Abstract
Lactose intolerance is exceedingly common, reportedly affecting up to 70% of the world’s population, leading to both abdominal and systemic symptoms. Current treatment focuses predominantly on restricting dietary consumption of lactose. Given lactose is one of the most commonly used excipients in the pharmaceutical industry, consideration must be given to the lactose content and therefore safety of pharmaceutical preparations prescribed for patients with lactose intolerance. This article summarizes the current literature examining the likelihood of inducing adverse effects through the administration of lactose-containing pharmaceutical preparations in patients reporting lactose intolerance, describes how to assess this risk on an individual patient basis and reviews suitable analgesic options for this population.
A case study is presented detailing a patient reporting lactose intolerance who insists on treatment with the lactose-free product codeine/ibuprofen (Nurofen Plus) rather than other codeine-free analgesics. It is important to assess the likelihood of lactose as an excipient inducing symptoms in this scenario, as reluctance to cease codeine could suggest codeine dependence, an issue that is becoming increasingly common in countries such as Australia and Canada. Given codeine dependence is associated with serious sequelae including hospitalization and death, the patient must either be reassured the lactose component in their prescribed analgesics will not induce symptoms or an alternative treatment strategy must be confirmed. General recommendations applying theory from the literature to the management of acute pain in lactose-intolerant patients are discussed and specific treatment options are outlined.
Although large inter-individual variability is reported, most lactose-intolerant patients can tolerate the small quantities of lactose found in pharmaceutical preparations. Cumulative lactose exposure can be assessed in patients taking multiple medications while also consuming lactose in the diet. In those sensitive to small quantities of lactose, lactase supplements can be trailed. Additionally, for the analgesic drug classes employed for the management of acute pain, lactose-free formulations, including most oral liquids and dispersible tablets and some oral tablets and capsules, are available.
Keywords: acute pain, analgesics, codeine, excipient, lactase deficiency, lactose intolerance
Introduction
In pharmacy practice, it is common to encounter patients who report lactose intolerance and therefore request lactose-free medications. Doctors and other prescribers are likely to face similar scenarios. In this situation, the health practitioner must assess the extent of lactose exposure resulting from the preferred treatment option, the likelihood of this exposure triggering clinical symptoms of lactose intolerance and the appropriateness of the alternative treatments available to obtain optimal outcomes for their patient.
Case scenario
We recently encountered a patient (woman, 32 years old) who was regularly consuming Nurofen Plus (Reckitt Benckiser, Sydney NSW Australia), a combination analgesic containing ibuprofen 200 mg and codeine phosphate 12.8 mg,1 which she had self-initiated and was purchasing over the counter to treat shoulder pain. She presented to a medical practitioner with gastrointestinal complaints suggestive of ibuprofen overuse. When her doctor suggested an alternative analgesic the patient insisted she remain on Nurofen Plus as she cannot tolerate lactose and this product is lactose free. The doctor then sought pharmacist advice regarding the use of lactose-containing pharmaceutical preparations in patients with lactose intolerance and alternative lactose-free analgesics. We were particularly motivated to confirm a suitable medication regimen for this patient given the recent rise in reports of codeine dependence and serious associated harm due to dose escalation. Numerous case reports detail the potentially life-threatening outcomes of patients using excessive amounts of Nurofen Plus.2–10
Lactose is a naturally occurring disaccharide of galactose bound to glucose that is obtained from the whey of milk.11–14 Lactose presents as a free-flowing powder, the particle size and flow characteristics of which can be easily modified.11 Lactose is also water soluble, inert, inexpensive, nontoxic, chemically stable and very palatable,15 all desirable characteristics for a pharmaceutical excipient.11 Lactose is used primarily in capsules and tablets as a bulking agent, filler or diluent, but is also used in lyophilized products, as a drug carrier in dry-power inhalers and in combination with sucrose in sugar-coating solutions.11,16 Thus, lactose is one of the most commonly used excipients in the pharmaceutical industry17 with an estimated 20% of prescription medicines including this excipient.18
Pathophysiology, prevalence and diagnosis of lactose intolerance
