Abstract
Inflammation is an important event in ischemic injury. These immune responses begin with the expression of pro-inflammatory genes modulating transcription factors, such as nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and signal transducers and activator of transcription-1 (STAT-1). The 70-kDa heat shock protein (Hsp70) can both induce and arrest inflammatory reactions and lead to improved neurological outcome in experimental brain injury and ischemia. Since Hsp70 are induced under heat stress, we investigated the link between Hsp70 neuroprotection and phosphorylation of inhibitor of κB (IκB), c-Jun N-terminal kinases (JNK) and p38 through co-immunoprecipitation and enzyme-linked immunosorbent assay (ELISA) assay. Transcription factors and pro-inflammatory genes were quantified by immunoblotting, electrophoretic-mobility shift assay and reverse transcription-polymerase chain reaction assays. The results showed that heat stress led to Hsp70 overexpression which rendered neuroprotection after ischemia-like injury. Overexpression Hsp70 also interrupts the phosphorylation of IκB, JNK and p38 and bluntsDNA binding of their transcription factors (NF-κB, AP-1 and STAT-1), effectively downregulating the expression of pro-inflammatory genes inheat-pretreatedastrocytes. Takentogether, these results suggest that overexpression of Hsp70 may protect against brain ischemia via an anti-inflammatory mechanism by interrupting the phosphorylation of upstream of transcription factors.
Keywords: ischemic injury, inflammation, 70-kDa heat shock protein, transcription factors, phosphorylation
INTRODUCTION
Cerebral ischemia results in a number of hemodynamic, biochemical, and neurophysiologic alterations that can be clinically linked to behavioral and pathologic disturbances. With declining blood flow, neuronal activity is affected first, and as ischemia progresses, metabolic activity is suppressed in order to maintain the structural integrity of the brain cells (Hossmann, 1998). These events lead to glutamate-mediated excitotoxicity, calcium overload, oxidative stress, stress signaling, inflammation and cell death (Mehta et al., 2007).
Inflammatory events initiated at the blood-microvessel interface a few hours after the onset of ischemia mark the transition from ischemic to inflammatory injury. In the inflammatory response, major players are cytokines, as well as transcription factors, such as nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and signal transducers and activator of transcription-1 (STAT-1). The activation of NF-κB, AP-1 and STAT-1 is mediated through phosphorylation of their regulatory proteins and the activation of other kinases. These transcription factors regulate the transcription of many genes involved in immunity, inflammation, and protection from programed cell death (Ghosh et al., 1998).
NF-κB plays an important physiological and pathological role in a variety of tissues and cells, including brain cells (O’Neill and Kaltschmidt, 1997). In astrocytes, NF-κB activity is required for the inducible expression of various genes involved in post-ischemic inflammation. NF-κB complexes are mainly composed of p65 and p50 subunits (Karin, 1999; Stasiolek et al., 2000). These remain sequestered in the cytoplasm of resting cells by association with a family of inhibitor of κB (IκB) proteins. Following the appropriate stimuli, the IκB proteins are rapidly phosphorylated by the IκB kinase complex (IKK), ubiquitinaed, and degraded by the 26 S proteasome (Sun et al., 1993; Chen et al., 1995). As a result, NF-κB translocates to the nucleus where it binds specific transcription sites and promotes expression of target genes (Sun et al., 1993).
AP-1 also takes part in the regulation of several genes expressed in the brain in response to ischemic injury, including cytoskeletal proteins and growth factors that support regeneration and repair the destroyed brain tissues (Pennypacker et al., 2000; Akaji et al., 2003). AP-1 is a heterodimer consisting of proteins in the Fos and Jun families (i.e. c-Fos and c-Jun). Upon binding to specific AP-1 site in the promoter region of target genes, this associated c-Fos/c-Jun complex enhances gene transcription including expression of diverse inflammatory proteins (Shaulian and Karin, 2001). AP-1 is thus a key player in post-ischemic events that are mediated through phosphorylation of c-Jun N-terminal kinases (JNK) signaling pathways. JNK activity leads to immediate early gene AP-1 activation in RBA-1 cells (Wang et al., 2009).
