Abstract
AIM
To investigate the effect of internal limiting membrane transplantation and autologous blood on treating refractory giant macular hole.
METHODS
Thirty-seven eyes with giant macular hole of the smallest hole diameter >700 µm, the maximum diameter of the substrate >1000 µm and hole formation factor <0.6 underwent surgical treatment. The patients were randomly divided into two groups. Nineteen eyes with surgical flip of the internal limiting membrane in group A, 18 eyes with internal limiting membrane transplantation in group B who underwent the tamponade of internal limiting membrane into the hole, autologous plasma was used to seal the hole. The patients were followed up for 3mo, optical coherence tomography and best corrected visual acuity (BCVA) were recorded before and after operation, and the results were statistically analyzed.
RESULTS
At 3mo after operation, BCVA of the two groups was significantly improved compared with that before operation (tA=4.192, tB=4.374, P<0.05). But there was no significant difference in visual acuity between the two groups (χ2=0.128, P>0.05). At 3mo after operation, the closure rate of group A was 68.4%, and 100% in group B. (χ2=5.628, P<0.05). The defect diameter of inner segment/outer segment at 3mo after the operation was significantly lower than that before operation (tA=12.287, tB=15.481, P<0.05), and the difference was statistically significant (t=2.552, P<0.05).
CONCLUSION
Internal limiting membrane transplantation combined with autologous whole blood can improve the postoperative closure rate of the refractory large aperture, and can effectively improve the postoperative visual acuity.
Keywords: internal limiting membrane transplantation, autologous blood, refractory, macular hole
INTRODUCTION
Macular hole (MH) is a retinal neuroepithelial layer of macular tissue defect, the incidence rate is about 0.6%-0.7%, MH can occur in all ages, is a serious damage to eyesight. There may be many causes of MH, such as ocular trauma, high myopia, endophthalmitis, laser burns and cystoid macular edema, some of which are unknown, such as idiopathic macular hole (IMH). IMH is more common in middle-aged and older women, most of them are monocular disease, the incidence of binocular vision is about 12%, The prevalence rate was 3.3% in people over 55 years old[1]. The progressive MH need to be solved by timely vitrectomy surgery, in order to avoid secondary retinal detachment, resulting in further loss of vision. At present, vitrectomy combined with internal limiting membrane (ILM) peeling has improved the closure rate of IMH as high as 90%[2]–[3], but for the larger diameter of refractory MH underwent vitrectomy combined with ILM peeling, postoperative closed rate is low[4]. The reasons for this are not clear, and it maybe related to decreased retinal pigment epithelial (RPE) function. In order to improve closure rate of such IMH, we creatively use ILM transplantation combined with autologous whole blood treatment for which minimum diameter is >700 µm, the maximum base diameter is 1000 µm, hole formation factor (HFF) is >0.6, and observe the clinical effect after operation. To the end, we prospectively studied 37 patients with IMH of the minimum diameter of >700 µm, the maximum base diameter of 1000 µm, HFF>0.6 in the Department of Ophthalmology, No.474 Hospital of the PLA, from October 2016 to February 2017.
SUBJECTS AND METHODS
Subjects
This study collected 37 eyes of 37 cases with the minimum IMH diameter of >700 µm, the maximum base diameter of 1000 µm, HFF>0.6 in the hospital center in October 2016 to February 2017. There were 3 males and 34 females, aged 56-72 (mean 62.80±9.35) years old, and associated with decreased vision and metamorphopsia. All the patients were randomly divided into two groups: group A (19 eyes) and group B (18 eyes). Exclusion criteria: 1) with retinal detachment; 2) a history of vitreous body and retinal surgery; 3) with high myopia, vitreous body and retinal disease, cataract, glaucoma, ocular trauma and other eye diseases; 4) surgical contraindications to systemic and ocular vitreous body. The study was approved by the hospital ethics committee and all participants signed informed consent.
