Abstract
Clozapine, a dibenzodiazepine developed in 1961, is a multireceptorial atypical antipsychotic approved for the treatment of resistant schizophrenia. Since its introduction, it has remained the drug of choice in treatment-resistant schizophrenia, despite a wide range of adverse effects, as it is a very effective drug in everyday clinical practice. However, clozapine is not considered as a top-of-the-line treatment because it may often be difficult for some patients to tolerate as some adverse effects can be particularly bothersome (i.e. sedation, weight gain, sialorrhea etc.) and it has some other potentially dangerous and life-threatening side effects (i.e. myocarditis, seizures, agranulocytosis or granulocytopenia, gastrointestinal hypomotility etc.). As poor treatment adherence in patients with resistant schizophrenia may increase the risk of a psychotic relapse, which may further lead to impaired social and cognitive functioning, psychiatric hospitalizations and increased treatment costs, clozapine adverse effects are a common reason for discontinuing this medication. Therefore, every effort should be made to monitor and minimize these adverse effects in order to improve their early detection and management. The aim of this paper is to briefly summarize and provide an update on major clozapine adverse effects, especially focusing on those that are severe and potentially life threatening, even if most of the latter are relatively uncommon.
Keywords: adverse effects, clozapine, life threatening, management, resistant schizophrenia
Introduction
Schizophrenia is a chronic and debilitating disease affecting approximately 0.5% of the population.1 Antipsychotics are the mainstay of treatment of such a severe disorder, but it has been widely demonstrated that between 20% and 60% of patients with schizophrenia do not respond sufficiently to conventional treatments.2
Clozapine, a dibenzodiazepine developed in 1961, is a multireceptorial atypical antipsychotic approved for the treatment of resistant schizophrenia.3 Since the pivotal study of Kane and colleagues4 it has remained the drug of choice in treatment-resistant schizophrenia despite a wide range of adverse effects.5 It has been demonstrated that clozapine is more effective than any other first-generation (FGA) or second-generation antipsychotics (SGA) in the treatment of resistant schizophrenia and it is also useful in other conditions6,7 (Table 1). It has been estimated that almost two thirds of those patients who do not respond adequately to treatment with FGAs or other SGAs may respond adequately to treatment with clozapine.8 Undoubtedly, clozapine is a very effective drug in everyday clinical practice and many patients who tolerate it will experience remarkable symptom relief that is often protracted in time and gives them the opportunity to achieve a satisfactory quality of life.9
Table 1.
Benefits of clozapine use in clinical practice (modified from De Berardis et al.10)
| Established effectiveness in treatment-resistant schizophrenia, both in the short and the long term |
| Effective on both positive and negative symptoms of treatment-resistant schizophrenia |
| Effective in improving patients’ functioning and quality of life in treatment-resistant schizophrenia |
| Effective in reducing aggressive behavior, hostility and excitement in schizophrenia |
| Effective in reducing the comorbid use of alcohol and drugs in patients with schizophrenia, possibly by a reduction in craving |
| Effective in reducing suicidality in schizophrenia |
| Efficacious in severe and treatment-resistant mood disorders |
| Efficacious in drug-induced psychosis in Parkinson’s disease, without worsening Parkinsonism |
| Effective in reducing drug-induced tardive dyskinesia |
Clozapine treatment may decrease mortality from natural or disease-related causes in treatment-resistant schizophrenia
However, clozapine is not considered as a first-line treatment because it may often be difficult for some patients to tolerate, as some adverse effects may be particularly bothersome (i.e. sedation, weight gain, sialorrhea etc.) and some others are potentially dangerous and life threatening.11 Specifically, the risk of agranulocytosis is one of the main reasons why clozapine is not a first-line agent for schizophrenia.12
Moreover, clozapine may have several interactions with other drugs as it is metabolized by the hepatic cytochrome P450 (CYP) system.13 Clozapine is transformed to norclozapine by CYP3A4 and 1A2 and to clozapine N-oxide by CYP3A4.14 Nevertheless, CYP2C19 is also significant at clozapine therapeutic concentration (24%) while the influences of CYP2C9 (12%) and 2D6 (6%) are more modest.15 CYP1A2 is the most significant form at a therapeutic concentration (30%), while CYP3A4 plays an important role at higher concentrations (37%) than at therapeutic concentrations (22%).16,17 All considered, blood-level monitoring of clozapine may be needed both when inhibitors (such as antifungals, oral contraceptives, fluvoxamine, ciprofloxacin, caffeine, disulfiram) or inducers (such as rifampicin, omeprazole, phenytoin, phenobarbital, tobacco smoke) of CYP1A2 and both inhibitors (such as cimetidine, erythromycin, clarithromycin etc.) or inducers (such as carbamazepine and rifampicin) of CYP13A4 are being used.18 It is important to note that tobacco smoking may affect clozapine metabolism through CYP1A2 induction, thus resulting in lower clozapine levels in smokers. This may cause adverse effects if a patient under clozapine treatment quits smoking because clozapine blood levels may rise up to 50–70%, potentially leading to toxic concentrations and development of adverse effects.19
Therefore, it is important to conduct careful therapeutic drug monitoring (TDM) for any patient on clozapine. As reported by Bastiampillai and colleagues,20 clozapine blood levels should usually be evaluated at least a week after a stable clozapine dose has been reached as a trough level. There is evidence that clozapine levels above 600 μg/ml may increase the risk of seizures, and prophylactic antiepileptics should be considered.21 Clozapine serum-level monitoring may also be useful to evaluate clinical efficacy, in addition to checking for side effects and toxicity, with levels between 350 and 400 μg/ml significantly associated with improved clinical response rates, but also with a significantly increased side-effect burden.21–23
As poor treatment adherence in patients with resistant schizophrenia may increase the risk of psychotic relapse,24 which may further lead to impaired social and cognitive functioning, psychiatric hospitalizations, suicide risks and increased treatment costs,25,26 clozapine adverse effects are a common reason for discontinuing this medication and this is a cause of concern.27,28 Therefore, all efforts should be made to monitor and minimize adverse effects, improve their early detection and conduct proper management, also to reduce the risk of people being harmed.29 This must be done considering that, when clozapine fails, there are no effective therapeutic strategies that can be put in place.30
The aim of this narrative review is to briefly summarize and provide an update on major clozapine adverse effects, especially focusing on those that are severe and potentially life threatening, even if most of the latter are relatively uncommon. Adverse reactions were divided by frequency regarding patients and treatment: common (⩾1/100), uncommon (⩾1/1000) and rare (⩾1/10,000) (see Table 2).
Table 2.
Adverse effects of clozapine (in brackets potentially life-threatening adverse effects if not recognized early, LT). Adverse reactions were divided by frequency regarding patients and treatment: common (⩾1/100), uncommon (⩾1/1000) and rare (⩾1/10,000).
| Common | Uncommon | Rare |
|---|---|---|
| Sedation | Agranulocytosis (LT) | Myocarditis/pericarditis (LT) |
| Hypertension | Diabetes mellitus/hyperglycemia/DKA (LT) | Cardiomyopathy (LT) |
| Weight gain | Metabolic syndrome | Heat stroke (LT) |
| Seizures (LT) | Delirium | Hepatic failure (LT) |
| Hypersalivation or sialorrhea | Liver enzyme abnormalities | Colitis (LT) |
| Benign fever | Interstitial nephritis | Intestinal obstruction/paralytic ileus (LT) |
| Hypotension | Stuttering | Pancreatitis (LT) |
| Nausea | Thrombocytopenia | Pneumonia (LT) |
| Nocturnal enuresis | Dysphonia | Respiratory failure (LT) |
| Gastroesophageal reflux | Neuroleptic malignant syndrome (LT) | Vasculitis |
| Constipation | Skin rash | |
| Tachycardia | Ocular pigmentation | |
| Dizziness | Priapism | |
| Blurred vision | Parotid gland swelling | |
| Dysarthria | Rhabdomyolysis | |
| Blood dyscrasias such as leukopenia/decreased WBC/neutropenia/eosinophilia/leukocytosis | QT prolongation (LT) | |
| Sudden death (LT) |
DKA, diabetic ketoacidosis; WBC, white blood cell.
Materials and methods of the review
Searches of the Medline database from 1980 to December 2017 and the PsycInfo/Embase database from 1980 to December 2017 were conducted and restricted to the English language. The search term ‘clozapine’ was combined with ‘adverse effects’, ‘side effects’ and ‘toxicity’ to identify relevant original research and review articles. All citations were screened and the full texts of peer-reviewed journal articles that were considered relevant for the purposes of the review were obtained. Bibliographies were scanned to locate additional relevant publications, even those prior to 1980. However, this paper is a narrative review. This type of review is useful when the aggregation of data is difficult because different studies or fields are being analyzed, but the methodology is less strict than in a systematic review.
Clozapine mechanism of action and relationships with adverse effects
Clozapine is a multireceptorial atypical antipsychotic approved for the treatment of resistant schizophrenia.31 Concerning DA receptors, clozapine has a higher affinity for D1 and D4 than D2 receptors.32 Moreover, clozapine is characterized by a rapid dissociation from D2 receptors (the ‘fast off’ phenomenon), which accounts for a more robust antipsychotic effect and a lower propensity to cause extrapyramidal symptoms and hyperprolactinemia.33,34
Clozapine has an affinity for several other receptors, including serotonergic 5-HT1A, 5-HT1C, 5-HT2A, 5-HT2C,5-HT3, 5-HT6, 5-HT7 receptors, adrenergic α1 and α2 receptors, histaminergic H1, H3, H4 receptors, and muscarinic M1 and M5 receptors.35 These affinities, especially for adrenergic, histaminergic and muscarinic receptors, may explain some adverse effects commonly seen during clozapine administration, whereas some other adverse effects do not necessarily depend on clozapine-related receptor agonism or antagonism.20
Furthermore, some populations, that is the elderly or adolescents, may be particularly vulnerable to clozapine adverse effects despite its proven efficacy.36–38
Besides clozapine activity on brain neurotransmitters and their receptors, a potential immune-mediated mechanism of action has been suggested.39,40 Clozapine may influence the cytokine system, especially during the first months of treatment, by increasing proinflammatory cytokines and C-reactive protein (CRP), and acting as an immunomodulatory drug in some circumstances.41–44 In fact, both in vitro and in vivo studies confirmed the immunomodulatory actions of clozapine.45–49 These effects of clozapine on the immune system may account for its efficacy in the treatment of schizophrenia in accordance with an immunological hypothesis of disorder etiology,50,51 but also for some adverse effects (i.e. eosinophilia, benign hyperthermia, cardiovascular and hematological adverse effects etc.).39,52
Common adverse effects
Orthostatic hypotension, often seen when initiating treatment, especially in the case of a rapid titration, and sexual dysfunction are mostly related to adrenergic α blockade,53–55 whereas sedation and constipation (see below for the latter) may be mainly due to H1 blockade.56,57 Clozapine-induced orthostatic hypotension and sedation may increase the risk of falls, especially in the elderly.36,58 Clozapine antagonism on muscarinic M1 receptors is responsible for anticholinergic effects such as constipation, tachycardia, blurred vision and urinary retention.59 Other common adverse effects include dizziness, transient eosinophilia, sialorrhea, benign hyperthermia, leukocytosis and nausea.60 Among the latter, sialorrhea may be particularly bothersome, although it is benign, dose related and often transient. However, there are some cases of aspiration pneumonia in patients treated with clozapine who have sialorrhea, so such an effect must be carefully evaluated.61,62 The management of clozapine-related sialorrhea is often problematic, but some strategies have been tried with good results, such as a low-dose amisulpiride or amytriptiline, bupropion, sublingual atropine, scopolamine, trihexyphenydil and glycopyrrolate, with the latter shown to be the most effective.63–67
Among the common adverse effects, the most dangerous and potentially life threatening are seizures and weight gain or metabolic syndrome (MetS).
