Abstract
Introduction
We tested the hypothesis that the amyloid β (Aβ) peptide ratios are more stable than Aβ42 alone when biofluids are exposed to two preanalytical conditions known to modify measurable Aβ concentration.
Methods
Human cerebrospinal fluid (CSF) and culture media (CM) from human cortical neurons were exposed to a series of volumes and polypropylene surfaces. Aβ42, Aβ40, and Aβ38 peptide concentrations were measured using a multiplexed electrochemiluminescence immunoassay. Data were analyzed using mixed models in R.
Results
Decrease of measurable Aβ peptide concentrations was exaggerated in longer peptides, affecting the Aβ42:Aβ40 and Aβ42:Aβ38 ratios. However, the effect size of surface treatment was reduced in Aβ peptide ratios versus Aβ42 alone. For Aβ42:Aβ40, the effect was reduced by approximately 50% (volume) and 75% (transfer) as compared to Aβ42 alone.
Discussion
Use of Aβ ratios, in conjunction with concentrations, may mitigate confounding factors and assist the clinical diagnostic process for Alzheimer's disease.
Keywords: Alzheimer's disease, Amyloid β ratio, Preanalytical factors, Cerebrospinal fluid, Cell culture media, Surface adsorption
1. Background
The cerebrospinal fluid (CSF) concentrations of the 42 amino acid form of amyloid β (Aβ42), total tau protein (T-tau), and phosphorylated tau (P-tau) are core biomarkers of Alzheimer's disease (AD) [1] and are incorporated in the clinical diagnostic process [2]. Largely constituting the neuropathological hallmarks of AD [3], these proteins are also integral to the validation and study of AD models in a research context.
While tau is soluble and concentrations remain relatively stable over a range of conditions [4], [5], [6], Aβ42 is well known to be highly labile and prone to aggregate, a property that underpins a range of dynamic structures and their contribution to the disease process [7]. These properties make Aβ42 concentrations susceptible to variation in the preanalytical process. Factors potentially include CSF collection technique [8], diurnal collection time [9], interval between collection and freezing [10], [11], temperature [12], pH [13], sample matrix composition [14], [15], sample exposure to storage surfaces [6], [16], [17], [18], [19], [20], [21], [22], and assay measurement variation [23], [24], [25], [26], [27]. Several articles have presented studies providing data from assessments of multiple factors [5], [28], [29], [30], [31], [32].
Further to preanalytical factors, concentrations of CSF Aβ also vary between individuals [33]; thus, individuals with constitutively high or low quantities of Aβ42 relative to chosen diagnostic “cut points” may be vulnerable to misinterpretation of test results. Recent meta-analysis shows that when comparing AD versus nondemented controls and non-AD dementias, CSF Aβ42 has a pooled sensitivity of 0.80 (95% confidence interval = 0.78–0.82) and a pooled specificity of 0.76 (95% confidence interval = 0.74–0.78) [34]. Increasingly, reports suggest improved diagnostic and mild cognitive impairment–AD conversion predictive power when Aβ42 is considered in ratio to Aβ40 [1], [15], [16], [31], [35], [36]. Aβ40 has been shown to be the most abundant Aβ peptide in the adult human brain [37] and does not generally have strong aggregative tendencies under physiological conditions or in vitro. Owing to this, CSF Aβ40 has been proposed as a proxy for total Aβ production [33], [38]. Another relatively abundant peptide, Aβ38, has also demonstrated utility in ratio with Aβ42 [35]. This is welcome news as, although biomarkers have facilitated earlier and more accurate diagnosis, improved patient group enrichment in current clinical trial design (i.e. more accurate, earlier disease phase biomarkers) is urgently required [39].
The aim of the present study was to extend previous work on the effect of surface exposure on Aβ peptide concentration by assessing the impact on ratios of Aβ42:Aβ40, Aβ42:Aβ38, and Aβ40:Aβ38. Aβ40:Aβ38 is not immediately relevant to the clinic, but better understanding of the relationship between production and interaction of different Aβ fragments may prove useful to future understanding of AD pathobiology. In addition to CSF, we also examined cell culture media (CM) from human glutamatergic cortical neurons derived from induced pluripotent stem cells (iPSCs). Neurons differentiated from the iPSCs of AD and non-AD donors can act as disease-relevant models with a fully human genetic background. Aβ secreted from these cells into the CM represents a key biomarker for AD-relevant neurobiology. In this context, ratios of Aβ are increasingly used to understand nuances of amyloidogenic pathways [40], and it is important to expand knowledge of preanalytical factors affecting Aβ measurement in this medium.
2. Methods
2.1. Preparation of CSF
This study used de-identified CSF from patients of unknown disease status. Ethical approval was received from the regional ethics board at the University of Gothenburg for the CSF pools used in this study. Samples were collected by lumbar puncture. All lumbar punctures were conducted before 13:00, between L3/L4 and L4/L5 in a sitting or side-laying position. Ten milliliters of CSF was collected at ambient room temperature into a 10-mL PP tube (Sarstedt, Nümbrecht, Germany; cat. 62.9924.284). In the case of visible blood contamination, the CSF was discarded and tap continued in a new tube once bleeding had stopped. Samples selected for inclusion had no erythrocyte contamination visible to the eye. Samples were centrifuged at 2200 relative centrifugal force for 10 minutes at 20°C, transferred to another 10-mL tube (Sarstedt; cat. 62.9924.284), and stored at −80°C. CSF samples were then thawed at 21°C for 1 hour to pool CSF to sufficient volume for experiment in Sarstedt 2-mL PP tubes (cat. 72.694.406), refrozen at −80°C, and thawed (21°C for 1 hour) for assay. CSF was transported on dry ice by international courier and received, frozen, within 24 hours of sending, and immediately stored at −80°C on reception.
2.2. Preparation of cell CM
The N2B27 cell CM used in this study was composed of a 1:1 solution of DMEM/F12 + GlutaMax-l (1×) (Life Technologies; cat. 10565018) and Neurobasal Medium (1×) (Life Technologies; cat. 12348017) with the following supplements: 1× N2 supplement (Life Technologies; cat. 17502-048), 1× B27 supplement (Life Technologies; cat. 17504-044), 100-μM MEM nonessential amino acids (Life Technologies; cat. 11140-050), 100-μM 2-mercaptoethanol (Life Technologies; cat. 31350-010), 50-U/mL penicillin and 50-μg/mL streptomycin (Life Technologies; cat. 15070063), 5-μg/mL insulin (Sigma-Aldrich; cat. I9278), and 1-mM glutamine (Life Technologies; cat. no. 25030-024). Fresh N2B27 was made every 7 days and stored at 4°C.
