Abstract
Purpose
To identify a clinically meaningful cut-point for the single item dry mouth question of the MD Anderson Symptom Inventory-Head and Neck Module (MDASI-HN).
Methods
Head and neck cancer survivors who had received radiation therapy (RT) completed the MDASI-HN, the University of Michigan Hospital xerostomia questionnaire (XQ), and the health visual analog scale (VAS) of the EuroQol Five Dimension Questionnaire (EQ-5D). Bayesian information criteria (BIC) were used to test the prediction power of each tool for EQ-5D VAS. The modified Breiman recursive partitioning analysis (RPA) was used to identify a cut point of the MDASI-HN dry mouth score (MDASI-HN-DM) with EQ-5D VAS, using a ROC-based approach; regression analysis was used to confirm the threshold effect size.
Results
Two-hundred seven respondents formed the cohort. Median follow-up from the end of RT to questionnaire completion was 88 months. The single item MDASI-HN-DM score showed a linear relationship with the XQ composite score (ρ = 0.80, p <0.001). The MDASI-HN-DM displayed improved model performance for association with EQ-5D VAS as compared to XQ (BIC of 1803.7 vs. 2016.9, respectively). RPA showed that an MDASI-HN-DM score of ≥ 6 correlated with EQ-5D VAS decline (LogWorth 5.5).
Conclusion
The single item MDASI-HN-DM correlated with the multi-item XQ and performed favorably in the prediction of QOL. A MDASI-HN-DM cut point of ≥ 6 correlated with decline in QOL.
Keywords: Head and Neck, Quality of Life, Dry Mouth, Patient reported outcomes, Prediction Model, Survivors
Introduction
Despite significant improvements in radiation therapy (RT) treatment planning and delivery, RT-induced xerostomia still represents one of the main morbidities affecting many head and neck cancer (HNC) survivors and can result in discomfort and difficulty chewing, swallowing, and maintaining adequate dental hygiene [1, 2] and poorer quality of life (QOL) [3]. Objective measures of xerostomia (e.g. sialometry) and physician ratings (e.g. CTC-AE) are commonly used in both clinical and research settings, yet applicability of these is limited by in their reproducibility and relying solely on physician ratings may underestimate the extent of this treatment-related toxicity and the impact on a patient’s function [4, 5]. Furthermore, improved treatment outcomes and increasing rates of long term survival for many patients with HNC have brought to focus the importance of comprehensive assessment of patients’ overall well-being[6, 7].
Given the growing focus on patients’ perception of their disease,[8, 9] there is a need to develop, validate, and implement reliable, easily administered patient-reported outcome (PRO) assessment tools on a larger scale to allow clinicians to actively address clinically relevant cancer- and treatment-related symptoms [10]. Additionally, clinically relevant and validated thresholds for understanding the severity of specific symptoms need to be established to best guide clinicians on when, if feasible, interventions to alleviate these symptoms should be undertaken. To address the need to define clinically relevant instrument-specific symptom severity thresholds, and as part of our larger goal to implement routine multi-symptom assessment across the HNC care continuum, this study was conducted to validate the dry mouth question of the MD Anderson Symptom Inventory - Head and Neck module (MDASI-HN) in a cohort of long term HNC survivors.
The aims of this study were to:
Characterize long term patient-reported dry mouth and QOL using simultaneously administered PRO tools: the MDASI-HN, the University of Michigan Hospital 8-item self-reported Xerostomia Questionnaire (XQ), and the health visual analog scale (VAS) of EuroQol five dimension questionnaire (EQ-5D);
Correlate and assess performance of the single item patient-reported dry mouth question of the MDASI-HN (MDASI-HN-DM) and composite XQ score with QOL (VAS score);
Identify a clinically meaningful cut-point for the MDASI-HN-DM in order to screen for those with long term xerostomia who may need additional assessment or intervention and to stratify patient subgroups for comparison in future studies.
Materials and Methods
Study population
Following approval from our Institutional Review Board, adults (≥18 years old) previously treated for HNC without evidence of active disease and who completed initial therapy more than 6 months previous were eligible for this prospective symptom assessment study. Study-specific informed consent was provided by all participants, who completed the MDASI-HN, XQ, and VAS of the EQ-5D via telephone interview, conducted using study-specific IRB approved script and questionnaires were delivered verbatim. The PRO data analyzed in this study was cross-sectional in nature and were those collected at the time the patients entered the specific survivorship study. Patient demographic, tumor, and treatment characteristics were extracted from their medical records.