Seizures
Seizures may be a relatively common and potentially life-threatening adverse effect of clozapine.68 In fact, clozapine may lower the seizure threshold not only in individuals at risk of epilepsy, but also in apparently healthy subjects.69 The seizure risk during clozapine treatment has been estimated at roughly 1–6%, is usually dose dependent and may appear more frequently in younger patients.70,71 It has been reported that rapid titration and higher dosages of clozapine (⩾600 mg/day) were associated with greater risk than medium (300–600 mg/day) or low dosages (<300 mg/day).72
Moreover, rapid clozapine titration may further increase seizure onset.70 Even if a higher risk of seizures during clozapine titration has been reported, this adverse effect may appear during all phases of treatment, even after several years of therapy.73 Patients who develop seizures during clozapine titration or maintenance treatment may continue clozapine with dose reduction, or better still, the addition of an antiepileptic medication. Valproate may be the best choice, even if this combination must be carefully monitored.74,75 Electroencephalography and clozapine plasma blood-level monitoring are recommended to avoid or minimize seizure risk.76,77
Weight gain and the risk of MetS
Second-generation antipsychotics are generally associated with weight gain, particularly olanzapine and clozapine, and with related risks of metabolic disturbances such as insulin resistance, type 2 diabetes, dyslipidemia and diabetic ketoacidosis (DKA).78
Weight gain is a common adverse effect of clozapine that, per se, may be not potentially life threatening if properly managed and treated.77 If not, the consequent development of MetS may be a major health concern which highly increases the risk of developing type 2 diabetes mellitus and cardiovascular diseases.79,80 A relationship between weight gain and clozapine doses has been proposed, but this point remains controversial.81
Clozapine metabolic abnormalities are mostly linked to 5-HT2A and 5-HT2C receptor blockade, but the effects on histaminergic and adrenergic receptors are also thought to play a significant role.82–84
Even if the prevalence of MetS in patients with schizophrenia is higher compared with the general population, going up to 40%, the effect of clozapine is remarkable with a prevalence rate varying from 11% to 64% among treated patients.85,86 Allison and colleagues reported that clozapine treatment was associated with an average gain of 9.8 lb over 10 weeks and this was the highest figure of all antipsychotics.87 The incidence of clozapine-related weight gain is considered to be the most significant at 5–35%, and almost half of patients may gain 20% or more of their initial body weight, especially if they are on polypharmacy, have a sedentary lifestyle or are not overweight at baseline.88 Several studies have also associated clozapine treatment with the development of dyslipidemia and hypertension,77,89–91 all factors that may affect MetS or make it worse. The metabolic risk associated with clozapine treatment may be increased in the short term after treatment initiation. In fact, it has been demonstrated that the increased short-term metabolic risk associated with clozapine may be diminished over time because multiple other variables are also likely to affect the metabolic risk during the lifespan.92
Clozapine treatment may also be associated with diabetes and DKA.93,94 Regarding DKA, Cohen and colleagues95 found a 1-year incidence rate of 1.2–3.1% and a case fatality rate of 20–31%, making DKA one of the riskiest side effects, with higher mortality than agranulocytosis with an incidence of 3.8–8.0% and case fatality rate of 2.2–4.2%. Therefore, the authors proposed that the screening guidelines should be modified with an early detection of treatment-emergent hyperglycemia through an obligatory monthly measurement of fasting plasma glucose. It has also been demonstrated that clozapine-related DKA typically occurs early in the course of treatment, when clozapine treatment duration is short and doses are low.96
Especially with combined behavioral interventions consisting of both nutritional counseling and diet modification), aripiprazole, topiramate, orlistat and metformin additions have been shown to be beneficial in reducing clozapine-induced weight loss, even if the evidence is based on a limited number of randomized controlled trials and further studies are needed.88,97,98 Counseling for patients needing proper diet and exercise for weight and glucose control should always be recommended.99
Gastrointestinal hypomotility and constipation
The anticholinergic peripheral properties of clozapine as well as its H1 antagonism properties were related to several grades of impairment of intestinal peristalsis ranging from transient to obstinate constipation and paralytic ileus up to intestinal obstruction.100,101 It is known that even low or therapeutic doses of clozapine may prolong colonic transit time (CTT).102
These effects are mainly mediated by the clozapine antagonism on cholinergic and serotoninergic receptors in the gut wall.103 It has been demonstrated that clozapine, but not norclozapine, has potent effects on the motility of the colon of a rabbit, thus inhibiting neurogenic contractions at lower concentrations and myogenic contractions at higher concentrations.104 These effects may be mediated by cholinergic and serotonergic receptors, presumably via M1–M3 and 5HT-3 and 5HT-7 receptors. Moreover, de Alvarenga and colleagues105 showed that clozapine significantly reduced the frequency of cycles of contractions of zebrafish larvae gastrointestinal tracts through anticholinergic action.
These adverse effects are bothersome for several patients who develop mild constipation, which is often a treatment discontinuation cause, but it may become severe and potentially life threatening in some others who develop moderate to severe untreated constipation or even an adynamic ileus.103 In fact, constipation is very frequent in patients treated with clozapine with an estimate range varying from 15% to 60% in accordance with several studies.106,107 Moreover, Every-Palmer and Ellis108 reviewed all reports of serious clozapine-induced gastrointestinal hypomotility (CIGH, also known as clozapine-related ‘slow gut’) submitted to the Australian Therapeutic Goods Administration and New Zealand Pharmacovigilance Centre between 1992 and 2013 and found that the reported prevalence of serious CIGH was 37/10,000, likely an underestimation of the true prevalence. CIGH is relatively common as between 50% and 80% of patients treated with clozapine have unambiguous objective evidence of CIGH in colonic transit studies,102 with mean transit times that are four times longer than normal, affecting all regions of the colon.106 It is worth noting that studies which have relied on reporting of constipation are not very sensitive in diagnosing slow gut.
In fact, rare but potentially fatal clozapine-related adverse effects are intestinal obstructions and a paralytic ileus that occur in almost 2% of treated patients.109 In severe cases, constipation progresses to ileus and bowel ischemia, with multiple fatalities related to aspiration of feculent vomit, sepsis and perforation, as described in the literature.110 Prolonged gastrointestinal hypomotility and constipation may promote ischemia, increasing intraluminal pressure and compression of mucosal vessels. As a consequence, bacterial translocation, inflammation and ischemia of the segment of bowel proximal to the obstruction will develop.111–113 One case of clozapine-induced severe constipation leading to a silent presentation of pneumonia with a subsequent respiratory arrest114 has also been reported.
Clozapine-related ileus has been reported to be dose dependent and may occur several days after initiation of treatment as well as at any time during treatment, with the latter often triggered by concomitant medications known to affect the gastrointestinal system, especially those with anticholinergic properties.115 Therefore, early detection and treatment of constipation and ileus are mandatory to avoid fatalities.
The recommendation of an adequate intake of fluids, especially in the presence of hypersalivation, and regular physical activity is mandatory in such patients, as well as dose reduction or laxative prescriptions to avoid the progression of severe gastrointestinal adverse effects.107,116,117 Recently, Every-Palmer and colleagues118 demonstrated that patients with clozapine-related constipation treated with docusate and senna augmented by macrogol had reduced CTTs and this was beneficial in treating constipation. Also, orlistat was found to be helpful,119 but further studies are needed.
Uncommon adverse effects
Less common adverse effects associated with clozapine treatment may include delirium, liver enzymes abnormalities, interstitial nephritis, stuttering, thrombocytopenia and dysphonia.120
Moreover, several cases of clozapine-related neuroleptic malignant syndrome have been reported, often characterized by atypical features, that is, with less intense extrapyramidal symptoms or high fever.121,122 However, it should be noted that the majority of published cases were reported in subjects with comorbid conditions123 or under polypharmacy with different antipsychotics, especially those with higher affinity on dopamine D2 receptors, such as risperidone, amisulpride and olanzapine or other drugs.124–126
Blood dyscrasias and agranulocytosis/granulocytopenia
Blood dyscrasias, such as mild to moderate leukopenia decreased white blood cell count, mild neutropenia, anemia, eosinophilia and leukocytosis, are often seen with clozapine but, if cautiously monitored, in the majority of the cases this may be transient or benign.127,128
The most severe and potentially life-threatening clozapine-related blood dyscrasias is neutropenia, which may eventually develop into clozapine-induced agranulocytosis or granulocytopenia. This occurs in roughly 0.8–2% of patients and requires mandatory hematological monitoring.20,129 The occurrence of agranulocytosis or granulocytopenia even led to the partial withdrawal of clozapine from the market and strong restrictions to its use in the mid 1970s.130
Neutropenia was defined in the 2005 clozapine guidelines as an absolute neutrophil count (ANC) below 1500 and occurs in approximately 3% of treated patients,131 whereas agranulocytosis was defined as an ANC below 500/mm3.132,133 A transient neutropenia (1 week) or weekly variations of ANC are relatively common during clozapine treatment and do not necessarily require treatment discontinuation, but it is not known why some patients develop transient neutropenia whereas others progress to having agranulocytosis.134,135
Although the highest risk of agranulocytosis has been reported to occur during the first 12 weeks from clozapine titration and during the first 6 months of treatment, patients may develop severe blood dyscrasia anytime during the treatment, even if the risk decreases exponentially over time after the first 6 months of treatment.135 However, some cases of late-onset clozapine-induced agranulocytosis have been reported.136,137 The development of agranulocytosis may be independent of dosage and risk factors include older age, female sex, human leukocyte antigen haplotypes and concomitant treatment with other drugs known to cause agranulocytosis.52,138–142 Interestingly, in cases with severe clozapine-induced agranulocytosis, the occurrence did not depend on the clozapine dosage.143
To explain this dangerous adverse effect, several studies have hypothesized a potential interaction between clozapine and the immune system, suggesting an immune-mediated mechanism to be involved in its adverse effects or even in its clinical efficacy.144,145 It is also possible that some subjects may be genetically predisposed; oxidative clozapine degradation may cause the development of some metabolites with reactive nitrenium ions, which may covalently bind to human leukocytes, resulting in direct toxicity or leading to the development of aptenic formation of an antigenic structure that will stimulate a targeted immune response.146–148
The 2015 US Food and Drugs Administration guidelines for hematologic monitoring during clozapine therapy149–151 recommend that treatment is not discontinued in the case of mild neutropenia (⩾1000–1499/mm3), with ANC monitored three times weekly until it is at least 1500/mm3. However, in the case of moderate neutropenia (⩾500–999/mm3), they recommend that treatment is interrupted when clozapine-induced neutropenia is suspected, with daily monitoring performed until ANC is at least 1000/mm3 (when treatment may resume), and consequently monitoring carried out three times weekly until ANC is at least 1500/mm3, then proceeding with weekly checks for 4 weeks, and finally returning to the patient’s last normal range ANC monitoring plan.