2.3. Neuronal culture
Glutamatergic cortical neurons were generated from human iPSCs following a protocol previously described [41], [42], [43]. Briefly, iPSCs (cultured on Geltrex and fed with Essential 8) were induced toward a neuronal fate by treatment with N2B27 media supplemented with SMAD (a family of human protein homologues of the drosophila ‘mothers against decapentaplegic’ [Mad] and the proteins encoded by the C. elegens gene ‘small body size’ [Sma]) inhibitors, SB431542 (10 μM) and Dorsomorphin (1 μM), for 12 days. Neuronal precursor colonies were expanded on laminin-coated plates and maintained in N2B27 media until cultures of mature cortical neurons were generated, at least 80 days after induction. Five different cell lines were used in this study: CTRL and ND were fibroblast-derived iPSC lines from nondegenerative controls, generated using retroviral transduction (obtained from the laboratory of Dr Tilo Kunath). SHEF6 was a human embryonic stem cell line obtained from the UK Stem Cell Bank. APP (derived from an amyloid precursor protein V717I mutation patient) and PSEN (derived from a presenilin-1 T113-114ins intron 4 deletion mutation patient) were fibroblast-derived iPSC lines generated using retroviral transduction, obtained from StemBANCC. Cell CM were collected after 4-day incubation in VWR 15-mL PP tubes (cat: 21008-216), pooled and centrifuged at 2000 relative centrifugal force for 5 minutes at 21°C. Supernatant was aliquoted in Sarstedt 2-mL PP tubes (cat. 72.694.406), stored at −80°C, and thawed at 21°C immediately before assay.
2.4. Volume experiments
To investigate the effect of storage volume on the ratios of Aβ peptides, each CSF (n = 8) and CM (n = 6) sample was thawed and aliquoted into Sarstedt 2-mL PP tubes (cat. 72.694.406) in a volume series: 1000, 500, 250, 125, and 100 μL. Aliquots were refrozen at −80°C and later thawed at 21°C for 1 hour and assayed for Aβx-38/x-40/x-42 using a Meso Scale Discovery V-PLEX Aβ peptide kit (6E10). Assays were performed on a Meso Scale Discovery SECTOR 6000, according to manufacturer protocol, which is freely available.
To examine the contribution of the pipette to any effect, a separate group of samples (CSF n = 2 and CM n = 2) were aliquoted into four different volumes (100, 250, 500, and 1000 μL) and, immediately before sample dilution during assay, each volume for each sample was mixed with a varying number of pumps (0, 5, 10, and 20) with a pipette tip (TipOne; Starlab, Milton Keynes; cat. S1113-1700). Tips used for samples given the 0 pump treatment were therefore not prewetted.
All samples were added to the assay plate in duplicate by multichannel in randomized, double-blind order. All samples and reagents of volume <5 mL were mixed by vortex (Vortex-Genie 2; Scientific Industries) at speed setting 10 for 5 seconds, and all samples and reagents of volume >5 mL were mixed on a roller for 5 minutes. Pipette tips (TipOne; Starlab, Milton Keynes; cat. S1112-1720, cat. S1113-1700, and cat. S1110-3700) were prewetted with three pumps when aspirating all solutions unless otherwise stated. The same pipette tip was used to create the volume series of each sample.
2.5. Serial transfer experiments
Each CSF (n = 9) and CM (n = 5) sample was thawed, aliquoted into Sarstedt 2-mL PP tubes at a volume of 1000 μL, refrozen at −80°C, and later thawed for assay. The sample was mixed by vortex and transferred from the storage aliquot (tube 0) to seven consecutive Sarstedt 2-mL PP tubes (tubes 1–7), leaving 100 μL of sample in each tube. This process took approximately 10 minutes to complete. Samples were then assayed immediately for Aβx-38/x-40/x-42 as described for the volume experiment. All samples were added to the assay plate in duplicate by multichannel in randomized, double-blind order. Mixing practice and pipette tips used were as described in section 2.4. The same pipette tip was used to create the transfer series of each sample. Data from two CSF samples previously reported (S12 and S13 in this study, previously AD and CT [36]) were included in the analyzed data set. These samples were prepared according to the same protocol just described and were included to bolster the power of the data set.
2.6. Statistical analysis
The relationship between analyte measurement and sample treatment (volume or transfer step) was assessed by mixed model analysis. Data normality was assessed by histogram, qq-plot, and Shapiro-Wilk test, and linearity was assessed by a scatterplot of the residual variance. All analyses set α at 0.05 and confidence intervals at 95%. The formula used for the mixed model was lme(sample concentration ∼ treatment + X, random = ∼1|sample) + ε, where the dependent variable is “sample concentration”—the average of duplicate concentration (or ratio) values of a given Aβ peptide in pg/mL (numeric data), the fixed effect variable is “treatment”—the volume or transfer series as relevant (numeric data), X represents other fixed effects (such as disease status, cell type, assay plate, and sample pooling status) where these variables were relevant, and the random effect variable is “sample”—variation unaccounted for differences samples (factor data). “∼1|” specifies an independent intercept for each sample. ε represents residual variation not accounted for by the stated parameters of the model.
While data from the volume study met the mixed model's assumption of linearity, the data from the transfer study did not. To meet this requirement, a separate analysis was conducted wherein average concentration was transformed by the natural logarithm (ln) and used as the dependent variable. To calculate the proportional change per treatment unit, “e” was exponentiated to the power of the model's output coefficient. Graphs were composed using the ggplot2 package in R. Intra- and inter-assay percentage coefficients of variance were calculated according to ISO 5725-2 standards [44].
3. Results
3.1. Assay variation
Intra- and inter-assay variations were calculated from the concentrations of an internal control CSF sample included in assay plates in both volume and transfer studies. Levels of variation (intra- and inter-assay percentage coefficients of variance, respectively) for Aβ42 (4.3% and 9.9%), Aβ40 (4.5% and 9.5%), and Aβ38 (1.6% and 5.3%) were within what is generally considered acceptable range for research assays (<20%).
