Abstract
Objective
The aim of this study was to evaluate the efficacy and safety of the mesenchymal stem cell (MSC) therapy in patients with ischemic stroke (IS).
Materials and methods
Clinical trials involved in this research were searched from PubMed, Web of Science, Cochrane Library, Embase, Wanfang and CNKI database. Therapeutic effects of MSC therapy were assessed according to National Institutes of Health Stroke Scale (NIHSS), Barthel index (BI), Fugl-Meyer Assessment (FMA) and Functional Independence Measure (FIM), and its safety was evaluated based on adverse events.
Results
This research covered 23 trials including 1,279 IS patients. Based on our analysis, the overall condition of IS patients significantly improved after MSC therapy, indicated by decreased NIHSS and increased BI, FMA and FIM scores. Our analysis also showed that the treatment effects in the MSC transplantation group were superior to those in the control group (routine medication therapy) with statistical significance for NIHSS (1 month after therapy: odds ratio [OR]=−1.92, CI=−3.49 to −0.34, P=0.02; 3 months after therapy: OR=−2.65, CI=−3.40 to −1.90, P<0.00001), BI (1 month after therapy: OR=0.99, CI=0.19–1.79, P=0.02; 6 months after therapy: OR=10.10, CI=3.07–17.14, P=0.005), FMA (3 months after therapy: OR=10.20, CI=3.70–16.70, P=0.002; 6 months after therapy: OR=10.82, CI=6.45–15.18, P<0.00001) and FIM (1 month after therapy: OR=15.61, CI=−0.02 to 31.24, P=0.05; 6 months after therapy: OR=16.56, CI=9.06–24.06, P<0.0001). No serious adverse events were reported during MSC therapy.
Conclusion
MSC therapy is safe and effective in treating IS by improving the neurological deficits, motor function and daily life quality of patients.
Keywords: mesenchymal stem cells, routine medication, ischemic stroke, meta-analysis
Introduction
Stroke is the most lethal and second most incident disease, together with cancer and cardiovascular disease.1,2 Ischemic stroke (IS) and intracerebral hemorrhage account for about 85% and 15%, respectively, of all stroke events.3 The main pathological manifestation of IS is temporary brain tissue ischemia.1 Ischemia results in reduced neuron number and interrupted neural axon network, and formation of a defected environment around the ischemic region, which prohibits brain self-healing, eventually resulting in the permanent loss of nerve tissue or disabled brain function.1,4,5 Over 50,000,000 people are suffering from IS of various degrees in the world.6 The annual mortality rate is close to 10%, and nearly 50% of stroke survivors are left with disabling sequelae.1,2,4 Poststroke rehabilitation therapy is scant, and currently the most efficient medicine for IS in clinical settings is tissue plasminogen activator.1,7 However, it functions mainly at the early stage of ischemia with a short time window, which may increase the risk of cerebral hemorrhage, and therefore, its clinical application is severely limited.7
Stem cell therapy, using hematopoietic stem cells (HSCs),8,9 neural stem cells (NSCs),10,11 endothelial progenitor cells (EPCs)12 and other types of stem cells,13,14 was considered a promising treatment for IS as it may reduce injury and promote rehabilitation after stroke. Mesenchymal stem cells (MSCs) are derived from mesoderm and have the capacity of regeneration and differentiation. MSCs can differentiate into various lineages such as NSCs, which can further differentiate into neurons, astrocytes, oligodendrocytes and so on,4,15,16 with low immunogenicity and high histocompatibility.1,15 Compared with other types of stem cells, such as NSCs, EPCs and HSCs, MSCs are more accessible as they can be easily obtained from umbilical cord, bone marrow, fat and other tissues, and can proliferate rapidly in vitro with little ethical constraints.15 Preliminary preclinical studies using MSCs to treat IS have shown beneficial effects.17–19 In animal models, researchers found the transplanted cells migrated to lesions, secreted neurotrophic factors, remitted inflammatory response and promoted plasticity and revascularization thereby minimizing the damage.18–20
Although both preclinical studies and studies with experimental models regarding MSC transplantation therapies for IS have been carried out,17–19 the clinical application of MSCs still has a long way to go due to its unverified safety and therapeutic effects. To address this issue, we conducted a meta-analysis of published clinical trials treating IS with MSCs, to provide scientific references for upcoming research and future clinical application.
Materials and methods
Search strategy and selection criteria
Studies were identified from PubMed, Web of Science, Cochrane Library, Embase, Wanfang and CNKI database, with key terms (“mesenchymal stem cells” or “MSC”) and (“ischemic stroke” or “cerebral infarction” or “cerebral ischemia” or “brain ischemia”), without language restriction. The last search was performed in October 2017.
Studies were included if they fulfilled the following inclusion criteria: case-controlled trials involving IS patients; participants were diagnosed with IS and without malignant tumor, pregnancy and lactation; and patients in the experimental group received both MSC transplantation and IS routine treatment (RT; including conventional medical therapy and rehabilitation training treatment) combined therapy, and those in control group were treated by RT alone.
Data extraction and quality assessment
All data collection and extraction were performed by two authors (PX and MW) independently. The following information was summarized: author’s names, years of publication, locations, type of IS, samples sizes, patients’ ages, study parameter types, therapeutic regimens, administration routes, MSC dosages and adverse events during the MSC therapy. Trials’ quality was assessed for risk of bias following the instruction of Cochrane Handbook.21
Outcome definition
Treatment efficacy was assessed in terms of National Institutes of Health Stroke Scale (NIHSS), Barthel index (BI), Fugl-Meyer Assessment (FMA) and Functional Independence Measure (FIM). The frequencies of adverse events were gathered and assessed for MSC therapy safety.
Statistical analysis
This meta-analysis was performed using Review Manager 5.3 (Cochrane Collaboration). The therapeutic efficacy was evaluated by odds ratio (OR) and presented with 95% CI. P<0.05 indicates differences with statistical significance. The appropriate analysis model was determined by analyzing the heterogeneity between trials by Cochran’s Q test.22 Studies with I2<50% or P>0.1 were considered homogenous.
Publication bias was evaluated based on funnel plots. Sensitivity analysis on subgroups was also performed to assess the impact of MSC types, cell administration methods and patients’ characteristics on clinical outcomes.
Results
Search results
A total of 2,998 articles were initially identified, and 2,921 were excluded for lacking clinical trials (n=2,693), duplication and repetition (n=177) or being unrelated (n=51). The later detailed assessment further excluded 18 articles with insufficient data, 23 reviews or case reports or meta-analyses and 13 articles without control group. A total of 23 trials23–45 with 1,279 IS patients were finally identified meeting the restrict inclusion criteria of this research (Figure 1).
Figure 1.
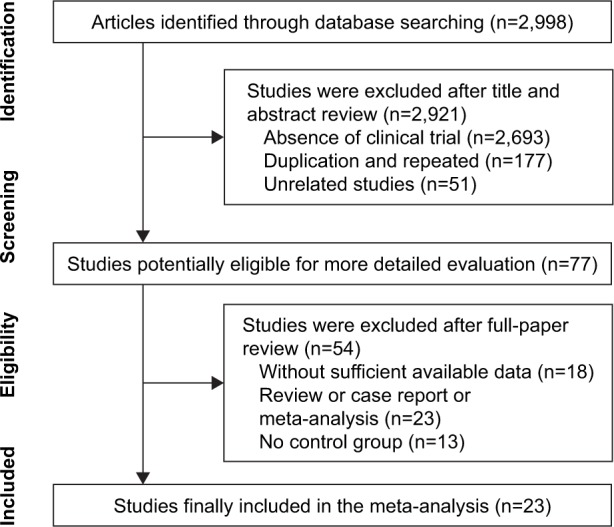
Flow diagram of the selection process.