Approximately 70% of the population worldwide are thought to suffer from lactose intolerance, due to primary lactase deficiency and lactose malabsorption.13,15 Lactose intolerance is a clinical nonimmunologic syndrome that occurs when a person cannot or inefficiently metabolizes lactose.14,19 In order for intestinal absorption of lactose to occur, this disaccharide must be hydrolysed into its monosaccharide components via lactase-phlorizin hydrolase, an intestinal brush border lactase enzyme.12,14,15 Lactase enzyme expression and activity tends to peak at birth as it is essential during breastfeeding and infancy.12,15 However, in approximately two thirds of the world’s population, after a few months of life lactase expression declines, as it is no longer essential for survival on a milk-based diet.12 This is known as lactase nonpersistence, a primary lactase deficiency.12–14 Secondary lactase deficiency occurs when gastrointestinal diseases such as gastroenteritis or inflammatory bowel disorder cause small bowel injury and a decline in lactase activity.12–14
Lactose intolerance is the clinical presentation of lactase deficiency and lactose malabsorption. Nonmetabolized and therefore unabsorbed, lactose increases the gastrointestinal osmotic load and subsequently increases intestinal water content. This leads directly to diarrhoea, and to fermentation of unabsorbed lactose by the colonic microbiome, which produces short chain fatty acids and gas.12,14 These processes result in an array of additional symptoms, such as abdominal discomfort/pain, bloating, stomach cramps, flatulence, and headache,13–15,20 which are often distressing to the patient. Numerous tests with a range of sensitivities are available to assess lactose malabsorption and tolerance, including hydrogen breath test, lactose tolerance test, small bowel biopsy, blood glucose testing, genetic testing and assessment of lactose activity at the jejunal brush border.12–14
Dietary lactose tolerance varies between patients.12,13,15 It is thought that numerous factors such as age, residual lactase expression, ingestion with other dietary components, gut transit time, small bowel expression, small bowel bacterial overgrowth, and composition of the enteric microbiome contribute to these inter-patient differences.12,13,15 Lactose tolerance is also associated with the amount of milk ingested by an individual, with those frequently ingesting milk less likely to display symptoms of lactose intolerance.21 Additionally, patients with irritable bowel syndrome present a greater self-reported risk of dairy/lactose intolerance.12 The frequency of lactose intolerance also varies between different populations around the world, ranging from 25% of Europeans, to 50–80% of those with a Hispanic background, 15–80% in the United States and almost 100% in American Indians and Asians.15
General treatment for lactose intolerance
The mainstay of treatment for lactose intolerance centres around dietary restriction to reduce lactose exposure, however most patients do not require a severely restricted or entirely lactose-free diet.12–15,22 Lactase enzyme replacement represents another potential treatment for lactose intolerance,12,13 with replacement providing both objective and subjective benefits.23 A systematic review found probiotic supplementation in general does not improve symptoms in patients with lactose intolerance, although data suggest particular strains at specific concentrations may provide a benefit.24
Assessing lactose tolerance and relative safety of lactose-containing pharmaceutical formulations in individual patients
The reported amount of ingested lactose required to produce clinical symptoms in a lactose-intolerant individual varies. In those with lactase nonpersistence, emergence of symptoms depends on the quantity of lactose that needs to be consumed before the lactase available becomes saturated. Results from a systematic review indicate patients with self-reported lactose intolerance can tolerate approximately 12 g of dietary lactose when consumed as a single dose without other nutrients, while 15–18 g could be tolerated as a single dose without symptoms if given with other nutrients.25–27 As the amount of lactose contained in 1 ml of milk is approximately 47 mg, this equates to a tolerance of around 254 ml of milk.15,28,29 However, a number of case reports exist in the literature detailing patients experiencing symptoms following lactose ingestion in pharmaceutical formulations.30–37 Notably, some individuals report gastrointestinal symptoms following exposure to as little as 100–200 mg of lactose,22 and the majority of the case reports include patients documented to be extremely sensitive to lactose-containing products.38
It must also be considered, however, that the symptoms documented in case reports may represent a negative placebo effect, or ‘nocebo’ effect, driven by psychological factors such as expectation. Patients who have experienced symptoms post ingestion of large quantities of milk may become psychologically sensitized, or conditioned, to anticipate symptoms following consumption of even very small amounts of lactose.39 In the practice setting it may be difficult to establish to what extent the nocebo effect contributes to a patient’s symptoms, and equally as difficult to explain this concept to the patient without appearing dismissive. While the treating clinician should inform the patient about the possible impact of expectation on symptoms, care must be taken to acknowledge the patient’s experience and minimize possible disruption to the patient–clinician relationship. In many cases, regardless of symptom aetiology, lactose-free treatment may be preferable, as this most often aligns with the patients’ wishes.