In the case of severe neutropenia (<500/mm3), all patients must interrupt treatment for suspected clozapine-induced neutropenia and clozapine rechallenge should not be considered unless the prescriber, along with a hematologist consultation, determines that the benefits outweigh the risks.152 Recently, some studies have pointed out that granulocyte colony-stimulating factor may be beneficial in the treatment of clozapine-induced agranulocytosis,153 but more evidence is needed and withdrawing clozapine is still mandatory in such cases.
Clozapine rechallenge is always a risk after an episode of agranulocytosis as several reports observed a new episode of blood dyscrasia.154,155 However, there are also some reports that observed no blood dyscrasia when clozapine was reintroduced after an episode of agranulocytosis with or without adjuvant therapies.156–158 Dunk and colleagues159 conducted clozapine rechallenge in 53 patients who developed leucopenia or neutropenia during previous clozapine treatment and found that 38% experienced a further blood dyscrasia whereas 55% did not. More recently, Prokopez and colleagues160 analyzed the results of a clozapine rechallenge after leucopenia or neutropenia in 19 patients and observed that almost 70% of the patients did not develop a new hematological adverse effect, whereas the remaining 30% had a faster but less serious neutropenia. In conclusion, clozapine rechallenge requires careful evaluation and should be taken into consideration on a case-by-case basis when the risks outweigh the benefits for all other strategies.
Uncommon adverse effects
Clozapine may be associated with several uncommon adverse effects such as vasculitis, skin rash, ocular pigmentation, priapism, parotid gland swelling and rhabdomyolysis that may not always be severe but require cautious monitoring.5 However, some of the (fortunately) rare adverse effects may be severe and potentially life threatening, and these include heat stroke, hepatic failure, colitis, pancreatitis, pneumonia and respiratory failure.7
QT prolongation
It has been reported that clozapine may be associated with QT prolongation in a dose-dependent manner.161,162 In most cases, drug-induced QT prolongation is not significant per se, whether it is symptomatic or malignant,163 but in some individuals it may lead to the life-threatening torsades de pointes, a sudden and often fatal arrhythmia.164
The effect on QTc is not peculiar to clozapine; it is often seen with other antipsychotics, especially FGAs, and other psychiatric drugs.165–167 Almost all of the drugs known to prolong the QT interval both directly or through one of their metabolites seem to preferentially block the rapid component of the delayed rectifier outward K+ current, IKr, of an ion channel in ventricular cardiomyocytes, whose α subunit is encoded by the human ether-a-go-go related gene (HERG).168
Clozapine has demonstrated the capability to block HERG currents and this may account for drug-induced QT prolongation.169 However, it is known that clozapine may induce an increased heart rate and this may influence the QTc determination (when using Bazett’s formula), leading to an overestimation of this effect.170
All considered, clozapine seems to rarely influence QT traits. However, electrocardiogram monitoring is always useful when clozapine is commenced or when administered together with other drugs, and in elderly patients.171–175 The QT interval should be calculated using methods involving linear regression, adjusting for age and sex, as recommended by the American Heart Association,176 and not with Bazett’s formula. Clozapine dose reductions may be useful for QT prolongation, especially in the case of polypharmacy.177
Clozapine-induced cardiomyopathy, myocarditis and pericarditis
Clozapine may be associated with the occurrence of severe cardiovascular side effects, including dilated cardiomyopathy, myocarditis and pericarditis, which may be fatal when not timely recognized and managed.10,170
Dilated cardiomyopathy is an uncommon, dose-independent, but potentially life-threatening adverse effect of clozapine treatment. It may occur even after a longer drug exposure and is often confused with myocarditis.178 The clinical manifestations of clozapine-induced cardiomyopathy range from subclinical forms to severe ones with fulminant pulmonary edema and cardiogenic shock.179,180 Often the symptoms are insidious and include persistent tachycardia at rest, palpitations, arrhythmias, dyspnea, chest pain, malaise and other signs and symptoms that may be underestimated by the clinicians until the symptoms resemble the clinical picture of heart failure or myocardial infarction.181
Myocarditis is an uncommon, dose-independent, life-threatening adverse effect and occurs in up to 3% of treated patients.182,183 Moreover, several sudden deaths reported with clozapine use may be due to an unrecognized or fulminant myocarditis.184 Although myocarditis has a tendency to appear at any time during treatment with clozapine, this has been reported more frequently from 4 days to 22 weeks after treatment initiation,185 with a particular ‘danger period’ during the first 4 weeks following initiation.186
The clinical presentation of clozapine-related myocarditis in adults may be highly variable, ranging from subclinical diseases to fulminant heart failure.187,188 Flu-like symptoms such as fatigue, fever, myalgias, dizziness, arthralgias, nasal congestion and sensations of ‘scratchy throat’ may often be the first to appear and are sometimes unrecognized or underestimated.189,190
Respiratory symptoms, such as dyspnea, cough, subjective sensations of chest discomfort and orthopnea, as well as cardiovascular symptoms, such as persistent resting tachycardia, increased heart rate, palpitations, chest pain, syncope, arrhythmias and hypotension, are the main symptoms that drive the diagnosis. However, in some cases, clozapine-related myocarditis may develop in an atypical form without accompanying symptoms and these cases are often fatal.191,192
It has been suggested that risk factors for any cardiac complications of clozapine may be the rapid dose titration, the presence of weight gain or metabolic adverse effects, the coadministration of selective serotonin reuptake inhibitors and the use of illicit substances.193–195 Moreover, concomitant treatment with medications that have been implicated as the cause of cardiovascular complications like myocarditis and heart failure, namely some antibacterials, cisapride, thyroxine, ranitidine, cyclophosphamide, lithium, phenotiazines and certain antidepressant agents like amytriptiline, imipramine and desipramine, may represent another risk factor when a patient is taking clozapine.196 Interestingly, some cases of clozapine-related myocarditis were detected in patients taking valproate, which is often prescribed to prevent seizures or as a mood stabilizer.184,195,197 Hence, when clozapine is administered together with valproate, patients should be more carefully and strictly evaluated for an eventual myocarditis onset.
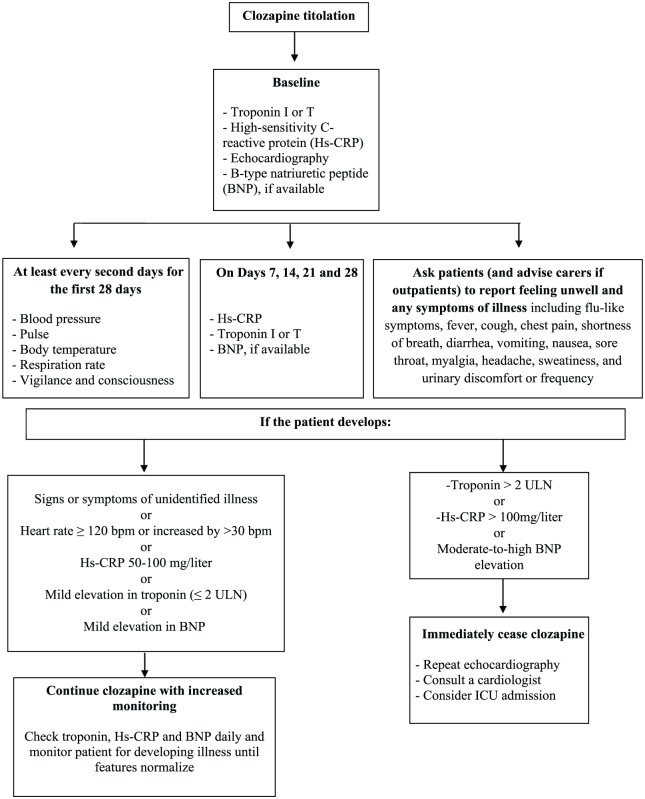
The early detection of clozapine-related myocarditis is mandatory as early recognition improves clinical outcome and reduces mortality.170,198 To date, the monitoring protocol adapted from De Berardis and colleagues10 and Ronaldson and colleagues185 seems to be scientifically sound and clinically useful in everyday clinical practice (see Figure 1).
Figure 1.
Proposed protocol for monitoring patients commenced on clozapine for clozapine-related cardiomyopathy, myocarditis and pericarditis (adapted and modified from De Berardis et al.10 and Ronaldson et al.185). ICU, intensive care unit; ULN, upper limit of normal.
