Abstract
Introduction
Patients with amnestic mild cognitive impairment (aMCI) are heterogeneous as regard to their amyloid status. The present study aimed at highlighting the neuropsychological, brain atrophy, and hypometabolism profiles of amyloid-positive (Aβpos) versus amyloid-negative (Aβneg) aMCI patients.
Methods
Forty-four aMCI patients and 24 Aβneg healthy controls underwent neuropsychological, structural magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography scans. Data were compared between groups in specific regions of interest and voxelwise with statistical parametric mapping.
Results
When directly comparing Aβpos to Aβneg aMCI, the former had lower performances in episodic memory tests (P = .02 to P < .001) while the latter had worse scores in working memory (P = .01) and language (P < .005). Compared to Aβneg healthy controls, both aMCI subgroups showed similar profiles of atrophy and hypometabolism, with no difference between both aMCI subgroups.
Conclusion
In a sample of aMCI patients recruited and scanned in the same center, the main difference at baseline between Aβpos and Aβneg aMCI concerned the neuropsychological profile, but not the structural magnetic resonance imaging or 18F-fluorodeoxyglucose positron emission tomography profiles of brain alterations.
Keywords: Amyloid status, Amnestic mild cognitive impairment, Alzheimer's disease, Cognition, Glucose metabolism, Gray matter volume
Highlights
-
•
Amyloid-positive (Aβpos) amnestic mild cognitive impairment (aMCI) had lower performances than amyloid-negative (Aβneg) aMCI in episodic memory.
-
•
Aβneg aMCI had lower performances than Aβpos aMCI in working memory and language.
-
•
Aβneg and Aβpos aMCI did not differ in terms of brain atrophy or metabolism.
-
•
Cognition is more efficient than neuroimaging to discriminate Aβneg from Aβpos aMCI.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder defined pathologically by the presence of amyloid β (Aβ) plaques and tau-rich neurofibrillary tangles [1], [2], [3], [4]. Cerebral Aβ pathology can be visualized in vivo using positron emission tomography (PET) imaging coupled with Aβ radioligands. Studies have shown increased brain Aβ load in AD patients compared to healthy elderly, with about 90% of patients with a clinical diagnosis of AD being classified as amyloid positive based on the PET scan [5]. Aβ is known to accumulate progressively 15 to 20 years before dementia and even years before the detection of clinical deficits [6]. Mild cognitive impairment refers to the clinical stage of cognitive decline that is greater than expected for a given age and educational attainment but does not interfere activities of daily living; such patients are generally considered to be in a predementia stage [7]. When memory deficits are predominant, patients are called as amnestic mild cognitive impairment (aMCI) and thought to be more specifically at the prodromal stage of AD [8], [9]. Yet, aMCI patients will not all progress to AD dementia [10], and they do not all present with an amyloid-positive PET scan [11], [12], [13], [14], [15]. Thus, 47%–75% of aMCI patients present with high cortical Aβ retention on amyloid PET [11], [13], [16], [17], [18], [19]. aMCI patients with an amyloid-positive PET scan are more likely to convert to AD dementia: the percentage of progression to AD dementia over 2 to 3 years is 45% to 82% within the amyloid-positive aMCI (Aβpos aMCI) patients versus 0% to 11% within the amyloid-negative aMCI (Aβneg aMCI) [11], [13], [16], [17], [18], [19]. A more extensive appraisal of aMCI patients who present with the same symptoms but differ as regard to the presence or absence of abnormal levels of amyloid deposition would help understanding the specific cognitive and brain changes associated with a particular molecular phenotype. This is of high relevance for clinical diagnosis, to screen patients for anti-amyloid clinical trials, and to improve our understanding of the role of amyloid deposition in the pathophysiology of AD.
Previous studies assessing differences in cognitive performances between Aβpos aMCI and Aβneg aMCI have consistently reported greater deficits in episodic memory in Aβpos patients [11], [12], [15]. Aβneg aMCI patients have been shown to be more impaired in nonepisodic memory domains compared to Aβpos aMCI [12], [20], although this was not found in all studies [11], [15]. As regard to brain atrophy, greater hippocampal atrophy in Aβpos aMCI than Aβneg aMCI was found in some [12], [14], [21] but not all [11], [22] studies. Cerebral 18F-fluorodeoxyglucose metabolism has been studied only in three studies that found differences between Aβpos and Aβneg aMCI but in different regions according to the study. Thus, a decrease was reported in Aβpos aMCI compared to Aβneg aMCI in the temporoparietal or only in the precuneus [15] or in the inferior parietal, inferior temporal, and precuneus [14]. However, cognition, atrophy, and hypometabolism were assessed separately in these previous studies therefore not allowing to identify which changes are the most specific, within a population presenting with the same symptoms, to a particular molecular phenotype—that is, the presence of amyloid deposition.
Landau et al.'s study [13] is the only previous study providing an overall picture of the profiles of neuropsychological changes, brain atrophy, and brain hypometabolism in Aβpos versus Aβneg aMCI patients. They reported higher hippocampal atrophy and temporoparietal hypometabolism in Aβpos compared to Aβneg aMCI. Moreover, Aβneg aMCI had higher performances than Aβpos aMCI on global cognition and on episodic memory. The present study is complementary as it compares neuropsychological, atrophy, and hypometabolism profiles between Aβpos and Aβneg aMCI from a more restricted but monocentric sample and also includes a group of healthy controls so that each subgroup of patients could also be compared to a same control group (see the Supplementary Material for further details). This comprehensive picture is yet needed to identify which changes are the most specific, within a population presenting with the same symptoms, to a particular molecular phenotype—that is, the presence of amyloid deposition.