For most pharmaceutical products the total dose of lactose contained is usually less than 2 g/day.16 A double-blind, placebo-controlled, crossover study found no statistical difference in symptom presence or breath hydrogen excretion between lactose-intolerant patients ingesting capsules containing 400 mg of lactose and placebo. Thus, the authors propose lactose intolerance should not be a contraindication to pharmaceutical products containing up to 400 mg of lactose.40
Importantly, when prescribing or recommending a treatment, the amount of lactose per tablet should be considered in the context of the number of tablets per day, the number of other lactose-containing medications the patient is taking and the amount of lactose consumed in the diet. For reference, a study investigating lactose exposure resulting from medication regimens to treat gastrointestinal disorders found the maximum amount of tablet-derived lactose consumed by patients ranged from 4 mg/day, equivalent to 0.2 ml of milk, up to 10.2 g/day, equivalent to 216 ml of milk, depending on the specific medication regimen.15 For some patients this exposure range, particularly at the higher end, could be sufficient to illicit symptoms.
Implications for clinicians managing acute pain
The choice of an analgesic in an acute pain setting is largely patient and condition specific. Given the variation in lactose tolerance between patients, the difficulty in establishing the contribution of the nocebo effect and the potential for lactose as an excipient to cause clinically significant symptoms as discussed above, empirically it is reasonable to consider lactose-free options when providing analgesia to a patient reporting lactose intolerance. Most commonly used analgesics are available as tablets or capsules in both lactose-containing and lactose-free brands (see Table 1), thus in the case of a lactose-intolerant individual it may be simplest to select a lactose-free formulation of the desired analgesic. Effervescent and liquid formulations rarely contain lactose and may be considered a viable alternative, and consideration of other administration routes such as sublingual, transdermal or rectal may also be appropriate.
Table 1.
Table of commonly available tablet and capsule analgesic formulations, by lactose containing and lactose-free brands.
| Analgesic class | Drug name | Lactose-containing products (formulation type) | Lactose-free products (formulation type) |
|---|---|---|---|
| NSAIDs | Aspirin | Alka-Seltzer (effervescent tablets) Aspro Clear (effervescent tablets) Disprin Direct (effervescent tablets) Disprin Original (effervescent tablets) |
|
| Aspirin + codeine | Aspalgin (effervescent tablets) Codis (effervescent tablets) Disprin Forte (effervescent tablets) |
||
| Celecoxib | APO-Celecoxib (capsules) Celaxib (capsules) Celebrex (capsules) Celecoxib Actavis (capsules) Celecoxib AN (capsules) Celecoxib Sandoz (capsules) Celexi (capsules) Kudeq (capsules) |
||
| Diclofenac | APO-diclofenac (tablets) Clonac (tablets) Diclofenac AN (tablets) Diclofenac Sandoz (tablets) Dinac (tablets) Fenac (25, 50 mg tablets) Imflac (tablets) Viclofen (tablets) Voltaren Rapid (12.5 mg tablets) |
Voltaren Rapid (liquid capsules, 50 mg tablets, 25 mg tablets) | |
| Ibuprofen | Brufen (tablets) Gold Cross Ibuprofen (tablets) Nurofen (caplets, liquid capsules, tablets) Nurofen Zavance (caplets, tablets, liquid capsules) Rafen (tablets) |
Advil (liquid capsules, tablets) Chemists’ own ibuprofen (tablets) |
|
| Ibuprofen + codeine | Chemists’ own ibuprofen plus codeine (tablets) Panafen Plus (tablets) Pharmacor ibuprofen plus codeine (tablets) |