When myocarditis is suspected or diagnosed, immediate clozapine cessation is mandatory as usually this may resolve the case within a few days or weeks.60 However, some patients may require treatment with a β blocker or angiotensin-converting enzyme inhibitor, whereas severe cases will need mechanical ventilation.199,200 Corticosteroids have been administered in some cases, but their efficacy is uncertain.201,202
As far as clozapine rechallenge after an episode of myocarditis is concerned, it should be noted that this strategy may be dangerous, is not recommended, and should be employed after careful evaluation of the pros and cons together with a consultation with a cardiologist,170,203,204 even though there are some positive reports.191,205–207
Moreover, several cases of clozapine-related pericarditis have been reported in the literature in patients on stable clozapine regimens for several weeks, months or even years, or during the titration phase.208–211 In the majority of published cases, clozapine treatment was immediately discontinued, resulting in complete resolution of symptoms.212 In some cases, clozapine rechallenge was tried, but this strategy is controversial as pericarditis may develop again, even though this is not the rule.213,214
Venous thromboembolism
There are several reports on clozapine-related venous thromboembolism (VTE), but luckily it seems to be relatively rare and dose independent.215–217 The mechanism of this dangerous adverse effect remains unknown.218–220 Axelsson and colleagues221 compared several antipsychotics and found that clozapine was the only compound that increased platelet adhesion and aggregation, and shortened activated partial thromboplastin time, even at therapeutic concentrations. Moreover, in a case report, clozapine induced an allergic vasculitis that resulted in VTE.222
Nevertheless, the risk of VTE seems to be an adverse effect of several antipsychotics including clozapine,223,224 and may be higher in elderly patients and in women taking high doses or receiving parenteral antipsychotics.225–228 However, it has been suggested that VTE may be more a consequence of several risk factors for VTE present in patients with psychiatric disorders, such as MetS, inactivity, low levels of water intake and other medical illnesses, than the treatment with antipsychotics itself.229–231
All considered, caution is needed to recognize VTE symptoms early, for example painful edema of the lower limbs, chest pain and dyspnea in patients taking clozapine, especially in those with known risk factors for VTE, like patients over 65, with previous VTE episodes, obesity, MetS, inactivity, lower limb trauma, estrogen treatment, among others.170,218,225
Conclusion
Despite its potentially life-threatening adverse effects which deserve special attention, clozapine remains the gold standard in the treatment of treatment resistant schizophrenia (TRS) with, to date, inimitable results compared with other available antipsychotics. However, regardless of the evidence, clozapine is still underprescribed or utilized too late and this may be due to, but not limited to, blood monitoring because it is often tedious for the patients,129 and the psychiatrists’ fear of severe adverse effects.232,233
Some psychiatrists do not prescribe clozapine when required as they are anxious about its management and perceive a lack of knowledge and a negative attitude toward this drug.234,235 Such discomfort with clozapine is a major cause of concern that the patients will pay in terms of a lack of a therapeutic possibility and, therefore, efforts should be made to overcome this.3,236 This brief review was written not to scare prescribers, but to increase knowledge on the adverse effects of clozapine and to improve their early identification and management. Despite the fear of death of clozapine-treated patients with its possible legal consequences, it is important to emphasize that clozapine treatment may decrease mortality in treated patients by reducing self harm, risk of suicide and attempted suicide.237,238 Furthermore, Hayes and colleagues220,239 reported an association between clozapine and reduced risk of mortality from natural causes in a sample of 14,754 individuals with serious mental illness.
Last, but not least, clozapine is the only drug able to restore satisfactory functioning in patients with TRS by improving their lives and self care, even in the long term.240–243 This should always be kept in mind. Therefore improving our knowledge of clozapine is important to increase the possibility of prescribing it to those in need. Also, improving our knowledge on the adverse effects of clozapine and their management may help to reduce the understandable apprehension of physicians and help them to be more confident when prescribing clozapine.
Acknowledgments
The authors thank Prof. Roberta Polimanti for checking and editing the English language.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: No author or immediate family member has financial relationships with commercial organizations that might appear to represent a potential conflict of interest with the material presented.
Contributor Information
Domenico De Berardis, National Health Service, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, ‘G. Mazzini’ Hospital, p.zza Italia 1, 64100 Teramo, Italy.
Gabriella Rapini, National Health Service, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, ‘G. Mazzini’ Hospital, Teramo, Italy.
Luigi Olivieri, National Health Service, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, ‘G. Mazzini’ Hospital, Teramo, Italy.
Domenico Di Nicola, National Health Service, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, ‘G. Mazzini’ Hospital, Teramo, Italy.
Carmine Tomasetti, Polyedra Research Group, Teramo, Italy Department of Neuroscience, Reproductive Science and Odontostomatology, School of Medicine ‘Federico II’ Naples, Naples, Italy.
Alessandro Valchera, Polyedra Research Group, Teramo, Italy Villa S. Giuseppe Hospital, Hermanas Hospitalarias, Ascoli Piceno, Italy.
Michele Fornaro, Department of Neuroscience, Reproductive Science and Odontostomatology, School of Medicine ‘Federico II’ Naples, Naples, Italy.
Fabio Di Fabio, Polyedra Research Group, Teramo, Italy Department of Neurology and Psychiatry, Sapienza University of Rome, Rome, Italy.
Giampaolo Perna, Hermanas Hospitalarias, FoRiPsi, Department of Clinical Neurosciences, Villa San Benedetto Menni, Albese con Cassano, Como, Italy Department of Psychiatry and Neuropsychology, University of Maastricht, Maastricht, The Netherlands Department of Psychiatry and Behavioral Sciences, Leonard Miller School of Medicine, University of Miami, Florida, USA.
Marco Di Nicola, Institute of Psychiatry and Psychology, Catholic University of Sacred Heart, Rome, Italy.
Gianluca Serafini, Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, Section of Psychiatry, University of Genoa, Genoa, Italy.
Alessandro Carano, National Health Service, Department of Mental Health, Psychiatric Service of Diagnosis and Treatment, Hospital ‘Madonna Del Soccorso’, San Benedetto del Tronto, Italy.
Maurizio Pompili, Department of Neurosciences, Mental Health and Sensory Organs, Suicide Prevention Center, Sant’Andrea Hospital, Sapienza University of Rome, Rome, Italy.
Federica Vellante, Department of Neuroscience, Imaging and Clinical Science, Chair of Psychiatry, University ‘G. D’Annunzio’, Chieti, Italy.
Laura Orsolini, Polyedra Research Group, Teramo, Italy Psychopharmacology, Drug Misuse and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, Herts, UK.
Giovanni Martinotti, Department of Neuroscience, Imaging and Clinical Science, Chair of Psychiatry, University ‘G. D’Annunzio’, Chieti, Italy.
Massimo Di Giannantonio, Department of Neuroscience, Imaging and Clinical Science, Chair of Psychiatry, University ‘G. D’Annunzio’, Chieti, Italy.
References
- 1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990–2013: a systematic literature review. BMC Psychiatry 2015; 15: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry 2017; 174: 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry 2014; 14: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45: 789–796. [DOI] [PubMed] [Google Scholar]
- 5. Remington G, Lee J, Agid O, et al. Clozapine’s critical role in treatment resistant schizophrenia: ensuring both safety and use. Exp Opin Drug Saf 2016; 15: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 6. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2016; 209: 385–392. [DOI] [PubMed] [Google Scholar]
- 7. De Berardis D, Serroni N, Campanella D, et al. Safety and efficacy of combined clozapine-azathioprine treatment in a case of resistant schizophrenia associated with Behcet’s disease: a 2-year follow-up. Gen Hosp Psychiatry 2013; 35: 213e9–213e11. [DOI] [PubMed] [Google Scholar]
- 8. Essali A, Al-Haj Haasan N, Li C, et al. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev 2009; 1: CD000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orsolini L, Tomasetti C, Valchera A, et al. An update of safety of clinically used atypical antipsychotics. Exp Opin Drug Saf 2016; 15: 1329–1347. [DOI] [PubMed] [Google Scholar]
- 10. De Berardis D, Serroni N, Campanella D, et al. Update on the adverse effects of clozapine: focus on myocarditis. Curr Drug Saf 2012; 7: 55–62. [DOI] [PubMed] [Google Scholar]
- 11. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag 2017; 13: 757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wicinski M, Weclewicz MM. Clozapine-induced agranulocytosis/granulocytopenia: mechanisms and monitoring. Curr Opin Hematol 2018; 25: 22–28. [DOI] [PubMed] [Google Scholar]
- 13. Cadeddu G, Deidda A, Stochino ME, et al. Clozapine toxicity due to a multiple drug interaction: a case report. J Med Case Rep 2015; 9: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buur-Rasmussen B, Brosen K. Cytochrome P450 and therapeutic drug monitoring with respect to clozapine. Eur Neuropsychopharmacol 1999; 9: 453–459. [DOI] [PubMed] [Google Scholar]
- 15. Fang J, Coutts RT, McKenna KF, et al. Elucidation of individual cytochrome P450 enzymes involved in the metabolism of clozapine. Naunyn Schmiedeberg’s Arch Pharmacol 1998; 358: 592–599. [DOI] [PubMed] [Google Scholar]