3.2. Effect of storage volume on Aβ peptide ratio
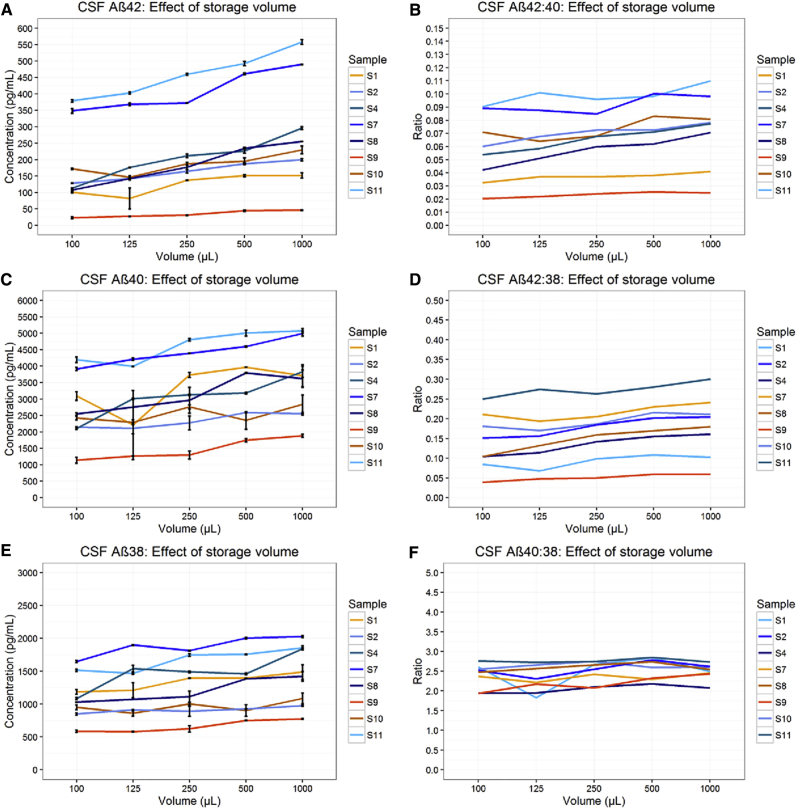
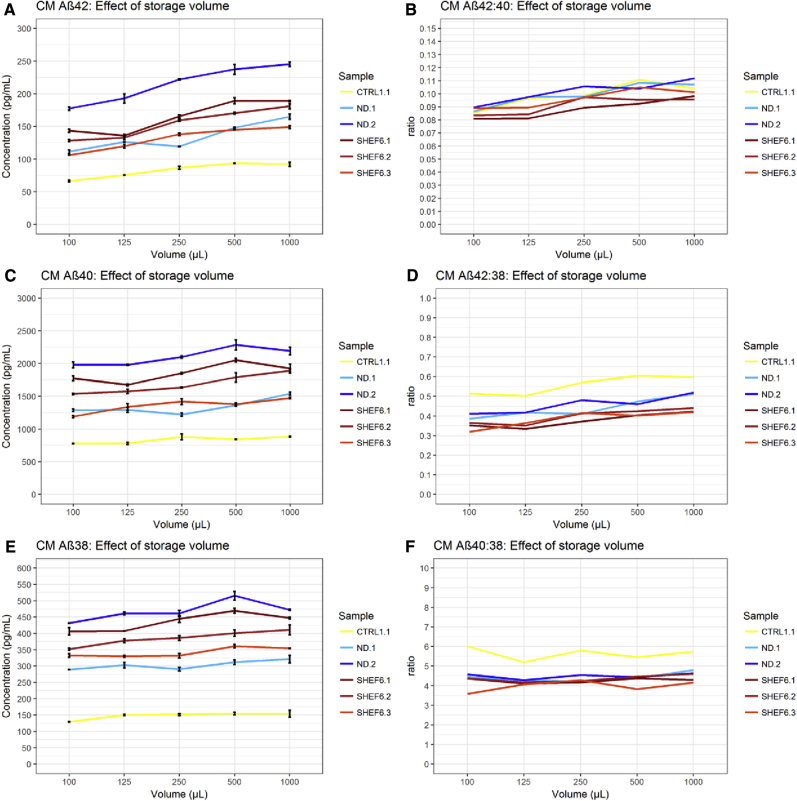
Detectable Aβ42/Aβ40/Aβ38 concentration was observed to be significantly lower (all P < .001) in samples of smaller storage volumes in both CSF and CM (Figs. 1 and 2). Results from these data predict a concentration change of Aβ42: 1.1 pg/mL (0.6%), Aβ40: 9.2 pg/mL (0.3%), and Aβ38: 3.1 pg/mL (0.2%), for every 10-μL change in CSF storage volume, and a concentration change of Aβ42: 0.5 pg/mL (0.3%), Aβ40: 2.5 pg/mL (0.2%), and Aβ38: 0.4 pg/mL (0.1%), for every 10 μL change in CM storage volume (Table 1). Results for CSF Aβ42 are highly consistent with those previously reported for control CSF (a change of 0.95 pg/mL per 10 μL) [6].
Fig. 1.
Effect of storage volume on CSF Aβ. Results in CSF show the concentration of Aβ42, Aβ40, and Aβ38 decreased with decreased storage volume (A, C, E). Concentration of Aβ42 decreased proportionally more than Aβ40 (B) and Aβ38 (D) with lower storage volume, resulting in a decrease in the Aβ42:Aβ40 and Aβ42:Aβ38 ratios. (F) Aβ40 showed a nonsignificant tendency to decrease more than Aβ38, (P = .05). Data for sample S8 at 125 μL were excluded due to insufficient volume in the assay well; the line interpolates through this point. Error bars represent standard error of the mean. Abbreviations: CSF, cerebrospinal fluid; Aβ, amyloid β.
Fig. 2.
Effect of storage volume on cell media Aβ. Results in CM show the concentration of Aβ42, Aβ40, and Aβ38 decreased with decreased storage volume (A, C, E). Concentration of Aβ42 decreased proportionally more than Aβ40 (B) and Aβ38 (D) with lower storage volume, resulting in a decrease in the Aβ42:Aβ40 and Aβ42:Aβ38 ratios. (F) Aβ40 showed a tendency to decrease more than Aβ38, although this was only weakly significant (P = .03). Abbreviations: CM, culture media; Aβ, amyloid β.
Table 1.
Summary of results
| Type | Peptide | Study | % Change per unit | P (α = 0.05) | 95% confidence interval | |
|---|---|---|---|---|---|---|
| CSF | Aβ38 | Vol | 0.245 | <.001 | 0.168 | 0.322 |
| CSF | Aβ40 | Vol | 0.318 | <.001 | 0.202 | 0.435 |
| CSF | Aβ42 | Vol | 0.555 | <.001 | 0.403 | 0.707 |
| CSF | Aβ42:Aβ40 | Vol | 0.237 | <.001 | 0.160 | 0.313 |
| CSF | Aβ42:Aβ38 | Vol | 0.310 | <.001 | 0.205 | 0.415 |
| CSF | Aβ40:Aβ38 | Vol | 0.073 | .054 | −0.001 | 0.148 |
| CSF | Aβ38 | Tra | 15.756 | <.001 | 14.818 | 16.695 |
| CSF | Aβ40 | Tra | 17.497 | <.001 | 16.734 | 18.260 |
| CSF | Aβ42 | Tra | 22.359 | <.001 | 21.177 | 23.541 |
| CSF | Aβ42:Aβ40 | Tra | 4.862 | <.001 | 3.990 | 5.734 |
| CSF | Aβ42:Aβ38 | Tra | 6.603 | <.001 | 5.409 | 7.796 |
| CSF | Aβ40:Aβ38 | Tra | 1.741 | <.001 | 1.111 | 2.372 |
| CM | Aβ38 | Vol | 0.105 | <.001 | 0.053 | 0.156 |
| CM | Aβ40 | Vol | 0.162 | <.001 | 0.106 | 0.217 |
| CM | Aβ42 | Vol | 0.331 | <.001 | 0.235 | 0.427 |
| CM | Aβ42:Aβ40 | Vol | 0.169 | <.001 | 0.104 | 0.234 |
| CM | Aβ42:Aβ38 | Vol | 0.226 | <.001 | 0.160 | 0.293 |
| CM | Aβ40:Aβ38 | Vol | 0.057 | .028 | 0.007 | 0.108 |
| CM | Aβ38 | Tra | 2.411 | <.001 | 1.676 | 3.205 |
| CM | Aβ40 | Tra | 4.789 | <.001 | 4.103 | 5.711 |
| CM | Aβ42 | Tra | 7.786 | <.001 | 6.993 | 9.219 |
| CM | Aβ42:Aβ40 | Tra | 3.148 | <.001 | 2.315 | 4.082 |
| CM | Aβ42:Aβ38 | Tra | 5.508 | <.001 | 4.532 | 6.799 |
| CM | Aβ40:Aβ38 | Tra | 2.436 | <.001 | 1.599 | 3.334 |
Abbreviations: CM, culture media; Aβ, amyloid β; CSF, cerebrospinal fluid.
