Abstract
Neuroimaging is advancing a new definition of Alzheimer's disease (AD). Using imaging biomarkers, clinicians may begin to diagnose the disease by identifying pathology and neurodegeneration in either cognitively impaired or unimpaired adults. This “biomarker-based” diagnosis may allow clinicians novel opportunities to use interventions that either delay the onset or slow the progression of cognitive decline, but it will also bring novel challenges. How will changing the definition of AD from a clinical to a biomarker construct change the experience of living with the disease? Knowledge of AD biomarker status can affect how individuals feel about themselves (internalized stigma) and how others judge them (public stigma). Following a review of AD stigma, we appraise how advances in diagnosis may enable or interrupt its transfer from clinical to preclinical stages and then explore conceptual and pragmatic challenges to addressing stigma in routine care.
Keywords: Stigma, Alzheimer's disease, Early diagnosis
1. Introduction
In medicine, stigma describes how, after a person is labeled with a disease that has negative social connotations, their social status and sense of self may be tainted and devalued [1], [2], [3]. In persons with Alzheimer's disease (AD), stigma can affect how they perceive themselves, such as feeling they have little worth or are incompetent and how others treat them, such as acting in ways that discriminate, patronize, or isolate [4], [5], [6], [7]. This stigma can lead to problems such as economic hardships, loneliness, and depression [8]. To date, the experience of stigma has been grounded in a disease label that is based on diagnosis of disabling cognitive and behavioral impairments, that is, dementia caused by AD. Advances in neuroimaging and other biomarker assays are changing our understanding of AD from a disease defined clinically to one defined biologically, and, in this article, we argue that a biological definition will change the experience of stigma.
A biomarker-based definition—using, for example, structural magnetic resonance imaging that measures neurodegeneration and positron emission tomography studies that measure brain metabolism or the presence of pathologic markers—is a departure from the historic, and still quite common, understanding of AD as a clinicopathologic state, defined by observable signs and symptoms. This definition is often referred to as “dementia due to Alzheimer's disease” or “Alzheimer's disease dementia.”
Researchers are using a biomarker-based definition to test in biomarker positive persons interventions to prevent or slow cognitive and functional declines [9]. Should these trials succeed, clinicians will use biomarker tests and these interventions to diagnose and treat patients before the onset of clinical signs and symptoms. This “preclinical” diagnosis is a novel opportunity to slow cognitive decline, but, it will also bring challenges. The stigma experience of the clinical stages of the disease may spillover to individuals diagnosed in preclinical stages.
A biomarker-based definition of the disease will also change the experience of living with the diagnosis. Persons who have dementia that fits the clinical criteria of AD but a biomarker result that excludes AD will have doubt cast on their personal narratives of a symptom experience and their families' caregiving narratives. Persons in the “preclinical stages” and their families will have to interpret what symptoms mark the transition from asymptomatic to symptomatic AD. In short, biomarkers will change what it means for a person to live with AD.
We report a narrative review of what is known about AD stigma and its effects on persons living with dementia and their caregivers. This literature was derived from a search of PubMed and Google Scholar with the key terms of “stigma” and “Alzheimer's disease.” We then discuss how advances in diagnosis and their translation into clinical practice may change stigma and thereby the experience of the disease. We offer research recommendations and considerations for practice and public policy.
2. Alzheimer's disease stigma defined, its context and its effects
Stigma is a complex social experience, referring to the reaction of others when a person was thought to deviate from “normal” [2]. Stigma is often described as a process in which a label, such as a diagnosis, links the person to discrediting characteristics associated with that label [10]. This process has three features [10], [11]. First, there is an authority who has the power to apply a label to others. In medicine, this is typically a physician or other clinician who makes a diagnosis. Second, the label must relate to negative or deviant qualities, such as a disease marked by physiologic pathology, functional impairments, and symptoms outside of normal functioning. Third, the person receiving the label must have less social power than the individual assigning the label, which is typically the case in the patient-clinician relationship.
When a clinician diagnoses a person with AD, the person is transformed into a patient with the disease. The person is a member of a patient group associated with behaviors, abilities, and experiences related both formally and informally to the diagnosis [10]. The diagnostic label implies what signs and symptoms the person may have and may be expected to develop [2]. It is also linked to predictions about the patient's future, such as prognosis and life expectancy.
The connection between observable characteristics of a disease and its diagnosis reflects the medical convention of using a symptom-based system of classification to define and diagnose disease. This approach can contribute to reifying stereotypes and biases about the disease. It can lend to presumptions that, even in the absence of a diagnosis, individuals fit stereotypes of the disease because they are seen to have symptoms that are strongly associated with the disease.
Stereotypes about AD center on it being a chronic and debilitating neurodegenerative disease. The diagnosis is strongly associated with the loss of capacity, suffering, disability, economic losses, and other undesirable features [8]. These associations inform widely held ideas about the characteristics of a person who has AD. These ideas lead to stereotypes that focus on the later stages of disease when a person is most impaired and fully dependent for care [12], [13], [14], [15]. These ideas lead people to act in ways that undermine a person's competency, identity, sense of normality, self-control, and social capital. This may include pressuring an individual to retire prematurely or habitually interrupting to finish the individual's sentences. This can have deleterious effects on how persons living with dementia and caregivers feel about themselves and what they choose to do or not do [6].
2.1. Types of stigma
Many models have been put forward to conceptually describe the AD stigma experience [3]. Three models that describe the patient, caregiver, and public experience of AD stigma are public stigma, self-stigma, and spillover stigma.
Public stigma, also referred to as “enacted” stigma, describes how the general population may carry negative or pejorative beliefs that cause them to act in discriminatory, exclusionary, or patronizing ways toward persons who either have or are closely associated with persons with AD [7], [16].
Self-stigma, also referred to as “felt” or “internalized” stigma, describes when a person cognitively or emotionally absorbs negative beliefs, attitudes, assumptions, and stereotypes related to the disease, such as feeling ashamed and inferior because of being closely linked to the disease [4]. Self-stigma is associated with depression, avoidant coping, social avoidance, low self-esteem, hopelessness, relatively worse psychiatric symptoms, and decreased help-seeking behaviors [5].
Spillover stigma, also referred to as “stigma by association,” describes how people who do not have AD are nonetheless affected by the stigma related to the disease [5]. Spillover stigma often affects individuals who share close social proximity to those who have the disease, such as family members and caregivers [17], [18]. It can also affect individuals who have a different but similar condition, such as mild cognitive impairment (MCI) [19].
2.2. Cultural context
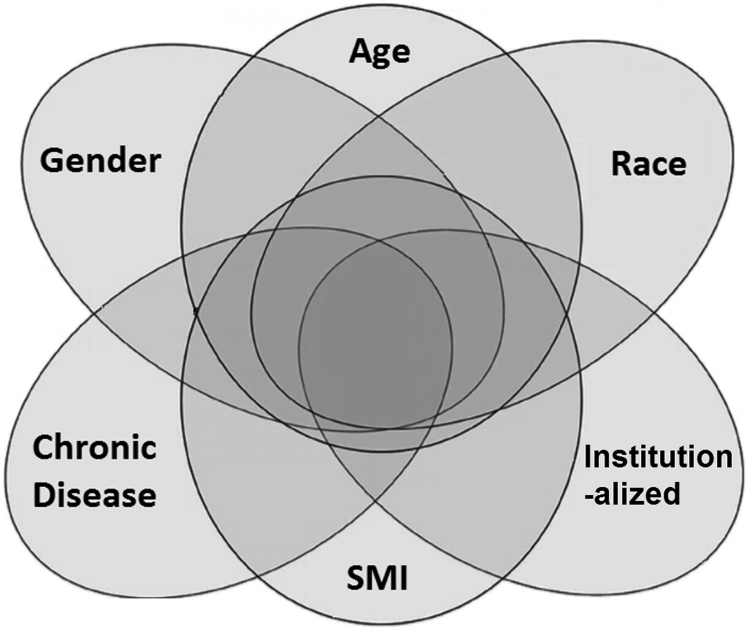
Stigma associated with the clinical form of the disease—that is, AD dementia—has been well documented across many cultures, but its presentation varies by cultural context [20], [21]. In this section, we describe how disease characteristics together with culturally influenced aspects of social identity—such as age, race, and gender—can interact (Fig. 1). When they do, they can make AD stigma more frequent and intense.
Fig. 1.
Aspects of personal identity and disease beliefs intersect to alter stigma of Alzheimer's disease dementia. Abbreviation: SMI, serious mental illness.
2.2.1. Characteristics of the disease
Cultural beliefs about illness and disease affect the presentation and consequences of AD stigma. In general, physical illness tends to garner public pity and support as it is viewed, with some exceptions, as being outside the individual's control [22]. Debilitating physical illness can also lend to paternalistic worries about the person's welfare. In contrast, mental illnesses are known to intensify negative judgments and social distance [2], [23], [24]. Thus, AD stigma has the unique characterization of including dimensions that are both “positive” (compassion and paternalism) and “negative” (inflated doubts about competence) [25].
Although AD is not officially categorized as a mental illness [25], it shares many psychiatric symptoms in common with serious mental illnesses—apathy, agitation, depression, and delusions. Research in the United States (U.S.) has shown that the public can judge a person's symptoms to be more severe when they believe AD dementia is a mental illness [7]. This may be bound to culturally ascribed ideas of controllability introduced previously, whereby negative reactions toward mental illness relate to misconceptions that symptoms manifest due to laziness or unwillingness of the person to control their condition [22].
2.2.2. Effects of personal identity
Features of the disease are not the only contributors to the stigma experienced by persons living with AD dementia. Their age, gender, and race can intensify the stigma experience. Individuals living with dementia must contend with both the stereotypes about the disease and stereotypes and beliefs about the competency and abilities of older adults [8], [26], [27], [28], [29], [30].