APO Health ibuprofen plus codeine (tablets) Nurofen Plus (tablets) Rafen Plus (tablets) Trust ibuprofen plus codeine (tablets) |
|
| Meloxicam | APO-Meloxicam (capsules, tablets) Melox (capsules) Meloxiauro (tablets) Meloxibell (tablets) Meloxibindo (tablets) Meloxicam Sandoz (capsules, tablets) Mobic (capsules, tablets) Movalis (capsules, tablets) |
||
| Paracetamol + NSAID | Paracetamol + ibuprofen | Maxigesic (tablets) | Combigesic (tablets) Nuromol (tablets) |
| Paracetamol | Paracetamol | Panadol (gel capsules, mini capsules gel tablets) Panamax (tablets) Paracetamol Sandoz (tablets) |
Panadol Osteo (tablets) Panadol Ostezorb (tablets) |
| Paracetamol + codeine | APO Health paracetamol plus codeine 15 (tablets) Chemists’ own pain (tabsules) Codapane Xtra (tablets) Mydol (tablets) |
APO-paracetamol/codeine 500/30 (tablets) APO Health paracetamol plus codeine (10 mg tablets) Chemists’ own pain (tablets) Codalgin and Codalgin Forte (tablets) Codapane and Codapane Forte (tablets) Comfarol Forte (tablets) Dolaforte (tablets) Febridol Plus (tablets) Mersyndol DayStrength (tablets) Panadeine (caplets, rapid soluble tablets, tablets) Panadeine Extra (caplets) Panadeine Forte (tablets) Panamax Co (tablets) Prodeine and Prodeine-15 (caplets) Prodeine Forte (tablets) |
|
| Paracetamol + codeine + doxylamine | Chemists’ own Dolased analgesic-calmative (tablets) Codalgin Plus (tablets) Dolased Forte (tablets) |
Codagesic (tablets) Fiorinal (capsules, tablets) and Fiorinal Dental (capsules) Maxydol (tablets) Mersyndol (capsules, tablets) and Mersyndol Forte (tablets) Tensodeine (caplets) |
|
| Opioids | Codeine | Aspen codeine phosphate (tablets) | |
| Oxycodone | Endone (tablets) Mayne Pharma Oxycodone IR (tablets) Oxycodone Sandoz (CR tablets) OxyContin (CR tablets) Targin (CR tablets in combination with naloxone) |
OxyNorm (capsules) | |
| Tramadol | Tramal (CR tablets) Tramadol Sandoz (SR tablets) Tramedo (capsules) |
APO-Tramadol (capsules, SR tablets) Durotram XR (tablets) Tramadol Actavis (capsules) Tramadol AN (capsules, SR tablets) Tramadol Sandoz (capsules) Tramedo (SR tablets) Zydol (capsules) Tramal (capsules) |
Information extracted from MIMS (current at time of submission).
Abbreviations: CR = Controlled release, NSAIDs = Non-steroidal anti-inflammatory drugs, SR = Slow release. 1. Bayer Consumer Care, Pymble, NSW; 2. Reckitt Benckiser, Sydney, NSW; 3. Aspen Pharma Pty Ltd, St Leonards, NSW; 4. Apotex Pty Ltd, Macquarie Park, NSW; 5. Alphapharm Pty Ltd, Millers Point, NSW; 6. Pfizer Australia Pty Ltd, West Ryde, NSW; 7. Actavis Australia Pty Ltd, Sydney, NSW; 8. Amneal Pharma Australia Pty Ltd, South Yarra, VIC; 9. Sandoz Pty Ltd, Macquarie Park, NSW; 10. Arrow Pharma Pty Ltd, Cremorne, VIC; 11. Novartis Consumer Health Australasia Pty Ltd, Mulgrave, VIC; 12. Mylan Health Pty Ltd, Millers Point, NSW; 13. Biotech Pharmaceuticals Pty Ltd, Laverton North, VIC; 14. GlaxoSmithKline Consumer Healthcare, Ermington, NSW; 15. Pharmacor Pty Ltd, Dee Why, NSW; 16. Medis Pharma Pty Ltd, North Sydney, NSW; 17. Aurobindo Pharma (Australia) Pty Ltd, South Melbourne, VIC; 18. Generic Health Pty Ltd, Camberwell, VIC; 19. Boehringer Ingelheim Pty Ltd, North Ryde, NSW; 20. AFT Pharmaceuticals Pty Ltd, North Ryde, NSW; 21. Sanofi-Aventis Australia Pty Ltd, Macquarie Park, NSW; 22. Mayne Pharma International Pty Ltd, Salisbury South, SA; 23. Mundipharma Pty Ltd, Sydney, NSW; 24. Seqirus Pty Ltd, Parkville, VIC; 25. iNova Pharmaceuticals Australia Pty Ltd, Chatswood, NSW.