Surgical Methods
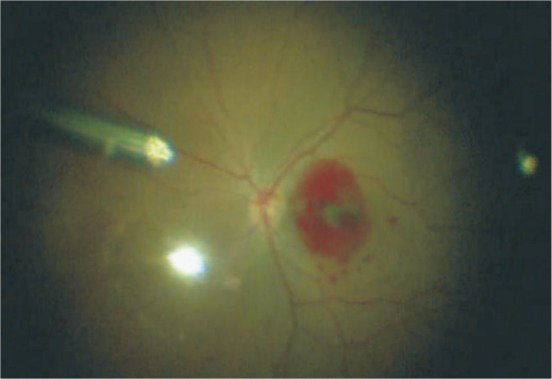
All operations were performed by the same surgeon who had extensive experience in vitreous surgery. In group A, all patients underwent conventional 23G vitrectomy through pars plana corporis ciliaris, and the hole of the macular area was covered with stripped ILM after indocyanine green staining. In group B, all patients underwent conventional 23G vitrectomy through pars plana corporis ciliaris, and annular detachment of ILM in macular after indocyanine green staining. The valve with the size of the hole in the central part of the left side of the nasal side (like a tennis racket), and ILM flap is turned, and filled in MH. Finally, the peripheral ILM is removed. Autologous peripheral blood about 1 mL extracted from the dorsal hand vein, drop hole and cover flap (Figure 1). After 3min, gas exchange and injection of inert gas SF6 about 0.6 mL was performed. After surgery, the patients remain in the prone position before gas is fully absorbed.
Figure 1. In group B, autologous blood was driped after ILM transplantation.
Observation Index
Best corrected visual acuity (BCVA), fracture closure, inner segment/outer segment (IS/OS) defect diameter were recorded at preoperative and 3mo after operation. Optical coherence tomography (OCT) was used to evaluate the MH closure: 1) complete closure: the nerve epithelium completely covers the naked pigment epithelium, and the neuroepithelial tissue can be seen in the macula, but can be partially replaced by scar; 2) part of the closure: nerve epithelium and pigment epithelium are clothing in the edge of the fissure, retinal subside in the hole periphery, the center of the macula is completely replaced by scar tissue; 3) unclosure: MH edge detachment[5].
Statistical Analysis
Statistical software SPSS18.0 was used to analyze the data, paired t-test was used to compare BCVA and the diameter of IS/OS before and after operation, application of chi square test to the closure of MH, the test level was 0.05, which was statistically significant at P<0.05.
RESULTS
Vision
There were 16 cases in group A with higher BCVA than preoperative, and the improvement rate was 84.2%. In group B, 16 cases were improved, the improvement rate was 88.9%. BCVA was significantly improved compared with preoperative in two groups 3mo after operation (tA=4.192, tB=4.374, P<0.05). But there was no significant difference in visual acuity between the two groups (χ2=0.128, P>0.05).
Macular Hole Closure
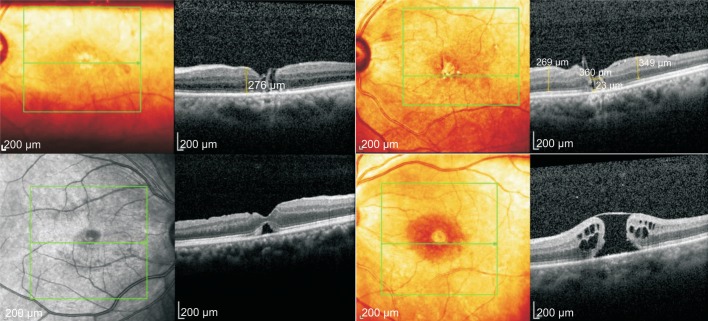
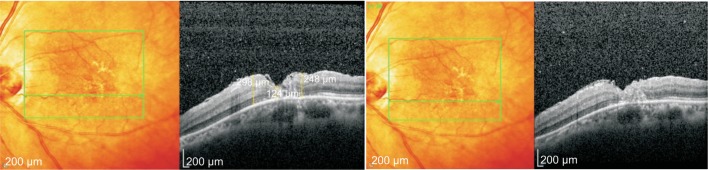
The macular area was observed 1d after operation through the gas on, procumbent macular area can be showed in group A; in group B, the whole blood was found in the macular 1d after operation. Blood absorbed gradually after 1wk and the MH could be observed by gas after 10d. At 1mo after operation, the OCT scan could clearly observe transplanted ILM tissue in MH. At 3mo after operation, there were 5 cases in group A appear neuroepithelial layer cavity defect in OCT. It is suggested that the MH is not complete closure (Figure 2). In 1 case, the MH was not closed and visible superior ILM flap in OCT, MH closure rate was 68.4%. In the group B, the epithelium of the nerve or ILM was completely covered the exposed pigment epithelium and MH closure rate was 100% (Figure 3). The difference between the two groups was statistically significant (χ2=5.628, P<0.05).