Study and patient characteristics
After selection, all included trials were found to have been conducted in Asia. Two studies were conducted in Korea,23,35 two in India24,25 and the rest of the included studies in China. MSCs were obtained from bone marrow in 18 studies,23–30,32, 35–37,40–45 from umbilical cord in three studies33,34,38 and from umbilical cord blood in two studies.31,39 Cells were administered through peripheral vein in 14 studies,23–27, 30,32,34,35,37,40–43 subarachnoid in five studies,28,29,36,44,45 intrathecal in three studies31,33,39 and intracarotid artery in one study.38 In total, 625 IS patients accepted MSC and RT combined therapy, and 654 patients were treated by RT alone. Detailed information about the involved studies and participants is summarized in Tables 1 and 2.
Table 1.
Clinical information from the eligible trials in the meta-analysis
| Included studies | Country | Type of stroke: acute/chronic | Patients: control/experimental | Age (years)
|
Parameter types | |
|---|---|---|---|---|---|---|
| Control | Experimental | |||||
| Bang et al23 | Korea | Acute | 25/5 | ND | ND | BI |
| Bhasin et al24 | India | Chronic | 20/20 | 45.2±11.8 | 45.1±12.1 | FMA, BI |
| Bhasin et al25 | India | Chronic | 6/6 | 46.5±6.3 | 42±9.3 | FMA, BI, AE |
| Cai et al26 | China | Chronic | 21/21 | 62.7±6.9 | 61.4±6.7 | FMA, FIM, BI |
| Cheng et al27 | China | Acute | 18/18 | 68.1±2.5 | 69.1±1.2 | FIM, BI |
| Chen et al28 | China | ND | 43/43 | ND | ND | FMA, FIM |
| Chen et al29 | China | ND | 30/30 | 57.4±9.6 | 49.3±20.8 | NIHSS |
| Deng et al30 | China | ND | 15/15 | ND | ND | NIHSS |
| Feng et al31 | China | ND | 50/50 | 60.2±11.8 | 61.4±11.3 | NIHSS |
| He32 | China | ND | 18/20 | 54.3±8.7 | 56.4±7.9 | NIHSS, BI |
| Hu et al33 | China | ND | 60/60 | 59.2±13.8 | 60.8±15.2 | FMA, FIM |
| Ji et al34 | China | ND | 60/60 | ND | ND | FMA, BI |
| Lee et al35 | Korea | Acute | 36/16 | 64.9±14.5 | 64.0±11.6 | AE |
| Liu et al36 | China | ND | 29/29 | 56.9±4.4 | 55.3±3.6 | NIHSS, FMA, BI |
| Meng et al37 | China | ND | 30/30 | 52.9±8.3 | 52.7±7.9 | FMA, FIM |
| Shen38 | China | Acute | 16/16 | ND | 52±10.4 | FIM |
| Song et al39 | China | ND | 28/28 | 65.4 | 63.2 | NIHSS |
| Sun et al40 | China | Acute | 22/20 | 58.9±7.4 | 57.8±8.9 | NIHSS, BI |
| Sun et al41 | China | ND | 15/20 | 30.9±16.9 | 29.5±9.4 | FMA |
| Tsang et al42 | Hong Kong | Chronic | 4/5 | 51.5 | 53.4 | FIM, BI, AE |
| Wang et al43 | China | ND | 60/60 | ND | ND | FIM |
| Xie et al44 | China | ND | 30/30 | 53.7±6.1 | 51.4±7.2 | NIHSS, BI |
| Zhao et al45 | China | ND | 18/23 | 53.3±18.9 | 50.2±20.0 | NIHSS |
Note: Data are presented as mean±SD or median.
Abbreviations: ND, nondetermined; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; FMA, Fugl-Meyer Assessment; FIM, Functional Independence Measure; AE, adverse event.
Table 2.
Information of MSC therapy
| Includedstudies | Therapeutic regimen
|
Administration route | Cell dose (cycles) | Enrollment period | Follow-up (months) | Adverse events | |
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Bang et al23 | Con Reg+BMSC | RM+G-CSF | IVE | 5×107 (2 cycles) | ND | 52 | No obvious adverse reactions |
| Bhasin et al24 | Con Reg+BMSC | RM | IVE | 5–6×107 (1 cycle) | ND | 6 | No obvious adverse reactions |
| Bhasin et al25 | Con Reg+BMSC | RM | IVE | 5–6×107 (1 cycle) | ND | 6 | Fever (1); pain (2) |
| Cai et al26 | Con Reg+BMSC | RM | IVE | 0.5–2×108 (3 cycles) | 2014.1–2015.1 | 6 | ND |
| Cheng et al27 | Con Reg+BMSC | RM | IVE | 0.5–2×108 (3 cycles) | 2011.1–2012.12 | 3 | ND |
| Chen et al28 | Con Reg+BMSC | RM | SUB | 1×106/kg (1 cycle) | 2009.12–2011.8 | 5 | ND |
| Chen et al29 | Con Reg+BMSC | RM | SUB | 3–5×106 (2 cycles) | 2009.1–2011.5 | 6 | Low-grade fever (3); headache (4) |
| Deng et al30 | Con Reg+BMSC | RM+SM | IVE | 1–5×107 (3 cycles) | ND | 1 | No obvious adverse reactions |
| Feng et al31 | Con Reg+UBMSC | RM | IT, IVE | 3×107 (6 cycles) | 2010.9–2013.2 | 3 | Low-grade fever (1) |
| He32 | Con Reg+BMSC | RM | IVE | 1×108 (1 cycle) | 2010.4–2012.2 | 3 | ND |
| Hu et al33 | Con Reg+UCMSC | RM | IT+IVE | 1×108 (1 cycle) | 2011.4–2012.6 | 3 | Low-grade fever (12); headache (5); flank soreness (10) |
| Ji et al34 | Con Reg+UCMSC | RM | IVE | 1×107 (1 cycle) | 2009–2010 | 6 | No obvious adverse reactions |
| Lee et al35 | Con Reg+BMSC | RM | IVE | 5×107 (2 cycles) | 2003.7–2005.12 | 60 | No obvious adverse reactions |
| Liu et al36 | Con Reg+BMSC | RM | SUB | 1×107/kg (4 cycles) | 2010.12–2012.12 | 3 | No obvious adverse reactions |
| Meng et al37 | Con Reg+BMSC | RM | IVE | 2.97×109 (1 cycle) | 2003.6–2008.6 | 6 | Low-grade fever (4); headache (3) |
| Shen38 | Con Reg+UCMSC | RM | IC | ND | 2012.1–2013.12 | 3 | ND |
| Song et al39 | Con Reg+UBMSC | RM | IT+IVE | ND | 2009–2010 | 1 | Low-grade fever (5) |
| Sun et al40 | Con Reg+BMSC +G-CSF | RM | IVE | 1.4±0.6×108 (1 cycle) | 2006.8–2007.6 | 3 | ND |
| Sun et al41 | Con Reg+BMSC | RM | IVE | ND (3 cycles) | 2011.8–2012.8 | 3 | Low-grade fever (2) |
| Tsang et al42 | Con Reg+BMSC | RM | IVE | 4.57×107 (1 cycle) | ND | 15 | No obvious adverse reactions |
| Wang et al43 | Con Reg+BMSC | RM | ND | 1–2×108 (1 cycle) | 2009.1–2010.6 | 6 | No obvious adverse reactions |
| Xie et al44 | Con Reg+BMSC | RM | SUB | 2×107 (1 cycle) | 2011.1–2012.7 | 6 | Low-grade fever (3); headache (4) |
| Zhao et al45 | Con Reg+BMSC | RM | SUB | ND | ND | 1 | Fever (1) |
Abbreviations: Con Reg, control group regimen; RM, routine medication; MSC, mesenchymal stem cell; BMSC, bone marrow mesenchymal stem cell; UBMSC, umbilical cord blood mesenchymal stem cell; UCMSC, umbilical cord mesenchymal stem cell; ND, nondetermined; SM, Salvia miltiorrhiza; G-CSF, granulocyte colony-stimulating factor; IVE, intravenous; IT, intrathecal; SUB, subarachnoid; IC, intracarotid.