Alternatively, if the patient’s lactose tolerance can be established (by assessing how much milk the patient can consume before presenting with symptoms), the amount of lactose in the treatment regimen of choice can be calculated for comparison. If the calculated treatment-related lactose exposure is less than the patient tolerates in terms of dietary exposure, the treatment can be employed, so long as the patient is provided with counselling to reduce dietary lactose intake accordingly. Even in circumstances where the degree of lactose intolerance cannot be firmly established, as the majority of lactose-intolerant individuals will not display symptoms in response to the amounts of lactose present in pharmaceutical preparations, a single-dose tolerance trial can be performed. The dose can then be titrated upwards while monitoring tolerance if multiple doses are required each day.
In patients known to be particularly sensitive to lactose and those who fail a tolerance trial, alternative analgesic drugs and lactose replacement therapy can be considered when no lactose-free formulations containing the drug of choice are available. Administration of a lactose-containing analgesic formulation with concurrent lactase enzyme replacement therapy and strict dietary lactose restriction may be a viable option, however lactase substitutes have not yet been studied in this setting.
For all patients with severe lactose intolerance, the specific lactose content of all medications prescribed can be ascertained. The pharmacist can provide assistance in this regard, and in some situations, it may be necessary to contact product manufacturers. If the lactose content of a pharmacological treatment regimen exceeds the patients’ lactase metabolic capacity, diarrhoea can result, potentially reducing absorption of the active ingredient, and in the setting of acute analgesia, hampering pain relief.
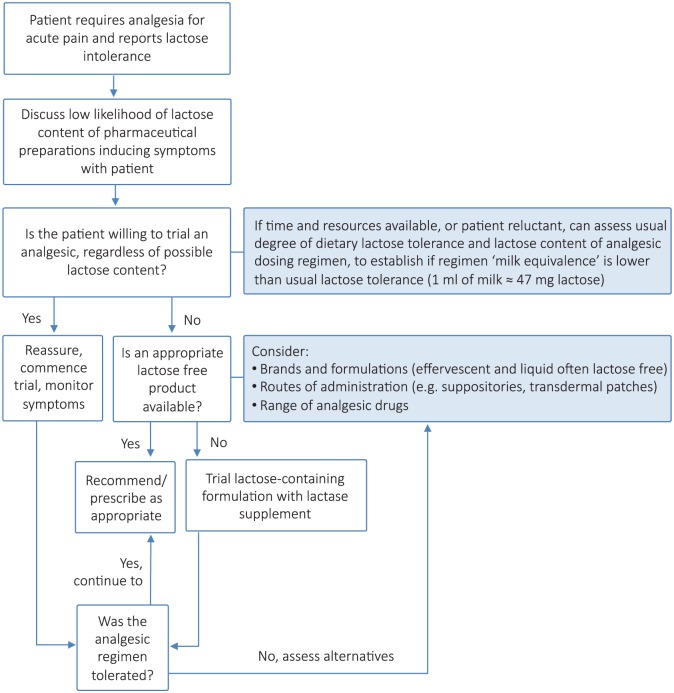
See Figure 1 for a flowchart summary to guideline management in a patient reporting lactose intolerance.
Figure 1.
Flowchart summary to guide management of acute pain in a patient reporting lactose intolerance.
Case scenario: Upon further questioning the patient reported complete inability to tolerate even small quantities of lactose, and insisted upon the need for a lactose-free treatment strategy. Although the patient denied codeine dependence, Nurofen Plus was ceased due to symptoms of gastritis. The patient agreed to a reducing dose of tramadol (lactose-free immediate release capsules) for a period of 2 weeks to replace codeine for her current pain exacerbation. The patient was also advised to take paracetamol (lactose-free Panadol Osteo or Panadol Optizorb formulation, GlaxoSmithKline Errington NSW Australia) instead of anti-inflammatory preparations as required to manage flares of her shoulder pain in the long term. Our institution does not require ethics approval for publication of de-identiifed case reports, thus no approval was sought in relation to the case scenario presented.
In all cases the need for therapy with a specific drug and formulation must be considered. In some cases a trial of a lactose-containing drug may be warranted, given the symptoms of lactose intolerance, while uncomfortable, are self limiting, unlikely to be severe and certainly not life threatening.