2. Material and methods
2.1. Participants
All participants were included in the Imagerie Multimodale de la maladie d'Alzheimer à un stade Précoce (IMAP+) study (Caen, France), and the inclusion and exclusion criteria are detailed in the Supplementary Materials and the previous publication [23], [24], [25], [26]. For the sake of the present study, 68 right-handed native French-speaking participants were included, comprising 44 patients with aMCI (20 Aβneg aMCI and 24 Aβpos aMCI; see section 2.4. Neuroimaging procedure) and 24 Aβneg healthy controls (HCs) (all amyloid negative; see section 2.4. Neuroimaging procedure). Participants were selected from the IMAP+ study database, if they were older than 55 years (inclusive), and had magnetic resonance imaging (MRI), FDG-PET, and florbetapir-PET imaging. We included in the present study all aMCI patients from the IMAP database meeting these criteria, but only the HCs who met these criteria and whose florbetapir PET scan was classified as amyloid negative (see section 2.4. Neuroimaging procedure). The two groups of participants were matched for age, gender, and education (Table 1).
Table 1.
Demographic and clinical data for the amyloid-negative healthy controls (Aβneg HC) and the patients with amnestic mild cognitive impairment (aMCI) categorized by amyloid status (Aβpos aMCI and Aβneg aMCI)
| Measure | Aβneg HC (n = 24) | Aβneg aMCI (n = 20) | Aβpos aMCI (n = 24) | Group comparison |
|---|---|---|---|---|
| Age: years ± SD | 71.4 ± 4.4 | 73.1 ± 7.6 | 73.3 ± 6.7 | PANOVA = .52 |
| Gender: % male | 46 | 50 | 58 | |
| Education: years ± SD | 11.8 ± 3.9 | 9.9 ± 2.8 | 11.9 ± 4.3 | PANOVA = .16 |
| MMSE: score ± SD | 28.7 ± 1.0 | 26.9 ± 1.8 | 26.8 ± 1.7 | PANOVA < .001; Aβneg HC > Aβneg aMCI, Aβpos aMCI |
| MADRS: score ± SD | 142.5 ± 1.3 | 134.3 ± 4.7 | 133.5 ± 6.1 | PANOVA < .001; Aβneg HC > Aβneg aMCI, Aβpos aMCI |
| APOE: % ε4 carriers | 4.2 | 20.0 | 77.3 | < .001; Aβpos aMCI > Aβneg aMCI, Aβneg HC |
Abbreviations: MMSE, Mini–Mental State Examination; MADRS, Mattis Dementia Rating Scale; SD, standard deviation; ANOVA, analysis of variance.
NOTE. All variables were compared using ANOVA and χ2 (Pearson) tests.
Within a few days from recruitment, each participant underwent (1) a detailed neuropsychological battery detailed below, (2) a structural MRI scan, (3) a PET scan using 18F-fluorodeoxyglucose, and (4) a PET scan using [18F] florbetapir (AV45). All participants were scanned on the same MRI and PET cameras. The IMAP study was approved by regional ethics committee (Comité de Protection des Personnes Nord-Ouest III) and registered with ClinicalTrial.gov (number NCT01638949). All participants gave written informed consent to the study before the investigation.
2.2. Neuropsychological assessment
Neuropsychological tests and scores have been selected among a more detailed neuropsychological battery that covered the main domains of cognition that are affected by AD and other dementias. All continuous raw scores were transformed into W-scores, that is, age- and education-adjusted Z-scores [27]. Eight different W-scores or composite W-scores were used to measure the following cognitive areas: episodic memory (free recall and recognition), verbal fluency, language, short-term memory, working memory, executive function, and visuospatial functions (see the Supplementary Material for further details).
2.3. Follow-up procedure
Using the same neuropsychological battery used at inclusion, all aMCI patients were evaluated every 6 months over a 36-month follow-up period to assess whether they met NINCDS-ADRDA criteria of probable AD or not; at the end of the follow-up period, patients were classified as converters to AD dementia, remained diagnosed as aMCI, or developed another pathology. Patients were declared as converters to AD if they had impaired performances (more than 1.65 SD below the normal means according to age and education when available) in at least one of the general global cognition scales as well as in at least two areas of cognition including memory, leading to impaired daily activities as judged by the clinicians from the consultation interviews. The decision was made by the clinicians blind to the amyloid status.
2.4. Neuroimaging procedure
Details on image acquisition and preprocessing are available in previous publications and in the Supplementary Material. Briefly, MRI data were prepared using the DARTEL toolbox implemented in SPM12. FDG-PET and florbetapir-PET images were preprocessed using MRI data for partial volume effect correction and spatial normalization. Individual florbetapir uptake values were extracted in a predetermined neocortical mask [22], [28] (including the entire gray matter except the cerebellum, occipital and sensory motor cortices, hippocampi, amygdala, and basal nuclei) in all participants. Then, these values were used to classify participants as amyloid positive or negative using a threshold of 1.08. The threshold for positivity was determined on the basis of the mean florbetapir uptake values in the neocortical mask of a group of 49 HCs (mean age = 69.5 ± 5.7) using an iterative outlier approach [29], [30]. Among the 44 aMCI patients, 20 were classified as Aβneg aMCI and 24 as Aβpos aMCI. Note that all analyses were also performed after removing the eight “intermediate” cases defined as those having an standardized uptake value ratio (SUVr) value ranging from 5% higher to 5% lower than the threshold (i.e., between 1.03 and 1.14).