Quality assessment
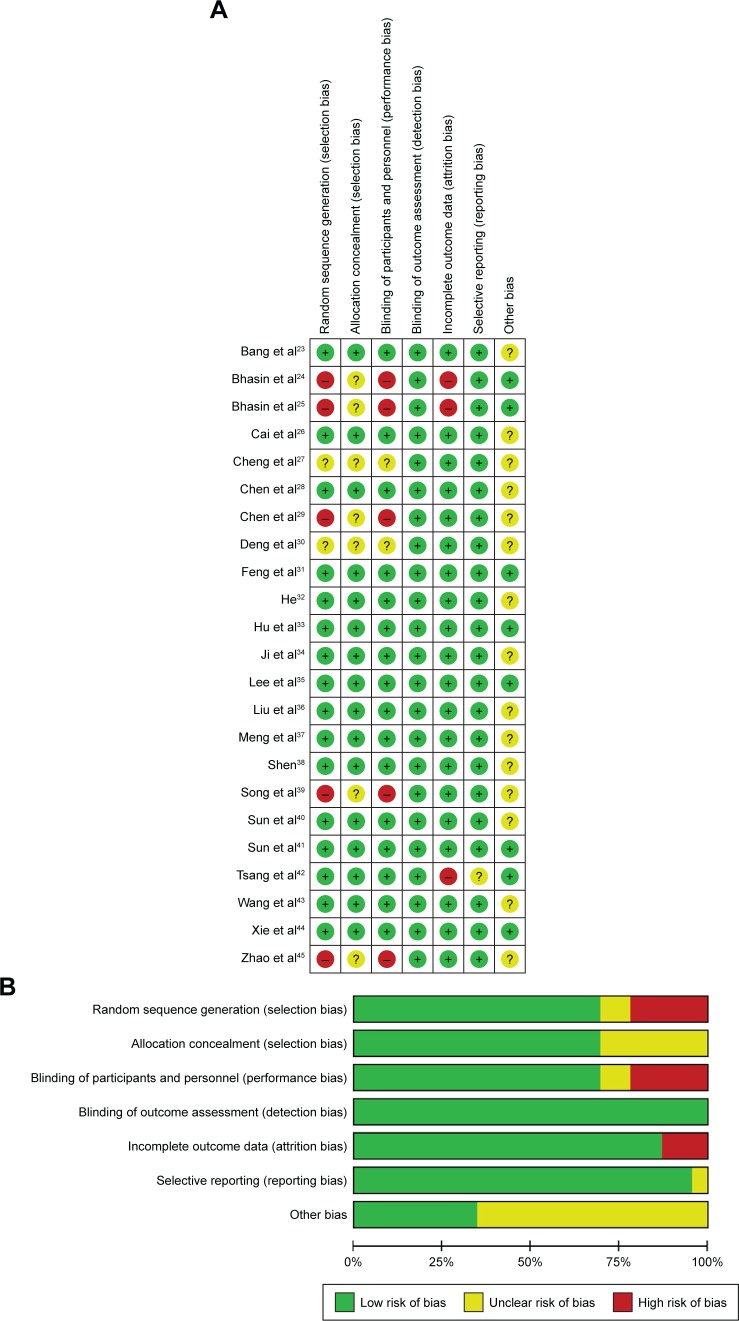
Bias risk of involved trials is shown in Figure 2. Sixteen studies were determined as low risk, five researches were not truly randomized controlled trials and the other two studies did not have clear illustration of randomization procedures. Seven trials did not provide clear description of allocation and performance concealment. All the included studies were free of detection risk. Three trials missing the follow-up study were considered as high risk, and one study with selective reporting was considered as unclear risk.
Figure 2.
(A) Risk-of-bias summary: review of authors’ judgments about each risk-of-bias item for included studies. (B) Risk-of-bias graph: review of authors’ judgments about each risk-of-bias item presented as percentages across all included studies.
Note: Each color represents a different level of bias.
Therapeutic efficacy assessments
Effectiveness of MSCs assessed by the NIHSS score
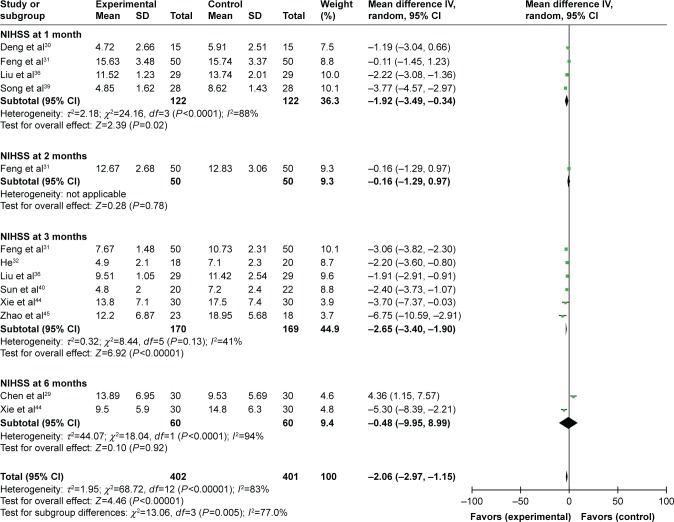
The analysis of involved trials showed that after MSC therapy, the NIHSS score was reduced in the first, second, third and sixth month after treatment (1 month: OR=−5.20, CI=−6.52 to −3.87, P<0.00001; 2 months: OR=−6.46, CI=−7.86 to −5.06, P<0.00001; 3 months: OR=−7.50, CI=−9.59 to −5.40, P<0.00001; 6 months: OR=−9.19, CI=−11.77 to −6.60, P<0.00001; Figure S1). As shown in Figure 3, compared to the control group, the NIHSS score of the experimental group was lower in the first (OR=−1.92, CI=−3.49 to −0.34, P=0.02) and third month (OR=−2.65, CI=−3.40 to −1.90, P<0.00001).
Figure 3.
Forest plot of the comparison of NIHSS scores between the experimental and control groups.
Notes: Control group, RT alone group; experimental group, RT plus MSC therapy. The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; NIHSS, National Institutes of Health Stroke Scale; RT, routine treatment; MSC, mesenchymal stem cell.