Study instruments
The MDASI-HN, is a previously validated, brief, patient-reported, diseases-site specific, multi-symptom assessment tool. It contains 13 “core symptom items” (symptoms common to all cancer types), 9 additional symptoms items specific to the MDASI-HN, and 6 items concerning how these symptoms interfere with activities of daily living. The 22 symptoms symptom items are rated on a 0–10 ordinal scale from “not present” to “as bad as you can imagine”, indicating the presence and severity of the symptom in the past 24 hours. The patient reported dry mouth item of the MDASI-HN asks patients to rate, “Your having a dry mouth at its worst”. Likewise, the symptom interference items are rated on a 0–10 ordinal scale from “did not interfere” to “interfered completely.” For this study, we analyzed only the single item patient reported dry mouth score of the MDASI-HN and symptom interference items.
The XQ is a validated patient reported xerostomia assessment tool that is frequently collected in cooperative group clinical trials. It contains 8 questions regarding dryness either during feeding or in the unstimulated state. Patients rate each item from 0 to 10, where 10 indicates the maximum dryness or discomfort due to dryness. The sum of these items produces a composite score with a maximum of 80, than can be normalized to 100 for comparative analyses [2]. Question selection had been performed after review of xerostomia-specific and overall QOL evaluation in HNC patients by investigators at the University of Michigan [11, 12].
The EQ-5D is a well-established tool for general assessment of an individual’s health state. The questionnaire is accompanied by a VAS, where patients provide an overall impression of their health status on the day of the assessment using a scale from 0 to 100, where 100 represents their best-imaginable health status [13]. For this study, we considered only the VAS component of the EQ-5D as a primary overall QOL outcome for correlation with xerostomia.
Statistical methods
Summary statistics were used to describe the clinical characteristics and questionnaire results. The MDASI-HN-DM and XQ scores were correlated using bivariate analysis using Spearman's correlation coefficients. Moreover, we investigated the direction of the association between xerostomia assessment tools and VAS score and the MDASI-HN-DM and MDASI-HN symptom interference items.
Bayesian information criteria [BIC] were used to test the prediction power of each xerostomia instrument performance with QOL (VAS score). A lower BIC was considered indicative of improved model performance and parsimony when applying the BIC evidence grades presented by Raftery [14], where the posterior probability of superiority of a lower BIC model is based on the difference (BICi - BICminimum). Per Raftery, a BIC difference of <2 is considered “Weak” (representing a 50–75% posterior probability of BICminimum model being superior to BICi), 2–6 denoted “Positive” (posterior probability of 75–95%), 6–10 as “Strong” (posterior probability of >95%), and >10, “Very strong” (posterior probability >99%).
To identify the possible cutoff score of the MDASI-HN-DM at which a change in the VAS scores could be observed, we used the modified Breiman recursive partitioning analysis (RPA) with an receiver operating characteristic (ROC)-based approach. Training and validation sets for optimization of the MDASI-HN-DM score RPA were conducted using MDASI-HN-DM as a continuous variable. The RPA (decision tree-based partitioning) was performed with 20% verification “holdback” and a minimum split size of 10% per split/partition. Post hoc K-fold cross validation (n=10) was performed to evaluate for over-fitting. Regression analysis was used to confirm the threshold effect size.
Results
Participants
The data from a total of 207 HNC survivors were included in this analysis. Median follow-up time from the end of RT to questionnaire completion was 88 months (range: 21–184) and 160 patients (77%) had greater than 5 years from the end of RT to questionnaire completion. Patient and previous treatment characteristics are listed in Table 1. Of the 140 patients with OPC, 50% had known HPV-association by either HPV or p16 testing. Of those tested, 91% were positive for HPV/p16. Of the 140 patients with OPC, 50% were never smokers. Intensity modulated radiation therapy (IMRT) was utilized in 90% of the patients. Median RT dose was 69.96 Gy (range 60–72), and conventional RT schedule was utilized in 91% of the patients. Mean±standard deviation(SD) RT dose delivered to ipsilateral parotid, ipsilateral submandibular, contralateral parotid, contralateral submandibular and extended oral cavity were 33.4±18.2, 58.8±21.5, 20.9±16.6, 44.8±24.3, and 41.4±19.2 Gy, respectively.