Women encounter disproportionate social burdens associated with aging [31] and caregiving [24], [32], [33]. These burdens can intensify the stigma experience of AD. For women living with dementia, stereotypes related to the loss of femininity and sexual power that are assumed to commence with age can increase the negative judgments they encounter as a result of the disease. Moreover, the presumed role as the de facto caregiver can result in women being subsumed into this role by default upon diagnosis of a spouse. Alternatively, upon their own diagnosis, they can be vulnerable to lack of support and encounter added stressors related to demands of caring for others in addition to tending to their needs.
The relationships between race and AD stigma are complex. In a small U.S. sample, African-Americans appeared less likely than whites to endorse negative attributions related to AD [34]. This finding raises the question of whether this might be due to the widespread belief in African-American communities that AD is not a pathology but rather a natural part of aging [35]. This misbelief may be a form of “silent stigma” [36], whereby it serves to protect individuals from the stigma associated with the disease. In addition, how broader issues of racism and disparity intersect with AD stigma remain largely unknown. However, it is critical to understand this to address the underrepresentation of African-Americans in research [37], which may compound existing health disparities that disproportionately affect them [38]. The absence of African-Americans and other socially disadvantaged groups from research that moves the practices of AD diagnosis and treatment to genetic and biological markers risks excluding sections of the population from interventions targeted on narrow biological data.
These personal characteristics—older age, being female, and being African-American—have significant roles in informing an individual's personal and social identities. They are also risk factors for AD [39]. The concurrent role as a marker of identity and as an indicator of disease risk solidifies their connections to the disease. The processes by which these connections are established and maintained warrant study to discover ways of modifying and limiting stigma.
2.3. Experiences of AD stigma
The next sections review how stigma affects patients, caregivers, the political climate, and social systems. For each, we distinguish among stigma associated with AD in general, in dementia, and in the preclinical forms of the disease. However, much of the evidence is from studies of stigma associated with AD dementia.
2.3.1. Patient experience of AD stigma
Stigma of AD is common. Across 54 countries, most people surveyed (75%) believed individuals with dementia face stigma [21]. Although the expression and intensity differ between countries, the most common negative association is that of being discounted or marginalized by others (28%) [21]. In Australia, half of adults surveyed discounted the ability of a person with dementia to be able to have a meaningful conversation [40], while in Britain, most (81%) adults surveyed believed they would be looked upon or treated differently if others knew they were diagnosed with dementia [41]. In the U.S., over half surveyed expected a person with AD dementia to be discriminated against by employers (55%) and be excluded from medical decision-making (55%) [34].
AD stigma affects persons both before and after diagnosis. Before a diagnosis, stigma impedes the willingness to seek care, to educate oneself about the disease, and to participate in research [5], [18], [30], [42], [43], [44], [45], [46]. In addition, people may conceal symptoms to avoid being associated with the disease [5].
In early stages of disease, a patient is often aware of their diagnosis and the stigma associated with it [47], [48], [49], [50], [51], [52], [53], [54], [55]. As a result, they may feel shame, anxiety, and depression [47], [48], [49], [50], [51]. This may make individuals hesitant to disclose their diagnosis or impairments, which can impede their access to care and result in worse health outcomes [47]. The stress of managing the psychosocial consequences of the disease can lead to development and exacerbation of existing symptoms [56]. This is sometimes misconstrued by patients or their caregivers as evidence of decline, increasing the burden of morbidity, likelihood of social withdrawal, and risk of institutionalization [57], [58]. It can also lend to relationship conflict and loss, particularly with family members [59]. Stigma can create financial difficulties, like being fired or forced into early retirement or being unable to secure insurance to cover the cost of care [60], [61].
Persons living with dementia worry about conforming to stereotypes of the disease [62]. When faced with this threat, some react by exhibiting signs that confirm that stereotype [63]. This phenomenon, called stereotyped threat, has been found to affect individuals even before the onset of cognitive symptoms; persons who learned their genetic risk for developing AD have been found to show lower performance on memory tasks than others who were at risk but uninformed [64].
Research with persons living with dementia and the emerging findings from studies of unimpaired persons who learned their risk of developing AD dementia raise important questions. These findings suggest that advances in AD science may affect the psychosocial wellbeing and daily lives of persons diagnosed early. The emerging research raises questions about whether they experience similar negative experiences as those with clinical forms of disease and what types of psychological effects may follow from learning one's risk of AD. To answer this, we need to understand what psychological processes (forecasting, stereotyped threat, etc.) may be engaged in how people react after learning their biomarker status. We summarize this and other recommendations for future study in Table 1.
Table 1.
Summary of areas for research in preclinical Alzheimer's disease
Research
Practice
Policy
|
Much of what is currently known about the experiences of unimpaired persons who learn their AD risk comes from those who learned gene-based risk estimates. Although gene- and biomarker-based risk estimates may both denote an elevated risk for dementia, they do differ. Biomarker-based estimates are not as well quantified and they more readily change over time as compared to gene-based estimates. It is important therefore to understand the processes individuals do or do not use to cope with uncertainty after learning an elevated or positive biomarker. How a biomarker is quantified and how it may change over time could influence how people react to their result. Research is needed to understand the experiences of persons who learn their AD risk and to understand how learning varied types of risk data—gene or biomarker—might affect their experiences. This will inform interventions to limit the social and psychological consequences of learning an Alzheimer's biomarker result.
2.3.2. The family and caregiver experience of AD stigma
Through close association with a person who has AD dementia, caregivers and family members are often affected by stigma [17], [65]. A large amount of the burden from stigma arises from the need to manage the social consequences of the disease. Because of negative associations and fear, wider family members tend to distance themselves from the patient and caregivers [65]. This adds to the physical, emotional, and social costs of the copious time and energy caregivers dedicate to care [66]. In addition to reducing the caregiver's support network, caregivers often invest psychological and emotional resources into what is called “impression management,” a term that describes strategies to manipulate or control situations in ways that minimize or avoid negative social reactions [65]. Caregivers, for example, may make up socially appropriate excuses for why the person living with dementia may be unavailable for a social engagement or frequently answer on the person's behalf to assure an image of competency or lucidity when moments of confusion unexpectedly arise.
Demands on caregivers to manage stigma shift based upon the severity of the disease, frequency and intensity of care, and availability and willingness of others to help or coparticipate [67], [68]. All of which are largely currently defined and informed by the individual's signs and symptoms and the stigma that accompany them [14]. In essence, the severity of disease determines the demands of caregiving both in terms of managing care and the social ramifications of the disease [21].
Efforts to minimize the effects of stigma can harm the person with dementia. For example, to protect the individual's dignity, caregivers may keep the individual's problems and needs as private as possible. They may choose to become the solitary bearer of all caregiving responsibilities and even delay or not seek help from health care professionals. Each of these behaviors can worsen an individual's health [69].
Caregivers can experience poor health as a result of caring for a person with AD dementia. A collection of factors likely contributes to the poor outcomes experienced by caregivers but stigma can exacerbate these problems. For instance, it can intensify the strain, stress, or injury that can arise from the physical demands of addressing mobility problems. Or, the loss of employment opportunities because of a persistent need to manage issues related elopement, falls, and basic needs. It can also increase difficulties related to the psychic and social burden of the disease, independent of the severity of the disease. These may include employment discrimination or other forms of structural discrimination as well as loss of social relationships and experiences of harsh social judgments.
Aspects of the caregiver's identity, particularly gender and familial relationship, can modify the effects of stigma. The responsibility for caregiving tends to fall disproportionately on women, usually a wife or adult daughter [24], [32], [33]. The gender disparity among caregivers adds to the social burden already faced by women due to ageism and sexism [8], [28], [29], [30]. In addition, as caregivers, women report contending with more stigma and greater caregiving burdens compared to men [70]. The burden women caregivers encounter due to stigma can differ based upon their relationship to the person with dementia, where adult children report greater burdens than spouses [70].
The experience of being a caregiver for a person with AD dementia is unique from that of caring for individuals with other diseases [71], [72]. The finding that clinical symptoms shape the caregiver experience raises questions about how the caregiver experience may be affected by diagnosis before the onset of symptoms and how the anticipation of symptom onset and then the experience of emergent symptoms may affect this experience. We return to this topic later to discuss the ways the caregiver experience may shift because of advances in neuroimaging and other biomarker-based diagnostics.
2.3.3. Public stigma of AD
Public stigma typically describes how members of the general population act in discriminatory, exclusionary, or patronizing ways toward persons who either have or are closely associated with AD [7], [16]. In this section, we broaden this definition to understand public stigma as a wide-spread social problem that manifests in social systems and has consequences for those systems.
Social systems, such as a neighborhood or work place, manifest the stigma of AD in ways that range from indifference to rejection. Surveys of the general public repeatedly show that the public responds in ways that discount, stereotype, infantilize, marginalize, and reject persons with dementia [21], [40], [73]. Typically, messages in mass media are consistent with those findings. A common characterization depicts persons with AD as “zombies,” dehumanizing them as mindless and lifeless [6]. The media's typical treatment of AD relies on stereotypes that promote ageism, gerontophobia, and negative emotions [12], [15], [74], [75]. Individuals living with dementia are typically shown in the later stages of disease when they are incapable of making autonomous decisions, are a burden to their family members and caregivers, and are unable to speak for themselves [12], [13], [14], [75]. The common negative depictions are useful for some purposes—such as motivating financial donation in fundraising campaigns—but they also promote and affirm stigma by emphasizing negative aspects of the condition [15].
Societal attitudes that are dismissive and devaluing can stifle the public momentum that is needed to enact policies, garner funding, develop programming, or advance research. This can lead to imbalances and injustices in social structure, political decisions, and legal regulations [17], [69]. The effects are visible over a range of areas, such as in the reluctance to accept new therapies [76], scrutiny of medicare-reimbursed services [77], diminished work force, and the difficulty most countries face in prioritizing public policy to address AD from among other competing demands [78].
Stigma can also affect how services are commissioned, designed, and provided [79]. The disparaging attitudes and beliefs can spill over to negatively affect how health care providers care for persons with AD [30], [79], [80], [81]. They can affect how people linked to the disease are treated within social systems, such as being patronized, isolated, excluded, and discriminated against [82], [83], [84]. All of which, in turn, can further limit a person's opportunities for employment and to otherwise live productively in the society. In more deleterious forms, they can contribute to social expectations and acceptance that persons living with AD should be sequestered in institutions.