NOTE. Volume unit is 10 μL, and transfer is 1 transfer. The table is divided into data generated for CSF and CM for storage volume (Vol) and serial transfer (Tra) treatments, respectively. Data are given for Aβ42, Aβ40, Aβ38, and the ratio of these peptides transformed by natural logarithm (ln). Values given in “% Change per unit” are exponentiated coefficients of the mixed model as percent of Aβ concentration (pg/mL) or ratio change per unit of treatment. For the volume treatment, the unit of change is 1 μL, that is, the amount of Aβ lost per 1 μL of decreased storage volume. For serial transfer treatment, the unit of change is one transfer, that is, the amount of Aβ lost per transfer of sample to another tube.
Concordantly, ratios of Aβ42:Aβ40 and Aβ42:Aβ38 changed significantly with storage volume. In CSF Aβ42:Aβ40, change was 0.2% of an initial ratio value per 10 μL (P < .001), and in CSF Aβ42:Aβ38, change was 0.3% per 10 μL (P < .001) (Table 1). In CM, change in the Aβ42:Aβ40 and Aβ42:Aβ38 ratios were 0.1% and 0.2% of the ratio per 10 μL (P < .001), respectively (Table 1). The magnitude of change per unit volume was reduced in both CSF and CM ratios versus Aβ42 alone. The ratio of Aβ40:Aβ38 showed a trend toward decreased Aβ40, which bordered on significance in CSF (P = .054) and CM (P = .028) (Table 1).
3.3. Effect of serial tube transfer on Aβ peptide ratio
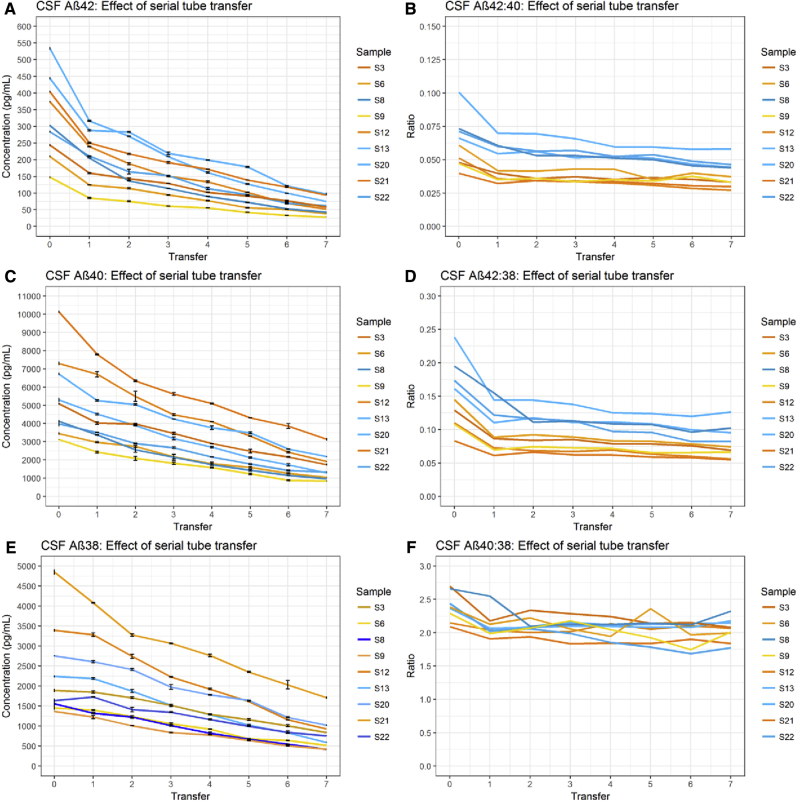
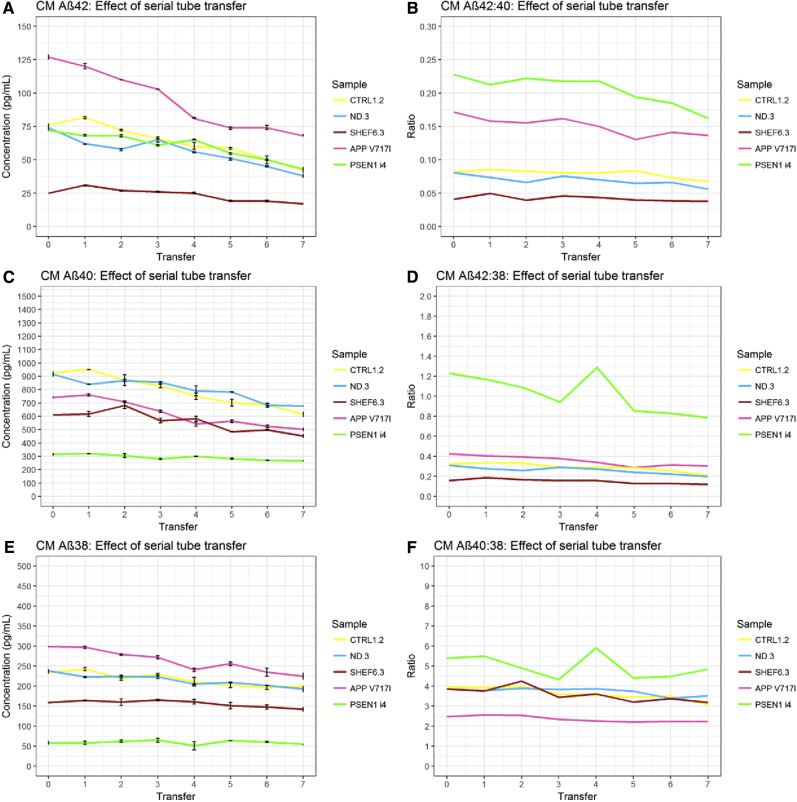
Detectable Aβ42/Aβ40/Aβ38 concentration was observed to decrease significantly (all P < .001) over the transfer series in both CSF and CM (Figs. 3 and 4). Results from untransformed CSF data were highly consistent with observations made of control CSF in a previous study [16]. However, these data violated the model's assumption of linearity. Concentration loss in CSF between transfer 0 and transfer 1 was particularly pronounced for Aβ42, an effect not observed in CM. The mean difference in Aβ42 between transfer 0 and transfer 1 was 95.8 pg/mL (paired, two-tailed t-test P < .001), whereas the mean difference between transfer 1 and the final concentration at transfer 7 was 149.7 pg/mL (paired, two-tailed t-test P < .001). This highlights that, in CSF, the first transfer accounted for 39% of the total Aβ42 lost (as compared to Aβ38 = 8.8% and Aβ40 = 24.1%). The decrease in all Aβ peptides remained significant between transfers 1 and 7 after transfer 0 was removed. This effect was not observed in CM samples.