Table 1.
Patient and disease characteristics
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 167 (81) |
| Female | 40 (19) |
| Age (years) | |
| Median (range) | 57 (32–79) |
| Ethnicity | |
| White | 188 (91) |
| African American | 3 (1) |
| Hispanic | 8 (4) |
| Others | 8 (4) |
| Smoking Status | |
| Current | 38 (18) |
| Former | 72 (35) |
| Never | 91 (44) |
| Unknown | 6 (3) |
| Primary Site | |
| Oropharynx | 144 (69) |
| Nasopharynx/Sinonasal | 20 (10) |
| Larynx/Hypopharynx | 42 (20) |
| T-Category | |
| Tx-2 | 143 (69) |
| T3-4 | 94 (31) |
| N-Category | |
| Nx-1 | 88 (43) |
| N2-3 | 119 (57) |
| Chemotherapy regimens | |
| Induction | 37 (18) |
| Concurrent | 58 (28) |
| Induction+ Concurrent | 39 (19) |
| No Chemotherapy (RT Alone) | 73 (35) |
Patient reported outcomes and model performance
The mean±SD for MDASI-HN-DM, individual XQ item and cumulative score, and EQ-5D VAS are shown in Supplementary Table 1. Mean±SD MDASI-HN-DM was 3.9±3.2, the composite XQ scores ranged from 0 to 79 with a mean±SD of 21±17, and the mean±SD EQ-5D VAS score was 81.1±18.
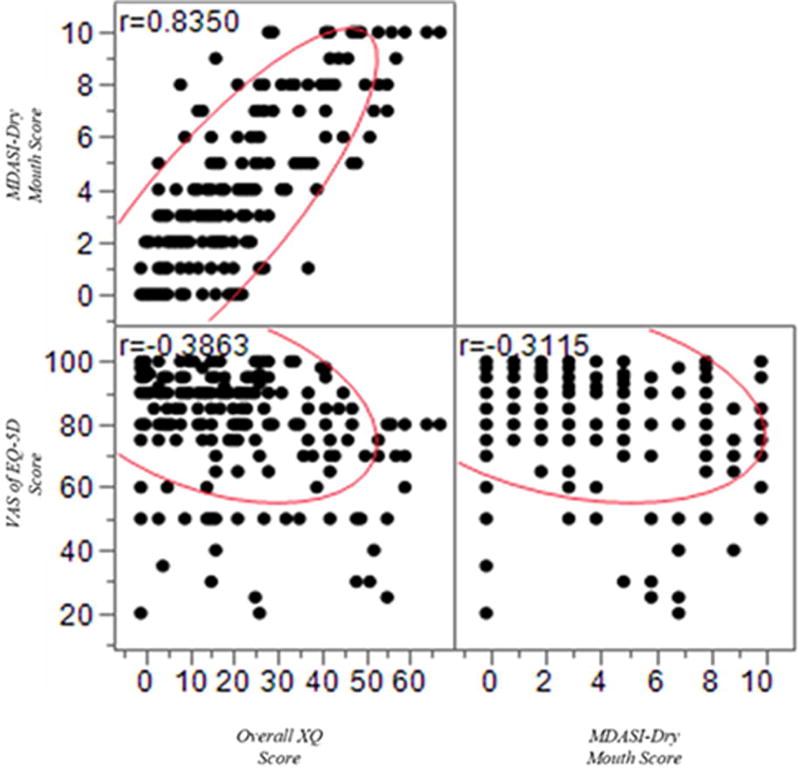
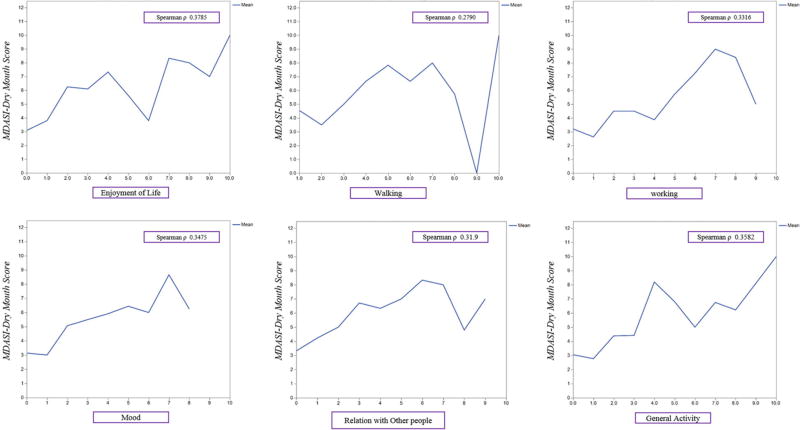
The correlations between MDASI-HN-DM, XQ, and EQ-5D VAS scores are shown in Figure 1. There was a very strong linear relationship between the MDASI-HN-DM and the composite XQ score, which indicate that both tools capture the similar symptom burden, (Spearman's ρ=0.8, p <0.0001). Bivariate analyses showed inverse relationships both between MDASI-HN-DM and EQ-5D VAS (Spearman's ρ=−0.31, p <0.001) and XQ and EQ-5D VAS (Spearman's ρ=−0.38, p <0.001). The correlation between MDASI-HN-DM and the 6 MDASI-HN symptom interference items is shown in Figure 2 with a Spearman's ρ range from 0.28–0.38, p <0.0001 for each. By utilizing the bivariate analyses, we tested the performance of each tool individually, and the results confirm that dry mouth scores, captured by either tool were correlate with QOL.