Our review of literature on public stigma underscores the importance of media portrayals of AD. Studies would be useful that can aid in informing efforts to improve public attitudes and beliefs about AD, including how to develop effective messages that promote the dignity of persons with the disease. In Section 4.0, we continue the discussion of the effects of public stigma in terms of disclosing risk data and gaps in existing public policies.
2.4. Interventions to reduce stigma of AD
Statewide and national programs have created strategies and opportunities to address and reduce stigma, for example, through national dementia strategies or legal frameworks like the U.S.'s National Alzheimer's Project Act [43], [85], [86]. These include public education and awareness about AD and effective and sensitive communication to limit passing on stigmatizing beliefs and negative stereotypes associated with the disease [21], [44], [85]. Efforts to reduce stigma face challenges and opportunities from advances in neuroimaging. Challenges include how these advances may expand the stigma of AD to affect more groups, particularly individuals who are unimpaired but at risk to develop dementia. Opportunities include that stigma may evolve or even become less potent.
3. The effects of advances in neuroimaging
A recent conceptual framework outlines how results from neuroimaging and other biomarker-based tests might be used to diagnose AD and how negative AD biomarker results may be used to rule out the disease as the cause of cognitive decline [87]. Biomarker tests will change what it means to live with the knowledge of “having” this disease, particularly the experience of stigma in those identified in preclinical stages of disease. They will also have important consequences for symptomatic individuals who learn a negative result. We focus only on the former group in the following discussion.
3.1. How advances in early diagnosis might cause a spillover of AD stigma from the clinical to the preclinical stage
Emerging evidence from people diagnosed with MCI and research volunteers in AD prevention trials—who learn their Alzheimer gene and biomarker results so they can participate—suggests that stigma currently associated only with the dementia disease stage may spill over to individuals with only mild or even no symptoms [88], [89], [90], [91], [92]. In other words, cognitively unimpaired persons identified in a “preclinical” stage of the disease based on biomarker results may experience stigma, such as social isolation, discrimination, and internalized distress.
Understanding how persons with MCI or other similar conditions contend with this may help anticipate the experience of living in the preclinical stage of the disease. Interviews with persons diagnosed with MCI found they expressed a strong desire to differentiate themselves from persons with dementia because of its negative associations [19]. However, they had difficulty doing this because of the challenge of distinguishing between diagnostic categories of MCI and AD. These findings suggest AD stigma can spill over to affect individuals with clinically distinct stages of the disease.
Being aware versus unaware of one's diagnosis affects quality of life in older adults with MCI and mild AD dementia [93]. Among patients who were either aware or unaware of their diagnosis but otherwise similar in cognitive functioning, awareness of diagnosis was associated with generally lower quality of life as measured by satisfaction, difficulty, basic functioning, and physical wellbeing. This finding raises the question of how learning one has “preclinical AD” may affect the psychosocial wellbeing of individuals.
Evidence from cognitively unimpaired persons shows there may be psychosocial consequences to learning a positive gene-based test result. Using a nested case-control design [64], 144 cognitively unimpaired older adults learned or did not learn their gene-based risk of developing AD dementia, then completed objective verbal and visual memory tests and self-report measures of memory function. The two groups did not differ in objective memory test performance, but those who knew they were at risk of AD performed worse on an objective verbal memory test and judged their memory more harshly than those who were similarly at risk but did not know this information. The finding raises the concern that, in addition to consequences that are already well known to affect individuals linked to AD dementia, stigma that spills over to affect individuals in the preclinical stage may raise unique psychosocial concerns. It could affect perceptions of cognitive functioning and could also inadvertently impact clinical evaluation.
3.1.1. Research recommendations
We need to understand how knowledge of one's biomarker status may change the experience of worsening symptoms and signs of cognitive and behavioral problems. This work should examine how longitudinal changes in neuroimaging and other biomarker-based tests affect how individuals perceive their abilities to function in daily life, how they evaluate their experience of symptoms, and how they may be judged by others. The timing of when a person learns his or her neuroimaging result may be a key factor for understanding the strategies individuals use to effectively cope with and accommodate this information. This knowledge will also help inform interventions to minimize the impact of stigma in preclinical stages of disease.
How learning one's biomarker-based risk of AD affects an individual's and their families' psychological and social functioning remains largely unknown. Questions in need of study include understanding the specific ways stigma of AD may spill over to affect persons in preclinical stages of disease such as the distancing and protective behaviors described in Section 2.3.2. This work should examine how these findings may vary across groups, such as those defined by age, gender, race, and other cultural contexts, and if these effects differ by the timing of biomarker disclosure and in what ways learning a biomarker result might be different than learning a gene-based result.
3.2. How advances in neuroimaging and other biomarker methods might reduce AD stigma
By changing the understanding of the disease, advances in neuroimaging and other biomarker methods of early diagnosis could help to reduce stigma associated with AD.
3.2.1. Shifting the definition of AD from clinical to biological
A biomarker diagnosis may reduce stigma. Persons with diseases believed to be biologically based are judged less harshly than those with diseases judged to be psychological or mental [7], [22]. In addition, the emerging biological definition may also give caregivers a better means to avoid and manage stigma by providing them use of a medicalized definition to account for disabilities and behavioral symptoms [47], [66].
A key factor that will influence how “biological evidence” affects stigma will be how this new knowledge is associated with disease risk factors. The notion that certain behaviors can modify the disease risk can create a sense of personal responsibility that, in turn, can increase stigma as a person who develops the disease can be judged as having not acted responsibly. In juxtaposition, the idea that nothing can be done to change one's risk may lead to complacency or, possibly, a sense of being a victim of circumstance. The development of recommendations for managing risk that are accurate, consistent, and help guide health promoting behaviors demands attention and study [94].
In addition to offering a means to discover novel therapies, advances in neuroimaging and other assays have the potential to improve diagnostic accuracy and patient management within the current standard of care [95]. They offer opportunities to improve both the course and prognosis of the disease. Consistent with prior research that has shown stigma is intensified by the expectation that a person's condition will worsen over time [16], improving expectations for treatment of persons diagnosed with the disease may help reduce stigma, including that related specifically to discrimination, pity, and social distance.
4. Considerations for practice
Successful translation of advances in AD diagnostics into routine clinical practice holds promise for reducing the stigma associated with AD. We examine pragmatic considerations of this undertaking related to the shifting role of the caregiver, public policy, and service design and delivery, as well as conceptual issues in measurement and evaluation.
4.1. Service design and delivery
Two challenges of a biomarker-based diagnosis of AD are the design and delivery of programs and services for persons who undergo these diagnostics. How the health care system addresses these challenges will affect patients' psychosocial wellbeing.
4.1.1. Service design
Advances in neuroimaging and other diagnostics for AD will require redesigning clinical services. Developing language to explain these services offers opportunities to reduce stigma. Because stigma can be conveyed and perpetuated in the language used to talk about ideas or experiences [27], it is critical to understand and intentionally shape the language that describes these technologies and their results [81]. Because these technologies will be linked to diagnosis, understanding how the language that describes them interacts with how patients make sense of their diagnosis will be important. Patients often understand an AD diagnosis through their expectations for normal aging and personal experiences with dementia [96]. A cognitively unimpaired individual who learns a diagnosis of “AD” may face greater challenges to their identity than someone who learns a diagnosis of “amyloidosis.” The effects of language require study to optimize terms that limit stigma while encouraging active engagement in health promoting activities.
Studies nested within Alzheimer's prevention trials are providing information about the impact of disclosing the results of gene and biomarker tests to people [91], [97], [98], [99], [100]. Of particular relevance is the “Study of Knowledge and Reactions to Amyloid Testing,” a companion study to the “Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4)” trial. This interview study follows persons who have learned the results of an amyloid positron emission tomography scan. The study's baseline interviews of those who learned they had an elevated level of brain amyloid showed they understood that the result conferred an increased but uncertain risk for later developing AD dementia [97]. Interviewees made clear that they wanted more information and education than had been offered about the result, particularly how a dimensional biomarker is turned into a categorical result (“elevated” or “not elevated”). These findings have direct implications for how advances in neuroimaging and other biomarker technologies are translated into routine clinical practice. They suggest the use of biomarker testing will require changes to the content and structure of a typical clinical encounter to meet demands for education, planning, and shared decision-making.
It will be crucial to study and understand how stigma affects efforts—services, programs, clinical practices—aimed at meeting the demands for education, planning, and shared decision-making that will accompany these advances in diagnosis. It could exacerbate existing gaps in patient-caregiver education, impede the ability to link individuals to appropriate resources, and serve as a barrier to engaging patients and other stakeholders, particularly in socioeconomically marginalized groups. [69], [101]. Moreover, stigma and fear of dementia pose risks as powerful motivating factors that could lead to the preemptive introduction of new diagnostic and therapeutic technologies. It will be crucial to study and attend to these issues as to help assure the translation of advanced diagnostics is not rushed before their value is established and their consequences are appropriately addressed.
4.1.2. Service delivery
As many as one-third of adults aged 65 years and older may have “elevated amyloid.” Routine use of biomarker testing in clinical practice could potentially overwhelm current systems, designed to manage relatively small groups of patients, with large numbers of cognitively unimpaired persons identified as at risk. These issues raise an important question about how to manage and “treat” individuals with preclinical forms of the disease as part of a continuum of care that includes those with symptomatic AD. These challenges could, if left unaddressed, exacerbate AD stigma.
One challenge is how to monitor individuals who are cognitively unimpaired but found to be at risk for developing AD dementia. Some may be prescribed therapies and require ongoing monitoring of these medications. Others may need monitoring that has assurances built in to identify emergent health needs and capabilities to link individuals with appropriate services while also, alternatively, assuring individuals are not entered prematurely into unnecessary treatment. The design of these care delivery systems for groups of unimpaired but at-risk individuals may pose unique challenges to clinical practice, which focuses on care for clinical symptoms [102]. These areas remain largely unstudied at present but appropriate procedures for this follow-up and monitoring need to be established. Central to this is the need to avoid translating a risk of dementia into a condition treated and regarded as symptomatic disease and the concomitant risk of overtreating worried adults. The success of these efforts will be essential for limiting the spillover of stigma from clinical to preclinical disease stages and for reducing stigma associated with preclinical stages.