Lastly, STAT proteins are latent cytoplasmic transcription factors that become activated by tyrosine phosphorylation. Phosphorylated STAT proteins dimerize and translocate to the nucleus, where they interact with DNA-binding elements and induce transcription (Jacobson et al., 1995; Takeda et al., 1996; Monteleone et al., 2003). Prior data suggest that STAT-1 also regulates early phases of T-cell differentiation in immune cells (Afkarian et al., 2002; Neurath et al., 2002). STAT-1 is induced by activation of p38 MAP kinase under hypoxic conditions (Bode et al., 1999).
During ischemia, the 70-kDa inducible heat shock protein (Hsp70) is thought to enhance cell survival by its chaperone functions: preventing protein aggregation and facilitating the refolding of partially denatured proteins (Giffard et al., 2004; Xu et al., 2006). The neuroprotective mechanism of Hsp70 is still not completely understood, particularly in the central nervous system. Prior studies from our group have established that overexpressing Hsp70 is protective against focal and global cerebral ischemia and neurotoxicity (Yenari et al., 1998; Lee et al., 2001). One of the earliest reports also described Hsps as capable of modulating immune responses either by potentiating or inhibiting them in brain ischemia or injury (Srivastava, 2002). To better understand the mechanisms by which Hsp70 interacts with inflammatory transcription factors after ischemia, we and others have investigated how Hsp70 inhibits NF-κB’s transcriptional activity by directly binding NF-kB, or how it may interfere with its inhibitory kinase (Ran et al., 2004; Zheng et al., 2008; Sheppard et al., 2014). These observations demonstrate that Hsp70 overexpression impacts inflammatory transcription factors in ischemic injury. Here we explored whether heat stress is related to the observed neuroprotection by Hsp70 overexpression, and how Hsp70 expression modulates transcription factors in an in vitro model of ischemic injury.
EXPERIMENTAL PROCEDURES
Animals and Primary astrocyte culture
Experiments were performed according to a protocol approved by the Yonsei University Animal Care and Use Committee in accordance with NIH guidelines. Primary cortical astrocytes were cultured from 1- to 3-day-old postnatal ICR mice and maintained in minimum essential medium (MEM, Gibco, USA) containing 10% fetal bovine serum and 10% equine serum (Hyclone, USA).
Heat Pretreatment and oxygen and glucose deprivation (OGD)
Primary astrocyte cultures were washed three times with balanced salt solution (BSS5.5) containing 5.5 mM glucose, 116 mM NaCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.4 mM KCl, 1 mM NaH2PO4, 14.7 mM NaHCO3, and HEPES at pH7.4. The culture medium was then exchanged with BSS5.5 and incubation continued at 43 °C for 30 min. The culture medium was then changed to BSS containing no glucose (BSS0.0). Astrocytes cultures were kept in an oxygen-free chamber at 37 °C for 6 h, thus depriving them of glucose and oxygen. Cultures were then transferred to a 37 °C incubator with 5% CO2 and reperfused with glucose at a concentration of 5.5 mmol/l (BSS5.5) at normoxia for 24 h. All experiments were performed in triplicate.
Hoeschst-PI nuclear staining
Cell death was evaluated by staining non-viable cells with propidium iodide (Sigma, St. Louis, Missouri, USA) and living cells with Hoechst 33,258 dye (Sigma, USA). Hoeschst dye (2–5 μg/ml) was added to the culture medium, and cells were kept at 37 °C for 30 min. Propidium iodide (2–5 μg/ml) was added immediately prior to observation in an Olympus microscope equipped for epifluorescence with UV filter block. PI-positive cells were counted as dead cells (Bokara et al., 2011).