Fig (1).
Correlation between Overall XQ, MDASI-DM and VAS of EQ-5D Scores
Fig (2).
Correlation between MDASI-DM and life interference symptoms
Subsequently, for the purpose of model comparison, we used the Bayesian information criteria (BIC), which indicate a difference of >10 between the in the BIC values of MDASI-HN-DM and XQ. The MDASI-HN-DM and EQ-5D VAS correlation showed improved model performance compared to XQ and EQ-5D VAS (Bayesian information criteria of 1803.7 vs. 2016.9, respectively) indicative of “very strong” (posterior Probability of > 99%) statistical evidence that MDAS-HN-DM was more parsimonious for prediction of QOL within this cohort (Supplementary Figure 1). The aforementioned data indicate that the dry mouth scores, captured by either tool were significantly correlated with QOL scores, however, the MDASI-HN-DM showed improved model performance for correlation with the QOL and compared favorably to XQ in term of QOL assessment. This is to say that the single item screen at least compares favorably to the more commonly used, widely accepted, multi-item XQ.
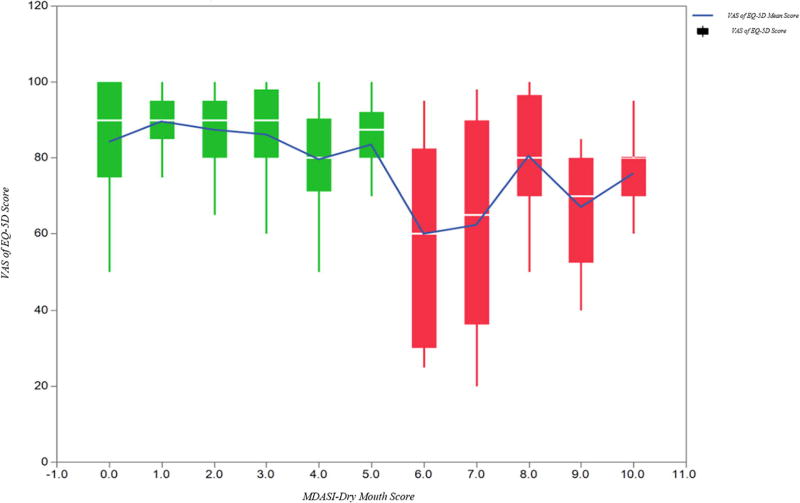
By using the modified Breiman recursive partitioning analysis (RPA), that uses an ROC-based classification and regression tree approach with test-training methodology with 20% “holdback” verification for discrimination optimization, a MDASI-HN-DM score of ≥ 6 was identified as the threshold for EQ-5D VAS decline in the training set (Log Worth 5.5), suggesting that at a cut-point of 6, there was a significant transition in QOL scores and could identify two distinct groups in terms of overall QOL. These results were maintained after 10 fold cross validation. We then tested this cut-point as a binary split across the entire cohort (n=207) using regression analysis yielding mean±SD EQ-5D VAS of 71.4±21 versus 84.9±15 for those with MDASI-HN-DM scores ≥6 versus <6, respectively (p<0.001).