Effectiveness of MSCs assessed by the BI score
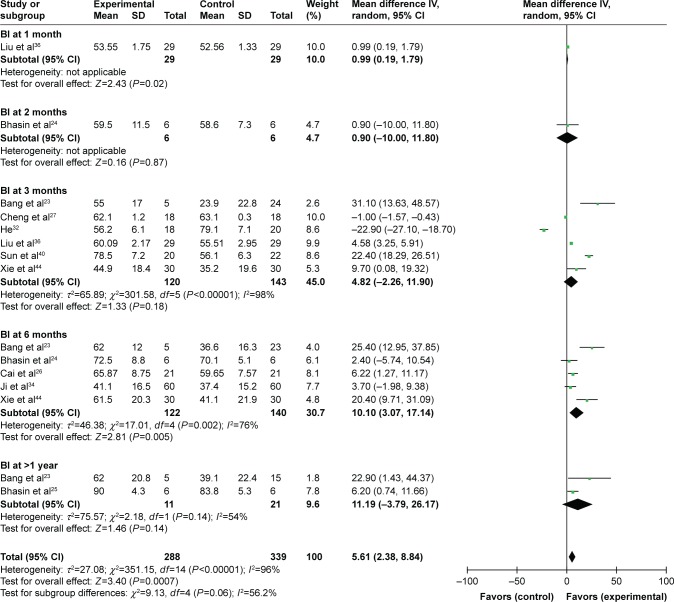
The postoperative BI score was increased after combined therapy in the first, second, third and sixth month and after 12 months (1 month: OR=30.14, CI=29.34–30.94, P<0.00001; 2 months: OR=15.50, CI=2.99–28.01, P=0.02; 3 months: OR=29.66, CI=24.12–35.20, P<0.00001; 6 months: OR=27.76, CI=13.24–42.28, P=0.0002; after 1 year: OR=45.79, CI=37.32–54.25, P<0.00001; Figure S2). In the comparison between patients treated by combined therapy and RT alone, the BI score in the combined therapy group was higher in the first and sixth month (1 month: OR=0.99, CI=0.19–1.79, P=0.02; 6 months: OR=10.10, CI=3.07–17.14, P=0.005; Figure 4).
Figure 4.
Forest plot of the comparison of the BI scores between the experimental and control groups.
Notes: Control group, RT alone group; experimental group, RT plus MSC therapy. The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; B1, Barthel index; RT, routine treatment; MSC, mesenchymal stem cell.
Effectiveness of MSCs assessed by the FMA score
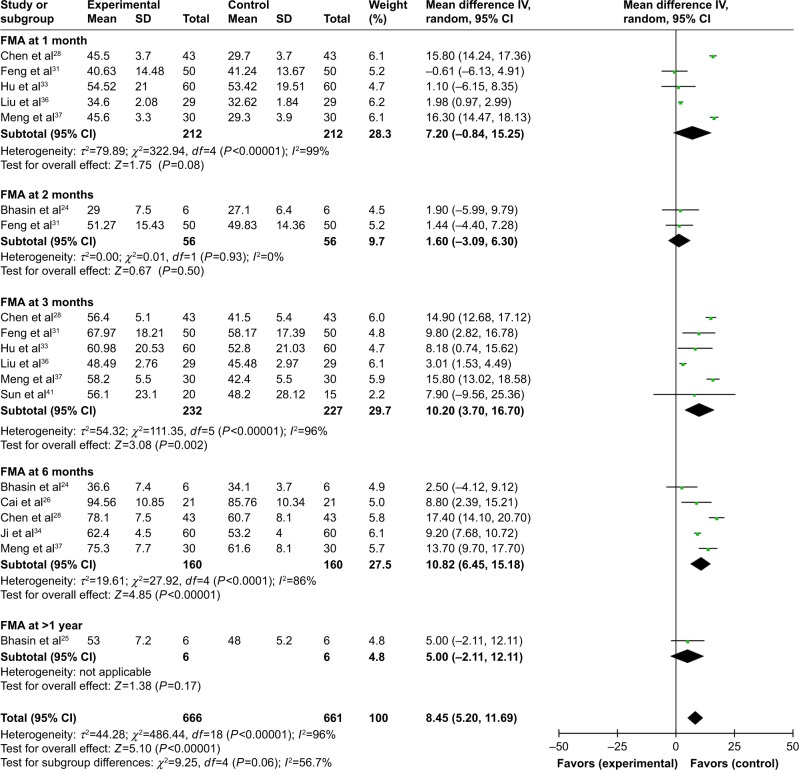
The FMA score after combined therapy was significantly increased in the first, second, third and sixth month, and after 12 months (1 month: OR=15.49, CI=7.51–23.47, P=0.0001; 2 months: OR=18.46, CI=7.11–29.82, P=0.001; 3 months: OR=27.00, CI=19.78–34.23, P<0.00001; 6 months: OR=39.26, CI=25.85–52.67, P<0.00001; after 1 year: OR=36.40, CI=29.31–43.49, P<0.00001; Figure S3). A comparison between the two groups indicated a significantly increased FMA score in the third and sixth month postoperation in the combined therapy group (3 months: OR=10.20, CI=3.70–16.70, P=0.002; 6 months: OR=10.82, CI=6.45–15.18, P<0.00001; Figure 5).
Figure 5.
Forest plot of the comparison of FMA scores between the experimental and control groups.
Notes: Control group, RT alone group; experimental group, RT plus MSC therapy. The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; FMA, Fugl-Meyer Assessment; RT, routine treatment; MSC, mesenchymal stem cell.
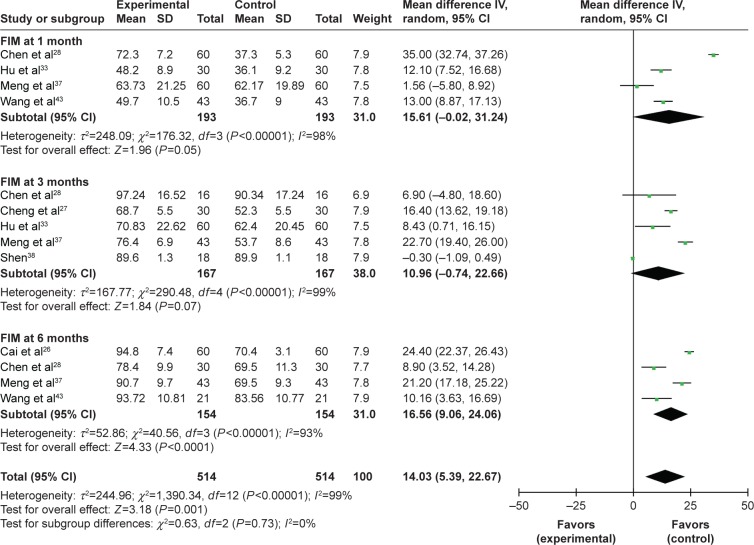
Effectiveness of MSCs assessed by the FIM score
As shown in Figure 3, the FIM score was increased after combined therapy, especially in the first, third and sixth month postoperation (1 month: OR=24.47, CI=7.14–41.80, P=0.006; 3 months: OR=24.05, CI=6.56–41.54, P=0.007; 6 months: OR=48.13, CI=32.04–64.23, P<0.00001; Figure S4). Meanwhile, the FIM score in the combined therapy group was higher than that of the control group in the first and sixth month (1 month: OR=15.61, CI=−0.02 to 31.24, P=0.05; 6 months: OR=16.56, CI=9.06–24.06, P<0.0001; Figure 6).
Figure 6.
Forest plot of the comparison of the FIM scores between the experimental and control groups.
Notes: Control group, RT alone group; experimental group, RT plus MSC therapy. The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; FIM, Functional Independence Measure; RT, routine treatment; MSC, mesenchymal stem cell.