Figure 2. In group A, there were no complete cover of ILM, and the empty defect appeared in the retinal neuroepithelial layer in five cases.
It be found that ILM flap in the MH and an empty defect in the cerebral cortex. There was an empty defect in the retinal neuroepithelial layer. The defect of retinal neuroepithelial layer was discontinuous, and ILM flap could be seen above. It is suggesting that the closure was incomplete.
Figure 3. In group B, the retinal nerve epithelium and ILM were completely covered the naked macular pigment epithelium at 1mo after operation. At 3mo after operation, it can be seen that ILM tissue is transplanted into the slit, and the hole is completely closed.
Optical Coherence Tomography
The defect diameter of IS/OS 3mo after the operation was significantly lower than that before operation (tA=12.287, tB=15.481, P<0.05), and the difference was statistically significant (t=2.552, P<0.05) between the two groups.
Complications
The posterior limiting membrane of vitreum and ILM were successfully identified with the assistance of triamcinolone acetonide and indocyanine green. None of the patients had iatrogenic hole formation. A patient had a glaucoma attack on the side of the surgery eye after the follow-up, and intraocular pressure (IOP) decreased to normal after drug treatment.
DISCUSSION
IMH is a primary eye disorder that threatens the vision of middle-aged patients. Timely treatment of IMH helps prevent the development of secondary retinal detachment. The aetiology and pathogenesis of IMH are not well understood. IMH may be linked to posterior vitreous traction, in which the tractional force is generated in the tangential or vertical direction during the vitreous liquefaction process[6].
Currently, many surgical methods are available for the treatment of IMH, but there is controversy about which method is the best. A large number of literatures have confirmed that vitrectomy combined with ILM peeling can effectively improve the postoperative closure rate of MH[7]–[10]. As shown in some reports, completely removing the posterior vitreous cortex and peeling ILM promotes the closure of MHs because the procedure stimulates the proliferation of RPE cells, which results in the formation of a fibrotic or scarring lesion that covers and closes the holes[11]. Many factors can affect the post-operative closure of MHs and the visual prognosis, including the minimum diameter of the hole, maximum diameter of the basal hole, height of the hole, and HFF. In particular, Woon et al[12] suggests that the minimum hole diameter and maximum basal hole diameter are the most important factors that affect postoperative MH closure and vision. Salter et al[13] suggest that postoperative therapeutic efficacy is best when an MH has a minimum diameter of <500 µm and a maximum basal diameter of <1000 µm. If an MH has a minimum diameter of >500 µm, its closure rate is lower after vitrectomy and ILM peeling, and the postoperative visual recovery is also worse. Further, the likelihood that a second surgery would be required significantly increasingly, and the hole closure rate following the second surgery is much lower than the first surgery[14]. Refractory MHs with an HFF value of <0.6 have only a 67% closure rate after vitrectomy and ILM peeling[14]. To enhance postoperative closure rate of refractory MHs and achieve better surgical outcomes, researchers worldwide have performed a variety of modified surgeries, including ILM peeling, partial ILM preservation, and inverted ILM insertion[15]. An IMH with a maximum diameter of <400 µm has a postoperative closure rate of more than 90% after vitrectomy and ILM peeling[16]. However, bigger holes have lower closure rates, with as many as 44% of MHs failing to be closed after the first surgery and requiring a second surgery[17]–[18]. Gass et al[19] modified MH surgery and applied an inverted ILM flap technique, achieving a 100% postoperative MH closure rate. In addition, following surgery, macular structures were stable and there were no reports of relapse. During actual practice, it was found that patients with refractory MHs with a minimum diameter of >700 µm, maximum basal diameter of >1000 µm, and HFF of <0.6 after vitrectomy using the inverted ILM flap technique appeared to have cavities and discontinuous RPE underneath ILM flap such that the MHs were not closed well.