EQ-5D VAS scores are displayed using box plots and mean values by MDASI-HN-DM groupings (score 0–10), and consistent with RPA results, shows that QOL scores were essentially stable in patients with MDASI-HN-DM scores between 0–5, while a decline in QOL is observed across patients with MDASI-HN-DM scores above ≥6 (Figure 3). The mean MDAS-HN-DM, mean cumulative XQ, and mean ED-5D VAS are shown in Supplementary Table 2, according to identified MDASI-HN-DM categories for those with ratings of “none” (MDASI-HN-DM = 0), “low” (1–5), and “high” (6–10).
Figure (3).
Box plots and mean VAS of EQ-5D values per each MDASI-HN-DM score category (0–10).
Discussion
The results of our study show that patient reported xerostomia as assessed by the MDASI-HN-DM, a single item question on an 11-point Likert scale, correlates with patient reported xerostomia by the popular multi-question XQ, overall patient QOL, and overall patient function as assessed by the symptom interference domain of the MDASI-HN. The MDASI-HN-DM compared favorably to XQ in prediction models for QOL, in that it showed improved model performance for correlation with the QOL (lower BIC) as assessed by the EQ-5D VAS and patient reported xerostomia results from the two separately validated tools were significantly correlated.
Overall, patients with MDASI-HN-DM ratings of 0–5 showed broadly stable QOL scores, suggesting that dry mouth, albeit present (for those with scores of 1–5), did not interfere with QOL, and thus unlikely to be a substantial burden to the patients. Conversely, those with a score of 6 or more could have clinically meaningful xerostomia and intervention could be indicated as this is translated to an observed decline in QOL across our cohort. Thus, a threshold of 6, which proved to be consistent across different statistical models, could be suggested as a cut-point for a simple and time-efficient screening of HNC patients at risk for experiencing long term or persistent xerostomia. Likewise, this could be used as a threshold for longitudinal tracking of symptom recovery or progression. While, multiple PRO instruments have been developed and validated, some with emphasis on specific symptoms experienced by HNC patients, such as the Head and Neck Distress Scale (HNDS) [15] and the Functional Assessment of Cancer Therapy-Head and Neck (FACT-H&N) [16], we have implemented routine patient reported symptom assessment using the MDASI-HN as part of clinical patient assessments and symptom management pathways. Importantly the MDASI-HN has been shown to accurately predict the severity of radiation-induced mucositis [17], the most impactful acute treatment-related reaction. Likewise, the present study highlights the ability of the MDASI-HN to identify important late treatment-related reactions, in this case, QOL-altering dry mouth. In contrast to XQ, the applicability of the single item MDASI-HN-DM questionnaire is not limited to patients with oral feeding, thus allowing for assessment of radiation-associated xerostomia, even in case of patients with nothing-per-os (NPO) diets.
Modern RT techniques and the use of parotid sparing IMRT, as was used in the far majority in this study, has proven to be effective in reducing the impact of RT on salivary flow which has translated to improved QOL compared to those treated with conventional techniques [18]. However, parotid sparing techniques have not translated to the same level of improvement in patient reported dry mouth [2]. This is likely due to the impact of the IMRT beam path adversely affecting other saliva producing tissues, namely the submandibular glands and the minor salivary glands of the oral cavity for example, which may track more closely with oral comfort [19]. Thus, the RT dose-response relationship is more likely to be elucidated by considering multiple saliva producing organs-at-risk and patient reports, rather than the previously used objective measures (e.g. parotid sialometry). Consistent with other contemporary reports [20, 21], only a minority of patients in the present study reported no xerostomia (i.e. rating of “0” on the MDASI-HN-DM), and approximately 29% reported potentially problematic levels. The limitations of the current study include those inherent to biases of a single-institution, cross-sectional, questionnaire-based series based in a tertiary care, cancer specialty hospital, most notably patient acceptance, recall and selection biases, and lack of data about other potential xerostomia causing agents and xerostomia specific interventions. Moreover, the results of RPA for the definition of a specific threshold in our single-item questionnaire are unavoidably affected by the low-frequency extreme scores such as 9 and 10 in the analyzed PROs.