- 16. Eiermann B, Engel G, Johansson I, et al. The involvement of CYP1A2 and CYP3A4 in the metabolism of clozapine. Br J Clin Pharmacol 1997; 44: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olesen OV, Linnet K. Contributions of five human cytochrome P450 isoforms to the N-demethylation of clozapine in vitro at low and high concentrations. J Clin Pharmacol 2001; 41: 823–832. [DOI] [PubMed] [Google Scholar]
- 18. Chetty M, Murray M. CYP-mediated clozapine interactions: how predictable are they? Curr Drug Metab 2007; 8: 307–313. [DOI] [PubMed] [Google Scholar]
- 19. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment. Ann Pharmacother 2010; 44: 727–732. [DOI] [PubMed] [Google Scholar]
- 20. Bastiampillai T, Allison S, Gupta A. The clinical utility of therapeutic drug monitoring for clozapine. Aust N Z J Psychiatry 2017; 51: 295–296. [DOI] [PubMed] [Google Scholar]
- 21. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res 2013; 147: 368–374. [DOI] [PubMed] [Google Scholar]
- 22. Iglesias Garcia C, Iglesias Alonso A, Bobes J. Concentrations in plasma clozapine levels in schizophrenic and schizoaffective patients. Rev Psiquiatr Salud Ment 2017; 10: 192–196. [DOI] [PubMed] [Google Scholar]
- 23. Spina E, Avenoso A, Facciola G, et al. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology 2000; 148: 83–89. [DOI] [PubMed] [Google Scholar]
- 24. Iasevoli F, Giordano S, Balletta R, et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuropsychopharmacol Biol Psychiatry 2016; 65: 34–48. [DOI] [PubMed] [Google Scholar]
- 25. Ebisch SJ, Salone A, Ferri F, et al. Out of touch with reality? Social perception in first-episode schizophrenia. Soc Cogn Affect Neurosci 2013; 8: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marasco V, De Berardis D, Serroni N, et al. Alexithymia and suicide risk among patients with schizophrenia: preliminary findings of a cross-sectional study. Riv Psichiatr 2011; 46: 31–37. [PubMed] [Google Scholar]
- 27. Raja M, Raja S. Clozapine safety, 40 years later. Curr Drug Saf 2014; 9: 163–195. [DOI] [PubMed] [Google Scholar]
- 28. Iqbal MM, Rahman A, Husain Z, et al. Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry 2003; 15: 33–48. [DOI] [PubMed] [Google Scholar]
- 29. Bak M. Monitoring clozapine adverse effects calls for the integration of protocol and good clinical practice. J Clin Psychiatry 2012; 73: 1313–1314. [DOI] [PubMed] [Google Scholar]
- 30. Miyamoto S, Jarskog LF, Fleischhacker WW. Schizophrenia: when clozapine fails. Curr Opin Psychiatry 2015; 28: 243–248. [DOI] [PubMed] [Google Scholar]
- 31. Meltzer HY, Bastani B, Ramirez L, et al. Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci 1989; 238: 332–339. [DOI] [PubMed] [Google Scholar]
- 32. Bunney BS. Clozapine: a hypothesised mechanism for its unique clinical profile. Br J Psychiatry Suppl 1992: 17–21. [PubMed] [Google Scholar]
- 33. Seeman P. Clozapine, a fast-off-D2 antipsychotic. ACS Chem Neurosci 2014; 5: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vauquelin G, Bostoen S, Vanderheyden P, et al. Clozapine, atypical antipsychotics, and the benefits of fast-off D2 dopamine receptor antagonism. Naunyn Schmiedeberg’s Arch Pharmacol 2012; 385: 337–372. [DOI] [PubMed] [Google Scholar]
- 35. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide. 5th ed. New York, NY: Cambridge University Press, 2014, p.xvi, 802. [Google Scholar]
- 36. Bishara D, Taylor D. Adverse effects of clozapine in older patients: epidemiology, prevention and management. Drugs Aging 2014; 31: 11–20. [DOI] [PubMed] [Google Scholar]
- 37. Sporn AL, Vermani A, Greenstein DK, et al. Clozapine treatment of childhood-onset schizophrenia: evaluation of effectiveness, adverse effects, and long-term outcome. J Am Acad Child Adolesc Psychiatry 2007; 46: 1349–1356. [DOI] [PubMed] [Google Scholar]
- 38. Grover S, Hazari N, Chakrabarti S, et al. Metabolic disturbances, side effect profile and effectiveness of clozapine in adolescents. Indian J Psychol Med 2016; 38: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roge R, Moller BK, Andersen CR, et al. Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far? Schizophr Res 2012; 140: 204–213. [DOI] [PubMed] [Google Scholar]
- 40. Pollmacher T, Schuld A, Kraus T, et al. On the clinical relevance of clozapine-triggered release of cytokines and soluble cytokine-receptors. Fortschr Neurol Psychiatr 2001; 69(Suppl. 2): S65–S74. [DOI] [PubMed] [Google Scholar]
- 41. De Berardis D, Campanella D, Gambi F, et al. The role of C-reactive protein in mood disorders. Int J Immunopathol Pharmacol 2006; 19: 721–725. [DOI] [PubMed] [Google Scholar]
- 42. Maes M, Bosmans E, Kenis G, et al. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res 1997; 26: 221–225. [DOI] [PubMed] [Google Scholar]
- 43. Kracmarova A, Pohanka M. The impact of clozapine on regulation of inflammation in murine macrophage cells. Neuro Endocrinol Lett 2014; 35(Suppl. 2): 175–179. [PubMed] [Google Scholar]
- 44. Klemettila JP, Kampman O, Seppala N, et al. Cytokine and adipokine alterations in patients with schizophrenia treated with clozapine. Psychiatry Res 2014; 218: 277–283. [DOI] [PubMed] [Google Scholar]
- 45. Heiser P, Enning F, Krieg JC, et al. Effects of haloperidol, clozapine and olanzapine on the survival of human neuronal and immune cells in vitro. J Psychopharmacol 2007; 21: 851–856. [DOI] [PubMed] [Google Scholar]
- 46. Hinze-Selch D, Becker EW, Stein GM, et al. Effects of clozapine on in vitro immune parameters: a longitudinal study in clozapine-treated schizophrenic patients. Neuropsychopharmacology 1998; 19: 114–122. [DOI] [PubMed] [Google Scholar]
- 47. Capannolo M, Fasciani I, Romeo S, et al. The atypical antipsychotic clozapine selectively inhibits interleukin 8 (IL-8)-induced neutrophil chemotaxis. Eur Neuropsychopharmacol 2015; 25: 413–424. [DOI] [PubMed] [Google Scholar]
- 48. O’Connell KE, Thakore J, Dev KK. Pro-inflammatory cytokine levels are raised in female schizophrenia patients treated with clozapine. Schizophr Res 2014; 156: 1–8. [DOI] [PubMed] [Google Scholar]
- 49. Ajami A, Abedian F, Hamzeh Hosseini S, et al. Serum TNF-α, IL-10 and IL-2 in schizophrenic patients before and after treatment with risperidone and clozapine. Iran J Immunol 2014; 11: 200–209. [PubMed] [Google Scholar]
- 50. Watkins CC, Andrews SR. Clinical studies of neuroinflammatory mechanisms in schizophrenia. Schizophr Res 2016; 176: 14–22. [DOI] [PubMed] [Google Scholar]
- 51. Muller N. Immunology of schizophrenia. Neuroimmunomodulation 2014; 21: 109–116. [DOI] [PubMed] [Google Scholar]
- 52. Guzelcan Y, Scholte WF. Clozapine-induced agranulocytosis: genetic risk factors and an immunologic explanatory model. Tijdschr Psychiatr 2006; 48: 295–302. [PubMed] [Google Scholar]
- 53. Testani M., Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry 1994; 55: 497–498. [PubMed] [Google Scholar]
- 54. Hummer M, Kemmler G, Kurz M, et al. Sexual disturbances during clozapine and haloperidol treatment for schizophrenia. Am J Psychiatry 1999; 156: 631–633. [DOI] [PubMed] [Google Scholar]
- 55. Mullen B, Brar JS, Vagnucci AH, et al. Frequency of sexual dysfunctions in patients with schizophrenia on haloperidol, clozapine or risperidone. Schizophr Res 2001; 48: 155–158. [DOI] [PubMed] [Google Scholar]
- 56. Perdigues SR, Quecuti RS, Mane A, et al. An observational study of clozapine induced sedation and its pharmacological management. Eur Neuropsychopharmacol 2016; 26: 156–161. [DOI] [PubMed] [Google Scholar]
- 57. Sauras RB, Ramos S, Mann L, et al. A study of clozapine-induced sedation. Actas Esp Psiquiatr 2016; 44: 244–252. [PubMed] [Google Scholar]
- 58. Gareri P, De Fazio P, Russo E, et al. The safety of clozapine in the elderly. Exp Opin Drug Saf 2008; 7: 525–538. [DOI] [PubMed] [Google Scholar]
- 59. Sethy VH, Ellerbrock BR, Wu H. Comparative dopaminergic and muscarinic antagonist activity of clozapine and haloperidol. Life Sci 1996; 58: 585–590. [DOI] [PubMed] [Google Scholar]
- 60. Citrome L, McEvoy JP, Saklad SR. Guide to the management of clozapine-related tolerability and safety concerns. Clin Schizophr Relat Psychoses 2016; 10: 163–177. [PubMed] [Google Scholar]
- 61. Saenger RC, Finch TH, Francois D. Aspiration pneumonia due to clozapine-induced sialorrhea. Clin Schizophr Relat Psychoses 2016; 9: 170–172. [PubMed] [Google Scholar]
- 62. Hinkes R, Quesada TV, Currier MB, et al. Aspiration pneumonia possibly secondary to clozapine-induced sialorrhea. J Clin Psychopharmacol 1996; 16: 462–463. [DOI] [PubMed] [Google Scholar]
- 63. Matos Santana TE, Capurso NA, Ranganathan M, et al. Sublingual atropine in the treatment of clozapine-induced sialorrhea. Schizophr Res 2017; 182: 144–145. [DOI] [PubMed] [Google Scholar]
- 64. Man WH, Colen-de Koning JC, Schulte PF, et al. The effect of glycopyrrolate on nocturnal sialorrhea in patients using clozapine: a randomized, crossover, double-blind, placebo-controlled trial. J Clin Psychopharmacol 2017; 37: 155–161. [DOI] [PubMed] [Google Scholar]
- 65. Sinha S, Simlai J, Praharaj SK. Very low dose amitriptyline for clozapine-associated sialorrhea. Curr Drug Saf 2016; 11: 262–263. [DOI] [PubMed] [Google Scholar]
- 66. Kulkarni RR. Low-dose amisulpride for debilitating clozapine-induced sialorrhea: case series and review of literature. Indian J Psychol Med 2015; 37: 446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother 2011; 45: 667–675. [DOI] [PubMed] [Google Scholar]
- 68. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure 2010; 19: 69–73. [DOI] [PubMed] [Google Scholar]
- 69. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf 2002; 25: 91–110. [DOI] [PubMed] [Google Scholar]
- 70. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs 2015; 29: 101–111. [DOI] [PubMed] [Google Scholar]
- 71. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat 2014; 10: 1973–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dumortier G, Mahe V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci 2001; 13: 302–303. [DOI] [PubMed] [Google Scholar]
- 73. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry 2016; 77: e573–e579. [DOI] [PubMed] [Google Scholar]
- 74. Balen RM, Procyshyn RM. Valproic acid for seizure prophylaxis during clozapine therapy: where’s the evidence? Int J Psychiatry Clin Pract 1999; 3: 249–251. [DOI] [PubMed] [Google Scholar]
- 75. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol 2005; 19: 93–96. [DOI] [PubMed] [Google Scholar]
- 76. Varma S, Bishara D, Besag FM, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol 2011; 1: 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry 2000; 157: 975–981. [DOI] [PubMed] [Google Scholar]