Fig. 3.
Effect of serial tube transfer on CSF Aβ. Results in CSF show the concentration of Aβ42, Aβ40, and Aβ38 decreased with consecutive transfer of sample to new storage tubes (A, C, E). Concentration of Aβ42 decreased proportionally more than Aβ40 (B) and Aβ38 (D) with each transfer, resulting in a decrease in the Aβ42:Aβ40 and Aβ42:Aβ38 ratios. (F) In turn, the rate of Aβ40 decrease with each transfer was greater than Aβ38. Error bars represent standard error of the mean. Abbreviations: Aβ, amyloid β; CSF, cerebrospinal fluid.
Fig. 4.
Effect of serial tube transfer on cell media Aβ. Results in CM show the concentration of Aβ42, Aβ40, and Aβ38 decreased with consecutive transfer of sample to new storage tubes (A, C, E). Concentration of Aβ42 decreased proportionally more than Aβ40 (B) and Aβ38 (D) with each transfer, resulting in a decrease in the Aβ42:Aβ40 and Aβ42:Aβ38 ratios. (F) In turn, the rate of Aβ40 decrease with each transfer was greater than Aβ38. Abbreviations: CM, culture media; Aβ, amyloid β.
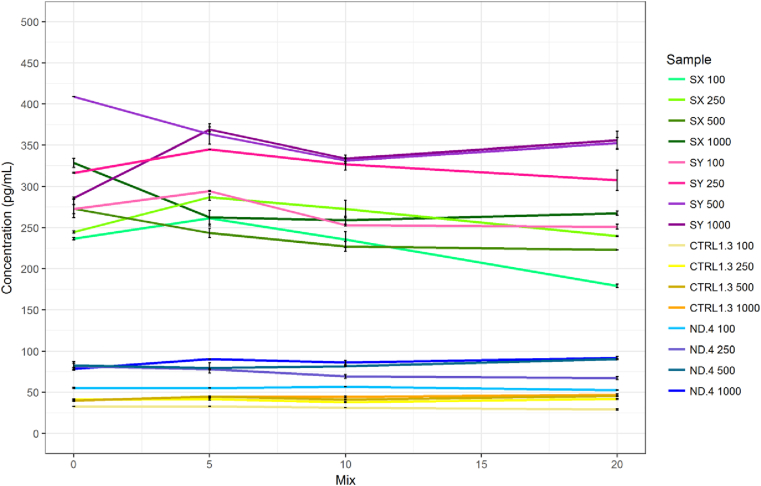
To test whether exaggerated Aβ42 loss at first transfer may have been due to adsorption to the pipette tip, we conducted a pilot experiment to measure Aβ42 peptide concentration change in response to a varying number of aspirations using the same tip. The number of fluid pumps had no effect on Aβ42 peptide concentration in either CSF or CM, indeed paired, two-tailed t-test showed no significant difference between 0 pumps and 5 pumps, although it was observed that measurement variability was greater in the 0 pump group (Fig. 5). The initial exaggerated decrease in Aβ42 cannot therefore be attributed to adsorption to the pipette tip.
Fig. 5.
CSF (SX and SY) and CM (CTRL and ND) Aβ42: Effect of mixing by pipette across different storage volumes. Results show the concentration of Aβ42 were not significantly affected by different levels of exposure to the pipette tip over a series of different volumes in either CSF or CM. Greater variability between measurements was observed in CSF measurements than those of CM. Error bars represent standard error of the mean. Abbreviations: CM, culture media; Aβ, amyloid β; CSF, cerebrospinal fluid.
To account for nonlinearity, data were transformed by ln and reanalyzed. After ln transformation, Aβ42 decrease over serial tube transfers remained exaggerated in relation to Aβ40 and Aβ38 in both CSF and CM (Table 1). In CSF, the Aβ42:Aβ40 ratio decreased by 4.9% per transfer, and the Aβ42:Aβ38 decreased by 6.6% per transfer (Table 1). In CM, these were 3.1% and 5.5%, respectively. The decrease per transfer of Aβ40:Aβ38 was 1.7% in CSF and 2.4% in CM; in both fluids, the decrease of Aβ40 relative to Aβ38 was significant (P < .001).
4. Discussion
In this study, we explored the effect of storage volume and serial between-tube transfer on the concentration of Aβ42/Aβ40/Aβ38 in human CSF and the CM of human cortical neurons derived from iPSCs. We report a novel finding: First, Aβ peptides are differentially affected by changes in sample surface exposure and raise the implication that subpopulations of Aβ peptide structures may be differentially vulnerable to surface exposure. Second, we show that ratios are less sensitive to surface exposure than peptides considered alone, although the effect is still significant.
4.1. Aβ peptides are differentially vulnerable to surface exposure
CSF and CM Aβ42/Aβ40/Aβ38 concentrations decreased as a result of two different PP surface exposure treatments (storage volume and serial tube transfer), closely replicating observations we previously reported in CSF [6], [16]. Results are consistent with irreversible peptide adsorption to the tube surface, although the experiments did not test for this mechanism directly. Importantly, this decrease did not occur at the same rate for each peptide. Ratios of Aβ42:Aβ40 and Aβ42:Aβ38 demonstrated that decrease in Aβ42 concentration per treatment unit was consistently greater than that observed in Aβ40 and Aβ38, in both CSF and CM. In addition, Aβ40:Aβ38 ratios indicated that Aβ40 may demonstrate a tendency toward greater concentration loss per treatment than Aβ38.