The volume of care resultant from routine use of biomarker testing and its reliance on potentially expensive testing modalities could be resource intensive. The real or perceived competition for resources could intensify stigma, particularly in terms of feelings of exclusion or marginalization of those in the less served or underserved groups. It could also intensify stigma toward clinically impaired groups by establishing a connotation that they are beyond help or otherwise discountable.
Disclosing biomarker results to research volunteers involves patient-provider interactions like tailored education, helping the patient plan for the future, and shared decision-making [103], [104]. Clinical encounters that focus on education, planning, and shared decision-making will be formative for helping patients interpret brain imaging results and the associated risk for AD in ways that are accurate, affirming, and, as needed, corrective to stigmatizing expectations. The safe and effective disclosure of the results of this testing to a patient needs to address social relationships that affect an individual's health and wellbeing and acknowledge how this information may affect their personhood. These emphases on relationships and personhood are core to the clinician-patient relationship and effective clinical management. They are also what characterize dignity in clinical care [79]. Enhancing dignity, the antithesis of stigma, tends to lead to decreases in stereotyping and an increased value for the individual. However, large gaps remain in the knowledge and methods that are needed to effectively accomplish these tasks. Particularly, given the demands embedded in routine clinical practice, new methods may need to be developed to accommodate the resource requirements of delivering this care, such as mixed modality interventions that would distribute efforts across in-person and virtual interactions.
4.2. The caregiver role in preclinical AD
A diagnosis of dementia initiates caregiving attitudes and roles [105], but we do not know what attitudes and roles a family member experiences after a diagnosis of preclinical AD. Being the partner or child of an adult who is at present unimpaired but who later may begin to experience problems in memory and cognition will define a novel element to what it means to be a caregiver of a person diagnosed with AD.
Critical questions remain unanswered about how individuals undergoing testing and their families navigate the emergence of the caregiver role. Of particular importance is understanding what social burdens, including isolation, discrimination, and social rejection, may affect persons assuming this role and how these vary along the lines of age, race, and gender. Understanding this may help anticipate how advances in early diagnosis could add to existing disparities in caregiving and help to inform strategies for intervention. In addition, it will be important to understand how a caregiver becomes designated and the responsibility of a health care professional in guiding this process. Moreover, as part of understanding the “patient-caregiver” relationship, it will be necessary to learn how early diagnosis may affect the individual's autonomy, social opportunities, and willingness to plan for the possibility of future declines, particularly given that the person undergoing testing may be mildly impaired or unimpaired.
4.3. Assessing stigma
Patient-reported outcomes (PROs) are likely to be valuable in the care of persons with preclinical AD as patient-reported data are less likely to be biased by the disease process [52], [53], [54], [55]. PROs that can measure stigma and other psychosocial factors will improve our understanding of how knowledge of AD biomarkers affects individuals and in developing and testing interventions to reduce stigma.
Using PROs routinely may help improve delivery of clinical care, such as monitoring those who are at risk and helping guide efforts to improve patient endpoints. Strategic development and implementation of psychosocial PROs may be particularly useful for addressing the novel demands for education, planning, and shared decision-making that will be needed to deploy advances in neuroimaging and other methods of early diagnosis into routine clinical practice.
4.3.1. Existing stigma measures
To inform development of a measure that assesses the psychosocial effects of early diagnosis, we reviewed existing instruments to understand what might be needed. We focused only on measures that were developed specifically for AD or later adapted for this purpose because AD—unlike other conditions [106]—invokes both positive (compassion and warmth) and negative dimensions (inflated doubts about competence and paternalism) of stigma [25]. Although several studies have measured AD stigma [107], all have been for the purpose of understanding the phenomenon in the dementia stages of the disease. Our review focuses largely on the eight that have been evaluated for their psychometric properties or at least have been pilot tested for their appropriateness and validity (Table 2).
Table 2.
Summary of measures that have been validated or pilot tested in samples with Alzheimer's disease dementia
| Scale | Description | Content assessed | Reliability (Cronbach's α)∗ | Items assess clinical symptoms |
|---|---|---|---|---|
| Family Stigma in Alzheimer's Disease Scale (FS-ADS) [17] | 42 items, five-point Likert scales | Stereotypes; prejudice (emotional reactions); discrimination. Includes three scales: caregiver, lay person, and structural | 0.97 to 0.41 for 8 theoretical subscales† | True |
| The Social Impact Scale [114], [115] | 24 items, four-point Likert scale | Experience of social rejection; social and psychological feelings regarding experience of stigma | 0.87 Full scale; subscales 0.76 to 0.87 | True |
| The Stigma Scale for Chronic Illness (SSCI) Revised [113] | Eight items, five-point Likert scale; standardized t-scores | Six items about experience of treatment by others; two items about experience of embarrassment | 0.89 | False |
| Stigma Experience Scale [116], [125] | 19 items; yes or no responses | Nine items about experiences of stigma and 10 items about experiences with discrimination | 0.67 Full scale; subscales 0.70 and 0.50 | False |
| Weiner et al. scale [22] | 13 items, nine-point Likert scale | Beliefs about stability-controllability of disease; affective and behavioral consequences of disease; and stability of the disease (improvability) | DNR | True |
| Fernando et al. [41], [108] | Eight items, five-point Likert scale | Stigmatizing attitudes including those related to being unpredictable, to blame, hard to talk to | DNR | False |
| Kubiak et al. [110], [111], [112] | 11 items, six-point Likert scale | Medical Condition Regard Scale assessing clinicians' views of patients with a given medical condition as enjoyable, treatable, and worthy of medical resources | 0.86 | False |
| DNR, six-point Likert scale | Attributions of stability and pity toward clients with the four conditions. Items obtained from the Psychiatric Disability Attribution Questionnaire (PDAQ) | DNR | True | |
| Low et al. 2010 [109] | Seven items, agreed (coded 1) or disagreed (coded 2) | Attitudes toward people with dementia were assessed by asking them whether they agreed or disagreed with a series of statements | DNR | True |
Abbreviation: DNR, data not reported.
In samples with Alzheimer's disease.
Modified version of the FS-ADS was used to derive seven empirical scales: Structural discrimination; negative severity attributions; negative esthetic attributions; antipathy; supportiveness; pity; and social distance [16].
4.3.2. What domains to assess
A paramount challenge to addressing AD stigma and its spillover to novel stages being defined by biomarkers is determining which dimensions to measure. Existing metrics capture multiple dimensions such as attitudes toward responsibility [41], [108], [109], beliefs about the mutability of the condition [22], [110], [111], [112], [113], and emotional reactions [17], [113], [114], [115]. They also tend to ask directly about experiences of “stigma,” which has been shown to result in poor psychometric properties [115], [116].
Measures informed by existing literature and interviews with patients elicited aspects of stigma connected to physical, psychological, and social wellbeing. The Family Stigma in AD Scale, which was informed by existing literature on family stigma of mental illness [17], assesses cognitive attributions (stereotypes), emotional reactions (prejudice), and behavioral consequences (discrimination). These are domains known to be relevant to understanding stigma of AD as understood via labeling theory [17]. Similarly, the Social Impact Scale was developed for persons with HIV/AIDS and cancer and then later adapted and tested with patients with AD dementia [114], [115]. This scale assesses two domains: experiences of social rejection and social psychological feelings regarding stigma. It also allows for estimation of four subscales: social rejection, financial insecurity, internalized shame, and social isolation.
Instruments developed to assess stigma across a range of diseases, of which AD dementia was one, measure factors common to the experience of having chronic conditions. Weiner et al. developed a scale to compare stigma across a range of diagnoses [22]. The instrument assesses three domains: belief that the cause of the disease is stable and controllable (responsibility, blame, changeability); affective and behavioral reactions (liking, pity, anger, charitable donations, and personal assistance); and beliefs about the stability of the condition (e.g., likelihood of improvement with medical treatment, psychotherapy, etc.). The Stigma Scale for Chronic Illness was originally developed to assess internalized and experienced stigma across chronic illnesses [113], [117]. These measures can be helpful for understanding how stigma can differ across conditions, but they tend to omit domains that may be critical for understanding the psychosocial effects specific to AD.
There is no one measure that assesses all domains that may be relevant to understanding the consequences of AD stigma. Key domains commonly recognized in clinical practice include family and other relationship loss, social isolation, mental wellbeing, and personal loss of social capital and domains of quality of life including the experience of subjective cognitive problems, activities of daily living, physical functioning and wellbeing, mood and psychological wellbeing, and perceptions of functioning and satisfaction in daily life.
In addition to these domains, there is a need for measures that capture aspects that may be unique to emerging disease stages where individuals may be clinically unimpaired, such as beliefs about and expectations for the future and changes to social capital such as planning for the future related to occupation, finances, and independent living. The capacity to understand both the short-term and longer term social effects on individuals who learn biomarker results will also be essential. Thus, it will be of importance to develop a measure that is sensitive and specific enough to capture these effects.
Because existing measures were developed to assess stigma in clinical forms of the disease, they often rely at least in part on assessing respondents' experiences or beliefs about symptoms. As AD diagnosis shifts from a symptom-based understanding of the disease to a biologically defined stage, measures focused on outward-presenting characteristics related to symptoms, esthetics, and functioning may not adequately capture experiences of unimpaired or mildly impaired persons who learn they are at risk for developing AD dementia. In fact, a heavy emphasis could appear tone-deaf and reify stereotypes in asymptomatic populations. Measures to assess and understand stigma and other psychosocial experiences of the disease may need to shift away from reliance on symptoms. Instead, queries framed around behaviors related to concealing and sharing information may be more useful. When symptoms are queried, the framing may need to be conceptualized along a continuum to capture experiences of a disease that occur as a progression rather than a static state.