2.5. Statistical analyses
Statistica v.10 was used for all non-voxelwise statistical analyses, whereas SPM12 was used for all voxelwise analyses.
2.5.1. Demographic, clinical, and neuropsychological data
Continuous demographic and clinical data (age, education level, Mini–Mental State Examination) were compared between groups (Aβneg HC, Aβneg aMCI, and Aβpos aMCI) with single-factor (group) analyses of variance and post hoc comparisons when the effect of group was significant. Categorical variables (gender and apolipoprotein E (APOE) genotype) were compared using proportional χ2 tests (Pearson). All results were considered significant at P < .05.
Neuropsychological W-scores were compared between Aβneg aMCI and Aβpos aMCI using two-sample t-tests, except for language (categorical variable) where proportional χ2 tests were performed. Bonferroni correction was applied to control for multiple comparisons so that a threshold of P < .00625 (.05/8) was required for results to be considered as significant.
2.5.2. Neuroimaging data analyses
2.5.2.1. Voxelwise analyses
The smoothed normalized gray matter segments issued from the DARTEL toolbox and the smoothed preprocessed FDG-PET images were entered in voxelwise full-factorial analyses with three groups (Aβneg HC, Aβpos aMCI, and Aβneg aMCI) and including age and education (and total intracranial volume for MRI data) as nuisance variables. The total intracranial volume was obtained by summing the volumes of the gray matter, white matter, and cerebrospinal fluid obtained from the T1-weighted images using the VBM12 toolbox implemented in SPM12. To address the issue of multiple comparisons, a voxel-level P (uncorrected) < .001 threshold was combined with a cluster size allowing to achieve a corrected statistical significance for multiple comparisons of P < .05 (as determined through Monte Carlo simulations using the AlphaSim program), that is, k > 50 for MRI and k > 120 for FDG-PET.
2.5.2.2. Region of interest (ROI) analyses
In addition to voxelwise analyses, we also conducted ROI-based analyses in regions specifically sensitive to AD, that is, the hippocampus for structural MRI and the posterior association cortex for FDG-PET (see Supplementary Materials for details).
All ROI measures were transformed into W-scores using the Aβneg HC as the reference, that is, age- and education-adjusted (and total intracranial volume for hippocampal volumes) Z-scores [27]. Then they were compared between groups (Aβneg HC, Aβneg aMCI, and Aβpos aMCI) with single-factor (group) analyses of variance and post hoc comparisons when the effect of group was significant.
2.5.3. Discriminant analyses
Discriminant analyses were performed to assess the ability of each neuropsychological score and imaging biomarker to distinguish Aβneg aMCI from Aβpos aMCI. Areas under the receiver operating characteristic curve (AUC) of each neuropsychological score and each imaging biomarker were then compared to assess which measure was the most accurate to discriminate Aβneg from Aβpos aMCI. ROC analyses were conducted in Easy-ROC version 1.3 (http://www.biosoft.hacettepe.edu.tr/easyROC/).
3. Results
3.1. Demographic, clinical, and neuropsychological data
As illustrated in Table 1, the three groups did not differ in age, gender, and education level. The Mini–Mental State examination score was significantly lower in both aMCI patient subgroups compared to Aβneg HC but did not differ between the two aMCI subgroups. The proportion of apolipoprotein ε4 carriers was significantly higher in Aβpos aMCI compared to both Aβneg aMCI and Aβneg HC.
Aβneg aMCI showed lower baseline performance in nonepisodic memory tests compared to Aβpos aMCI, with a significant difference between groups for language and a trend effect for working memory. By contrast, Aβpos aMCI showed lower performance in episodic memory compared to Aβneg aMCI with a significant between-group difference for recognition and a trend effect for free recall (Table 2).
Table 2.
Neuropsychological data for patients with amyloid-negative (Aβneg aMCI) and amyloid-positive (Aβpos aMCI) amnestic mild cognitive impairment
| Measure | Aβneg aMCI, mean ± SD | Aβpos aMCI, mean ± SD | Group comparison |
|---|---|---|---|
| Free recall (episodic memory) | −2.0 ± 1.3 | −3.0 ± 1.5 | Pt-test = .02; Aβneg aMCI > Aβpos aMCI |
| Recognition (episodic memory) | −3.5 ± 3.2 | −8.2 ± 5.1 | Pt-test< .001; Aβneg aMCI > Aβpos aMCI |
| Verbal fluency | −1.1 ± 1.5 | −0.9 ± 1.7 | Pt-test = .67 |
| Languagea | 6/13 | 18/6 | Pχ2< .005; Aβpos aMCI > Aβneg aMCI |
| Short-term memory | −0.02 ± 1.1 | −0.1 ± 1.3 | Pt-test = .90 |
| Working memory | −0.7 ± 0.7 | −0.1 ± 0.9 | Pt-test = .01; Aβpos aMCI > Aβneg aMCI |
| Executive functions | −1.0 ± 3.1 | −0.2 ± 1.9 | Pt-test = .31 |
| Visuospatial functions | −1.4 ± 1.8 | −2.0 ± 4.8 | Pt-test = .64 |
NOTE. Scores and composite scores are expressed as W-scores relative to Aβneg HC (by definition, Aβneg HC mean ± SD = 0.0 ± 1.0).