Nevertheless, to the best of our knowledge, our work represents the largest prospective cohort investigating patient-reported persistent xerostomia following RT for HNC. Specifically, no previous study has tested the clinical performance of a dedicated single-item questionnaire such as MDASI-HN-DM nor a direct comparison with other validated tools. We specifically targeted in this study longer term HNC survivors, more than two years after treatment completion, a time point at which recovery of salivary function after RT would be expected to have reached near full recovery. Moreover, using this time point could avoid the expected fluctuations during the acute/subacute phases of recovery and reduce the influence of other resolving acute treatment related toxicities (e.g. mucositis and pain). This is further supported when we restricted our analyses to those with more than 5-years of follow-up and the cut-point identified remained consistent. Similar studies should be performed in the acute and subacute recovery phases, as we would expect overall higher xerostomia scores, poorer QOL, and variable recovery trajectories.
Beyond needed multi-institutional validation of the cut-point identified within our cohort and longitudinal study, we plan to explore the patient reported xerostomia-dose-response relationship for saliva producing tissues, in order to define the clinical applicability of these findings and inform clinical treatment planning decisions and plan optimization. Furthermore, once the potentially clinically meaningful cut-point for each MDASI-HN item has been identified, we plan to develop an integrated predictive model for QOL after RT based on multi-symptom burden and the clinical characteristics. In conclusion, the single item MDASI-HN-DM scores correlated with multi-question instrument scores and QOL and performed favorably in terms of predicting QOL change. We were able to identify a symptom severity cut-point for the MDASI-HN-DM that correlated with decline in QOL, which can serve as a simple screening tool for persistent and QOL-altering xerostomia.
Supplementary Material
Fig (S1) Model Performance Assessment of MDASI-HN-DM and XQ and Correlation with QOL.
Acknowledgments
Funding sources and financial disclosures: Collective research efforts of the MD Anderson Head and Neck Cancer Symptom Working Group are accomplished with infrastructure support of the multidisciplinary Stiefel Oropharyngeal Research Fund of the University of Texas MD Anderson Cancer Center Charles and Daneen Stiefel Center for Head and Neck Cancer. This study is supported by donations made by the Family of Paul W. Beach to Dr. Gunn for the execution of symptom research efforts, providing direct salary support for Dr. Kamal. Drs. Hutcheson, Mohamed and Fuller received funding support from the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248-01/R56DE025248-01). Dr. Hutcheson and Fuller are supported via the NIH/National Cancer Institute (NCI) Small Grants Program for Cancer Research (R03 CA188162). Dr. Fuller is an MD Anderson Cancer Center Andrew Sabin Family Fellow, and received project support in this role from the Andrew Sabin Family Foundation. Drs. Fuller and Mohamed receive funding and/or salary support from the National Institutes of Health (NIH), including: a Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825-01); NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148-01); an NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672) and an NIH/NCI Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50 CA097007-10); Paul Calabresi Clinical Oncology Program Award (K12 CA088084–06); the National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679); General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research (CROR) at MD Anderson Cancer Center Seed Grant; and the MD Anderson Institutional Research Grant (IRG) Program. Dr. Fuller has received direct industry grant funding and speaker travel from Elekta AB for unrelated technical projects. These entities played no role in designing the study; collecting, analyzing, or interpreting its data; writing the manuscript; or making the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjordal K, Kaasa S, Mastekaasa A. Quality of life in patients treated for head and neck cancer: A follow-up study 7 to 11 years after radiotherapy. International Journal of Radiation Oncology*Biology*Physics. 1994;28:847–56. doi: 10.1016/0360-3016(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 2.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. International Journal of Radiation Oncology*Biology*Physics. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 3.Almståhl A, Alstad T, Fagerberg-Mohlin B, Carlén A, Finizia C. Explorative study on quality of life in relation to salivary secretion rate in patients with head and neck cancer treated with radiotherapy. Head & neck. 2016;38:782–91. doi: 10.1002/hed.23964. [DOI] [PubMed] [Google Scholar]