4.3.3. Who to assess
Most existing measures were designed to assess stigma in the general public or among those diagnosed with the disease. One notable exception is the Family Stigma in AD Scale, whose creators recognized the import of assessing stigma of AD across multiple groups. The scale has versions tailored to patients, caregivers, and a general public [17]. As biomarker-based diagnosis broadens the range of disease stages, there will be a need for measures that can capture effects on family members, such as genetic heritability that could differ by familial and biological relationship and for individuals impacted by novel relationships, such as the early designation of “care partner” for an unimpaired adult.
4.3.4. The scale score
All but one existing measure of AD stigma offers a grand score that is either a mean or sum of item responses. Both calculations are useful as descriptors and comparators. However, without the ability to transform them to standardized scores, such as theta or T-scores, they lack the benefits of Item Response Theory scoring, such as added precision and standardized interpretation. A measure that permits easy and routine calculation of T-scores, such as the Stigma Scale for Chronic Illness-8 [113], would allow for ease in interpreting clinical significance. It would be useful to develop a robust measure of AD stigma that has the benefits of these psychometric properties. Moreover, a scale score that was developed and tested to have predictive and prognostic utility could help guide clinical decision-making. This, in turn, would help align interventions to individuals who could most benefit from them.
4.3.5. Cultural validity
Although biomarker technologies are bringing the understanding of AD closer to its biological and molecular pathways, clinical and psychosocial outcomes remain heavily influenced by sociocultural factors. As AD stigma and its effects can differ based on cultural factors, including race, ethnicity, and economic status [118], it is crucial that new instruments attend to these factors. This will avoid limitations related to inappropriate wording, unsuitable response sets, and oversight of key cultural nuances. In addition to improved psychometric properties, instruments that attend to cultural variation can generate results that offer information that better informs culturally relevant programs, policies, and interventions [86].
5. Public policy
Stigma has the potential to undermine the uptake of advances in neuroimaging and other biomarker-based methods of AD diagnosis and to compromise the wellbeing of people who undergo this testing. Public policy changes may be warranted to protect individuals' social rights and privileges, assure access to support, and guarantee the fair allocation of services. This will help ensure the successful introduction of biomarker-based diagnostic tools and models of care.
5.1. Protection of social rights
Social, employment, and economic policies need to consider the potential harms caused by the stigmatization of people with preclinical AD, notably the potential for discrimination in employment, insurance, and driving. In the case of genetic testing, national regulations and international agreements protect the rights of individuals to genetic privacy and prohibit discrimination [119], including the U.S. Genetic Information Nondiscrimination Act of 2008 [120]. However, as in the case of Genetic Information Nondiscrimination Act of 2008, such legislation may not extend to biomarker testing, such as brain imaging results [121] nor does it address protections for long-term care insurance, which is often a key factor for persons undergoing AD gene and biomarker testing in the U.S. [122], [123].
The absence of social protections regarding the access and use of an individual's data about dementia risk poses potential harms to individuals. In the U.S., 46.6% of adults surveyed worried a person would have his health insurance limited because of having documentation about AD in the medical record, and about 45.6% expressed strong concerns that results from a brain imaging test could result in capitations in health insurance, which was similar to estimates for genetic testing (44.7%, P > .05) [34]. These estimates are in addition to the more than half of respondents that expected a person with AD dementia to be discriminated against by employers (55%) and be excluded from medical decision-making (55%) [34]. These concerns may reflect personal experiences with affected friends or immediate family and their knowledge of the challenges people with AD can face. They may also indicate a barrier that may limit interest in and uptake of new diagnostic techniques. It is thus important to consider how and by whom information about AD biomarkers is accessed, and what, if any, constitute appropriate uses of this information for individuals, health care systems, and insurers.
5.2. Access to resources and support
The support needed after learning a diagnosis of preclinical AD is unknown. Patients' organizations, such as the Alzheimer's Association and Alzheimer Europe, could offer a valuable resource for connecting with others who have similar shared experiences; however, their engagement could also reinforce a disease state or activate internalized stigma, particularly given their strong affiliations with clinical forms of the disease. The ways patient's organizations choose to engage persons identified in preclinical disease stages will be an important aspect of establishing the framework through which individuals view themselves and are viewed by others.
Guidelines should be established to ensure access to appropriate support services while distinguishing between individuals who do and do not have clinical disease. These guidelines should be developed in collaboration with patients' organizations and the public. Rather than active clinical intervention, support may involve enabling conversation and action related to a possible future with AD dementia. Dementia is among the most feared aspects of aging. The stigma associated with it can prevent open discussion of its implications. Here, patients' organizations and health care providers have a role in communicating the meaning of changing diagnostic criteria and associated disease categories. This includes establishing frameworks and training for clinical communication and raising public awareness about AD and dementia. A first step in the latter involves establishing clear guidance on communication about new diagnostic categories and the role of neuroimaging biomarkers [91], [124]. Furthermore, both public health programs and patients' organizations have a role in supporting conversations about future health and financial planning, while national and state legislatures may be better positioned to ensure development of clear frameworks related to powers of attorney and advance directives related to care and research participation.
5.3. Allocation of services
To ensure the just introduction of new diagnostic technologies, health care must be fairly allocated. This includes balancing the requirements of the population that is unimpaired but at-risk for developing the disease with individuals living with AD dementia. This balance between caring for the unimpaired and impaired is particularly salient, given the potential impact of stigma on political and social decision-making. For instance, as a result of the stigma associated with dementia—including the relative exclusion of the older population and people with symptomatic dementia from the public sphere—public discourse and funding could become skewed toward relatively younger “charismatic” or telegenic groups diagnosed with the latest technology as having “preclinical Alzheimer's disease.” This would detract both resources and attention from older, more impaired groups. By studying and illuminating the role of stigma in social and political discussions around health care priority setting, it might be possible to avoid or limit biased resolutions.
The fair introduction and use of new diagnostic technologies also requires equitable access to these resources. The provision of new diagnostic tests based on neuroimaging is currently localized in centers with the requisite equipment and expertise. This has the potential to perpetuate and exacerbate inequalities in access to timely diagnosis and health care, not least as the population considered eligible for testing grows. Providing a high standard of care to people who are privileged by virtue of either wealth or location risks increasing the stigmatization of those who are not treated early, such that having symptomatic dementia becomes seen as an individual failure to seek care or as a consequence of lower socioeconomic status. Health policy actions are needed to ensure fair access to diagnoses and associated therapies.
6. Conclusion
Neuroimaging and other methods to measure AD biomarkers are changing the definition of the disease. Clinicians may soon be able to routinely diagnose and treat AD in cognitively unimpaired adults. This “preclinical” diagnosis would allow clinicians novel opportunities to slow cognitive decline, but it would also bring challenges. It will fundamentally change the patient experience of the disease and thereby also the experiences of family and caregivers.
Knowledge of AD biomarker status can affect how individuals feel about themselves (internalized stigma) and how others judge them (public stigma). Emerging evidence suggests this, in part, reflects the spillover of stigma experienced by persons with AD dementia. However, individuals in preclinical stages may also experience unique psychosocial concerns, such a stereotyped threat, burdens of planning for the future, and anticipatory disease-specific distress. The effects may impact on an individual's sense of mental and physical wellbeing as well as impede their ability to live and work productively in society—be that due to, for example, change to personal identity, responsibility, and authority or the result of being discriminated against by others.
The psychosocial effects of early diagnosis warrant study to better support, reduce stigma, and shape accurate and appropriate expectations for individuals with AD and their families. Several important areas for study are emerging. These include the need to discover how individuals are affected by learning their biomarker result, how these effects may differ based on the type of test result individuals learn and when they learn it, and how individuals cope with the uncertainty that comes with learning these facts about themselves. This line of inquiry may help inform the safe and effective translation of advances in early diagnosis into routine clinical practice, including development of the approaches that are needed to safely monitor and follow individuals after disclosure and aid in developing interventions to optimize outcomes for individuals who learn their risk of AD. There also remains a need to understand how, if at all, individuals engage behaviors and activities to manage their risk or to plan for the future.
To accomplish these goals, PRO measures must be developed to assess the effects of stigma on individuals who learn their biomarker-based risk of AD and their family members. Improving measurement will aid in the development of interventions to reduce the deleterious effects of living with the knowledge that one has preclinical AD. In addition, measures to appraise the public's views and expectations associated with AD may help inform messaging campaigns and other population-based interventions to reduce public stigma of the disease.
Advances in neuroimaging and other biomarker technologies are shifting the definition of AD away from a symptom-based definition and toward a biologically defined stage. These developments could have dramatic effects on undoing the conflation of AD with other stigmatized states, such as mental illness and natural aging, by changing expectations that symptoms may be mutable and shifting stereotypes that currently tend to rely heavily on the disease's signs and symptoms. It will be important to understand how these changes affect individuals, including caregivers, particularly over time as symptoms may begin and to understand how social aspects of identity—such as age, race, and gender—interact with these effects, perhaps in ways that could exacerbate the burden of caregiving for women or, alternatively, could offer opportunities to reduce disparities in the participation of African-Americans in research trials.
The advances emerging in AD are offering novel opportunities to improve the prognosis and clinical management of patients diagnosed with AD. It is critical to understand and intentionally shape the language used when ushering in these new clinical technologies. The decisions made around choices in language may directly affect stigma that related to both an internalized sense of self and how the general public judges individuals associated with the disease. Decisions around language will also impact how individuals make meaning of learning they may have an early diagnosis. It will be essential to engage clinicians, researchers, and individual stakeholders in finding consensus on terminology that conveys accuracy, dignity, and personhood.
Engagement of state and national regulatory and advocacy agencies as well as patients' organizations is needed to help support individuals diagnosed early through both individual-level resources and broad messaging about what it means and does not mean to be diagnosed early with AD in terms of individuals' social capital and wellbeing. These efforts will also be essential for developing public policies that protect the rights and privileges of those who undergo testing to learn their biomarker risk of AD.
Advances in diagnostics may allow stigma related to clinical forms of the disease to spill over to affect individuals in preclinical disease stages. Alternatively, neuroimaging and other advances in diagnostics are presenting new opportunities to interrupt AD stigma by changing the scientific and medical understanding of the disease. Strategic use of these changes may aid to reduce stigma by altering the public image of the disease and improving clinical care for persons who are diagnosed.