Co-immunoprecipitation and immunoblotting
Co-immunoprecipitation was performed by following a protocol from Stressgen Biotechnologies with minor modifications. Astrocyte cell lysates were pre-cleared by adding 50-ml Protein A/G PLUS-Agarose (Santa Cruz, Dallas, Texas, USA), 2 mg of tissue lysate in 1 mL of complete RIPA buffer. Precleared lysates (200 ml) were then incubated with 2.5 mg of mouse monoclonal Anti-Hsp70 antibody (Stressgen, San Diego, California, USA) or an IgG isotype control (2.5-mg normal mouse IgG, Santa Cruz) at 4 °C overnight. The Protein A/G PLUS-Agarose was then collected and the supernatant was aspirated off by microcentrifuging the mixture for 2 min at 4300g. After washing all reactions five times, samples were boiled for 5 min and then microcentrifuged briefly to pellet Protein A/G PLUS-Agarose. For the transcription factor immunoblots, cytoplasmic and nuclear protein subfractions were prepared as described previously (Zheng et al., 2008). 10–20-μg aliquots of protein were run in 10% SDS–PAGE electrophoresis, then transferred to PVDR membrane (Millipore, Billerica, Massachusetts, USA), and probed for the protein of interest by incubating in mouse anti-Hsp70 (1:1000, Stressgen) and phospho-IκB (1:1000, Santa Cruz) or rabbit anti-NF-κB (1:1000, Millipore), phospho-p38 mitogen-activated protein kinase (MAPK) (1:1000, Santa Cruz), phospho-STAT-1 (1:1000, Cell Signaling, Danvers, Massachusetts, USA), phospho-SAPK/JNK (1:1000, Cell Signaling), phospho-c-Jun (1:1000, Cell Signaling), β-actin (1:1000, Sigma), and anti-histone H1 antibodies (1:1,000, Santa Cruz). The membrane was then incubated with the secondary antibody and thoroughly washed. Immunoreactive bands were visualized using SuperSignal (Thermo, Waltham, Massachusetts, USA).
Phosphorylation ELISA assay
An Immunoassay Kit (Biosource) was used to assess cell extracts according to the manufacturer’s instructions, using anti-rabbit IgG horseradish peroxidase (Biosource). The absorbance was quantified at 450 nm and cell extract samples were compared with a standard curve.
Electrophoretic-Mobility Shift Assay (EMSA)
Nuclear extracts were subjected to the EMSA “Gel Shift” Kit (Panomics) according to the manufacturer’s specifications. This assay enabled the simultaneous detection and semiquantitative comparison of the DNA-binding activity of NF-κB (5′-AGTTGAGGGGAC TTTCCCAGGC-3′), AP-1 (5′-GCCTTGATGACTCAG CCGGAA) and STAT-1 (5′-CATGTTATGCA TATTCCTGTA AGTG-3′) from nuclear extracts in mouse brain and primary cultured astrocytes. Biotin-labeled DNA-binding oligonucleotides were incubated with 10 mg of nuclear extract at 15 °C for 30 min to allow the formation of NF-κB/DNA, AP-1/DNA and STAT-1/DNA complexes. Complexes were separated from the free probes by 6% non-denaturing gel electrophoresis in 0.5× Tris/Borate/EDTA buffer (TBE) at 120V for 15 min. The probes in the complexes were then extracted, ethanol-precipitated, and hybridized to an EMSA “Gel Shift” Kit array. Signals were detected using SuperSignal (Thermo).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated and purified with Trizol Reagent (Invitrogen). RNA was quantified by measuring the absorbance at 260 nm. cDNA synthesis of mRNA was carried out by reverse transcription (RT). Samples were normalized using RT-PCR. PCR amplification for MMP-9, interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α) and GAPDH was performed at 94 °C for 30 s, at 53 °C for 30 s and at 72 °C for 30 s for 35 cycles. The sequences of the specific primers were as follows: sense, 5′-AAATGTGGGTGTACACAGGC-3′ and antisense 5′-TTCACCTCATTTTGGAAACT-3′ for MMP-9; sense, 5′-CTCCATTGAGCTTTGTACAAGC-3′ and antisense, 5′-GGGGTTGACCATGTAGTCGT-3′ for IL-1β; sense, 5′-TCAGCCTCTTCTCATTCCTGC-3′ and antisense, 5′-TTGGTGGTTTGCTACGACGTG-3′ for TNF-α; sense, 5′-ACCACAGTCCATGCCATCAC-3′ and antisense, 5′-TCCACCACCTGTTGCTGTA-3′ for GAPDH.