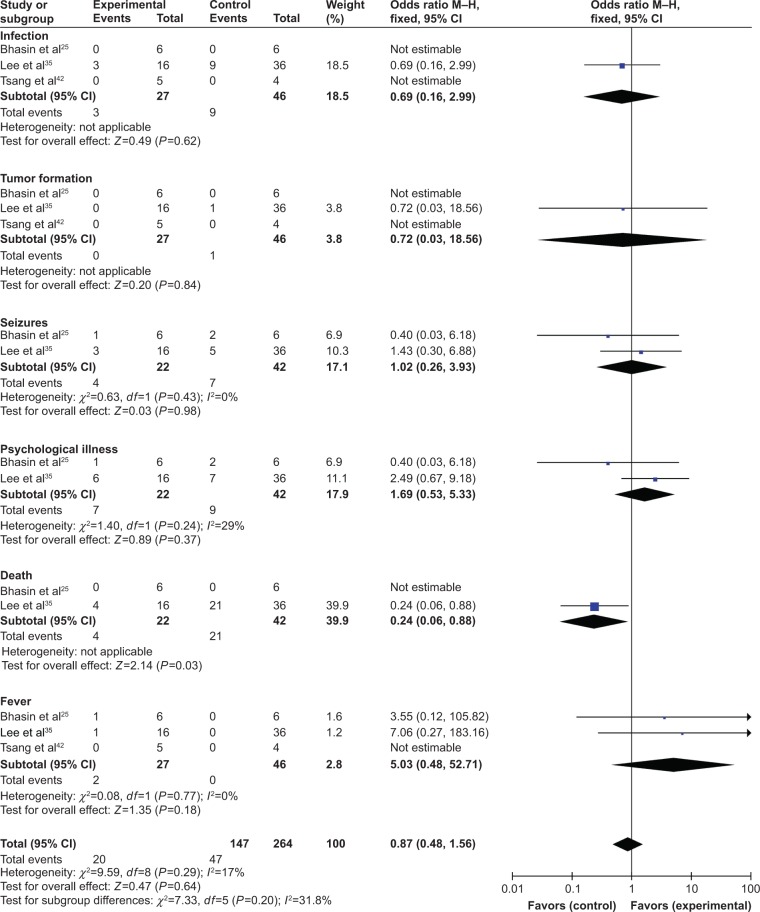
Adverse event assessment
We evaluated the safety of MSC therapy in this meta-analysis. The most common side effects of MSC treatment were headache and fever, which usually subsided within 24 hours without treatment. No serious adverse events were reported in the involved studies (Table 1). However, the incidence of side effects in experimental and control groups was not compared in most included trials. Three studies25,35,42 conducted the comparison of adverse events including infection, tumor formation, seizures, psychological illness, death and fever. Except death, no significant difference was found for other indicators between the two groups (infection: OR=0.69, CI=0.16–2.99, P=0.62; tumor formation: OR=0.72, CI=0.03–18.56, P=0.84; seizures: OR=1.02, CI=0.26–3.93, P=0.98; psychological illness: OR=1.69, CI=0.53–5.33, P=0.37; death: OR=0.24, CI=0.06–0.88, P=0.03; fever: OR=5.03, CI=0.48–52.71, P=0.18; Figure 7).
Figure 7.
Forest plot of the comparison of adverse events between the experimental and control groups.
Notes: Control group, RT alone group; experimental group, RT plus MSC therapy. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Abbreviations: M–H, Mantel–Haenszel; RT, routine treatment; MSC, mesenchymal stem cell.
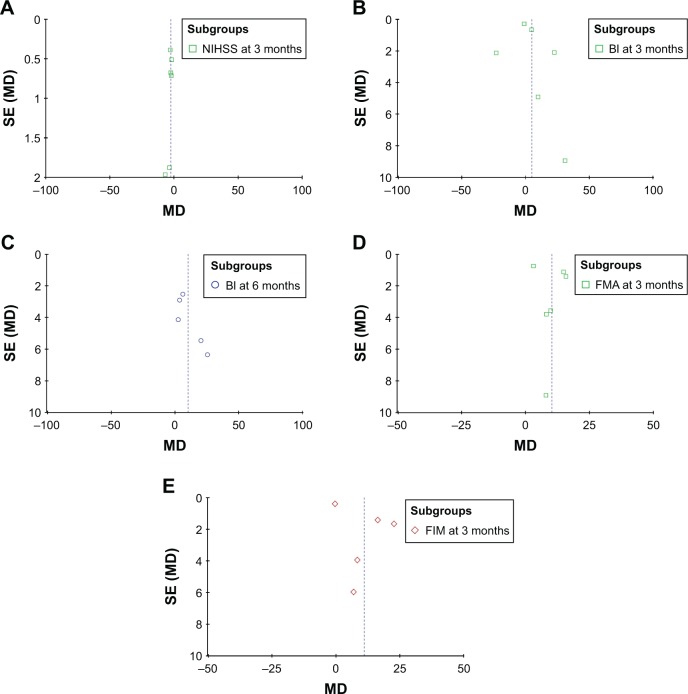
Publication bias
Based on the NIHSS,31,32,36,40,44,45 BI,23,24,26,27,32,34,36,40,42,44 FMA28,31,33,36,37,41 and FIM27,28,33,37,38,42 data, funnel plots were drawn for the studies. The funnel plots were symmetrical, indicating no existence of publication bias (Figures 8 and S5).
Figure 8.
Funnel plot of the NIHSS (A), BI (B and C), FMA (D) and FIM (E) scores between the experimental and control groups.
Note: Parameters were discussed in over five studies which were included in bias analyses.
Abbreviations: SE, standard error; MD, mean difference; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; FMA, Fugl-Meyer Assessment; FIM, Functional Independence Measure.
Sensitivity analysis
To further evaluate the effects of clinical variables including cell types and different administration routes on clinical efficacy of patients with different characteristics, we performed subgroup analysis. Results showed that MSC therapy was more effective when infusion was performed through vein, and autogenous MSCs were superior to those derived from other sources, indicated by increased BI, FMA and FIM scores (Table 3).
Table 3.
Subgroup analyses of NIHSS, BI, FMA and FIM between the experimental and control groups
| Parameter (TP after surgery) | Factors at study level | Experimental group
|
Control group
|
Analysis method | Heterogeneity
|
OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| No. of patients (n) | No. of patients (n) | I2 (%) | P-value | ||||||
| NIHSS (Month 3) | Cell type | ||||||||
| Auto-MSC | 120 | 119 | Random | 37 | 0.18 | −2.49 | −3.45 to −1.54 | <0.00001 | |
| Allo-MSC | 50 | 50 | Random | −3.06 | −3.82 to −2.30 | <0.00001 | |||
| Route of delivery | |||||||||
| Subarachnoid | 82 | 77 | Random | 68 | 0.04 | −3.66 | −6.53 to −0.80 | 0.01 | |
| Intravenous | 38 | 42 | Random | 0 | 0.84 | −2.30 | −3.27 to −1.34 | <0.00001 | |
| BI (Month 3) | Route of delivery | ||||||||
| Subarachnoid | 59 | 59 | Random | 6 | 0.30 | 4.83 | 2.66–7.01 | <0.0001 | |
| Intravenous | 61 | 84 | Random | 99 | ,0.00001 | 6.02 | −10.58 to 22.63 | 0.48 | |
| BI (Month 6) | Cell type | ||||||||
| Auto-MSC | 62 | 80 | Random | 80 | 0.002 | 12.48 | 3.01–21.94 | 0.010 | |
| Allo-MSC | 60 | 60 | Random | 3.70 | −1.98 to 9.38 | 0.20 | |||
| Route of delivery | |||||||||
| Subarachnoid | 30 | 30 | Random | 20.40 | 9.71–31.09 | 0.0002 | |||
| Intravenous | 92 | 110 | Random | 72 | 0.01 | 7.70 | 0.96–14.44 | 0.03 | |
| Patients’ characteristics | |||||||||
| Acute stroke | 5 | 23 | Random | 25.40 | 12.95–37.85 | <0.0001 | |||
| Chronic stroke | 27 | 27 | Random | 0 | 0.43 | 5.19 | 0.96–9.42 | 0.02 | |
| FMA (Month 3) | Cell type | ||||||||
| Auto-MSC | 122 | 117 | Random | 97 | <0.00001 | 10.76 | 2.38–19.14 | 0.01 | |
| Allo-MSC | 110 | 110 | Random | 0 | 0.76 | 9.04 | 3.95–14.13 | 0.0005 | |
| Route of delivery | |||||||||
| Subarachnoid | 72 | 72 | Random | 99 | <0.00001 | 8.92 | −2.73 to 20.58 | 0.13 | |
| Intravenous | 50 | 45 | Random | 0 | 0.38 | 15.60 | 12.86–18.35 | <0.00001 | |
| FIM (Month 3) | Cell type | ||||||||
| Auto-MSC | 89 | 89 | Random | 84 | 0.002 | 17.28 | 11.01–23.55 | <0.00001 | |
| Allo-MSC | 78 | 78 | Random | 79 | 0.03 | 3.19 | −5.19 to 11.57 | 0.46 | |
| Route of delivery | |||||||||
| Subarachnoid | 16 | 16 | Random | 6.90 | −4.80 to 18.60 | 0.25 | |||
| Intravenous | 73 | 73 | Random | 88 | 0.004 | 19.49 | 13.31–25.66 | <0.00001 | |
Abbreviations: TP, time point; OR, odds ratio; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; FMA, Fugl-Meyer Assessment; FIM, Functional Independence Measure; auto-MSC, autogenous mesenchymal stem cell; allo-MSC, allogenic mesenchymal stem cell.