Conclusion
Lactose intolerance is a highly variable, patient-dependent, clinical condition. For most patients the lactose content of medications will not be sufficient to induce symptoms and can be considered safe, however adverse effects can occur in patients who report being particularly sensitive to lactose and in those taking multiple doses of several medications all containing lactose. When managing acute pain in a lactose-intolerant individual, likely tolerability can be determined based upon the patient’s usual tolerance to dietary lactose and the calculated total lactose exposure in the medication regimen. If unable to establish usual lactose tolerance or in a highly sensitive individual, a range of options exist, including selecting a lactose-free brand of the desired formulation; changing to an alternative lactose-free dose form, such as an oral liquid or effervescent tablet; use of an alternative route of administration, such as rectal; or trialling the addition of concurrent lactase replacement therapy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Deanna Mill, University of South Australia, Adelaide, South Australia, Australia.
Jessica Dawson, Flinders Medical Centre, Bedford Park, South Australia, Australia.
Jacinta Lee Johnson, University of South Australia Division of Health Sciences, City East Campus, Corner Frome Rd and North Tce, Adelaide, South Australia, 5001, Australia.
References
- 1. MIMS Australia Issuing Body. MIMS online. St Leonards, NSW: MIMS Australia, 1996. [Google Scholar]
- 2. Blackstock MJ, Lee A. Hypokalaemia and renal tubular acidosis due to abuse of Nurofen Plus. Case Rep Crit Care. Epub ahead of print 29 August 2012. DOI: 10.1155/2012/141505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chetty R, Baoku Y, Mildner R, et al. Severe hypokalaemia and weakness due to nurofen misuse. Ann Clin Biochem 2003; 40: 422–423. [DOI] [PubMed] [Google Scholar]
- 4. Dutch MJ. Nurofen Plus misuse: an emerging cause of perforated gastric ulcer. Med J Aust 2008; 188: 56–57. [DOI] [PubMed] [Google Scholar]
- 5. Dyer BT, Martin JL, Mitchell JL, et al. Hypokalaemia in ibuprofen and codeine phosphate abuse. Int J Clin Pract 2004; 58: 1061–1062. [DOI] [PubMed] [Google Scholar]
- 6. Ernest D, Chia M, Corallo CE. Profound hypokalaemia due to Nurofen Plus and Red Bull misuse. Crit Care Resusc 2010; 12: 109–110. [PubMed] [Google Scholar]
- 7. Frei MY, Nielsen S, Dobbin MD, et al. Serious morbidity associated with misuse of over-the-counter codeine-ibuprofen analgesics: a series of 27 cases. Med J Aust 2010; 193: 294–296. [DOI] [PubMed] [Google Scholar]
- 8. Lambert AP, Close C. Life-threatening hypokalaemia from abuse of Nurofen Plus. J R Soc Med 2005; 98: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medani S, Short S, Wall C. Nurofen Plus toxicity – what should nephrologists be alert to? Rare complications of an increasingly misused medication. NDT Plus 2010; 3: 343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng JL, Morgan DJ, Loh NK, et al. Life-threatening hypokalaemia associated with ibuprofen-induced renal tubular acidosis. Med J Aust 2011; 194: 313–316. [DOI] [PubMed] [Google Scholar]
- 11. Bayfield AE. Martindale: the complete drug reference. London: Pharmaceutical Press, 2015. [Google Scholar]
- 12. Deng Y, Misselwitz B, Dai N, et al. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015; 7: 8020–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heyman M, Committee ON. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006; 118: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 14. Tomar B. Lactose intolerance and other disaccharidase deficiency. Indian J Pediatr 2014; 81: 876–880. [DOI] [PubMed] [Google Scholar]
- 15. Eadala P, Waud J, Matthews S, et al. Quantifying the ‘hidden’ lactose in drugs used for the treatment of gastrointestinal conditions. Aliment Pharmacol Ther 2009; 29: 677–687. [DOI] [PubMed] [Google Scholar]