Statistical analysis
All data were analyzed with standard statistical methods (t test, Systat Software, Inc., San Jose, CA, USA). P value<0.05 was considered significant. All data are expressed as mean±SEM.
RESULTS
Protective effect of Hsp70 overexpression on OGD in primary cultured astrocytes by heat stress
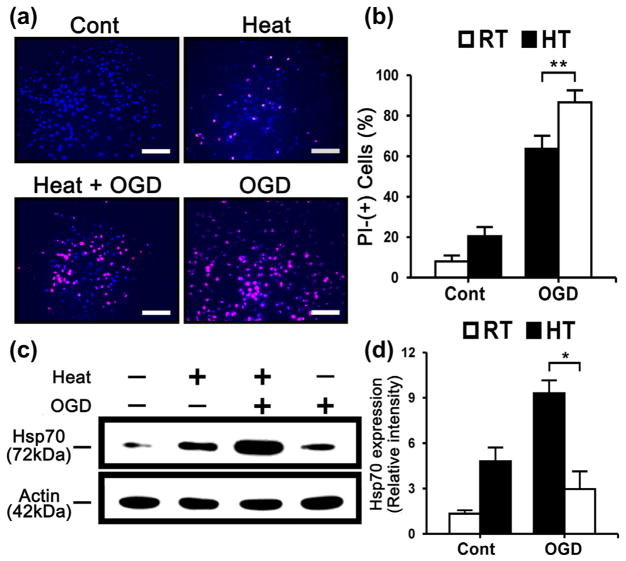
To investigate the protective effect of Hsp70 overexpression in OGD, we examined the degree of Hoechst-PI nuclear staining in primary cultured astrocytes subjected to OGD. After OGD for 4 h and reperfusion up to 20 h, PI-stained nuclei increased. However, a comparative analysis showed that heat-stressed astrocytes had significantly decreased PI-stained nuclei (Fig. 1a, b). Astrocytes exposed to heat stress showed three- to fourfold higher expression in the protein level of Hsp70 compared to untreated controls (Fig. 1c, d).
Fig. 1.
Hsp70 overexpression reduces cell death in primary cultured astrocytes after OGD injury. (a) Control (Cont), Heat and OGD treatment images after Hoechst-PI with primary cultured astrocytes. (Scale bar=100 μm) (b) Cell counts of PI-positive neurons in the primary cultured astrocytes of control, heat and OGD treatment show that heat stress reduced PI-positive cells detected after OGD. (RT: room temperature, HT: heat treatment; **P<0.05; n=6/group) (c) Immunoblots show expression of Hsp70 proteins from heat treatment. β-Actin is shown as a housekeeping control. (d) Relative intensities of Hsp70 protein was quantified by NIH ImageJ software, and normalized to the intensity of β-actin. Hsp70 was increased in heat-treated cells with OGD compared to OGD only. (RT: room temperature, HT: heat treatment; *P<0.01; n=3/group).