To our knowledge experiments such as these have not previously been published on CM. However, a body of work has been growing on the impact of preanalytical surface adsorption in CSF. Vanderstichele et al. [32] observed significant decreases in Aβ1–42 (−13.6%), Aβ1–40 (−15.5%), and Aβ1–38 (−10.6%) between CSF stored at 1500 μL (Sarstedt; cat. 72.706) and 500 μL (Sarstedt; cat. 72.730.006) in PP tubes, but not in Eppendorf LoBind tubes. They found that the Aβ42:Aβ40 ratio was not significantly altered by the difference in volume, whereas Aβ42:Aβ38 was altered by 3.4%. This is in contrast to our model that predicts larger, significant, changes in Aβ42:Aβ40 (23.7%) and Aβ42:Aβ38 (30.9%). It is worth noting that the volume effect is closely related to tube dimension [6], [21], and our results represent the difference between 1000 and 100 μL rather than 1500 and 500 μL, which have different relative surface area exposure to the conical portion of the tube. In addition, the tubes used by Vanderstichele et al. for each volume were not the same, and neither matched the tube we tested (Sarstedt; cat. 72.694.007), which may reduce the direct comparability of results. With regard to the preanalytical effects of tube transfer, the observations between this group and our own align more closely. Vanderstichele et al. [32] observed significantly lower concentrations of Aβ42 (11.0%), Aβ40 (7.3%), and Aβ38 (2.7%) in CSF collected into PP tubes. In addition, they report a concentration decrease of Aβ42 (42.5%), Aβ40 (27.8%), and Aβ38 (16.7%) after one transfer between PP tubes (Sarstedt; cat 62.554.502 and either 72.706 or 72.730.006). In comparison, at the first transfer, our results showed a similar decrease of Aβ42 (39.0%) and Aβ40 (24.1%), but smaller decrease of Aβ38 (8.8%).
Willemse et al. [21] reported a 5% decrease (up to 10% in small volume samples) in Aβ42 and Aβ40 per transfer over four transfers between tubes and that Aβ42:Aβ40 therefore remained constant over transfer treatment. An effect size of 5%–10% is at odds with the Aβ42 (22.3%) and Aβ40 (17.5%) decrease per transfer that we observed over equivalent transfers. The tubes used by Willemse et al. (Sarstedt; cat. 72.694.007) are identical to the tubes we studied (Sarstedt; cat. 72.694.406), except that cat. 72.694.406 is certified DNA and RNase free. It is not clear why our data should be divergent, other factors are seemingly involved, and a degree of interlaboratory variation should be taken into consideration until these factors are identified.
Repeated aspirations and ejections from the same tip did not significantly alter Aβ concentration in either CSF or CM. Indeed even when the tip was not prewetted, no effect was seen, contrary to what others have described [21]. Therefore, this cannot account for the initial exaggerated decrease we observed at the first transfer step. Given this, we hypothesize the existence of a subpopulation of Aβ42 that is more readily adsorbed to PP and rapidly depleted from solution. This interpretation would fit the work of Vanderstichele et al. [32], but not Willemse et al. [21]. A similar, adsorption-attributed, initial effect on fluorescein-labeled bovine serum albumin (BSA), also found in the B27 fraction of the CM used in our study, has been reported [45]. It is possible that competition for surface binding sites by this and other proteins of the CM matrix might explain why the first transfer step effect was not observed in these samples, although we did not examine fluid protein content as a variable.
Although still not well understood, Aβ monomers, oligomers, and fibrils adopt a range of conformations in solution, and indeed current models highlight the importance of C-terminal sequence for multimeric stability and predict the presence of a laterally exposed hydrophobic “patch” unique to certain Aβ42 fibrils [46], [47]. These properties may contribute to differences observed in Aβ42 versus Aβ40 nucleation rate constants [48], [49] and the range of adsorption dynamics at different polymer surfaces [50], [51]. Our results, which are preliminary, highlight the risk that potentially disease-relevant peptide subpopulations may be differentially vulnerable to loss during preanalytical processes.
4.2. Aβ ratios are less vulnerable to preanalytical surface exposure
Despite disparities in the size of transfer and volume effects between studies on PP tubes, our data and those of others [21], [32] converge on the reduction of preanalytical surface exposure effect when a ratio of Aβ peptides is used. For Aβ42:Aβ40, the disparity between initial and treatment subsequent result was reduced by approximately 50% (volume) and 75% (transfer) as compared to Aβ42 alone (Table 1). It is difficult to give a hard estimate for the amount of concentration loss necessary to mislead interpretation of the ratio, and this will depend on how close the individual peptide values are to a chosen diagnostic threshold. However, it is reasonable to expect reduction in the range, or “gray zone”, of diagnostic uncertainty if preanalytically derived “noise” can be mitigated. This is also relevant to cell models in AD research, where Aβ measurement variability within and between cell lines presents a hindrance to experiment repeatability, which use of ratios may help reduce.
4.3. Limitations
This study was limited by the relatively low number of independent samples used.
4.4. Summary
Loss of Aβ42 following differential exposure to polypropylene surfaces was significantly greater than Aβ40 and Aβ38 in both human CSF and neuronal cell CM. In addition, there was a tendency toward a pattern of greater loss of Aβ40 relative to Aβ38. Despite differences between peptides, Aβ ratios were less strongly affected by storage volume and tube transfer treatments than peptides considered individually. We conclude that the Aβ42:Aβ40 and Aβ42:Aβ38 ratios may predispose toward a risk of a false-negative diagnostic result for AD if samples are not treated in a standardized manner, though risk of misinterpretation may be less attendant than to Aβ42 alone. Reporting Aβ ratios in concert with individual peptides may reduce misinterpretation of Aβ assay results.
Research in Context.
-
1.
Systematic review: Amyloid β 42 (Aβ42):Aβ40 ratio has attracted interest as a biomarker for Alzheimer's disease (AD) with a number of recent clinical studies demonstrating high sensitivity and specificity. Work on identifying preanalytical factors relevant to the ratio is ongoing. The authors have comprehensively cited this literature.
-
2.
Interpretation: Our findings add to growing evidence supporting Aβ42:Aβ40 and Aβ42:Aβ38 ratios as reliable biomarkers for AD and expand the experimental perspective beyond cerebrospinal fluid (CSF) to cell media. Significantly we highlight that longer peptides adsorb to polypropylene surfaces, in a context relevant to clinical diagnostics, to a greater extent than shorter peptides.
-
3.
Future directions: The manuscript forms a platform for further characterization of peptide-surface interaction for a wider range of Aβ fragments, which may have disease relevance or biomarker utility, and also for identification of factors affecting Aβ measurement from in vitro model fluids.
Acknowledgments
The authors gratefully acknowledge the support of the Leonard Wolfson Experimental Neurology Centre, the NIHR Queen Square Dementia BRU. The Dementia Research Centre is an Alzheimer's Research UK Coordinating Centre.
The authors have declared that no conflict of interest exists.