- 78. Smith M, Hopkins D, Peveler RC, et al. First-v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2008; 192: 406–411. [DOI] [PubMed] [Google Scholar]
- 79. Choong E, Bondolfi G, Etter M, et al. Psychotropic drug-induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res 2012; 46: 540–548. [DOI] [PubMed] [Google Scholar]
- 80. Hirsch L, Yang J, Bresee L, et al. Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Saf 2017; 40: 771–781. [DOI] [PubMed] [Google Scholar]
- 81. Citrome L, McEvoy JP, Saklad SR. A guide to the management of clozapine-related tolerability and safety concerns. Clin Schizophr Relat Psychoses 2016; 10:163-177. [PubMed] [Google Scholar]
- 82. Hong CJ, Lin CH, Yu YW, et al. Genetic variant of the histamine-1 receptor (glu349asp) and body weight change during clozapine treatment. Psychiatr Genet 2002; 12: 169–171. [DOI] [PubMed] [Google Scholar]
- 83. Humbert-Claude M, Davenas E, Gbahou F, et al. Involvement of histamine receptors in the atypical antipsychotic profile of clozapine: a reassessment in vitro and in vivo. Psychopharmacology 2012; 220: 225–241. [DOI] [PubMed] [Google Scholar]
- 84. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry 1994; 55(Suppl. B): 47–52. [PubMed] [Google Scholar]
- 85. Rojo LE, Gaspar PA, Silva H, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol Res 2015; 101: 74–85. [DOI] [PubMed] [Google Scholar]
- 86. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015; 14: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156: 1686–1696. [DOI] [PubMed] [Google Scholar]
- 88. Whitney Z, Procyshyn RM, Fredrikson DH, et al. Treatment of clozapine-associated weight gain: a systematic review. Eur J Clin Pharmacol 2015; 71: 389–401. [DOI] [PubMed] [Google Scholar]
- 89. Henderson DC, Daley TB, Kunkel L, et al. Clozapine and hypertension: a chart review of 82 patients. J Clin Psychiatry 2004; 65: 686–689. [DOI] [PubMed] [Google Scholar]
- 90. Lund BC, Perry PJ, Brooks JM, et al. Clozapine use in patients with schizophrenia and the risk of diabetes, hyperlipidemia, and hypertension: a claims-based approach. Arch Gen Psychiatry 2001; 58: 1172–1176. [DOI] [PubMed] [Google Scholar]
- 91. Dursun SM, Szemis A, Andrews H, et al. The effects of clozapine on levels of total cholesterol and related lipids in serum of patients with schizophrenia: a prospective study. J Psychiatry Neurosci 1999; 24: 453–455. [PMC free article] [PubMed] [Google Scholar]
- 92. Kelly AC, Sheitman BB, Hamer RM, et al. A naturalistic comparison of the long-term metabolic adverse effects of clozapine versus other antipsychotics for patients with psychotic illnesses. J Clin Psychopharmacol 2014; 34: 441–445. [DOI] [PubMed] [Google Scholar]
- 93. Lafayette JM, Pirl WF, Henderson DC. Low-dose clozapine and diabetic ketoacidosis. Psychosomatics 2003; 44: 249–252. [DOI] [PubMed] [Google Scholar]
- 94. Pierides M. Clozapine monotherapy and ketoacidosis. Br J Psychiatry 1997; 171: 90–91. [DOI] [PubMed] [Google Scholar]
- 95. Cohen D, Bogers JP, van Dijk D, et al. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry 2012; 73: 1307–1312. [DOI] [PubMed] [Google Scholar]
- 96. Nihalani ND, Tu X, Lamberti JS, et al. Diabetic ketoacidosis among patients receiving clozapine: a case series and review of socio-demographic risk factors. Ann Clin Psychiatry 2007; 19: 105–112. [DOI] [PubMed] [Google Scholar]
- 97. Cooper SJ, Reynolds GP; With Expert Co-authors et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016; 30: 717–748. [DOI] [PubMed] [Google Scholar]
- 98. Dayabandara M, Hanwella R, Ratnatunga S, et al. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat 2017; 13: 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tso G, Kumar P, Jayasooriya T, et al. Metabolic monitoring and management among clozapine users. Australas Psychiatry 2017; 25: 48–52. [DOI] [PubMed] [Google Scholar]
- 100. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry 1995; 152: 298. [DOI] [PubMed] [Google Scholar]
- 101. Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry 1997; 31: 149–150. [PubMed] [Google Scholar]
- 102. Baptista T, Carrizo E, Fernandez E, et al. Colonic transit diagnostic test shows significant gastrointestinal hypomotility in clozapine-treated patients in comparison with subjects treated with other antipsychotics. Schizophr Res 2015; 166: 207–211. [DOI] [PubMed] [Google Scholar]
- 103. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry 2008; 69: 759–768. [DOI] [PubMed] [Google Scholar]
- 104. Every-Palmer S, Lentle RG, Reynolds G, et al. Spatiotemporal mapping techniques show clozapine impairs neurogenic and myogenic patterns of activity in the colon of the rabbit in a dose-dependent manner. Front Pharmacol 2017; 8: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. de Alvarenga KAF, Sacramento EK, Rosa DV, et al. Effects of antipsychotics on intestinal motility in zebrafish larvae. Neurogastroenterol Motil 2017; 29. [DOI] [PubMed] [Google Scholar]
- 106. Every-Palmer S, Nowitz M, Stanley J, et al. Clozapine-treated patients have marked gastrointestinal hypomotility, the probable basis of life-threatening gastrointestinal complications: a cross sectional study. EBioMedicine 2016; 5: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci 2016; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘Slow Gut’ reactions, and comparison with international drug safety advice. CNS Drugs 2017; 31: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Flanagan RJ, Ball RY. Gastrointestinal hypomotility: an under-recognised life-threatening adverse effect of clozapine. Forensic Sci Int 2011; 206: e31–e36. [DOI] [PubMed] [Google Scholar]
- 110. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull 2012; 38: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baptista T. A fatal case of ischemic colitis during clozapine administration. Rev Bras Psiquiatr 2014; 36: 358. [DOI] [PubMed] [Google Scholar]
- 112. Rodriguez-Sosa JT, Lazaro-Archilla J, Trujillo-Cubas A. Apropos of a case: relationship of ischemic colitis with clozapine. Actas Esp Psiquiatr 2014; 42: 325–326. [PubMed] [Google Scholar]
- 113. Shah V, Anderson J. Clozapine-induced ischaemic colitis. BMJ Case Rep 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Galappathie N, Khan S. Clozapine-associated pneumonia and respiratory arrest secondary to severe constipation. Med Sci Law 2014; 54: 105–109. [DOI] [PubMed] [Google Scholar]
- 115. De Hert M, Hudyana H, Dockx L, et al. Second-generation antipsychotics and constipation: a review of the literature. Eur Psychiatry 2011; 26: 34–44. [DOI] [PubMed] [Google Scholar]
- 116. John JP, Chengappa KN, Baker RW, et al. Assessment of changes in both weight and frequency of use of medications for the treatment of gastrointestinal symptoms among clozapine-treated patients. Ann Clin Psychiatry 1995; 7: 119–125. [DOI] [PubMed] [Google Scholar]
- 117. Legge SE. Examining treatment response and adverse effects of clozapine. Doctoral Dissertation, Cardiff University, 2015. [Google Scholar]
- 118. Every-Palmer S, Ellis PM, Nowitz M, et al. The Porirua protocol in the treatment of clozapine-induced gastrointestinal hypomotility and constipation: a pre- and post-treatment study. CNS Drugs 2017; 31: 75–85. [DOI] [PubMed] [Google Scholar]
- 119. Chukhin E, Takala P, Hakko H, et al. In a randomized placebo-controlled add-on study orlistat significantly reduced clozapine-induced constipation. Int Clin Psychopharmacol 2013; 28: 67–70. [DOI] [PubMed] [Google Scholar]
- 120. Werner FM, Covenas R. Safety of antipsychotic drugs: focus on therapeutic and adverse effects. Exp Opin Drug Saf 2014; 13: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 121. Wang Y, He R, Zhang H. Case report on clozapine-associated neuroleptic malignant syndrome. Shanghai Arch Psychiatry 2012; 24: 116–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Leonardo QF, Juliana GR, Fernando CJ. Atypical neuroleptic malignant syndrome associated with use of clozapine. Case Rep Emerg Med 2017; 2017: 2174379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mesquita J, Siva L. Fatal neuroleptic malignant syndrome induced by clozapine in Parkinson’s psychosis. J Neuropsychiatry Clin Neurosci 2014; 26: E34. [DOI] [PubMed] [Google Scholar]
- 124. Szota A, Oglodek E, Araszkiewicz A. Fever development in neuroleptic malignant syndrome during treatment with olanzapine and clozapine. Pharmacol Rep 2013; 65: 279–287. [DOI] [PubMed] [Google Scholar]
- 125. Cherry S, Siskind D, Spivak V, et al. Fever, confusion, acute kidney injury: is this atypical neuroleptic malignant syndrome following polypharmacy with clozapine and risperidone? Australas Psychiatry 2016; 24: 602–603. [DOI] [PubMed] [Google Scholar]
- 126. Bonnet U, Taazimi B, Montag M, et al. Severe acute pancreatitis, neuroleptic malignant syndrome and grand mal seizures associated with elevated amisulpride and low clozapine serum levels. Psychiatr Danub 2015; 27: 424–425. [PubMed] [Google Scholar]
- 127. Demler TL, Morabito NE, Meyer CE, et al. Maximizing clozapine utilization while minimizing blood dyscrasias: evaluation of patient demographics and severity of events. Int Clin Psychopharmacol 2016; 31: 76–83. [DOI] [PubMed] [Google Scholar]
- 128. Fabrazzo M, Prisco V, Sampogna G, et al. Clozapine versus other antipsychotics during the first 18 weeks of treatment: a retrospective study on risk factor increase of blood dyscrasias. Psychiatry Res 2017; 256: 275–282. [DOI] [PubMed] [Google Scholar]
- 129. Mohapatra S. New food and drug administration recommendations for clozapine prescribing and monitoring requirements. Indian J Psychol Med 2016; 38: 488–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hippius H. The history of clozapine. Psychopharmacology 1989; 99: S3–S5. [DOI] [PubMed] [Google Scholar]
- 131. Atkin K, Kendall F, Gould D, et al. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry 1996; 169: 483–488. [DOI] [PubMed] [Google Scholar]
- 132. Ingimarsson O, MacCabe JH, Haraldsson M, et al. Neutropenia and agranulocytosis during treatment of schizophrenia with clozapine versus other antipsychotics: an observational study in Iceland. BMC Psychiatry 2016; 16: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ratanajamit C, Musakopas C, Vasiknanonte S, et al. Incidence and risk for neutropenia/agranulocytosis among clozapine users: a retrospective cohort study. Int J Psychiatry Clin Pract 2010; 14: 109–115. [DOI] [PubMed] [Google Scholar]