Between-group differences were assessed using t-tests and for categorical variable, χ2 test. P values < .00625 (significant P values after Bonferroni correction for multiple comparisons) are in bold.
Indicated is number of no error cases/number of one error or more cases.
The clinical follow-up was available in 38 of the 44 aMCI (18 Aβpos and 20 Aβneg) within an average of 31.2 ± 8.3 months (ranging from 7 to 43 months). Four (22%) of the Aβpos aMCI and none (0%) of the Aβneg aMCI converted to AD dementia. The remaining 14 Aβpos aMCI (78%) and 15 (80%) of the Aβneg aMCI remained diagnosed as aMCI. The diagnosis changed in the remaining four Aβneg aMCI patients (20%) for “reversion to normal,” corticobasal degeneration dementia, suspected hippocampal sclerosis, and Lewy body dementia, respectively.
3.2. Neuroimaging data
3.2.1. Voxelwise analyses
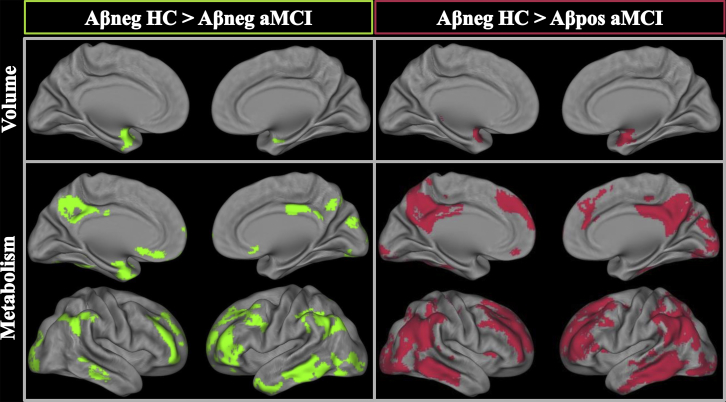
Compared to Aβneg HC, both Aβpos aMCI and Aβneg aMCI showed significant atrophy in the anterior part of the hippocampal region (including the hippocampal head and amygdala extending to the limen insula; see Fig. 1 and Supplementary Fig. 1 for effect sizes). Atrophy also concerned the posterior part of the left hippocampus in Aβpos aMCI. No significant difference was found between Aβpos aMCI and Aβneg aMCI.
Fig. 1.
Voxelwise gray matter and FDG-PET comparisons between amyloid-negative healthy controls (Aβneg HC) and amyloid-negative (Aβneg) and amyloid-positive (Aβpos) amnestic mild cognitive impairment (aMCI) patients. The threshold was set at P uncorrected < .001, k > 50 for MRI, and k > 120 for FDG-PET. Effect sizes are presented in Supplementary Fig. 1. Abbreviations: FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography; MRI, magnetic resonance imaging.
Compared to Aβneg HC, both Aβpos aMCI and Aβneg aMCI showed significant hypometabolism in the posterior cingulate precuneus, angular gyrus, and middle frontal cortex (Fig. 1 and Supplementary Fig. 1 for effect sizes). Hypometabolism was present also in the middle temporal gyrus in the Aβpos aMCI and the inferior temporal gyrus in the Aβneg aMCI. No significant difference was found between Aβpos aMCI and Aβneg aMCI.
The results were unchanged when excluding the “intermediate” aMCI cases with an SUVr value around 5% of the threshold.
3.2.2. ROI analyses
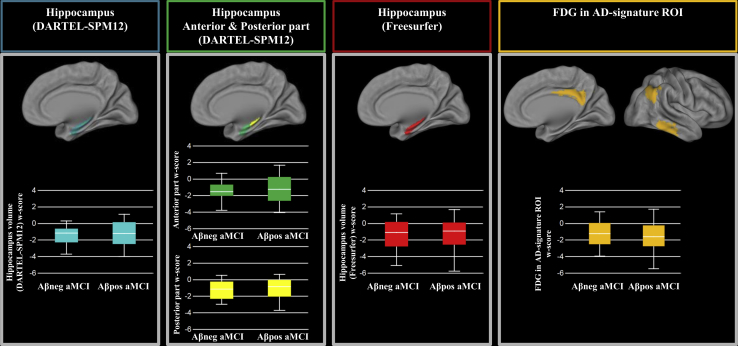
For the whole hippocampus volume (DARTEL-SPM12), both Aβpos aMCI and Aβneg aMCI showed smaller hippocampi than Aβneg HC while there was no difference between the two aMCI subgroups (Fig. 2 and Supplementary Table 1). The same results were found when assessing the anterior and posterior hippocampus and on hippocampal volumes obtained with FreeSurfer.
Fig. 2.
Regions of interest comparisons between amyloid-negative (Aβneg) and amyloid-positive (Aβpos) amnestic mild cognitive impairment (aMCI) patients. Volumes and FDG SUVr are expressed as W-scores. Abbreviations: FDG, 18F-fluorodeoxyglucose; SUVr, standardized uptake value ratio.
In the AD-signature ROI, both aMCI subgroups had lower metabolism compared to Aβneg HC while no difference was found between Aβneg aMCI and Aβpos aMCI (Fig. 2 and Supplementary Table 1). The results were unchanged when excluding the “intermediate” aMCI cases with an SUVr value around 5% of the threshold.