References
- 1.Blennow K., Zetterberg H., Fagan A.M. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: The IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Perl D.P. Neuropathology of Alzheimer's disease. Mount Sinai J Med. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandelkow E.M., Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillart degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoonenboom N.S.M., Mulder C., Vanderstichele H., Van Elk E.-J., Kok A., Van Kamp G.J. Effects of processing and storage conditions on amyloid beta (1-42) and tau concentrations in cerebrospinal fluid: Implications for use in clinical practice. Clin Chem. 2005;51:189–195. doi: 10.1373/clinchem.2004.039735. [DOI] [PubMed] [Google Scholar]
- 6.Toombs J., Paterson R.W., Lunn M.P., Nicholas J.M., Fox N.C., Chapman M.D. Identification of an important potential confound in CSF AD studies: Aliquot volume. Clin Chem Lab Med. 2013;51:2311–2317. doi: 10.1515/cclm-2013-0293. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z.-X.X., Tan L., Liu J., Yu J.-T.T. The essential role of soluble Aβ oligomers in Alzheimer's disease. Mol Neurobiol. 2015:1905–1924. doi: 10.1007/s12035-015-9143-0. [DOI] [PubMed] [Google Scholar]
- 8.Lucey B.P., Gonzales C., Das U., Li J., Siemers E.R., Slemmon J.R. An integrated multi-study analysis of intra-subject variability in cerebrospinal fluid amyloid-β concentrations collected by lumbar puncture and indwelling lumbar catheter. Alzheimers Res Ther. 2015;7:53. doi: 10.1186/s13195-015-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicognola C., Chiasserini D., Parnetti L. Preanalytical confounding factors in the analysis of cerebrospinal fluid biomarkers for Alzheimer's disease: The issue of diurnal variation. Front Neurol. 2015;6:143. doi: 10.3389/fneur.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser E., Schönknecht P., Thomann P.A., Hunt A., Schröder J. Influence of delayed CSF storage on concentrations of phospho-tau protein (181), total tau protein and beta-amyloid (1-42) Neurosci Lett. 2007;417:193–195. doi: 10.1016/j.neulet.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Le Bastard N., Aerts L., Sleegers K., Martin J.J., Van Broeckhoven C., De Deyn P.P. Longitudinal stability of cerebrospinal fluid biomarker levels: Fulfilled requirement for pharmacodynamic markers in Alzheimer's disease. J Alzheimer's Dis. 2013;33:807–822. doi: 10.3233/JAD-2012-110029. [DOI] [PubMed] [Google Scholar]
- 12.Sancesario G.M.G., Esposito Z., Nuccetelli M., Bernardini S., Sorge R., Martorana A. Aβ1-42 Detection in CSF of Alzheimer's disease is influenced by temperature: Indication of reversible Aβ1-42 aggregation? Exp Neurol. 2010;223:371–376. doi: 10.1016/j.expneurol.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Murphy B.M., Swarts S., Mueller B.M., van der Geer P., Manning M.C., Fitchmun M.I. Protein instability following transport or storage on dry ice. Nat Methods. 2013;10:278–279. doi: 10.1038/nmeth.2409. [DOI] [PubMed] [Google Scholar]
- 14.Slemmon J.R., Meredith J., Guss V., Andreasson U., Andreasen N., Zetterberg H. Measurement of Abeta1-42 in cerebrospinal fluid is influenced by matrix effects. J Neurochem. 2012;120:325–333. doi: 10.1111/j.1471-4159.2011.07553.x. [DOI] [PubMed] [Google Scholar]
- 15.Slemmon J.R., Shapiro A., Mercken M., Streffer J., Romano G., Andreasen N. Impact of cerebrospinal fluid matrix on the detection of Alzheimer's disease with Abeta42 and influence of disease on the total-Abeta42/Abeta40 ratio. J Neurochem. 2015;135:1049–1058. doi: 10.1111/jnc.13297. [DOI] [PubMed] [Google Scholar]
- 16.Toombs J., Paterson R.W., Schott J.M., Zetterberg H. Amyloid-beta 42 adsorption following serial tube transfer. Alzheimers Res Ther. 2014;6:5. doi: 10.1186/alzrt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perret-Liaudet A., Pelpel M., Tholance Y., Dumont B., Vanderstichele H., Zorzi W. Risk of Alzheimer's disease biological misdiagnosis linked to cerebrospinal collection tubes. J Alzheimer's Dis. 2012;31:13–20. doi: 10.3233/JAD-2012-120361. [DOI] [PubMed] [Google Scholar]
- 18.Lewczuk P., Lelental N., Spitzer P., Maler J.M., Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer's disease: Validation of two novel assays. J Alzheimer's Dis. 2014;43:183–191. doi: 10.3233/JAD-140771. [DOI] [PubMed] [Google Scholar]
- 19.Murray A.N., Palhano F.L., Bieschke J., Kelly J.W. Surface adsorption considerations when working with amyloid fibrils in multiwell plates and Eppendorf tubes. Protein Sci. 2013;22:1531–1541. doi: 10.1002/pro.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewczuk P., Beck G., Esselmann H., Bruckmoser R., Zimmermann R., Fiszer M. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid B peptides. Clin Chem. 2006;52:331–334. doi: 10.1373/clinchem.2005.058776. [DOI] [PubMed] [Google Scholar]
- 21.Willemse E., van Uffelen K., Brix B., Engelborghs S., Vanderstichele H., Teunissen C. How to handle adsorption of cerebrospinal fluid amyloid-beta (1-42) in laboratory practice? Identifying problematic handlings and resolving the issue by use of the Aβ42/Aβ40 ratio. Alzheimer's Dement. 2017;13:885–892. doi: 10.1016/j.jalz.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Toombs J., Foiani M.S., Paterson R.W., Heslegrave A., Wray S., Schott J.M. Effect of spinal manometers on cerebrospinal fluid amyloid-β concentration. J Alzheimer's Dis. 2017;56:885–891. doi: 10.3233/JAD-161126. [DOI] [PubMed] [Google Scholar]
- 23.Reijn T.S.M., Rikkert M.O., Van Geel W.J.A., De Jong D., Verbeek M.M. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid beta42 and tau proteins. Clin Chem. 2007;53:859–865. doi: 10.1373/clinchem.2006.081679. [DOI] [PubMed] [Google Scholar]
- 24.Fagan A.M., Shaw L.M., Xiong C., Vanderstichele H., Mintun M.A., Trojanowski J.Q. Comparison of analytical platforms for cerebrospinal fluid measures of beta-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis T.A., Li J., Leblond D., Waring J.F. The relationship between different assays for detection and quantification of amyloid beta 42 in human cerebrospinal fluid. Int J Alzheimers Dis. 2012;2012:984746. doi: 10.1155/2012/984746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vos S.J.B., Visser P.J., Verhey F., Aalten P., Knol D., Ramakers I. Variability of CSF Alzheimer's disease biomarkers: Implications for clinical practice. PLoS One. 2014;9:e100784. doi: 10.1371/journal.pone.0100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattsson N., Andreasson U., Persson S., Carrillo M.C., Collins S., Chalbot S. CSF biomarker variability in the Alzheimer's Association Quality Control Program. Alzheimer's Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjerke M., Portelius E., Minthon L., Wallin A., Anckarsäter H., Anckarsäter R. Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis. 2010;2010:1–12. doi: 10.4061/2010/986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.del Campo M., Mollenhauer B., Bertolotto A., Engelborghs S., Hampel H., Simonsen A.H. Recommendations to standardize preanalytical confounding factors in Alzheimer's and Parkinson's disease cerebrospinal fluid biomarkers: an update. Biomark Med. 2012;6:419–430. doi: 10.2217/bmm.12.46. [DOI] [PubMed] [Google Scholar]