In order to explore a better surgical protocol for the treatment of refractory MHs and enhance their postoperative closure rate, we selected patients with MHs with a minimum diameter of >700 µm, maximum basal diameter of >1000 µm, and HFF value of <0.6, all of which were measured at the edge of the MH using OCT. These MHs were clinically referred to as refractory MHs. Group A underwent a vitrectomy with the inverted ILM flap technique and group B underwent a vitrectomy with ILM transplantation. During surgery, the MHs were closed with autologous whole blood, and following surgery, all patients underwent inert-gas injection. Three months after surgery, all patients had a follow-up visit to observe the hole closure status and visual acuity.
During surgery, ILM peeling eliminated the traction caused by ILM acting on the periphery of the MH. After surgery, ILM flap transplanted within the MH provided a scaffold for glial cells and thus promoted the proliferation of neuroglial cells, which draw peripheral photoreceptor cells toward the centre of MHs. During surgery, the autologous whole blood covered and closed the MHs by which locally forming a closed cavity causing the peripheral areas of the MH to be in a relatively confined space. Moreover, the active transport of RPE moved the fluid underneath the retina to the periphery of the holes, causing the retinal neurosensory layer (RNL) to attach tightly to the RPE layer, ultimately promoting hole closure. In addition, we easily obtained the autologous whole blood with a low contamination rate. The blood contained multiple growth factors which could promote collagen synthesis and fibroblast proliferation, increase retinal adhesion, reduce prone postoperative positioning, enhance postoperative comfort, and facilitate hole closure; this was especially important for elderly patients who have difficulty maintaining a prolonged prone position. The results of this study showed 3mo after the operation, BCVA of the two groups was significantly improved compared with that before operation. But the difference was not statistically significant between the two groups.
Three months after surgery, group A included 5 cases with an incomplete covering of ILM and a cavity-like RNL defect (indicated by an incomplete closure of the hole) and 1 case with a fairly large cavity underneath ILM and a hole that failed to close (Figure 2). The other cases had complete hole closure, with a closure rate of 68.4%. This phenomenon was likely a result of poor prone position compliance and decreased RPE function. The RPEs at the holes in group B were all covered by the RNL or ILM, with a 100% hole closure rate (Figure 3). OCT scanning suggested that the defect diameter of the photoreceptor IS/OS junction was significantly smaller than that before surgery (tA=12.287, tB=15.481, P<0.05). During the postoperative follow-up, one patient had a glaucoma attack in the contralateral eye, but IOP dropped to normal after drug therapy. IOP of the operated eye was normal throughout the follow-up, therefore, this patient likely had predisposition for glaucoma. The results of this study imply that ILM transplantation with autologous whole blood application could promote postoperative visual recovery in patients with refractory MHs. Covering the hole with autologous whole blood and filling the vitreous chamber with inert gas were both beneficial to fixing ILM transplanted in the MH. During this surgery, great care should be taken during the process of fluid-gas exchange to prevent sucking out the introduced whole blood and the transplanted ILM.
The findings of this study indicate that vitrectomy with both ILM transplantation and autologous whole blood may significantly increase postoperative hole closure rate in patients with refractory MHs (minimum diameter >700 µm, maximum basal diameter >1000 µm, and HFF <0.6) and may dramatically enhance the visual functions of patients, rendering this surgical approach safe and effective. Moreover, we demonstrated that intraoperative blood collection is easily performed and the probability contamination is extremely low. However, further exploration is necessary to determine whether this surgical approach is applicable to other forms of refractory MHs and if indocyanine green-stained ILM would result in adverse side effects in the retina.
Acknowledgments
Conflicts of Interest: Lyu WJ, None; Ji LB, None; Xiao Y, None; Fan YB, None; Cai XH, None.