Discussion
MSC transfusion has been considered as a promising option to treat IS due to its unique biological characteristics. Transfused MSCs can migrate to infarction area and induce angiogenesis,46,47 reduce neuron apoptosis,48,49 enhance axonal regeneration and rebuild synapses. Upon stimulating the release of cytokines and neurotrophic factors,3,50 such as brain-derived neurotrophic factor,3 basic fibroblast growth factor15 and vascular endothelial growth factor,3,51 MSCs also promote the differentiation of endogenous neural stem and progenitor cells. Most importantly, the low immunogenicity of MSCs reduces the possibility of graft-versus-host reaction.1,15
In recent years, several studies reported that MSC therapy is a safe and feasible treatment option for IS,23–45 but different clinical protocols among studies may bring different therapeutic effects. In this study, we performed an extensive and systematic analysis of published clinical trials to assure statistical reliability. Our meta-analysis revealed that compared to IS patients treated by RT alone, those treated by MSC and RT combined therapy exhibited more favorable therapeutic efficacy, indicated by decreased NIHSS and increased BI, FMA and FIM scores.
MSC therapy has been applied to treat refractory diseases for years with satisfied safety record,52–55 and our analysis showed that MSCs were safe in treating IS as well. No serious adverse events have been reported during MSC therapy. Most common side effects, including fever and headache, usually resolved naturally. However, relevant studies were insufficient, and the potential long-term toxicity and the risk of tumor formation are unknown, which usually take years to occur. More research evidence will be required to support the safety of combined therapy.
Therapeutic effects of MSC therapy may be affected by infusion routes, cell dosages, cell types and patients’ characteristics. We found that intravenous infusion is generally superior to subarachnoid injection in therapeutic effects, but there were also contradicted conclusions drawn from different researches. There are articles that claimed that local subarachnoid injection may deliver a larger number of transplanted MSCs to the stroke lesion thereby promoting nerve recovery and regeneration.2,56 However, the different routes of cell infusion did not make big difference in other researches,57 which speculated that MSCs treat IS through releasing growth factors and antiapoptotic factors instead of homing to the nerve system.3,58 The treatment effect varies at different time points of detection, and dosages of transfused MSCs are a key factor in therapeutic strategy optimization. There are studies that showed that increased number of infused cells contributed to favorable clinical efficacy,57 but currently published literature is still not sufficient to perform reliable statistical analysis. Sources of MSC may also associate with treatment outcomes. Based on our extracted data, autogenous MSCs were associated with increased BI and FIM score, indicating a better therapeutic effect than allogenic MSCs for IS. However, our data were not sufficient, and more research evidence is needed to support this conclusion. The optimal conduction time of cell delivery is also undetermined yet. Preclinical studies showed that early intervention leads to an obvious relief of neurological defects.2,59 Our subgroup analysis suggested no significant difference in outcomes between the acute and chronic phases of stroke.
Limitations
There are some limitations in this analysis. First of all, the numbers of involved studies and patients were small and the follow-up period was short, which may cause publication bias. Second, all trials included in this paper were mainly conducted in Asian countries. There were indeed several trials conducted in non-Asian countries included upon the first retrieve. However, no paper meeting our inclusion criteria has been produced based on these trials, and studies were excluded due to insufficient data, and being case reports, unrelated to MSC therapy or without control group. We will keep paying close attention to global studies in this field and carry out further analyses in our later studies. Third, our data were partly extracted from published papers rather than original patient records, which means we were not able to avoid the analytical bias based on the information presented in them. In addition, different trials evaluated the treatment efficacy by different outcomes, which have to be summarized using various scales when assessed in this study, leading to small sample sizes in each statistical analysis. Due to above limitations, future studies and generated data will be valuable to further verify the safety and efficacy of MSC therapy.
Conclusion
In summary, our analysis verified the safety and efficacy of MSC therapy for IS. It significantly mitigated neurological defects and improved life quality of IS patients, without causing serious adverse events. Therefore, MSC therapy is a promising treatment option for IS patients.
Supplementary materials
Forest plot of the comparison of NIHSS scores pre-and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; NIHSS, National Institutes of Health Stroke Scale.
Forest plot of the comparison of BI scores pre- and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; BI, Barthel index.
Forest plot of the comparison of FMA scores pre- and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; FMA, Fugl-Meyer Assessment.
Forest plot of the comparison of FIM scores in pre- and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; FIM, Functional Independence Measure.
Funnel plot of the NIHSS (A), BI (B and C), FMA (D) and FIM (E) scores pre- and post-therapy.
Note: Parameters were discussed in over five studies which were included in bias analyses.
Abbreviations: SE, standard error; MD, mean deviation; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; FMA, Fugl-Meyer Assessment; FIM, Functional Independence Measure.