3.3. Discriminant analyses
For the discrimination between Aβneg and Aβpos aMCI, the AUCs of free recall (episodic memory), recognition (episodic memory), language, and working memory scores were all significantly different from 0.5 (Table 3), which means that these measures were able to distinguish Aβneg from Aβpos aMCI patients (significant P values ranging from .002 to .02). By contrast, none of the neuroimaging measures had an AUC significantly different from 0.5 (P values ranging from .3 to .8). When directly comparing the AUC of the neuropsychological versus the neuroimaging measures (Supplementary Table 2), all differences were in the same direction, that is, showing higher accuracy for neuropsychological scores to discriminate between Aβneg and Aβpos aMCI compared to neuroimaging biomarkers.
Table 3.
Receiver operating characteristic curves for discriminating patients with amyloid-negative (Aβneg aMCI) from those with amyloid-positive (Aβpos aMCI) amnestic mild cognitive impairment
| Measure | Area under the curve | Lower limit–upper limit | P value |
|---|---|---|---|
| Imaging biomarkers | |||
| Hippocampus volume (DARTEL-SPM12) | 0.48 | 0.29–0.67 | .82 |
| Anterior part | 0.46 | 0.28–0.64 | .67 |
| Posterior part | 0.53 | 0.35–0.72 | .72 |
| Hippocampus volume (FreeSurfer) | 0.54 | 0.35–0.72 | .70 |
| FDG in AD-signature ROI | 0.60 | 0.41–0.78 | .30 |
| Neuropsychological scores | |||
| Free recall (episodic memory) | 0.73 | 0.57–0.90 | .004 |
| Recognition (episodic memory) | 0.74 | 0.58–0.89 | .002 |
| Verbal fluency | 0.45 | 0.26–0.64 | .60 |
| Language | 0.28 | 0.10–0.46 | .02 |
| Short-term memory | 0.55 | 0.37–0.74 | .56 |
| Working memory | 0.31 | 0.14–0.49 | .04 |
| Executive functions | 0.49 | 0.29–0.67 | .89 |
| Visuospatial functions | 0.41 | 0.23–0.59 | .34 |
Abbreviations: FDG, 18F-fluorodeoxyglucose; AD, Alzheimer's disease; ROI, region of interest.
4. Discussion
This work explored differences between Aβpos and Aβneg aMCI patients in terms of cognitive deficits and brain patterns of atrophy and hypometabolism assessing all patients recruited and scanned in the same center. Our findings showed that differences essentially concerned the profile of cognitive deficits with more pronounced episodic memory deficits in Aβpos aMCI and greater language deficits (and working memory deficits as a tendency) in Aβneg aMCI patients. By contrast, both aMCI subgroups showed similar patterns of atrophy and hypometabolism in regions known to be sensitive to AD, that is, the hippocampus and posterior associative brain areas, respectively, with no substantial difference between subgroups.
Our findings of differential impairment of Aβpos versus Aβneg aMCI patients are in line with previous studies that reported greater episodic memory impairment in Aβpos aMCI [11], [12], [15] and greater deficits in nonepisodic memory domains such as working memory and language in Aβneg aMCI [12], [20]. The neuropsychological and neuroimaging profiles of alterations of the Aβneg aMCI highlighted in the present study would be consistent with several neurological conditions or diseases. Thus, psychiatric conditions such as subclinical depression, vascular diseases, or age-related nonamyloid neurodegenerative diseases such as frontotemporal dementia and dementia with Lewy bodies could contribute or lead to similar cognitive [31], [32], [33], [34], [35], [36] and brain [36], [37], [38], [39] alteration profiles. As regard to possible neuropathological causes of Aβneg aMCI, TDP 43 proteinopathy might be involved in some Aβneg aMCI cases as it is known to be associated with episodic memory, language, and working memory deficits [40], [41]. Tangle-predominant pathology or, as more recently termed, primary age-related tauopathy, defined by AD-type neurofibrillary changes without, or with few, Aβ plaques are other likely pathological etiologies for Aβneg aMCI. Finally, hippocampal sclerosis and argyrophilic grain disease were found to be the main etiologies of Aβneg aMCI in a neuropathological study assessing seven Aβneg aMCI patients, none of which meeting the neuropathologic criteria for AD [42]. These hippocampal-specific pathologies might also explain neurodegeneration in the absence of amyloid deposition in aMCI patients [43], [44]. Thus, Aβneg aMCI is likely a heterogeneous group with multiple possible etiologies.
As regard to neuroimaging, the profiles of atrophy and hypometabolism of Aβpos and Aβneg aMCI compared to controls were consistent with those found in the prodromal stage of AD [45]. More specifically, previous studies consistently reported hippocampal atrophy and posterior associative hypometabolism in Aβpos aMCI in comparison to controls [11], [12], [14], [15], [22]. Results are less consistent for Aβneg aMCI patients as hippocampal atrophy [12], [14] or hypometabolism [15] has been reported in some but not all [11], [14] studies.
