind to invertebrate tropomyosin.
Fig. S6. Invertebrate tropomyosin abrogates chitin-induced airway inflammation.
Fig. S7. Allelic variation in rs58677678 in 1000 Genomes populations.
Table S1. Location, frequency, and effect size of rs58677678.
Table S2. Association between CLEC7A SNP (rs58677678) and expression of CLEC7A gene in multiple tissues.
Table S3. Patient information for CRS.
Table S4. Patient information for asthmatics.