- 16. Cook W, Me F, Rowe R, et al. (Eds). Pharmaceutical excipients, lactose monohydrate. London: Pharmaceutical Press, American Pharmacists Association, 2016. May 11. [Google Scholar]
- 17. Gunsel W, Lachman L. Comparative evaluation of tablet formulations prepared from conventionally-processed and spray-dried lactose. J Pharm Sci 1963; 52: 178–182. [DOI] [PubMed] [Google Scholar]
- 18. Rusynyk R, Still C. Lactose intolerance. J Am Osteopath Assoc 2001; 101(4 Suppl. Pt 1): S10–S12. [PubMed] [Google Scholar]
- 19. Bahna SL. Cow’s milk allergy versus cow milk intolerance. Ann Allergy Asthma Immunol 2002; 89: 56–60. [DOI] [PubMed] [Google Scholar]
- 20. Zheng X, Chu H, Cong Y, et al. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices. Neurogastroenterol Motil 2015; 27: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 21. Qiao R, Huang C, Du H, et al. Milk consumption and lactose intolerance in adults. Biomed Environ Sci 2011; 24: 512–517. [DOI] [PubMed] [Google Scholar]
- 22. Pawar S, Kumar A. Issues in the formulation of drugs for oral use in children: role of excipients. Paediatr Drugs 2002; 4: 371–379. [DOI] [PubMed] [Google Scholar]
- 23. Montalto M, Nucera G, Santoro L, et al. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur J Clin Nutr 2005; 59: 489–493. [DOI] [PubMed] [Google Scholar]
- 24. Levri K, Ketvertis K, Deramo M, et al. Do probiotics reduce adult lactose intolerance? A systematic review. J Fam Pract 2005; 54: 613–620. [PubMed] [Google Scholar]
- 25. Savaiano D, Boushey C, Mccabe G. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration J Nutr 2006; 136: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 26. Shaukat A, Levitt M, Taylor B, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 2010; 152: 797–803. [DOI] [PubMed] [Google Scholar]
- 27. Suarez F, Savaiano D, Levitt M. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995; 333: 1–4. [DOI] [PubMed] [Google Scholar]
- 28. Campbell A, Waud J, Matthews S. The molecular basis of lactose intolerance. Sci Prog 2005; 88: 157–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomons N. Fermentation, fermented foods and lactose intolerance. Eur J Clin Nutr 2002; 56(Suppl. 4): S50–S55. [DOI] [PubMed] [Google Scholar]
- 30. Brandstetter R, Conetta R, Glazer B. Lactose intolerance associated with intal capsules. N Engl J Med 1986; 315: 1613–1614. [DOI] [PubMed] [Google Scholar]
- 31. Craveiro BS, Gomes P, Leitao MA. Severe lactose intolerance in a patient with coronary artery disease and ischemic cardiomyopathy. Rev Port Cardiol 2012; 31: 821–824. [DOI] [PubMed] [Google Scholar]
- 32. Lieb J, Kazienko D. Lactose filler as a cause of ‘drug-induced’ diarrhea. N Engl J Med 1978; 299: 10. [DOI] [PubMed] [Google Scholar]
- 33. Malen D. Parnate formulation change. J Clin Psychiatry 1992; 53: 328–329. [PubMed] [Google Scholar]
- 34. Manka R. Exogenous lactase in the treatment of oral acyclovir intolerance. Am J Ophthalmol 1989; 108: 733. [DOI] [PubMed] [Google Scholar]
- 35. Pao M. Lactose in buspirone. J Am Acad Child Adolesc Psychiatry 1999; 38: 1327. [DOI] [PubMed] [Google Scholar]
- 36. Petrini L, Usai P, Caradonna A, et al. Lactose intolerance following antithyroid drug medications. J Endocrinol Invest 1997; 20: 569–570. [DOI] [PubMed] [Google Scholar]
- 37. Yagoda A. Flutamide-induced diarrhea secondary to lactose intolerance. J Natl Cancer Inst 1989; 81: 1839–1840. [DOI] [PubMed] [Google Scholar]
- 38. Schilit S, Rosenberg J, Joseph P, et al. Clinical Q&A: can lactose-intolerant patients use lactose-containing medications? Drug Topics 2008; 1. [Google Scholar]
- 39. Suarez F, Savaiano D, Arbisi P, et al. Tolerance to the daily ingestion of two cups of milk by individuals claiming lactose intolerance. Am J Clin Nutr 1997; 65: 1502–1506. [DOI] [PubMed] [Google Scholar]
- 40. Montalto M, Gallo A, Santoro L, et al. Low-dose lactose in drugs neither increases breath hydrogen excretion nor causes gastrointestinal symptoms. Aliment Pharmacol Ther 2008; 28: 1003–1012. [DOI] [PubMed] [Google Scholar]