When directly comparing Aβpos to Aβneg aMCI patients, we did not find any significant difference in terms of atrophy or hypometabolism using both ROI and voxel-based analyses. Previous studies reported inconsistent findings with some showing greater atrophy [12], [13], [14], [21] or hypometabolism in Aβpos aMCI [13], [14], [15], while other studies reported same degree of brain alterations between these two groups of patients [11], [22]. Similarly, it has been shown that hippocampal volume was not able to predict brain amyloidosis in aMCI (AUC = 0.56; P > .05; [46]). Methodological differences, including the methods used to measure hippocampal volume or to define amyloid positivity, could explain these discordances. However, we used different approaches to measure hippocampal volume (voxelwise, ROI-based with DARTEL-SPM12 versus FreeSurfer approaches, whole hippocampus vs. anterior/posterior segmentation), and they all lead to the same findings, so that the lack of difference in the present study is unlikely due to the method. Similarly, our findings remained unchanged when excluding the patients with an SUVr within 5% of the threshold (n = 8). Interestingly, voxelwise analyses showed that, despite an overall negative rating, the Aβneg aMCI had higher amyloid load than the Aβneg HC in the precuneus and frontal regions (see Supplementary Material), suggesting that some of them already showed increased accumulation in selected brain regions compared to the controls. This has no impact on the conclusion of the article, especially as our results remained unchanged when excluding the “intermediate” cases (who did not differ anymore from the controls in terms of florbetapir binding when compared voxelwise; see Supplementary Material). Yet, this further supports the relevance of subthreshold amyloid accumulation [47], [48], [49], [50], [51]. Differences in the samples (sample size or degree of cognitive impairment) could also explain the discordances between studies. More specifically, the limited sample size in the present study might have prevented us from detecting neuroimaging differences due to limited statistical power. However, the differences were not even close to significance (all P values > .40). Moreover, this limitation was partly compensated by the fact that all data were acquired in the same center on the same scanners (increasing homogeneity and thus sensitivity). Finally, apart from Landau et al. [13], sample sizes were similar or lower in previous studies (Supplementary Table 3). On the other hand, in two previous studies where hippocampal volume was significantly more atrophied in Aβpos aMCI compared to Aβneg aMCI, the former were also significantly more cognitively impaired (i.e., had lower Mini–Mental State Examination) than the latter [13], [21] (Supplementary Table 3). It is thus possible that the greater hippocampal atrophy reflected the more severe cognitive deficits in the Aβpos aMCI in these previous studies, while in the present study, both aMCI subgroups had similar degree of cognitive impairment. As a whole, discordances in previous studies comparing neuroimaging data between Aβpos and Aβneg aMCI might reflect the heterogeneity in the size or degree of cognitive impairment of the samples or other methodological differences that limit the interpretations and comparisons between studies. The use of a control group (the Aβneg HC) seems of particular relevance so that both subgroups could be further characterized by comparison to a common reference group.
The lack of differences in volume and metabolism suggests that these specific profiles of atrophy and hypometabolism would not be due to the presence of Aβ and/or that two different processes (Aβ vs. another pathological process) could lead to the same patterns of alteration. Interestingly, at the AD dementia stage, the comparison between Aβneg and Aβpos patients led to similar cognitive findings (with greater deficits in language and executive functions in the former and in episodic memory in the latter), while neuroimaging findings differed from those found here in aMCI patients. Indeed, strong differences were found between groups with Aβpos AD being significantly more atrophied and hypometabolic than Aβneg AD patients in posterior associative cortical areas [52].
The clinical follow-up revealed that, among the 38 aMCI patients followed over 7 to 43 months, 4 of the 18 Aβpos aMCI group converted to AD while none among the 20 Aβneg aMCI did. Moreover, three Aβneg aMCI patients developed another pathology (i.e., corticobasal degeneration dementia, suspected hippocampal sclerosis, and Lewy body dementia) and one reverted to normal cognition with memory complaint (subjective cognitive decline; [53]). This further illustrates that aMCI is a heterogeneous entity that can be due to various underlying neurological or psychiatric etiologies [11], [12], [54]. These differences in terms of clinical evolution between Aβpos and Aβneg aMCI suggest that Aβpos aMCI patients are on the way to AD dementia while Aβneg aMCI patients are progressively differentiating to another dementia.
Research in Context.
-
1.
Systematic review: Among amnestic mild cognitive impairment (aMCI) patients, 50% to 75% are classified as amyloid positive (Aβpos). A better understanding of what differentiates Aβpos from amyloid-negative aMCI patients would be useful for enrichment of clinical trials and to improve our knowledge of the role of amyloid deposition in the physiopathology of Alzheimer's disease.
-
2.
Interpretation: Aβpos aMCI had lower performances in episodic memory while Aβneg aMCI had worse scores in nonmemory tasks, but the two subgroups did not differ in their profiles of atrophy or hypometabolism.
-
3.
Future directions: In a sample of aMCI patients recruited and scanned in the same center, the main difference between Aβpos and Aβneg aMCI concerned the neuropsychological profile. The lack of differences in volume and metabolism suggests that these specific profiles of atrophy and hypometabolism would not be due to the presence of Aβ and/or that two different processes (Aβ vs. another pathological process) could lead to the same patterns of alteration.
Acknowledgments
The authors thank J. Gonneaud, B. Desgranges, B. Landeau, F. Mézenge, J. Mutlu A. Perrotin, G. Poisnel, M. Leblond, T. Anquetil, K. Mevel, N. Villain, M. Fouquet, A. Quillard, C. Schupp, J. Dayan, A. Chocat, L. Barre, A. Manrique, and the Cyceron MRI-PET staff members for their help with data acquisition. The authors are grateful to C. Andre, R. La Joie, and A. Bejanin for their insightful comments and to the participants of the IMAP+ study.