Hsp70 overexpression interacts and blunts the phosphorylated upstream regulatory proteins of transcription factors in OGD after heat pretreatment
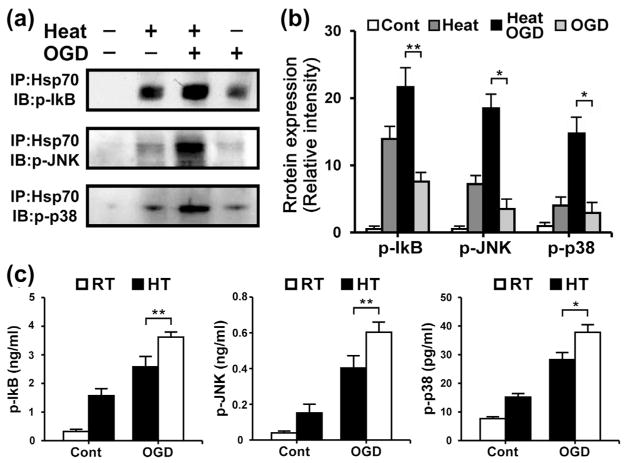
We performed co-immunoprecipitation and enzyme-linked immunosorbent assay (ELISA) to observe phosphorylation and other interactions between Hsp70 and upstream regulators (IκB, JNK, p38) of transcription factors. Hsp70 appeared to co-immunoprecipitate with these regulatory factors in primary cultured astrocytes after heat pretreatment (Fig. 2a, b). Untreated astrocytes exposed to OGD also showed greater quantities of phosphorylated IκB, JNK and p38. In contrast, phosphorylated IκB, JNK and p38 were markedly reduced in astrocytes exposed to OGD after heat pretreatment (Fig. 2c).
Fig. 2.
Hsp70 overexpression associates with phosphorylated IκB (p-IκB), JNK (p-JNK) and p38 (p-p38). Astrocyte lysates were first co-immunoprecipitated using anti-Hsp70. (a) Immunoblots (IB) of the co-immunoprecipitates (IP) showed significant levels of p-IκB, p-JNK and p-p38 as indicated. Hsp70 was found to associate with p-IκB, p-JNK and p-p38 in primary cultured astrocytes after heat stress. (b) Relative intensities of p-IκB, p-JNK and p-p38 were quantified by NIH ImageJ software. Hsp70 highly associated with these proteins in heat-treated OGD cultures compared to OGD injury only. (Cont: Control; *P<0.01, **P<0.05; n=3/group). (c) Phospho-ELISA assay of whole astrocyte lysate detected significantly decreased levels of p-IκB, p-JNK and p-p38 in cultures exposed to heat stress and OGD compared to OGD only. (RT: room temperature, HT: heat treatment; *P<0.01, **P <0.05; n=6/group).
Overexpression of Hsp70 interrupts nuclear translocation of transcription factors and blunts the expression of several transcription factor-regulated genes
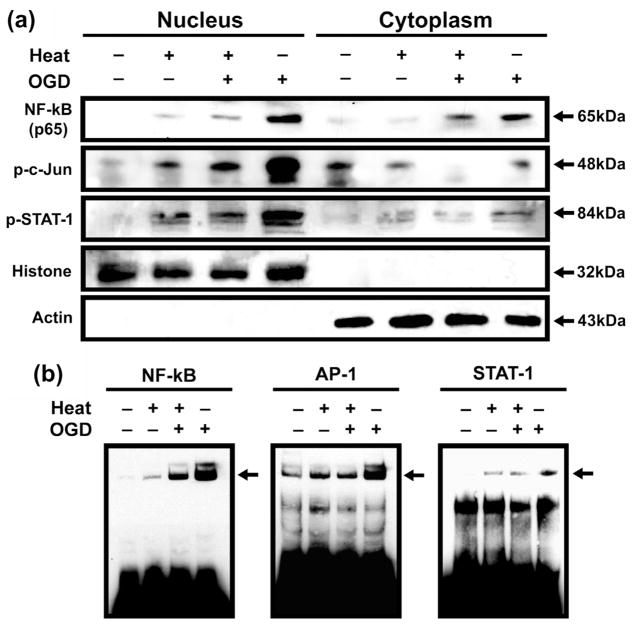
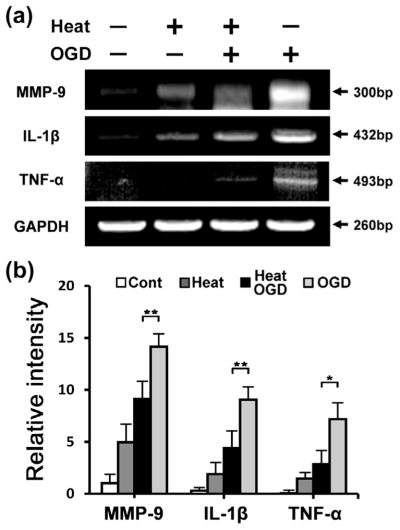
Overexpression of Hsp70 interrupted nuclear translocation of transcription factors (Fig. 3a), as shown in Western blots of cytosolic and nuclear subfractions. Transcription factors were found in the nuclear subfractions from cells given only OGD injury. These factors appeared in decreased concentrations in astrocytes exposed to OGD after heat stress, with little nuclear expression. To estimate the DNA-binding capacity of astrocytes after transcription factor activation, we performed an EMSA assay, which showed that transcription factor activity was decreased by Hsp70 overexpression in heat-stressed cells (Fig. 3b). We then performed RT-PCR of proinflammatory transcription factor-regulated genes using primary cultured astrocytes exposed to OGD with and without heat stress. RT-PCR analysis showed several-fold higher expression of TNF-α, IL-1β and MMP-9 mRNA in OGD-only astrocytes compared with astrocytes exposed to OGD after heat stress (Fig. 4a, b).
Fig. 3.