- 134. Rajagopal S. Clozapine, agranulocytosis, and benign ethnic neutropenia. Postgrad Med J 2005; 81: 545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol 2011; 26: 112–119. [DOI] [PubMed] [Google Scholar]
- 136. Singh A, Grover S, Malhotra P, et al. Late onset agranulocytosis with clozapine associated with HLA DR4 responding to treatment with granulocyte colony-stimulating factor: a case report and review of literature. Clin Psychopharmacol Neurosci 2016; 14: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Velayudhan R, Kakkan S. Late onset clozapine induced agranulocytosis. Indian J Psychol Med 2014; 36: 425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Demler TL, Trigoboff E. Are clozapine blood dyscrasias associated with concomitant medications? Innov Clin Neurosci 2011; 8: 35–41. [PMC free article] [PubMed] [Google Scholar]
- 139. de With SAJ, Pulit SL, Staal WG, et al. More than 25 years of genetic studies of clozapine-induced agranulocytosis. Pharmacogenomics J 2017; 17: 304–311. [DOI] [PubMed] [Google Scholar]
- 140. Opgen-Rhein C, Dettling M. Clozapine-induced agranulocytosis and its genetic determinants. Pharmacogenomics 2008; 9: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 141. Lahdelma L, Appelberg B. Clozapine-induced agranulocytosis in Finland, 1982–2007: long-term monitoring of patients is still warranted. J Clin Psychiatry 2012; 73: 837–842. [DOI] [PubMed] [Google Scholar]
- 142. Dettling M, Cascorbi I, Opgen-Rhein C, et al. Clozapine-induced agranulocytosis in schizophrenic Caucasians: confirming clues for associations with human leukocyte class I and II antigens. Pharmacogenomics J 2007; 7: 325–332. [DOI] [PubMed] [Google Scholar]
- 143. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 1993; 329: 162–167. [DOI] [PubMed] [Google Scholar]
- 144. Liu ZC, Uetrecht JP. Clozapine is oxidized by activated human neutrophils to a reactive nitrenium ion that irreversibly binds to the cells. J Pharmacol Exp Ther 1995; 275: 1476–1483. [PubMed] [Google Scholar]
- 145. Regen F, Herzog I, Hahn E, et al. Clozapine-induced agranulocytosis: evidence for an immune-mediated mechanism from a patient-specific in-vitro approach. Toxicol Appl Pharmacol 2017; 316: 10–16. [DOI] [PubMed] [Google Scholar]
- 146. Uetrecht JP. Metabolism of clozapine by neutrophils. Possible implications for clozapine-induced agranulocytosis. Drug Saf 1992; 7(Suppl. 1): 51–56. [DOI] [PubMed] [Google Scholar]
- 147. Ng W, Kennar R, Uetrecht J. Effect of clozapine and olanzapine on neutrophil kinetics: implications for drug-induced agranulocytosis. Chem Res Toxicol 2014; 27: 1104–1108. [DOI] [PubMed] [Google Scholar]
- 148. Williams DP, Pirmohamed M, Naisbitt DJ, et al. Induction of metabolism-dependent and -independent neutrophil apoptosis by clozapine. Mol Pharmacol 2000; 58: 207–216. [DOI] [PubMed] [Google Scholar]
- 149. Sultan RS, Olfson M, Correll CU, et al. Evaluating the effect of the changes in FDA guidelines for clozapine monitoring. J Clin Psychiatry 2017; 78: e933–e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mouaffak F, Foulon S, Smail F, et al. Clozapine: latest FDA recommendations and our practice. L’Encephale 2016; 42: 600–601. [DOI] [PubMed] [Google Scholar]
- 151. Bastiampillai T, Gupta A, Allison S. FDA changes clozapine monitoring guidelines: implications for worldwide practice. Asian J Psychiatr 2016; 21: 19–20. [DOI] [PubMed] [Google Scholar]
- 152. Leiderman DB. Risk management of drug products and the U.S. Food and Drug Administration: evolution and context. Drug Alcohol Depend 2009; 105(Suppl. 1): S9–S13. [DOI] [PubMed] [Google Scholar]
- 153. Lally J, Malik S, Whiskey E, et al. Clozapine-associated agranulocytosis treatment with granulocyte colony-stimulating factor/granulocyte-macrophage colony-stimulating factor: a systematic review. J Clin Psychopharmacol 2017; 37: 441–446. [DOI] [PubMed] [Google Scholar]
- 154. Hazewinkel AW, Bogers JP, Giltay EJ. Add-on filgrastim during clozapine rechallenge unsuccessful in preventing agranulocytosis. Gen Hosp Psychiatry 2013; 35: 576e11–576e12. [DOI] [PubMed] [Google Scholar]
- 155. Lertxundi U, Sanchez P, Hernandez R, et al. A case of agranulocytosis secondary to rechallenge with clozapine following severe neutropenia during previous therapy. J Clin Psychiatry 2011; 72: 1659. [DOI] [PubMed] [Google Scholar]
- 156. Safferman AZ, Lieberman JA, Alvir JM, et al. Rechallenge in clozapine-induced agranulocytosis. Lancet 1992; 339: 1296–1297. [DOI] [PubMed] [Google Scholar]
- 157. McKnight C, Guirgis H, Votolato N. Clozapine rechallenge after excluding the high-risk clozapine-induced agranulocytosis genotype of HLA-DQB1 6672G>C. Am J Psychiatry 2011; 168: 1120. [DOI] [PubMed] [Google Scholar]
- 158. Joffe G, Eskelinen S, Sailas E. Add-on filgrastim during clozapine rechallenge in patients with a history of clozapine-related granulocytopenia/agranulocytosis. Am J Psychiatry 2009; 166: 236. [DOI] [PubMed] [Google Scholar]
- 159. Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry 2006; 188: 255–263. [DOI] [PubMed] [Google Scholar]
- 160. Prokopez CR, Armesto AR, Gil Aguer MF, et al. Clozapine rechallenge after neutropenia or leucopenia. J Clin Psychopharmacol 2016; 36: 377–380. [DOI] [PubMed] [Google Scholar]
- 161. Kang UG, Kwon JS, Ahn YM, et al. Electrocardiographic abnormalities in patients treated with clozapine. J Clin Psychiatry 2000; 61: 441–446. [DOI] [PubMed] [Google Scholar]
- 162. Dhillon R, Bastiampillai T, Tee K, et al. Clozapine and associated QTc prolongation. Aust N Z J Psychiatry 2011; 45: 1098–1099. [DOI] [PubMed] [Google Scholar]
- 163. Olsen RE, Kroken RA, Bjorhovde S, et al. Influence of different second generation antipsychotics on the QTc interval: a pragmatic study. World J Psychiatry 2016; 6: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Warner B, Hoffmann P. Investigation of the potential of clozapine to cause torsade de pointes. Adverse Drug React Toxicol Rev 2002; 21: 189–203. [DOI] [PubMed] [Google Scholar]
- 165. Carra G, Crocamo C, Bartoli F, et al. First-generation antipsychotics and QTc: any role for mediating variables? Hum Psychopharmacol 2016; 31: 313–318. [DOI] [PubMed] [Google Scholar]
- 166. Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs 2014; 28: 887–920. [DOI] [PubMed] [Google Scholar]
- 167. Acciavatti T, Lupi M, Santacroce R, et al. Novel psychoactive substance consumption is more represented in bipolar disorder than in psychotic disorders: a multicenter-observational study. Hum Psychopharmacol 2017; 32. [DOI] [PubMed] [Google Scholar]
- 168. Hazell L, Raschi E, De Ponti F, et al. Evidence for the hERG liability of antihistamines, antipsychotics, and anti-infective agents: a systematic literature review from the ARITMO project. J Clin Pharmacol 2017; 57: 558–572. [DOI] [PubMed] [Google Scholar]
- 169. Lee SY, Kim YJ, Kim KT, et al. Blockade of HERG human K+ channels and IKr of guinea-pig cardiomyocytes by the antipsychotic drug clozapine. Br J Pharmacol 2006; 148: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ronaldson KJ. Cardiovascular disease in clozapine-treated patients: evidence, mechanisms and management. CNS Drugs 2017; 31: 777–795. [DOI] [PubMed] [Google Scholar]
- 171. Agelink MW, Majewski T, Wurthmann C, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol 2001; 21: 8–13. [DOI] [PubMed] [Google Scholar]
- 172. Sala M, Vicentini A, Brambilla P, et al. QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry 2005; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Alexopoulos GS, Streim J, Carpenter D, et al. ; Expert Consensus Panel for Using Antipsychotic Drugs in Older P. Using antipsychotic agents in older patients. J Clin Psychiatry 2004; 65(Suppl. 2): 5–99; discussion 100–102; quiz 103–104. [PubMed] [Google Scholar]
- 174. Takeuchi H, Suzuki T, Remington G, et al. Antipsychotic polypharmacy and corrected QT interval: a systematic review. Can J Psychiatry 2015; 60: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Piersanti M, Capannolo M, Turchetti M, et al. Increase in mortality rate in patients with dementia treated with atypical antipsychotics: a cohort study in outpatients in Central Italy. Riv Psichiatr 2014; 49: 34–40. [DOI] [PubMed] [Google Scholar]
- 176. Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119: e241–e250. [DOI] [PubMed] [Google Scholar]
- 177. Kim DD, White RF, Barr AM, et al. Clozapine, elevated heart rate and QTc prolongation. J Psychiatry Neurosci 2018; 43: 71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol 2014; 176: 315–320. [DOI] [PubMed] [Google Scholar]
- 179. Longhi S, Heres S. Clozapine-induced, dilated cardiomyopathy: a case report. BMC Res Notes 2017; 10: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Finsterer J, Stollberger C. Noncompaction and dilated cardiomyopathy in a patient with schizophrenia. Case Rep Cardiol 2016; 2016: 7384264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Makhoul B, Hochberg I, Rispler S, et al. Dilated cardiomyopathy: an unusual complication of clozapine therapy. Nat Clin Pract Cardiovasc Med 2008; 5: 566–570. [DOI] [PubMed] [Google Scholar]
- 182. Ronaldson KJ, Fitzgerald PB, McNeil JJ. Clozapine-induced myocarditis, a widely overlooked adverse reaction. Acta Psychiatr Scand 2015; 132: 231–240. [DOI] [PubMed] [Google Scholar]
- 183. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Clozapine-induced myocarditis and baseline echocardiography. Aust N Z J Psychiatry 2012; 46: 1006–1007. [DOI] [PubMed] [Google Scholar]
- 184. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Clinical course and analysis of ten fatal cases of clozapine-induced myocarditis and comparison with 66 surviving cases. Schizophr Res 2011; 128: 161–165. [DOI] [PubMed] [Google Scholar]
- 185. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry 2011; 45: 458–465. [DOI] [PubMed] [Google Scholar]
- 186. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf 2007; 30: 47–57. [DOI] [PubMed] [Google Scholar]
- 187. Molina Martin de Nicolas J, Parra Fuertes JJ, Gomez Mariscal E, et al. Clozapine-associated myocarditis. Med Clin 2015; 145: e19–e20. [DOI] [PubMed] [Google Scholar]
- 188. Rostagno C, Domenichetti S, Pastorelli F, et al. Clozapine associated cardiomyopathy: a cluster of 3 cases. Intern Emerg Med 2011; 6: 281–283. [DOI] [PubMed] [Google Scholar]