References
- 1.Deng YG, Zhang ZP, Yu W. Clinical efficacy of salvia miltiorrhiza injection combined with marrow stem cells in treatment of cerebral infarction. Pract J Cardiac Cereb Pneumal Vasc Dis. 2012;20(3):424–425. [Google Scholar]
- 2.Feng Y, Tian GP, Li L, Zhou J. Effect of human umbilical cord blood-derived mesenchymal stem cells in the treatment of cerebral infarction. Pract J Cardiac Cereb Pneumal Vasc Dis. 2014;22(1):28–30. [Google Scholar]
- 3.Liu DH, Han BJ, Hong SS, et al. Transplanting autologous mesenchymal nerve stem cells in the treatment of cerebral infarction. Chin J Phys Med Rehabil. 2014;36(6):425–428. [Google Scholar]
- 4.Song CW, Wang P, Hu XQ, et al. Clinical efficacy of umbilical cord blood mesenchymal stem cells transplantation in the treatment of cerebral infarction. J Clin Ration Drug Use. 2013;6(2):69–70. [Google Scholar]
- 5.He ZD. The functional mechanism of mesenchymal stem cell transplantation therapy for patients with cerebral infarction. Asia Pac Tradit Med. 2012;8(12):126–127. [Google Scholar]
- 6.Sun HB, Xia SM, Yang SS. The clinical effect of rhG-CSF combined transplantation of autologous bone marrow mesenchymal stem cells on acute cerebral infarction. J Chin Phys. 2008;10(4):441–443. [Google Scholar]
- 7.Sun SC, Wang C, Luo JS. Bone marrow mesenchymal stem cell transplantation improved the neurological function of 35 patients with sequelae of stroke. China Med Eng. 2013;21(6):14–17. [Google Scholar]
- 8.Xie XF, Liu SY, Jin GH, Qu XH, Zhang KN, Wu XM. Clinical analysis of autologous bone marrow mesenchymal stem cell transplantation for treating cerebral infarction. Lab Med Clin. 2014;11(21):2955–5957. [Google Scholar]
- 9.Zhao XL, Wang Y, Zhang CX, Tan J. Study of bone marrow mesenchymal stem cells in the treatment of stroke. Global Chin Med. 2013;6(S2):157. [Google Scholar]
- 10.Chen WM, Zou QY, Lu JJ, et al. Reinfusion of autologous bone marrow mesenchymal stem cells for treatment of stroke in 30 cases. Chin J Tissue Eng Res. 2012;16(32):6071–6075. [Google Scholar]
- 11.Bhasin A, Padma Srivastava MV, Mohanty S, Bhatia R, Kumaran SS, Bose S. Stem cell therapy: a clinical trial of stroke. Clin Neurol Neurosurg. 2013;115(7):1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Wang D, Liu HB. Bone marrow mesenchymal stem cells transplantation in the recovery phase of ischemic cerebral stroke patients. Chin J Transplant. 2013;7(3):141–144. [Google Scholar]
- 14.Tsang KS, Ng CPS, Zhu XL, et al. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9(8):133–143. doi: 10.4252/wjsc.v9.i8.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai MS, Shen CL, Zeng LH, Huang XQ, Song CW. Effect of stem cell transplantation on serum homocysteine, CRP and BDNF in patients with ischemic stroke. Chin J Biochem Pharm. 2015;35(9):91–93. [Google Scholar]
- 16.Ji X, Zhao HT, Zhang XB, et al. Clinical analysis of mesenchymal stem cells for treatment of stroke in 60 cases. J Xianning Univ. 2012;26(1):31–32. [Google Scholar]
- 17.Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13(1):14–19. doi: 10.46582/jsrm.1301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WD, Li JT, Zhang XB, et al. Clinical analysis of bone marrow mesenchymal stem cells transplantation in the treatment of cerebral infarction. Jilin Med J. 2012;33(21):4522. [Google Scholar]
- 19.Hu Q, Cao MY, Li RF, Jiang HW, Ge LT. Safety and efficacy on the treatment of cerebral infarction with umbilical cord mesenchymal stem cells. Med J Wuhan Univ. 2013;34(1):57–70. [Google Scholar]
- 20.Meng XG, Zhu SW, Gao H, et al. Treatment of cerebral infarction using autologous marrow mesenchymal stem cells transplantation: a six-month follow-up. J Clin Rehabil Tissue Eng Res. 2009;13(32):6374–6378. [Google Scholar]
- 21.Wang X, Zhang ZB, Jia FR, Yang H. A clinical study on treatment of cerebral infarction using autologous marrow mesenchymal stem cells intervening transplantation. Jilin Med J. 2014;35(2):237–239. [Google Scholar]
- 22.Shen DP. Umbilical cord mesenchymal stem cell early single transplantation in treatment of neurological recovery in acute cerebral infarction. Chin J Trauma Disabil Med. 2015;23(2):26–28. [Google Scholar]
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen L, Zhang G, Khan AA, Guo X, Gu Y. Clinical efficacy and meta-analysis of stem cell therapies for patients with brain ischemia. Stem Cells Int. 2016;2016:6129579. doi: 10.1155/2016/6129579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Prasad M, Jali VP, et al. Bone marrow mononuclear cell therapy in ischaemic stroke: a systematic review. Acta Neurol Scand. 2017;135(5):496–506. doi: 10.1111/ane.12666. [DOI] [PubMed] [Google Scholar]
- 3.Cao W, Li P. Effectiveness and safety of autologous bone marrow stromal cells transplantation after ischemic stroke: a meta-analysis. Med Sci Monit. 2015;21:2190–2195. doi: 10.12659/MSM.895081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis C, Wilkinson M, Reis H, et al. A look into stem cell therapy: exploring the options for treatment of ischemic stroke. Stem Cells Int. 2017;2017:3267352. doi: 10.1155/2017/3267352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsuan YC, Lin CH, Chang CP, Lin MT. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016;6(10):e00526. doi: 10.1002/brb3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 7.Cramer SC, Stradling D, Brown DM, et al. Organization of a United States county system for comprehensive acute stroke care. Stroke. 2012;43(4):1089–1093. doi: 10.1161/STROKEAHA.111.635334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S, Bentley P, Hamady M, et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 2014;3(11):1322–1330. doi: 10.5966/sctm.2013-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boy S, Sauerbruch S, Kraemer M, et al. Mobilisation of hematopoietic CD34+ precursor cells in patients with acute stroke is safe – results of an open-labeled non randomized phase I/II trial. PLoS One. 2011;6(8):e23099. doi: 10.1371/journal.pone.0023099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 11.Qiao LY, Huang FJ, Zhao M, et al. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014;23(Suppl 1):S65–S72. doi: 10.3727/096368914X684961. [DOI] [PubMed] [Google Scholar]
- 12.Liao S, Luo C, Cao B, et al. Endothelial progenitor cells for ischemic stroke: update on basic research and application. Stem Cells Int. 2017;2017:2193432. doi: 10.1155/2017/2193432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess DC, Wechsler LR, Clark WM, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 14.Prasad K, Sharma A, Garg A, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Yang B, Tian Y, et al. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272(1):33–38. doi: 10.1016/j.cellimm.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghorbani S, Tiraihi T, Soleimani M. Differentiation of mesenchymal stem cells into neuron-like cells using composite 3D scaffold combined with valproic acid induction. J Biomater Appl. 2018;32(6):702–715. doi: 10.1177/0885328217741903. [DOI] [PubMed] [Google Scholar]
- 17.Sarmah D, Agrawal V, Rane P, et al. Mesenchymal stem cell therapy in ischemic stroke: a meta-analysis of preclinical studies. Clin Pharmacol Ther. 2017 Nov 1; doi: 10.1002/cpt.927. Epub. [DOI] [PubMed] [Google Scholar]
- 18.Sammali E, Alia C, Vegliante G, et al. Intravenous infusion of human bone marrow mesenchymal stromal cells promotes functional recovery and neuroplasticity after ischemic stroke in mice. Sci Rep. 2017;7(1):6962. doi: 10.1038/s41598-017-07274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki Y, Sasaki M, Kataoka-Sasaki Y, et al. Synergic effects of rehabilitation and intravenous infusion of mesenchymal stem cells after stroke in rats. Phys Ther. 2016;96(11):1791–1798. doi: 10.2522/ptj.20150504. [DOI] [PubMed] [Google Scholar]