- 30.Le Bastard N., De Deyn P.P., Engelborghs S. Importance and impact of preanalytical variables on Alzheimer disease biomarker concentrations in cerebrospinal fluid. Clin Chem. 2015;61:734–743. doi: 10.1373/clinchem.2014.236679. [DOI] [PubMed] [Google Scholar]
- 31.Vanderstichele H., Bibl M., Engelborghs S., Le Bastard N., Lewczuk P., Molinuevo J.L. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: A consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimer's Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Vanderstichele H.M.J., Janelidze S., Demeyer L., Coart E., Stoops E., Herbst V. Optimized standard operating procedures for the analysis of cerebrospinal fluid Ab42 and the ratios of Ab isoforms using low protein binding tubes. J Alzheimer's Dis. 2016;53:1121–1132. doi: 10.3233/JAD-160286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiltfang J., Esselmann H., Bibl M., Hüll M., Hampel H., Kessler H. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low- and high-CSF Aβ40 load. J Neurochem. 2007;101:1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]
- 34.Mo J.-A., Lim J.-H., Sul A.-R., Lee M., Youn Y.C., Kim H.-J. Cerebrospinal fluid β-Amyloid1–42 levels in the differential diagnosis of Alzheimer's disease—systematic review and meta-analysis. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janelidze S., Zetterberg H., Mattsson N., Palmqvist S., Vanderstichele H., Lindberg O. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3:154–165. doi: 10.1002/acn3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorey A., Perret-Liaudet A., Tholance Y., Fourier A., Quadrio I. Cerebrospinal fluid Aβ40 improves the interpretation of Aβ42 concentration for diagnosing Alzheimer's disease. Front Neurol. 2015;6:247. doi: 10.3389/fneur.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schieb H., Kratzin H., Jahn O., Möbius W., Rabe S., Staufenbiel M. Beta-amyloid peptide variants in brains and cerebrospinal fluid from amyloid precursor protein (APP) transgenic mice: comparison with human Alzheimer amyloid. J Biol Chem. 2011;286:33747–33758. doi: 10.1074/jbc.M111.246561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansson O., Zetterberg H., Buchhave P., Andreasson U., Londos E., Minthon L. Prediction of Alzheimer's disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 39.Fiandaca M.S., Mapstone M.E., Cheema A.K., Federoff H.J. The critical need for defining preclinical biomarkers in Alzheimer's disease. Alzheimer's Dement. 2014;10:S196–212. doi: 10.1016/j.jalz.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Moore S., Evans L.D.B., Andersson T., Portelius E., Smith J., Dias T.B. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Rep. 2015;11:689–696. doi: 10.1016/j.celrep.2015.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y., Kirwan P., Smith J., Robinson H.P.C., Livesey F.J. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–486. doi: 10.1038/nn.3041. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sposito T., Preza E., Mahoney C.J., Setó-Salvia N., Ryan N.S., Morris H.R. Developmental regulation of tau splicing is disrupted in stem cell-derived neurons from frontotemporal dementia patients with the 10 + 16 splice-site mutation in MAPT. Hum Mol Genet. 2015;24:5260–5269. doi: 10.1093/hmg/ddv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludtmann M.H.R., Arber C., Bartolome F., De Vicente M., Preza E., Carro E. Mutations in valosin-containing protein (VCP) decrease ADP/ATP translocation across the mitochondrial membrane and impair energy metabolism in human neurons. J Biol Chem. 2017;292:8907–8917. doi: 10.1074/jbc.M116.762898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.1994. ISO 5725-2:1994(en), Accuracy (trueness and precision) of measurement methods and results — Part 2: Basic method for the determination of repeatability and reproducibility of a standard measurement method [Internet]https://www.iso.org/obp/ui/#iso:std:11834:en Available at: [Google Scholar]
- 45.Natascha W., Wente W., Müller P. Eppendorf AG; Hamberg, Germany: 2010. Application Note 180 – Eppendorf LoBind®: Evaluation of protein recovery in Eppendorf Protein LoBind Tubes and Plates [Internet] pp. 1–5.https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=0ahUKEwjClOWPrIDaAhVqCMAKHR95CJ8QFgg1MAE&url=https%3A%2F%2Fonline-shop.eppendorf.us%2FUS-en%2Feshopdownload%2Fdownloadbykey%2F118990_Application-Note_186&usg=AOvVaw2PpGq2UXAzCc1nrwJrZfbm Available at: [Google Scholar]
- 46.Colvin M.T., Silvers R., Ni Q.Z., Can T.V., Sergeyev I., Rosay M. Atomic resolution structure of monomorphic Ab42 amyloid fibrils. J Am Chem Soc. 2016;138:9663–9674. doi: 10.1021/jacs.6b05129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wälti M.A., Ravotti F., Arai H., Glabe C.G., Wall J.S., Böckmann A. Atomic-resolution structure of a disease-relevant Aβ(1-42) amyloid fibril. Proc Natl Acad Sci U S A. 2016;113:E4976–E4984. doi: 10.1073/pnas.1600749113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meisl G., Yang X., Hellstrand E., Frohm B., Kirkegaard J.B., Cohen S.I.A. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc Natl Acad Sci U S A. 2014;111:9384–9389. doi: 10.1073/pnas.1401564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esbjörner E.K., Chan F., Rees E., Erdelyi M., Luheshi L.M., Bertoncini C.W. Direct observations of amyloid beta self-assembly in live cells provide insights into differences in the kinetics of Abeta(1-40) and Abeta(1-42) aggregation. Chem Biol. 2014;21:732–742. doi: 10.1016/j.chembiol.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moores B., Drolle E., Attwood S.J., Simons J., Leonenko Z. Effect of surfaces on amyloid fibril formation. PLoS One. 2011;6:e25954. doi: 10.1371/journal.pone.0025954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocha S., Krastev R., Thünemann A., Pereira M., Möhwald H., Brezesinski G. Adsorption of amyloid beta-peptide at polymer surfaces: a neutron reflectivity study. Chemphyschem. 2005;6:2527–2534. doi: 10.1002/cphc.200500158. [DOI] [PubMed] [Google Scholar]