Neuroimaging and other advances that allow an early diagnosis of AD are creating opportunities to reduce stigma through research, clinical practice, and public policy. They offer hope but also raise concern for how individuals may be affected by this knowledge. The effects on the psychological and social functioning of those who receive an early diagnosis, to date, remain largely unknown and unstudied.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Neuroimaging is advancing a new definition of Alzheimer's disease (AD). Using imaging biomarkers, clinicians will diagnose the disease based on the identification of pathology and neurodegeneration in either cognitively impaired or unimpaired adults. Changing the definition of AD from a clinical to a biomarker construct will change the experience of living with the disease. Relevant literature of AD stigma and AD early diagnosis is reviewed.
-
2.
Interpretation: Advances in diagnosis may enable or interrupt the transfer of stigma from clinical to preclinical stages of disease. These advances may also shift ways stigma of AD presents.
-
3.
Future directions: The article discusses conceptual and pragmatic challenges to addressing stigma as advances in early AD diagnosis move into routine care. Areas for further investigation are described.
Acknowledgments
The authors thank Maya Cantu for her diligent and thorough assistance in the literature search.
This work was supported by grants from the Alzheimer's Association (AARF-17-528934) and the Alzheimer's Disease Center (NIA P30 AG 010124). This publication is the result of work conducted by the CDC Healthy Brain Research Network. The CDC Healthy Brain Research Network is a Prevention Research Centers program funded by the CDC Healthy Aging Program-Healthy Brain Initiative. Efforts were supported in part by cooperative agreements from CDC's Prevention Research Centers Program: U48 DP 005006, 005002, 005053, 005000, and 005013. The views of this publication are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
References
- 1.Goffman E. Prentice-Hall; Englewood Cliffs, N.J: 1963. Stigma; Notes on the Management of Spoiled Identity. [Google Scholar]
- 2.Scambler G. Health-related stigma. Sociol Health Illn. 2009;31:441–455. doi: 10.1111/j.1467-9566.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 3.Pescosolido B.A., Martin J.K. The stigma complex. Annu Rev Sociol. 2015;41:87–116. doi: 10.1146/annurev-soc-071312-145702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrigan P.W. How clinical diagnosis might exacerbate the stigma of mental illness. Soc Work. 2007;52:31–39. doi: 10.1093/sw/52.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Mukadam N., Livingston G. Reducing the stigma associated with dementia: approaches and goals. Aging Health. 2012;8:377–386. [Google Scholar]
- 6.Behuniak S.M. The living dead? The construction of people with Alzheimer's disease as zombies. Ageing Soc. 2011;31:70–92. [Google Scholar]
- 7.Stites S.D., Johnson R., Harkins K., Sankar P., Xie D., Karlawish J. Identifiable characteristics and potentially malleable beliefs predict stigmatizing attributions toward persons with Alzheimer's disease dementia: results of a survey of the U.S. General Public. Health Commun. 2018;33:264–273. doi: 10.1080/10410236.2016.1255847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham N., Lindesay J., Katona C., Bertolote J.M., Camus V., Copeland J.R. Reducing stigma and discrimination against older people with mental disorders: a technical consensus statement: reducing stigma and discrimination against older people with mental disorders. Int J Geriatr Psychiatry. 2003;18:670–678. doi: 10.1002/gps.876. [DOI] [PubMed] [Google Scholar]
- 9.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;228:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link B.G., Phelan J.C. Conceptualizing stigma. Annu Rev Sociol. 2001;27:363–385. [Google Scholar]
- 11.Link B.G., Phelan J.C. Labeling and Stigma. In: Aneshensel C.S., Phelan J.C., Bierman A., editors. Handbook of the Sociology of Mental Health. Springer Netherlands; Dordrecht: 2013. pp. 525–541. [Google Scholar]
- 12.Kirkman A.M. Dementia in the news: the media coverage of Alzheimer's disease. Australas J Ageing. 2006;25:74–79. [Google Scholar]
- 13.Pin Le Corre S., Scodellaro C., Arwidson P. The image of patients and caregivers in the social perception of Alzheimer's disease–results from a literature review and a qualitative study considered with this target. Alzheimers Dement. 2009;5:P233. [Google Scholar]
- 14.Werner P., Goldstein D., Buchbinder E. Subjective experience of family stigma as reported by children of Alzheimer's disease patients. Qual Health Res. 2010;20:159–169. doi: 10.1177/1049732309358330. [DOI] [PubMed] [Google Scholar]
- 15.Van Gorp B., Vercruysse T. Frames and counter-frames giving meaning to dementia: A framing analysis of media content. Soc Sci Med. 2012;74:1274–1281. doi: 10.1016/j.socscimed.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R., Harkins K., Cary M., Sankar P., Karlawish J. The relative contributions of disease label and disease prognosis to Alzheimer's stigma: A vignette-based experiment. Soc Sci Med. 2015;143:117–127. doi: 10.1016/j.socscimed.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Werner P., Goldstein D., Heinik J. Development and validity of the family stigma in Alzheimer's disease scale (FS-ADS) Alzheimer Dis Assoc Disord. 2011;25:42–48. doi: 10.1097/WAD.0b013e3181f32594. [DOI] [PubMed] [Google Scholar]
- 18.Garand L., Lingler J.H., Conner K.O., Dew M.A. Diagnostic labels, stigma, and participation in research related to dementia and mild cognitive impairment. Res Gerontol Nurs. 2009;2:112–121. doi: 10.3928/19404921-20090401-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beard R.L., Neary T.M. Making sense of nonsense: experiences of mild cognitive impairment: Sense-making in mild cognitive impairment experiences. Sociol Health Illn. 2013;35:130–146. doi: 10.1111/j.1467-9566.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen L. University of California Press; Berkeley, Calif: 2000. No Aging in India: Alzheimer's, The Bad Family, and Other Modern Things. [Google Scholar]
- 21.Batsch N.L., Mittleman M.S. Alzheimer's Disease International; London, UK: 2012. World Alzheimer Report 2012, Overcoming the Stigma of Dementia. Available at: http://www.alz.co.uk/research/world-report-2012. [Google Scholar]
- 22.Weiner B., Perry R.P., Magnusson J. An attributional analysis of reactions to stigmas. J Pers Soc Psychol. 1988;55:738–748. doi: 10.1037//0022-3514.55.5.738. [DOI] [PubMed] [Google Scholar]
- 23.Pescosolido B.A., Medina T.R., Martin J.K., Long J.S. The ‘Backbone’ of stigma: identifying the global core of public prejudice associated with mental illness. Am J Public Health. 2013;103:853–860. doi: 10.2105/AJPH.2012.301147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D., Hinton L., Tran C., Hinton D., Barker J.C. Reexamining the relationships among dementia, stigma, and aging in immigrant Chinese and Vietnamese family caregivers. J Cross Cult Gerontol. 2008;23:283–299. doi: 10.1007/s10823-008-9075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiske S.T., Cuddy A.J.C., Glick P., Xu J. A model of (often mixed) stereotype content: Competence and warmth respectively follow from perceived status and competition. J Pers Soc Psychol. 2002;82:878–902. [PubMed] [Google Scholar]
- 26.Kane M.N. Awareness of ageism, motivation, and countertransference in the care of elders with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2002;17:101–109. doi: 10.1177/153331750201700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caspermeyer J.J., Sylvester E.J., Drazkowski J.F., Watson G.L., Sirven J.I. Evaluation of stigmatizing language and medical errors in neurology coverage by US newspapers. Mayo Clin Proc. 2006;81:300–306. doi: 10.4065/81.3.300. [DOI] [PubMed] [Google Scholar]
- 28.Handbook of Mental Health and Aging–2nd Edition. https://www.elsevier.com/books/handbook-of-mental-health-and-aging/birren/978-0-12-101277-9 1992. Available at:
- 29.Dobbs D., Eckert J.K., Rubinstein B., Keimig L., Clark L., Frankowski A.C. An ethnographic study of stigma and ageism in residential care or assisted living. Gerontologist. 2008;48:517–526. doi: 10.1093/geront/48.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne A. The ‘D’ word: Reflections on the relationship between stigma, discrimination and dementia. J Ment Health. 2010;19:227–233. doi: 10.3109/09638231003728166. [DOI] [PubMed] [Google Scholar]
- 31.Calasanti T.M., Slevin K.F. Rowman Altamira; Walnut Creek, CA: 2001. Gender, Social Inequalities, and Aging. [Google Scholar]
- 32.Bouldin E., Andresen E. Caregiving across the United States: BRFSS data reveal the impact of Alzheimer's disease and dementia. Alzheimers Dement. 2012;8:599. [Google Scholar]
- 33.Freedman V.A., Spillman B.C. Disability and care needs among older Americans: disability and care needs of older Americans. Milbank Q. 2014;92:509–541. doi: 10.1111/1468-0009.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stites S.D., Rubright J.D., Karlawish J. What features of stigma do the public most commonly attribute to Alzheimer's disease dementia? results of a survey of the U.S. general public [published online ahead of print March 27, 2018] Alzheimers Dement. 2018 doi: 10.1016/j.jalz.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darnell K.R., McGuire C., Danner D.D. African American Participation in Alzheimer's Disease Research That Includes Brain Donation. Am J Alzheimers Dis Other Dement. 2011;26:469–476. doi: 10.1177/1533317511423020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heatherton T.F. Guilford Press; New York, NY: 2003. The Social Psychology of Stigma. [Google Scholar]
- 37.Shavers-Hornaday V.L., Lynch C.F., Burmeister L.F., Torner J.C. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2:31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 38.Noonan A.S., Velasco-Mondragon H.E., Wagner F.A. Improving the health of African Americans in the USA: an overdue opportunity for social justice. Public Health Rev. 2016;37:12. doi: 10.1186/s40985-016-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green R.C. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 40.Phillipson L., Magee D.C., Jones S., Skladzien D.E., Cridland E. Exploring dementia and stigma beliefs. Alzheimers Aust. 2012 Available at: https://www.dementia.org.au/files/20120712_US_28_Stigma_Report.pdf. Accessed March 20, 2018. [Google Scholar]
- 41.Crisp A.H. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177:4–7. doi: 10.1192/bjp.177.1.4. [DOI] [PubMed] [Google Scholar]
- 42.Burgener S.C., Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer's dementia and Parkinson's disease. Dementia. 2008;7:31–53. [Google Scholar]
- 43.Alzheimer's Association National Plan Milestone Workgroup 2014 Report on the Milestones for the US National Plan to Address Alzheimer's Disease. Alzheimers Dement. 2014;10(5 Suppl):S430–452. doi: 10.1016/j.jalz.2014.08.103. [DOI] [PubMed] [Google Scholar]
- 44.Anderson L.A., Egge R. Expanding efforts to address Alzheimer's disease: the Healthy Brain Initiative. Alzheimers Dement. 2014;10(5 Suppl):S453–456. doi: 10.1016/j.jalz.2014.05.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connell C.M., Shaw B.A., Holmes S.B., Foster N.L. Caregivers' Attitudes Toward Their Family Members' Participation in Alzheimer Disease Research: Implications for Recruitment and Retention. Alzheimer Dis Assoc Disord. 2001;15:137–145. doi: 10.1097/00002093-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Link BG, Cullen FT, Mirotznik J, Struening E. The consequences of stigma for persons with mental illness: Evidence from the social sciences. 1992.