REFERENCES
- 1.Wu P, Huang XD. Advances in the study of idiopathic macular hole. Guoji Yanke Zazhi (Int Eye Sci) 2014;14(2):259–262. [Google Scholar]
- 2.Bainbridge J, Herbert E, Gregor Z. Macular hole: vitreoretinal relationships and surgical approaches. Eye (Lond) 2008;22(10):1301–1309. doi: 10.1038/eye.2008.23. [DOI] [PubMed] [Google Scholar]
- 3.Wu TT, Kung YH. Comparison of anatomical and visual outcomes of macular hole surgery in patients with high myopia vs non-high myopia: a case-control study using optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):327–331. doi: 10.1007/s00417-011-1821-7. [DOI] [PubMed] [Google Scholar]
- 4.Dong FT, Yang ZK, Zou X. New surgery not with idiopathic macular hole: free ILM transplantation. Medical Journal of Peking Union Medical College Hospital. 2013;4(4):425–428. [Google Scholar]
- 5.Wu Y, Zhao PQ, Jiang CH. Evaluation of vitrectomy for retinal detachment with macular hole in high myopia by optical coherence tomography. Chinese Journal of Practical Ophthalmology. 2004;22(8):613–616. [Google Scholar]
- 6.Alsulaiman SM, Alrushood AA, Almasaud J, Alkharashi AS, Alzahrani Y, Abboud EB, Nowilaty SR, Arevalo JF, AL-Amry M, Alrashaed S, Ghazi NG. Full thickness macular hole secondary to high-power handheld blue laser: natural history and management outcomes. Am J Ophthalmol. 2015;160(1):107–113.e1. doi: 10.1016/j.ajo.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Briand S, Chalifoux E, Tourvlle E, Bourqault S, Caissie M, Tardif Y, Giasson M, Boivin J, Blanchette C, Cinq-Mars B. Prospective randomized trial: outcome of SF6 versus C3F8 in macular hole surgery. Can J Ophthalmol. 2015;50(2):95–100. doi: 10.1016/j.jcjo.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Wu LL, Ho TC, Yang CH, Yanq CM. Vitreo-retinal relationship and post-operative outcome of macular hole repair in eyes with high myopia. Graefes Arch Clin Exp Ophthalmol. 2016;254(1):7–14. doi: 10.1007/s00417-015-2986-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156(1):125–131.e1. doi: 10.1016/j.ajo.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Che X, He F, Lu L, Zhu D, Xu X, Song X, Fan X, Wang Z. Evaluation of secondary surgery to enlarge the peeling of the internal limiting membrane following the failed surgery of idiopathic macular hole. Exp Ther Med. 2014;7(3):742–746. doi: 10.3892/etm.2014.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, Tokoro T, Mochizuki M. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology. 2003;110(7):1297–1305. doi: 10.1016/S0161-6420(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 12.Woon WH, Greig D, Savage MD, Wilson MC, Grant CA, Bishop F, Mokete B. Asymmertric vitreomacular trection and symmetrical full thickness macular hole formation. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1851–1857. doi: 10.1007/s00417-014-2884-z. [DOI] [PubMed] [Google Scholar]
- 13.Salter AB, Folgar FA, Weissbrot J, Wald KJ. Macular hole surgery prognostic success rates based on macular hole size. Ophthalmic Surg Lasers Imaging. 2012;43(3):184–189. doi: 10.3928/15428877-20120102-05. [DOI] [PubMed] [Google Scholar]
- 14.Hillenkamp J, Kraus J, Framme C, Jackson TL, Roider J, Gabel VP, Sachs HG. Retreatment of full-thickness macular hole: predictive value of optical coherence tomography. Br J Ophthalmol. 2007;91(11):1445–1449. doi: 10.1136/bjo.2007.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Layana A, Garcia-Arumi J, Ruiz-Moreno JM, Arias-Barquet L, Cabrera-Lopez F, Figueroa MS. A review of current management of vitreomacular traction and macular hole. J Ophthalmol. 2015;2015:809640. doi: 10.1155/2015/809640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 18.Baba T, Hagiwara A, Sato E. Comparison of vitrectomy with brilliant blue G or ondocyanine green on retinal microstructure and function of eyes with macular hole. Ophthalmology. 2012;119(12):2609–2615. doi: 10.1016/j.ophtha.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 19.Gass CA, Haritoglou C, Schaumberger M, Kampik A. Functional outcome of macular hole surgery with and without indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2003;241(9):716–720. doi: 10.1007/s00417-003-0710-0. [DOI] [PubMed] [Google Scholar]