The study was supported by Fondation Plan Alzheimer (Alzheimer Plan 2008–2012); Programme Hospitalier de Recherche Clinique (PHRCN 2011-A01493-38 and PHRCN 2012 12-006-0347); Agence Nationale de la Recherche (LONGVIE 2007); Région Basse-Normandie; Association France Alzheimer et maladies apparentées AAP 2013. Funding sources were not involved in the study design, data acquisition, data analysis, or article writing.
Footnotes
The authors declare no conflict of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.02.008.
Supplementary data
References
- 1.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellinger K. Morphology of Alzheimer's disease and related disorders. In: Maurer K., Riederer P., Beckmann H., editors. Alzheimer's Disease. Epidemiology, Neuropathology, Neurochemistry, and Clinics. Key Topics in Brain Research. Springer; Vienna, Austria: 1990. [Google Scholar]
- 4.Masters C.L., Beyreuther K. The neuropathology of Alzheimer's disease in the year 2005. In: Beal M.F., Lang A.E., Ludolph A.C., editors. Neurodegenerative Diseases: Neurobiology, Pathogenesis and Therapeutics. Cambridge University Press; Cambridge, UK: 2005. pp. 433–440. [Google Scholar]
- 5.Ossenkoppele R., Jansen W.J., Rabinovici G.D., Knol D.L., van der Flier W.M., van Berckel B.N.M. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen R.C. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 8.Morris J.C., Price J.L. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 9.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 10.Petersen R.C. Mild cognitive impairment. Continuum (Minneap Minn) 2016;22:404–418. doi: 10.1212/CON.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolk D.A., Price J.C., Saxton J.A., Snitz B.E., James J.A., Lopez O.L. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye B.S., Seo S.W., Kim C.H., Jeon S., Kim G.H., Noh Y. Hippocampal and cortical atrophy in amyloid-negative mild cognitive impairments: comparison with amyloid-positive mild cognitive impairment. Neurobiol Aging. 2014;35:291–300. doi: 10.1016/j.neurobiolaging.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Landau S.M., Horng A., Fero A., Jagust W.J., Alzheimer's Disease Neuroimaging Initiative F the ADN Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86:1377–1385. doi: 10.1212/WNL.0000000000002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanseeuw B.J., Schultz A.P., Betensky R.A., Sperling R.A., Johnson K.A. Decreased hippocampal metabolism in high-amyloid mild cognitive impairment. Alzheimers Dement. 2016;12:1288–1296. doi: 10.1016/j.jalz.2016.06.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon S.Y., Yi D., Byun M.S., Choi H.J., Kim H.J., Lee J.H. Differential patterns of regional cerebral hypometabolism according to the level of cerebral amyloid deposition in patients with amnestic mild cognitive impairment. Neurosci Lett. 2016;632:104–108. doi: 10.1016/j.neulet.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 16.Forsberg A., Almkvist O., Engler H., Wall A., Långström B., Nordberg A. High PIB retention in Alzheimer's disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010;7:56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- 17.Okello A., Koivunen J., Edison P., Archer H.A., Turkheimer F.E., Någren K. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong K.T., Villemagne V.L., Bahar-Fuchs A., Lamb F., Langdon N., Catafau A.M. Aβ imaging with 18F-florbetaben in prodromal Alzheimer's disease: a prospective outcome study. J Neurol Neurosurg Psychiatry. 2015;86:431–436. doi: 10.1136/jnnp-2014-308094. [DOI] [PubMed] [Google Scholar]
- 19.Rowe C.C., Bourgeat P., Ellis K.A., Brown B., Lim Y.Y., Mulligan R. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 20.Lim Y.Y., Ellis K.A., Harrington K., Kamer A., Pietrzak R.H., Bush A.I. Cognitive consequences of high Aβ amyloid in mild cognitive impairment and healthy older adults: Implications for early detection of Alzheimer's disease. Neuropsychology. 2013;27:322–332. doi: 10.1037/a0032321. [DOI] [PubMed] [Google Scholar]
- 21.Huijbers W., Mormino E.C., Schultz A.P., Wigman S., Ward A.M., Larvie M. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138:1023–1035. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Joie R., Perrotin A., de La Sayette V., Egret S., Doeuvre L., Belliard S. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer's disease and semantic dementia. Neuroimage Clin. 2013;3:155–162. doi: 10.1016/j.nicl.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arenaza-Urquijo E.M., Gonneaud J., Fouquet M., Perrotin A., Mézenge F., Landeau B. Interaction between years of education and APOE ε4 status on frontal and temporal metabolism. Neurology. 2015;85:1392–1399. doi: 10.1212/WNL.0000000000002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Joie R., Perrotin A., Barre L., Hommet C., Mezenge F., Ibazizene M. Region-specific hierarchy between atrophy, hypometabolism, and β-Amyloid (Aβ) load in Alzheimer's disease dementia. J Neurosci. 2012;32:16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrotin A., de Flores R., Lamberton F., Poisnel G., La Joie R., de la Sayette V. Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. J Alzheimers Dis. 2015;48:S141–S150. doi: 10.3233/JAD-150087. [DOI] [PubMed] [Google Scholar]