- 47.Shifflett P.A., Blieszner R. Stigma and Alzheimer's disease: behavioral consequences for support groups. J Appl Gerontol. 1988;7:147–160. [Google Scholar]
- 48.Scheff T.J. University of Chicago Press; London, UK: 1994. Microsociology: Discourse, Emotion, and Social Structure. [Google Scholar]
- 49.Ownby R.L., Harwood D.G., Barker W.W., Duara R. Predictors of anxiety in patients with Alzheimer's disease. Depress Anxiety. 2000;11:38–42. doi: 10.1002/(sici)1520-6394(2000)11:1<38::aid-da6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 50.Mega M.S., Cummings J.L., Fiorello T., Gornbein J. The spectrum of behavioral changes in Alzheimer's disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 51.Riley R.J., Burgener S., Buckwalter K.C. Anxiety and Stigma in Dementia. Nurs Clin North Am. 2014;49:213–231. doi: 10.1016/j.cnur.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farias S.T., Mungas D., Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20:827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanetti O., Vallotti B., Frisoni G.B., Geroldi C., Bianchetti A., Pasqualetti P. Insight in dementia: when does it occur? Evidence for a nonlinear relationship between insight and cognitive status. J Gerontol B Psychol Sci Soc Sci. 1999;54:P100–P106. doi: 10.1093/geronb/54b.2.p100. [DOI] [PubMed] [Google Scholar]
- 54.McDaniel K.D., Edland S.D., Heyman A. Relationship between level of insight and severity of dementia in Alzheimer disease. CERAD Clinical Investigators. Consortium to Establish a Registry for Alzheimer's Disease. Alzheimer Dis Assoc Disord. 1995;9:101–104. doi: 10.1097/00002093-199509020-00007. [DOI] [PubMed] [Google Scholar]
- 55.Schmand B., Jonker C., Hooijer C., Lindeboom J. Subjective memory complaints may announce dementia. Neurology. 1996;46:121–125. doi: 10.1212/wnl.46.1.121. [DOI] [PubMed] [Google Scholar]
- 56.Jolley D.J., Benbow S.M. Stigma and Alzheimer's disease: causes, consequences and a constructive approach. Int J Clin Pract. 2000;54:117–119. [PubMed] [Google Scholar]
- 57.Cox C.B. 1st ed. Springer Publishing Company; New York: 2007. Dementia and Social Work Practice: Research and Interventions. [Google Scholar]
- 58.Aneshensel C.S., Pearlin L.I., Schuler R.H. Stress, role captivity, and the cessation of caregiving. J Health Soc Behav. 1993;34:54–70. [PubMed] [Google Scholar]
- 59.Park S., Park K.S. Family stigma: a concept analysis. Asian Nurs Res. 2014;8:165–171. [Google Scholar]
- 60.Francesca C., Ana L.-N., Jérôme M., Frits T. OECD Publishing; OECD Health Policy Studies: 2011. Help Wanted? Providing and Paying for Long-Term Care: Providing and Paying for Long-Term Care. [Google Scholar]
- 61.Allen M.N., Dublin S., Kimmerly P.J. Demos Med Publishing; New York, NY: 2012. A Look Inside Alzheimer's. [Google Scholar]
- 62.Ballard C., Boyle A., Bowler C., Lindesay J. Anxiety disorders in dementia sufferers. Int J Geriatr Psychiatry. 1996;11:987–990. [Google Scholar]
- 63.Steele C.M., Aronson J. Stereotype threat and the intellectual test performance of African Americans. J Pers Soc Psychol. 1995;69:797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- 64.Lineweaver T.T., Bondi M.W., Galasko D., Salmon D.P. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201–208. doi: 10.1176/appi.ajp.2013.12121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werner P., Mittelman M.S., Goldstein D., Heinik J. Family stigma and caregiver burden in Alzheimer's disease. Gerontologist. 2012;52:89–97. doi: 10.1093/geront/gnr117. [DOI] [PubMed] [Google Scholar]
- 66.MacRae H. Managing courtesy stigma: the case of Alzheimer's disease. Sociol Health Illn. 2008;21:54–70. [Google Scholar]
- 67.Carretero S., Garcés J., Ródenas F., Sanjosé V. The informal caregiver's burden of dependent people: Theory and empirical review. Arch Gerontol Geriatr. 2009;49:74–79. doi: 10.1016/j.archger.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Werner P. Social distance towards a person with Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20:182–188. doi: 10.1002/gps.1268. [DOI] [PubMed] [Google Scholar]
- 69.Drennan V.M., Cole L., Iliffe S. A taboo within a stigma? a qualitative study of managing incontinence with people with dementia living at home. BMC Geriatr. 2011;11:75. doi: 10.1186/1471-2318-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kahn P.V., Wishart H.A., Randolph J.S., Santulli R.B. Caregiver stigma and burden in memory disorders: an evaluation of the effects of caregiver type and gender. Curr Gerontol Geriatr Res. 2016;2016:8316045. doi: 10.1155/2016/8316045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinquart M., Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychol Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 72.Mannion E. Alzheimer's disease: the psychological and physical effects of the caregiver's role. Part 2. Nurs Older People. 2008;20:33–38. doi: 10.7748/nop2008.05.20.4.33.c8221. [DOI] [PubMed] [Google Scholar]
- 73.McParland P., Devine P., Innes A., Gayle V. Dementia knowledge and attitudes of the general public in Northern Ireland: an analysis of national survey data. Int Psychogeriatr. 2012;24:1600–1613. doi: 10.1017/S1041610212000658. [DOI] [PubMed] [Google Scholar]
- 74.Joyce M.L. The graying of America: implications and opportunities for health marketers. Am Behav Sci. 1994;38:341–350. [Google Scholar]
- 75.Van Gorp B., Vercruysse T., Van den Bulck J. Toward a more nuanced perception of Alzheimer's disease: designing and testing a campaign advertisement. Am J Alzheimers Dis Dementiasr. 2012;6:388–396. doi: 10.1177/1533317512454707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melzer D. New drug treatment for Alzheimer's disease: lessons for healthcare policy. BMJ. 1998;316:762–764. doi: 10.1136/bmj.316.7133.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartels S.J., Colenda C.C. Mental Health Services for Alzheimer's Disease: Current trends in reimbursement and public policy, and the future under managed care. Am J Geriatr Psychiatry. 1998;6:S85–S100. doi: 10.1097/00019442-199821001-00011. [DOI] [PubMed] [Google Scholar]
- 78.Khachaturian A.S., Hoffman D.P., Frank L., Petersen R., Carson B.R., Khachaturian Z.S. Zeroing out preventable disability: Daring to dream the impossible dream for dementia care. Alzheimers Dement. 2017;13:1077–1080. doi: 10.1016/j.jalz.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Benbow S.M., Jolley D. Dementia: stigma and its effects. Neurodegener Dis Manag. 2012;2:165–172. [Google Scholar]
- 80.Byrne P. Psychiatric stigma. Br J Psychiatry. 2001;178:281–284. [Google Scholar]
- 81.Taylor A. The Effects of Stigma on Access to Care and Services. Presented at the Research Summit on Dementia Care, 2017.