Immunoblotting and EMSA assay showed inhibited nuclear translocation of transcription factors in Hsp70 overexpression. (a) Overexpression of Hsp70 correlates with expression of major NF-κB subunit p65, p-c-Jun and p-STAT-1 and inhibits nuclear translocation of p65, p-c-Jun and p-STAT-1 in primary cultured astrocytes exposed OGD injury after heat stress. (b) EMSA assay showed DNA-binding capacity of transcription factors in primary cultured astrocytes. Decreased p65-, STAT-1- and AP-1-binding activity was observed in astrocytes exposed to OGD after heat stress compared with astrocytes exposed to OGD only.
Fig. 4.
Hsp70 overexpression reduces expression of several representative transcription factor-dependent pro-inflammatory genes in primary cultured astrocytes. (a) RT-PCR was used to estimate the expression of transcription factor-dependent pro-inflammatory genes. Compared with injury-only groups, expression of MMP-9, IL-1β and TNF-α was significantly inhibited at the mRNA level in primary cultured astrocytes exposed OGD injury after heat stress. GAPDH is shown as a housekeeping control. (b) Relative intensities of MMP-9, IL-1β and TNF-α were quantified by NIH ImageJ software. Expression of these genes was significantly decreased in cells heated prior to OGD injury compared to OGD injury only. (Cont: Control; *P<0.01, **P <0.05; n=3/group).
DISCUSSION
In this study, we explore the protection and anti-inflammatory effects of Hsp70 in ischemia-like injury. The cytotoxic properties of inflammation after brain ischemia have been well documented; we and other groups have shown that Hsp70 can protect by inhibiting various inflammatory mediators (Zheng et al., 2008; Wang et al., 2009; Kauppinen et al., 2009). Earlier studies from our lab indicate that overexpression of Hsp70 decreases infarct sizes in a middle cerebral artery occlusion model of stroke (MCAO) in mice and prevents cell death in neurons and astrocytes exposed to OGD (Lee et al., 2001, 2004). We applied heat stress to induce of Hsp family, which are known to function as cytoprotectants among various cell types in pathological states (Strauss et al., 2010; Wang et al., 2013; Oka et al., 2013; Li et al., 2013). Among the Hsps, Hsp70 is the best-studied because it is the most prominently induced after stress and has long been shown to contribute to cell survival in many conditions including ischemic stroke. Others have previously shown that incubation at elevated temperatures can increase Hsp70 expression in many different brain cell types, including primary cultured astrocytes from rats as early as 90 min after exposure (Thomas et al., 2002; Cheng et al., 2011). Hyperthermia has also been shown to induce Hsp70 expression in vivo in rats (Pavlik et al., 2003). In the present study, we present evidence that further illustrates one mechanism which may explain Hsp70’s anti-inflammatory and neuroprotective functions in ischemia-like injury. Specifically, our findings establish an important and previously unknown interaction between Hsp70 and upstream regulatory proteins (such as IκB, JNK and p38). We show that Hsp70 may impede phosphorylation of these proteins, thereby blocking activation of key pro-inflammatory transcription factors.