- 189. Power B, Williams H, Shymko G. The difficulties of diagnosing clozapine myocarditis: damned if you do, damned if you don’t. Australas Psychiatry 2012; 20: 165–166. [DOI] [PubMed] [Google Scholar]
- 190. Goodison G, Siskind D, Harcourt-Rigg C, et al. Clarifying the diagnosis of myocarditis in a patient on clozapine. Australas Psychiatry 2015; 23: 311–313. [DOI] [PubMed] [Google Scholar]
- 191. Cook SC, Ferguson BA, Cotes RO, et al. Clozapine-induced myocarditis: prevention and considerations in rechallenge. Psychosomatics 2015; 56: 685–690. [DOI] [PubMed] [Google Scholar]
- 192. Hatton JL, Bhat PK, Gandhi S. Clozapine-induced myocarditis: recognizing a potentially fatal adverse reaction. Tex Heart Inst J 2015; 42: 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Khan AA, Ashraf A, Baker D, et al. Clozapine and incidence of myocarditis and sudden death: long term Australian experience. Int J Cardiol 2017; 238: 136–139. [DOI] [PubMed] [Google Scholar]
- 194. Youssef DL, Narayanan P, Gill N. Incidence and risk factors for clozapine-induced myocarditis and cardiomyopathy at a regional mental health service in Australia. Australas Psychiatry 2016; 24: 176–180. [DOI] [PubMed] [Google Scholar]
- 195. Chopra N, de Leon J. Clozapine-induced myocarditis may be associated with rapid titration: a case report verified with autopsy. Int J Psychiatry Med 2016; 51: 104–115. [DOI] [PubMed] [Google Scholar]
- 196. Coulter DM, Bate A, Meyboom RH, et al. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ 2001; 322: 1207–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res 2012; 141: 173–178. [DOI] [PubMed] [Google Scholar]
- 198. Goldsmith DR, Cotes RO. An unmet need: a clozapine-induced myocarditis screening protocol. Prim Care Companion CNS Disord 2017; 19. [DOI] [PubMed] [Google Scholar]
- 199. Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 200. Howlett JG, McKelvie RS, Arnold JM, et al. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure, update 2009: diagnosis and management of right-sided heart failure, myocarditis, device therapy and recent important clinical trials. Can J Cardiol 2009; 25: 85–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pieroni M, Cavallaro R, Chimenti C, et al. Clozapine-induced hypersensitivity myocarditis. Chest 2004; 126: 1703–1705. [DOI] [PubMed] [Google Scholar]
- 202. Hagg S, Spigset O, Bate A, et al. Myocarditis related to clozapine treatment. J Clin Psychopharmacol 2001; 21: 382–388. [DOI] [PubMed] [Google Scholar]
- 203. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Observations from 8 cases of clozapine rechallenge after development of myocarditis. J Clin Psychiatry 2012; 73: 252–254. [DOI] [PubMed] [Google Scholar]
- 204. Reinders J, Parsonage W, Lange D, et al. Clozapine-related myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust N Z J Psychiatry 2004; 38: 915–922. [DOI] [PubMed] [Google Scholar]
- 205. Ittasakul P, Archer A, Kezman J, et al. Rapid rechallenge with clozapine following pronounced myocarditis in a treatment-resistant schizophrenia patient. Clin Schizophr Relat Psychoses 2016; 10: 120–122. [PubMed] [Google Scholar]
- 206. Chow V, Feijo I, Trieu J, et al. Successful rechallenge of clozapine therapy following previous clozapine-induced myocarditis confirmed on cardiac MRI. J Child Adolesc Psychopharmacol 2014; 24: 99–101. [DOI] [PubMed] [Google Scholar]
- 207. Ittasakul P, Archer A, Kezman J, et al. Rapid re-challenge with clozapine following pronounced myocarditis in a treatment-resistance schizophrenia patient. Clin Schizophr Relat Psychoses 2013: 1–11. [PubMed] [Google Scholar]
- 208. Paul I, Basavaraju V, Narayanaswamy JC, et al. Clozapine-induced pericarditis: an overlooked adverse effect. Clin Schizophr Relat Psychoses 2014; 8: 133–134. [PubMed] [Google Scholar]
- 209. Imon Paul MD, Basavaraju V, Narayanaswamy JC, et al. Clozapine induced pericarditis. Clin Schizophr Relat Psychoses 2014: 1–6. [PubMed] [Google Scholar]
- 210. De Berardis D, Campanella D, Serroni N, et al. Clozapine-related pericarditis during titration phase in a patient with resistant schizophrenia and concomitant valproate treatment: a case report. J Clin Psychopharmacol 2014; 34: 649–651. [DOI] [PubMed] [Google Scholar]
- 211. Markovic J, Momcilov-Popin T, Mitrovic D, et al. Clozapine-induced pericarditis. Afr J Psychiatry 2011; 14: 236–238. [DOI] [PubMed] [Google Scholar]
- 212. Rathore S, Masani ND, Callaghan PO. Clozapine-induced effuso-constrictive pericarditis. Case report and review of the literature. Cardiology 2007; 108: 183–185. [DOI] [PubMed] [Google Scholar]
- 213. Crews MP, Dhillon GS, MacCabe JH. Clozapine rechallenge following clozapine-induced pericarditis. J Clin Psychiatry 2010; 71: 959–961. [DOI] [PubMed] [Google Scholar]
- 214. Manu P, Sarpal D, Muir O, et al. When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res 2012; 134: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ 2010; 341: c4245. [DOI] [PubMed] [Google Scholar]
- 216. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry 2016; 173: 166–173. [DOI] [PubMed] [Google Scholar]
- 217. Gami RK, Mishra P, Sedlak T. Pulmonary embolism and clozapine use: a case report and literature review. Psychosomatics 2017; 58: 203–208. [DOI] [PubMed] [Google Scholar]
- 218. Jonsson AK, Spigset O, Hagg S. Venous thromboembolism in recipients of antipsychotics: incidence, mechanisms and management. CNS Drugs 2012; 26: 649–662. [DOI] [PubMed] [Google Scholar]
- 219. Liperoti R, Gambassi G. Antipsychotics and the risk of venous thromboembolism. BMJ 2010; 341: c4216. [DOI] [PubMed] [Google Scholar]
- 220. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology 1997; 8: 671–677. [DOI] [PubMed] [Google Scholar]
- 221. Axelsson S, Hagg S, Eriksson AC, et al. In vitro effects of antipsychotics on human platelet adhesion and aggregation and plasma coagulation. Clin Exp Pharmacol Physiol 2007; 34: 775–780. [DOI] [PubMed] [Google Scholar]
- 222. Voulgari C, Giannas R, Paterakis G, et al. Clozapine-induced late agranulocytosis and severe neutropenia complicated with Streptococcus pneumonia, venous thromboembolism, and allergic vasculitis in treatment-resistant female psychosis. Case Rep Med 2015; 2015: 703218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Hagg S, Bate A, Stahl M, et al. Associations between venous thromboembolism and antipsychotics. A study of the WHO database of adverse drug reactions. Drug Saf 2008; 31: 685–694. [DOI] [PubMed] [Google Scholar]
- 224. Jonsson AK, Horvath-Puho E, Hagg S, et al. Antipsychotics and risk of venous thromboembolism: a population-based case-control study. Clin Epidemiol 2009; 1: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Letmaier M, Grohmann R, Kren C, et al. Venous thromboembolism during treatment with antipsychotics: results of a drug surveillance programme. World J Biol Psychiatry 2017: 1–12. [DOI] [PubMed] [Google Scholar]
- 226. Wang MT, Liou JT, Huang YW, et al. Use of antipsychotics and risk of venous thromboembolism in postmenopausal women. A population-based nested case-control study. Thromb Haemost 2016; 115: 1209–1219. [DOI] [PubMed] [Google Scholar]
- 227. Chandele PH, Cholera R, Kale S, et al. Theoretical and practical issues related to the management of severe and refractory psychotic illness complicated by pulmonary embolism. Indian J Psychiatry 2015; 57: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Allenet B, Schmidlin S, Genty C, et al. Antipsychotic drugs and risk of pulmonary embolism. Pharmacoepidemiol Drug Saf 2012; 21: 42–48. [DOI] [PubMed] [Google Scholar]
- 229. Paciullo CA. Evaluating the association between clozapine and venous thromboembolism. Am J Health Syst Pharm 2008; 65: 1825–1829. [DOI] [PubMed] [Google Scholar]
- 230. Stein PD, Matta F, Goldman J. Obesity and pulmonary embolism: the mounting evidence of risk and the mortality paradox. Thromb Res 2011; 128: 518–523. [DOI] [PubMed] [Google Scholar]
- 231. Chapelle C, Quenet S, Delavenne X, et al. Antipsychotics: a real or confounding risk factor for venous thromboembolism? Pharmacopsychiatry 2013; 46: 36–37. [DOI] [PubMed] [Google Scholar]
- 232. Niehues GD, Balan AB, Pra VB, et al. Trends in the prescription of clozapine in a psychiatric hospital: a 5-year observational study. Trends Psychiatry Psychother 2017; 39: 158–164. [DOI] [PubMed] [Google Scholar]
- 233. Alessi-Severini S, Le Dorze JA, Nguyen D, et al. Clozapine prescribing in a Canadian outpatient population. PLoS One 2013; 8: e83539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Grover S, Balachander S, Chakarabarti S, et al. Prescription practices and attitude of psychiatrists towards clozapine: a survey of psychiatrists from India. Asian J Psychiatr 2015; 18: 57–65. [DOI] [PubMed] [Google Scholar]
- 235. Tungaraza TE, Farooq S. Clozapine prescribing in the UK: views and experience of consultant psychiatrists. Ther Adv Psychopharmacol 2015; 5: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv 2016; 67: 369–371. [DOI] [PubMed] [Google Scholar]
- 237. Wimberley T, MacCabe JH, Laursen TM, et al. Mortality and self-harm in association with clozapine in treatment-resistant schizophrenia. Am J Psychiatry 2017; 174: 990–998. [DOI] [PubMed] [Google Scholar]
- 238. Ringback Weitoft G, Berglund M, et al. Mortality, attempted suicide, re-hospitalisation and prescription refill for clozapine and other antipsychotics in Sweden-a register-based study. Pharmacoepidemiol Drug Saf 2014; 23: 290–298. [DOI] [PubMed] [Google Scholar]
- 239. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull 2015; 41: 644–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Bender S, Dittmann-Balcar A, Schall U, et al. Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double-blind comparison of olanzapine vs. clozapine. Int J Neuropsychopharmacol 2006; 9: 135–145. [DOI] [PubMed] [Google Scholar]
- 241. McGurk SR. The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry 1999; 60(Suppl. 12): 24–29. [PubMed] [Google Scholar]
- 242. Fujii DE, Ahmed I, Jokumsen M, et al. The effects of clozapine on cognitive functioning in treatment-resistant schizophrenic patients. J Neuropsychiatry Clin Neurosci 1997; 9: 240–245. [DOI] [PubMed] [Google Scholar]
- 243. Grace J, Bellus SB, Raulin ML, et al. Long-term impact of clozapine and psychosocial treatment on psychiatric symptoms and cognitive functioning. Psychiatr Serv 1996; 47: 41–45. [DOI] [PubMed] [Google Scholar]