- 4.Falchook AD, Green R, Knowles ME, et al. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngology–Head & Neck Surgery. 2016;142:517–23. doi: 10.1001/jamaoto.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein JB, Thariat J, Bensadoun R-J, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy. CA: A Cancer Journal for Clinicians. 2012;62:400–22. doi: 10.3322/caac.21157. [DOI] [PubMed] [Google Scholar]
- 6.Donovan K, Sanson-Fisher RW, Redman S. Measuring quality of life in cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7:959–68. doi: 10.1200/JCO.1989.7.7.959. [DOI] [PubMed] [Google Scholar]
- 7.Trotti A, Johnson DJ, Gwede C, Casey L, Sauder B, Cantor A, et al. Development of a head and neck companion module for the quality of life–radiation therapy instrument (QOL-RTI) International Journal of Radiation Oncology*Biology*Physics. 1998;42:257–61. doi: 10.1016/s0360-3016(98)00224-7. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient- Reported Outcomes (ASCPRO): searching for standards. Journal of pain and symptom management. 2010;39:1077–85. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Gunn GB, Mendoza TR, Fuller CD, Gning I, Frank SJ, Beadle BM, et al. High symptom burden prior to radiation therapy for head and neck cancer: a patient-reported outcomes study. Head Neck. 2013;35:1490–8. doi: 10.1002/hed.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15–17, 2002. Journal of the National Cancer Institute. 2003;95:1110–7. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 11.Eisbruch A, Dawson LA, Kim HM, Bradford CR, Terrell JE, Chepeha DB, et al. Conformal and intensity modulated irradiation of head and neck cancer: the potential for improved target irradiation, salivary gland function, and quality of life. Acta oto-rhino-laryngologica Belgica. 1999;53:271–5. [PubMed] [Google Scholar]
- 12.Henson BS, Inglehart MR, Eisbruch A, Ship JA. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral oncology. 2001;37:84–93. doi: 10.1016/s1368-8375(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 13.Balestroni G, Bertolotti G. [EuroQol-5D (EQ-5D): an instrument for measuring quality of life] Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. 2012;78:155–9. doi: 10.4081/monaldi.2012.121. [DOI] [PubMed] [Google Scholar]
- 14.Raftery AE. Bayesian Model Selection in Social Research. Sociological Methodology. 1995;25:111–63. [Google Scholar]
- 15.Jones HA, Hershock D, Machtay M, Chalian AA, Weber RS, Weinstein GS, et al. Preliminary investigation of symptom distress in the head and neck patient population: validation of a measurement instrument. American journal of clinical oncology. 2006;29:158–62. doi: 10.1097/01.coc.0000207424.62275.9d. [DOI] [PubMed] [Google Scholar]
- 16.List MA, D'Antonio LL, Cella DF, Siston A, Mumby P, Haraf D, Vokes E. The performance status scale for head and neck cancer patients and the functional assessment of cancer therapy-head and neck scale: A study of utility and validity. Cancer. 1996;77:2294–301. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2294::AID-CNCR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal DI, Mendoza TR, Chambers MS, Burkett VS, Garden AS, Hessell AC, et al. The M. D. Anderson symptom inventory-head and neck module, a patient-reported outcome instrument, accurately predicts the severity of radiation-induced mucositis. International journal of radiation oncology, biology, physics. 2008;72:1355–61. doi: 10.1016/j.ijrobp.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 18.Eisbruch A. Radiotherapy: IMRT reduces xerostomia and potentially improves QoL. Nat Rev Clin Oncol. 2009;6:567–8. doi: 10.1038/nrclinonc.2009.143. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NCS, Garcia J, Kies MS, et al. Beam Path Toxicities to Non-Target Structures During Intensity-Modulated Radiation Therapy for Head and Neck Cancer. International Journal of Radiation Oncology*Biology*Physics. 2008;72:747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. The Lancet Oncology. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn GB, Hansen CC, Garden AS, Fuller CD, Mohamed AS, Morrison WH, et al. Favorable patient reported outcomes following IMRT for early carcinomas of the tonsillar fossa: Results from a symptom assessment study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;117:132–8. doi: 10.1016/j.radonc.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig (S1) Model Performance Assessment of MDASI-HN-DM and XQ and Correlation with QOL.