Transcription factors play a pivotal role in controlling inflammatory gene expression. Microarray studies from other groups have shown the induction of many transcription factors after focal ischemia (Angstwurm et al., 1998; Raghavendra Rao et al., 2002; Satriotomo et al., 2006). Of these, activation of hypoxia inducible factor-1 (HIF-1), cAMP response element-binding protein (CREB), peroxisome proliferator-activated receptor alpha (PPARα), peroxisome proliferator-activated receptor gamma (PPARγ) and p53 is known to prevent ischemic neuronal damage and/or promote ischemic tolerance (Tanaka et al., 2000a; Cho et al., 2001; Lu et al., 2003), whereas the induction of NF-κB, AP-1, STAT-1, early growth response-1 (Egr1) and C/EBPβ leads to inflammation and neuronal death after cerebral ischemia (Iadecola et al., 1999; Johansson et al., 2000; Stephenson et al., 2000; Maeda et al., 2001; Akaji et al., 2003). These earlier findings from our group and others suggest that NF-κB, AP-1 and STAT-1 are key intermediates and useful indicators for neuroinflammation and outcome following ischemic stroke (Zheng et al., 2008; Dong et al., 2009; Hou et al., 2010).
Degradation of IκB induces NF-κB activation leading to the coordinated induction of multiple genes involved in many inflammatory and immune cascades. Genes induced by NF-κB include pro-inflammatory cytokines IL-1β, TNF-α and granulocyte–macrophage colony stimulating factor (GM-CSF), as well as chemokines IL-8, MIP-1α and MCP-1, which are largely responsible for attracting inflammatory cells into sites of inflammation (Nelson et al., 1993; Siebenlist et al., 1994; Tanaka et al., 2000b). Many NF-κB downstream genes product such as IL-1β and TNF-α also re-activate NF-κB itself, resulting in a positive regulatory loop that amplifies and perpetuates inflammatory responses (Iademarco et al., 1995). Our in vivo data from prior studies confirmed that activation of NF-κB occurs after MCAO, and that inhibiting NF-κB activity results in smaller infarcts (Zheng et al., 2008).
MAPKs are activated after ischemic stroke (Hayashi et al., 2000; Laher and Zhang, 2001; Alessandrini et al., 1999). Among the MAPKs, JNK and p38 are often involved in a variety of cell signaling, including inflammation (Xia et al., 1995; Dong et al., 2002). Some studies have demonstrated that phosphorylated JNK and p38 may contribute to neuronal death following ischemic stroke (Irving and Bamford, 2002; Borsello et al., 2003; Toledo-Pereyra et al., 2008). After ischemic stroke, phosphorylation of JNK can promote the transcription of AP-1 and pro-inflammation genes (Benakis et al., 2010; Zhang et al., 2006). AP-1 is a heterodimer of Fos and Jun oncoproteins, which includes a collection of transcription factors belonging to the Fos (cFos, FosB, Fra1, Fra2) and Jun (c-Jun, JunB, JunD) families. Fos and Jun proteins are able to dimerize in various combinations through their leucine zipper regions (Barnes and Adcock, 1998). AP-1 is activated by various cytokines, including TNF-α and IL-1β, via several protein tyrosine kinases and MAP kinases. Transcription factors Fos, c-Jun and JunB have been shown to be upregulated following cerebral ischemia (Kindy et al., 1991; Woodburn et al., 1993; Dragunow et al., 1994), and c-Jun is thought to play a role in apoptotic neuronal death (Raivich and Behrens, 2006). The STAT protein group is another family of cytoplasmic transcription factors involved in post-ischemic inflammation. STATs mediate intracellular signaling initiated at cytokine cell surface receptors and transmitted to the nucleus. They are activated by phosphorylation on conserved tyrosine and serine residues by the Janus kinases (JAKs) and MAP kinase families respectively, which allow STATs to dimerize and translocate to the nucleus where they regulate gene expression (Darnell, 1997). The C-terminal domains of STAT proteins contain a transcriptional transactivation domain (TAD), plus the phosphorylation site for JAKs and MAPK, which are essential for maximal STAT function. At present seven different STAT family members have been found to be encoded by distinct genes (STAT-1, STAT-2, STAT-3, STAT-4, STAT-5α, STAT-5β and STAT-6) (Levy and Darnell, 2002), each activated by distinct groups of cytokines.