- 82.Corner L., Bond J. Being at risk of dementia: Fears and anxieties of older adults. J Aging Stud. 2004;18:143–155. [Google Scholar]
- 83.Werner P., Giveon S.M. Discriminatory behavior of family physicians toward a person with Alzheimer's disease. Int Psychogeriatr. 2008;20:824–839. doi: 10.1017/S1041610208007060. [DOI] [PubMed] [Google Scholar]
- 84.Wortmann M. Dementia: a global health priority–highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4:40. doi: 10.1186/alzrt143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.National Plan to Address Alzheimer's Disease: 2014 Update. ASPE, 23-Nov-2015. https://aspe.hhs.gov/pdf-document/national-plan-address-alzheimers-disease-2014-update Available at:
- 86.Alzheimer Europe. 2013 Alzheimer Europe Report: The Ethical Issues Linked to the Perceptions and Portrayal of Dementia and People with Dementia. 2013. Available at: http://www.alzheimer-europe.org/Ethics/Ethical-issues-in-practice/2013-The-ethical-issues-linked-to-the-perceptions-and-portrayal-of-dementia-and-people-with-dementia. Accessed March 20, 2018. [Google Scholar]
- 87.Advisory Council July 2017 Meeting Presentation: Implications of a Biologically Based Definition. ASPE, 27-Jul-2017. https://aspe.hhs.gov/advisory-council-july-2017-meeting-presentation-implications-biologically-based-definition Available at:
- 88.Gauthier S., Leuzy A., Racine E., Rosa-Neto P. Diagnosis and management of Alzheimer's disease: Past, present and future ethical issues. Prog Neurobiol. 2013;110:102–113. doi: 10.1016/j.pneurobio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Lecouturier J., Bamford C., Hughes J.C., Francis J.J., Foy R., Johnston M. Appropriate disclosure of a diagnosis of dementia: identifying the key behaviours of ‘best practice’. BMC Health Serv Res. 2008;8:95. doi: 10.1186/1472-6963-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luck T., Luppa M., Sieber J., Schomerus G., Werner P., König H. Attitudes of the German General Population toward Early Diagnosis of Dementia – Results of a Representative Telephone Survey. PLoS One. 2012;7:e50792. doi: 10.1371/journal.pone.0050792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grill J.D., Apostolova L.G., Bullain S., Burns J.M., Cox C.G., Dick M. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther. 2017;9:35. doi: 10.1186/s13195-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roberts J.S., Dunn L.B., Rabinovici G.D. Amyloid imaging, risk disclosure and Alzheimer's disease: ethical and practical issues. Neurodegener Dis Manag. 2013;3:219–229. doi: 10.2217/nmt.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stites S.D., Karlawish J., Harkins K., Rubright J.D., Wolk D. Awareness of mild cognitive impairment and mild Alzheimer's disease dementia diagnoses associated with lower self-ratings of quality of life in older adults. J Gerontol B Psychol Sci Soc Sci. 2017;72:974–985. doi: 10.1093/geronb/gbx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Committee on Preventing Dementia and Cognitive Impairment, Board on Health Sciences Policy, Health and Medicine Division, and E . National Academies Press; Washington, D.C: 2017. National Academies of Sciences and Medicine, Preventing Cognitive Decline and Dementia: A Way Forward. [PubMed] [Google Scholar]
- 95.Zhong Y., Karlawish J., Johnson M.K., Neumann P.J., Cohen J.T. The Potential Value of β-Amyloid Imaging for the Diagnosis and Management of Dementia: A Survey of Clinicians. Alzheimer Dis Assoc Disord. 2017;31:27–33. doi: 10.1097/WAD.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 96.Lingler J.H., Nightingale M.C., Erlen J.A., Kane A.L., Reynolds C.F. 3rd, Schulz R. Making sense of mild cognitive impairment: a qualitative exploration of the patient's experience. Gerontologist. 2006;46:791–800. doi: 10.1093/geront/46.6.791. [DOI] [PubMed] [Google Scholar]
- 97.Mozersky J., Sankar P., Harkins K., Hachey S., Karlawish J. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75:44–50. doi: 10.1001/jamaneurol.2017.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Milne R., Diaz A., Bunnik E., Badger S., Fauria K., Wells K. At, with and beyond risk: expectations of living with the possibility of future dementia. Sociol Health Illn. 2017 doi: 10.1111/1467-9566.12731. (in press) [DOI] [PubMed] [Google Scholar]
- 99.Lim Y.Y., Maruff P., Getter C., Snyder P.J. Disclosure of positron emission tomography amyloid imaging results: A preliminary study of safety and tolerability. Alzheimers Dement J Alzheimers Assoc. 2016;12:454–458. doi: 10.1016/j.jalz.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 100.Burns J.M., Johnson D.K., Liebmann E.P., Bothwell R.J., Morris J.K., Vidoni E.D. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement J Alzheimers Assoc. 2017;13:1024–1030. doi: 10.1016/j.jalz.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holroyd S., Turnbull Q., Wolf A.M. What are patients and their families told about the diagnosis of dementia? Results of a family survey. Int J Geriatr Psychiatry. 2002;17:218–221. doi: 10.1002/gps.552. [DOI] [PubMed] [Google Scholar]
- 102.Ritchie C.W., Russ T.C., Banerjee S., Barber B., Boaden A., Fox N.C. The Edinburgh Consensus: preparing for the advent of disease-modifying therapies for Alzheimer's disease. Alzheimers Res Ther. 2017;9:85. doi: 10.1186/s13195-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kunneman M., Pel-Littel R., Bouwman F.H., Gillissen F., Schoonenboom N.S.M., Claus J.J. Patients' and caregivers' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: The ABIDE project. Alzheimers Dement (N Y) 2017;10:314–322. doi: 10.1016/j.trci.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Flier W.M., Kunneman M., Bouwman F.H., Petersen R.C., Smets E.M.A. Diagnostic dilemmas in Alzheimer's disease: Room for shared decision making. Alzheimers Dement Transl Res Clin Interv. 2017;3:301–304. doi: 10.1016/j.trci.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wadley V.G., Haley W.E. Diagnostic attributions versus labeling: impact of Alzheimer's disease and major depression diagnoses on emotions, beliefs, and helping intentions of family members. J Gerontol B Psychol Sci Soc Sci. 2001;56:P244–P252. doi: 10.1093/geronb/56.4.p244. [DOI] [PubMed] [Google Scholar]
- 106.Witte K., Allen M. A meta-analysis of fear appeals: implications for effective public health campaigns. Health Educ Behav. 2000;27:591–615. doi: 10.1177/109019810002700506. [DOI] [PubMed] [Google Scholar]
- 107.Werner P. Stigma and Alzheimer's disease: A systematic review of evidence, theory, and methods. In: Corrigan P.W., editor. The Stigma of Disease and Disability: Understanding Causes and Overcoming Injustices. American Psychological Association; Washington: 2014. pp. 223–244. [Google Scholar]
- 108.Fernando S.M., Deane F.P., McLeod H.J. “Sri Lankan doctors' and medical undergraduates' attitudes towards mental illness. Soc Psychiatry Psychiatr Epidemiol. 2010;45:733–739. doi: 10.1007/s00127-009-0113-6. [DOI] [PubMed] [Google Scholar]
- 109.Low L.F., Anstey K.J., Lackersteen S.M., Camit M., Harrison F., Draper B. Recognition, attitudes and causal beliefs regarding dementia in Italian, Greek and Chinese Australians. Dement Geriatr Cogn Disord. 2010;30:499–508. doi: 10.1159/000321667. [DOI] [PubMed] [Google Scholar]
- 110.Kubiak S.P., Ahmedani B.K., Rios-Bedoya C.F., Anthony J.C. Stigmatizing clients with mental health conditions: an assessment of social work student attitudes. Soc Work Ment Health. 2011;9:253–271. doi: 10.1080/15332985.2010.540516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corrigan P.W., River L.P., Lundin R.K., Penn D.L., Uphoff-Wasowski K., Campion J. Three strategies for changing attributions about severe mental illness. Schizophr Bull. 2001;27:187–195. doi: 10.1093/oxfordjournals.schbul.a006865. [DOI] [PubMed] [Google Scholar]
- 112.Corrigan P.W., River L.P., Lundin R.K., Uphoff Wasowski K., Campion J., Mathisen J. Stigmatizing attributions about mental illness. J Community Psychol. 2000;28:91–102. [Google Scholar]
- 113.Molina Y., Choi S.W., Cella D., Rao D. The Stigma Scale for Chronic Illnesses 8-item version (SSCI-8): development, validation and use across neurological conditions. Int J Behav Med. 2013;20:450–460. doi: 10.1007/s12529-012-9243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fife B.L., Wright E.R. The dimensionality of stigma: a comparison of its impact on the self of persons with HIV/AIDS and cancer. J Health Soc Behav. 2000;41:50–67. [PubMed] [Google Scholar]
- 115.Pan A.-W., Chung L., Fife B.L., Hsiung P.-C. Evaluation of the psychometrics of the Social Impact Scale: a measure of stigmatization. Int J Rehabil Res. 2007;30:235–238. doi: 10.1097/MRR.0b013e32829fb3db. [DOI] [PubMed] [Google Scholar]
- 116.Stuart H., Milev R., Koller M. The Inventory of Stigmatizing Experiences: its development and reliability. World Psychiatry. 2005;4:33–37. [Google Scholar]
- 117.Rao D., Choi S.W., Victorson D., Bode R., Peterman A., Heinemann A. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI) Qual Life Res. 2009;18:585–595. doi: 10.1007/s11136-009-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gray H.L., Jimenez D.E., Cucciare M.A., Tong H.-Q., Gallagher-Thompson D. Ethnic differences in beliefs regarding Alzheimer disease among dementia family caregivers. Am J Geriatr Psychiatry. 2009;17:925–933. doi: 10.1097/JGP.0b013e3181ad4f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Otlowski M., Taylor S., Bombard Y. Genetic discrimination: international perspectives. Annu Rev Genomics Hum Genet. 2012;13:433–454. doi: 10.1146/annurev-genom-090711-163800. [DOI] [PubMed] [Google Scholar]
- 120.Genetic Information Nondiscrimination Act of 2008. National Human Genome Research Institute (NHGRI) https://www.genome.gov/24519851/Genetic-Information-Nondiscrimination-Act-of-2008 Available at:
- 121.Stanford University; Stanford, CA: 2014. Predicting Alzheimer's: The Social and Policy Implications of Early Diagnosis. Stanford Journal of Public Health. [Google Scholar]
- 122.Zick C.D., Mathews C., Roberts J.S., Cook-Deegan R., Pokorski R.J., Green R.C. Genetic testing for Alzheimer's disease and its impact on insurance purchasing behavior. Health Aff (Millwood) 2005;24:483–490. doi: 10.1377/hlthaff.24.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arias J.J., Karlawish J. Confidentiality in preclinical Alzheimer disease studies When research and medical records meet. Neurology. 2014;82:725–729. doi: 10.1212/WNL.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alzheimer Europe 2016: Ethical issues linked to the changing definitions/use of the term Alzheimer's disease. http://www.alzheimer-europe.org/Ethics/Ethical-issues-in-practice/2016-Ethical-issues-linked-to-the-changing-definitions-use-of-terms-related-to-Alzheimer-s-disease/(language)/eng-GB 2016. Available at:
- 125.Lu Y., Wang X. Correlation between insight and internalized stigma in patients with schizophrenia. Shanghai Arch Psychiatry. 2012;24:91–98. doi: 10.3969/j.issn.1002-0829.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]