- 20.Rosado-de-Castro PH, Pimentel-Coelho PM, da Fonseca LM, de Freitas GR, Mendez-Otero R. The rise of cell therapy trials for stroke: review of published and registered studies. Stem Cells Dev. 2013;22(15):2095–2111. doi: 10.1089/scd.2013.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 22.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–3820. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 24.Bhasin A, Padma Srivastava MV, Mohanty S, Bhatia R, Kumaran SS, Bose S. Stem cell therapy: a clinical trial of stroke. Clin Neurol Neurosurg. 2013;115(7):1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin A, Kumaran SS, Bhatia R, Mohanty S, Srivastava MVP. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in Indian patients. A four-year follow up. J Stem Cells Regen Med. 2017;13(1):14–19. doi: 10.46582/jsrm.1301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai MS, Shen CL, Zeng LH, Huang XQ, Song CW. Effect of stem cell transplantation on serum homocysteine, CRP and BDNF in patients with ischemic stroke. Chin J Biochem Pharm. 2015;35(9):91–93. [Google Scholar]
- 27.Cheng Y, Wang D, Liu HB. Bone marrow mesenchymal stem cells transplantation in the recovery phase of ischemic cerebral stroke patients. Chin J Transplant. 2013;7(3):141–144. [Google Scholar]
- 28.Chen WD, Li JT, Zhang XB, et al. Clinical analysis of bone marrow mesenchymal stem cells transplantation in the treatment of cerebral infarction. Jilin Med J. 2012;33(21):4522. [Google Scholar]
- 29.Chen WM, Zou QY, Lu JJ, et al. Reinfusion of autologous bone marrow mesenchymal stem cells for treatment of stroke in 30 cases. Chin J Tissue Eng Res. 2012;16(32):6071–6075. [Google Scholar]
- 30.Deng YG, Zhang ZP, Yu W. Clinical efficacy of salvia miltiorrhiza injection combined with marrow stem cells in treatment of cerebral infarction. Pract J Cardiac Cereb Pneumal Vasc Dis. 2012;20(3):424–425. [Google Scholar]
- 31.Feng Y, Tian GP, Li L, Zhou J. Effect of human umbilical cord blood-derived mesenchymal stem cells in the treatment of cerebral infarction. Pract J Cardiac Cereb Pneumal Vasc Dis. 2014;22(1):28–30. [Google Scholar]
- 32.He ZD. The functional mechanism of mesenchymal stem cell transplantation therapy for patients with cerebral infarction. Asia Pac Tradit Med. 2012;8(12):126–127. [Google Scholar]
- 33.Hu Q, Cao MY, Li RF, Jiang HW, Ge LT. Safety and efficacy on the treatment of cerebral infarction with umbilical cord mesenchymal stem cells. Med J Wuhan Univ. 2013;34(1):57–70. [Google Scholar]
- 34.Ji X, Zhao HT, Zhang XB, et al. Clinical analysis of mesenchymal stem cells for treatment of stroke in 60 cases. J Xianning Univ. 2012;26(1):31–32. [Google Scholar]
- 35.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 36.Liu DH, Han BJ, Hong SS, et al. Transplanting autologous mesenchymal nerve stem cells in the treatment of cerebral infarction. Chin J Phys Med Rehabil. 2014;36(6):425–428. [Google Scholar]
- 37.Meng XG, Zhu SW, Gao H, et al. Treatment of cerebral infarction using autologous marrow mesenchymal stem cells transplantation: a six-month follow-up. J Clin Rehabil Tissue Eng Res. 2009;13(32):6374–6378. [Google Scholar]
- 38.Shen DP. Umbilical cord mesenchymal stem cell early single transplantation in treatment of neurological recovery in acute cerebral infarction. Chin J Trauma Disabil Med. 2015;23(2):26–28. [Google Scholar]
- 39.Song CW, Wang P, Hu XQ, et al. Clinical efficacy of umbilical cord blood mesenchymal stem cells transplantation in the treatment of cerebral infarction. J Clin Ration Drug Use. 2013;6(2):69–70. [Google Scholar]
- 40.Sun HB, Xia SM, Yang SS. The clinical effect of rhG-CSF combined transplantation of autologous bone marrow mesenchymal stem cells on acute cerebral infarction. J Chin Phys. 2008;10(4):441–443. [Google Scholar]
- 41.Sun SC, Wang C, Luo JS. Bone marrow mesenchymal stem cell transplantation improved the neurological function of 35 patients with sequelae of stroke. China Med Eng. 2013;21(6):14–17. [Google Scholar]
- 42.Tsang KS, Ng CPS, Zhu XL, et al. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9(8):133–143. doi: 10.4252/wjsc.v9.i8.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Zhang ZB, Jia FR, Yang H. A clinical study on treatment of cerebral infarction using autologous marrow mesenchymal stem cells intervening transplantation. Jilin Med J. 2014;35(2):237–239. [Google Scholar]
- 44.Xie XF, Liu SY, Jin GH, Qu XH, Zhang KN, Wu XM. Clinical analysis of autologous bone marrow mesenchymal stem cell transplantation for treating cerebral infarction. Lab Med Clin. 2014;11(21):2955–5957. [Google Scholar]
- 45.Zhao XL, Wang Y, Zhang CX, Tan J. Study of bone marrow mesenchymal stem cells in the treatment of stroke. Global Chin Med. 2013;6(S2):157. [Google Scholar]
- 46.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zacharek A, Shehadah A, Chen J, et al. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41(3):524–530. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakabayashi K, Nagai A, Sheikh AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88(5):1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 49.Lanza C, Morando S, Voci A, et al. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochem. 2009;110(5):1674–1684. doi: 10.1111/j.1471-4159.2009.06268.x. [DOI] [PubMed] [Google Scholar]
- 50.Hokari M, Kuroda S, Shichinohe H, Yano S, Hida K, Iwasaki Y. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J Neurosci Res. 2008;86(5):1024–1035. doi: 10.1002/jnr.21572. [DOI] [PubMed] [Google Scholar]
- 51.Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28(2):329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66(1):209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 53.Ahn SY, Chang YS, Kim JH, Sung SI, Park WS. Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr. 2017;185:49.e2–54.e2. doi: 10.1016/j.jpeds.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 54.Zhang YC, Liu W, Fu BS, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19(2):194–199. doi: 10.1016/j.jcyt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Li Y, Wu Q, Xu C, Liu Q. Combination of butylphthalide with umbilical mesenchymal stem cells for the treatment of delayed encephalopathy after carbon monoxide poisoning. Medicine. 2016;95(49):e5412. doi: 10.1097/MD.0000000000005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong H, Yim HW, Cho YS, et al. Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells. 2014;7(2):63–69. doi: 10.15283/ijsc.2014.7.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan H, Li F, Zhu L, Wang J, Yang Z, Pan Y. Update on therapeutic mechanism for bone marrow stromal cells in ischemic stroke. J Mol Neurosci. 2014;52(2):177–185. doi: 10.1007/s12031-013-0119-0. [DOI] [PubMed] [Google Scholar]
- 59.Yang B, Strong R, Sharma S, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89(6):833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot of the comparison of NIHSS scores pre-and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; NIHSS, National Institutes of Health Stroke Scale.
Forest plot of the comparison of BI scores pre- and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; BI, Barthel index.
Forest plot of the comparison of FMA scores pre- and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; FMA, Fugl-Meyer Assessment.
Forest plot of the comparison of FIM scores in pre- and post-therapy.
Note: The random-effects meta-analysis model (inverse variance method) was used.
Abbreviations: IV, inverse variance; FIM, Functional Independence Measure.
Funnel plot of the NIHSS (A), BI (B and C), FMA (D) and FIM (E) scores pre- and post-therapy.
Note: Parameters were discussed in over five studies which were included in bias analyses.
Abbreviations: SE, standard error; MD, mean deviation; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel index; FMA, Fugl-Meyer Assessment; FIM, Functional Independence Measure.