- 26.Tomadesso C., Perrotin A., Mutlu J., Mézenge F., Landeau B., Egret S. Brain structural, functional, and cognitive correlates of recent versus remote autobiographical memories in amnestic Mild Cognitive Impairment. Neuroimage Clin. 2015;8:473–482. doi: 10.1016/j.nicl.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack C.R., Dickson D.W., Parisi J.E., Xu Y.C., Cha R.H., O'Brien P.C. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besson F.L., La Joie R., Doeuvre L., Gaubert M., Mézenge F., Egret S. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. J Neurosci. 2015;35:10402–10411. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizenstein H.J., Nebes R.D., Saxton J.A., Price J.C., Mathis C.A., Tsopelas N.D. Impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwertman N.C., de Silva R. Identifying outliers with sequential fences. Comput Stat Data Anal. 2007;51:3800–3810. [Google Scholar]
- 31.Pantzar A., Laukka E.J., Atti A.R., Fastbom J., Fratiglioni L., Bäckman L. Cognitive deficits in unipolar old-age depression: a population-based study. Psychol Med. 2014;44:937–947. doi: 10.1017/S0033291713001736. [DOI] [PubMed] [Google Scholar]
- 32.Graham N.L., Emery T., Hodges J.R. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- 33.Desmond D.W. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004;226:3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Harciarek M., Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer's disease: a review. Neuropsychol Rev. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- 35.Boeve B.F. Mild cognitive impairment associated with underlying Alzheimer's disease versus Lewy body disease. Parkinsonism Relat Disord. 2012;18:S41–S44. doi: 10.1016/S1353-8020(11)70015-3. [DOI] [PubMed] [Google Scholar]
- 36.Kim H.J., Ye B.S., Yoon C.W., Noh Y., Kim G.H., Cho H. Cortical thickness and hippocampal shape in pure vascular mild cognitive impairment and dementia of subcortical type. Eur J Neurol. 2014;21:744–751. doi: 10.1111/ene.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon E., Rohrer J.D., Fox N.C. Advances in neuroimaging in frontotemporal dementia. J Neurochem. 2016;138:193–210. doi: 10.1111/jnc.13656. [DOI] [PubMed] [Google Scholar]
- 38.Kempton M.J., Salvador Z., Munafò M.R., Geddes J.R., Simmons A., Frangou S. Structural neuroimaging studies in major depressive disorder. Arch Gen Psychiatry. 2011;68:675. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 39.Tam C.W.C., Burton E.J., McKeith I.G., Burn D.J., O'Brien J.T. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64:861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- 40.Nag S., Yu L., Wilson R.S., Chen E.-Y., Bennett D.A., Schneider J.A. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology. 2017;88:653–660. doi: 10.1212/WNL.0000000000003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson R.S., Yu L., Trojanowski J.Q., Chen E.-Y., Boyle P.A., Bennett D.A. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70:1418. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen R.C., Parisi J.E., Dickson D.W., Johnson K.A., Knopman D.S., Boeve B.F. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 43.Duara R., Loewenstein D.A., Shen Q., Barker W., Potter E., Varon D. Amyloid positron emission tomography with 18F-flutemetamol and structural magnetic resonance imaging in the classification of mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2013;9:295–301. doi: 10.1016/j.jalz.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prestia A., Caroli A., van der Flier W.M., Ossenkoppele R., Van Berckel B., Barkhof F. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80:1048–1056. doi: 10.1212/WNL.0b013e3182872830. [DOI] [PubMed] [Google Scholar]
- 45.Chetelat G., Desgranges B., de la Sayette V., Viader F., Berkouk K., Landeau B. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126:1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- 46.Tosun D., Joshi S., Weiner M.W., Alzheimer's Disease Neuroimaging Initiative Neuroimaging predictors of brain amyloidosis in mild cognitive impairment. Ann Neurol. 2013;74:188–198. doi: 10.1002/ana.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonneaud J., Arenaza-Urquijo E.M., Mézenge F., Landeau B., Gaubert M., Bejanin A. Increased florbetapir binding in the temporal neocortex from age 20 to 60 years. Neurology. 2017;89:2438–2446. doi: 10.1212/WNL.0000000000004733. [DOI] [PubMed] [Google Scholar]
- 48.Grothe M.J., Barthel H., Sepulcre J., Dyrba M., Sabri O., Teipel S.J. In vivo staging of regional amyloid deposition. Neurology. 2017;89:2031–2038. doi: 10.1212/WNL.0000000000004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chételat G., Murray M.E. Amyloid PET scan. Neurology. 2017;89:2029–2030. doi: 10.1212/WNL.0000000000004678. [DOI] [PubMed] [Google Scholar]
- 50.Landau SM, Horng A, Jagust WJ. Memory decline accompanies subthreshold amyloid accumulation [published online ahead of print March 23, 2018]. Neurology. Available at: 10.1212/WNL.0000000000005354. [DOI] [PMC free article] [PubMed]
- 51.McMillan CT, Chételat G. Amyloid “accumulators”: The next generation of candidates for amyloid-targeted clinical trials? [published online March 23, 2018]. Neurology. Available at: 10.1212/WNL.0000000000005362. [DOI] [PubMed]
- 52.Chételat G., Ossenkoppele R., Villemagne V.L., Perrotin A., Landeau B., Mézenge F. Atrophy, hypometabolism and clinical trajectories in patients with amyloid-negative Alzheimer's disease. Brain. 2016;139:2528–2539. doi: 10.1093/brain/aww159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes T.F., Snitz B.E., Ganguli M. Should mild cognitive impairment be subtyped? Curr Opin Psychiatry. 2011;24:237–242. doi: 10.1097/YCO.0b013e328344696b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.