Accumulating evidence now indicates that Hsp70 are known to have significant modulating roles in both acting as pro-inflammatory cytokines and mediating regulatory immune responses. As an anti-inflammatory molecule, Hsp70 suppresses the release of proinflammatory factors, including NF-kB, matrix metalloproteinases (MMPs), and reactive oxygen species (ROS) (Zhang et al., 2006; Sinn et al., 2007; Voloboueva et al., 2008). Intracellular Hsp70 overexpression by heat stress has been shown to reduce inflammatory cell production of NO and iNOS expression while decreasing NF-κB activation in astrocytes (Feinstein et al., 1996). Hsp70 can also decrease responses to inflammatory cytokines such as TNF-α and IL-1 (Van Molle et al., 2002), while overexpression of Hsp70 blocked LPS-induced increases in TNF, IL-1, IL-10, and IL-12 in macrophages (Ding et al., 2001). In a model of intracerebral hemorrhage, upregulation of Hsp70 decreased TNF-α expression and attenuated BBB disruption, edema formation, and neurological dysfunction (Manaenko et al., 2010). Heat shock induction of Hsp70 reduces nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in neutrophils and increases superoxide dismutase, which scavenges superoxide, in phagocytes (Polla et al., 1995).
The current study focuses primarily on Hsp70’s role in early inflammatory events that are thought to exacerbate ischemic brain injury. Our data indicate that Hsp70 overexpression can inhibit neuroinflammatory pathways by physically binding and inactivating proteins which regulate their transcription factors. Based on our results, Hsp70 overexpression interacts with IκB, JNK, and p38 in the cytosol, thus hampering their phosphorylation. Unphosphorylated, these proteins are unable to active their transcription factors (NF-κB, AP-1 and STAT-1), ultimately limiting transcription of pro-inflammatory genes. Through RT-PCR analysis we were finally able to confirm that Hsp70 overexpression by heat stress decreased levels of pro-inflammatory mRNA (MMP-9, IL-1β and TNF-α). These observations establish an important and previously unknown correlation between Hsp70 neuroprotection and inactivation of upstream proteins which regulate pro-inflammatory transcription factors.
Closer examination may be necessary to draw a definitive connection, since another possible explanation for the decline in these transcription factors may be increased cell death in heat-stressed cells. Further, our approach is limited to the heat stress approach, rather than specific overexpression of Hsp70. However, prior work using specific Hsp70 overexpression approaches has shown similar anti-inflammatory effects in vivo (Zheng et al., 2008). Thus, observations in this heat stress model likely reflect an effect of Hsp70 overexpression. Our results provide not only a mechanistic basis for the neuroprotective effects of Hsp70 in ischemic brain injury, but further support the development of Hsp70 as a therapeutic against a range of conditions linked to neuroinflammation.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (NRF-2014R1A2A2A01006556) which was awarded to JEL. This work was also sponsored by a grant from National Institutes of Health (NS40516) and the Veteran’s Merit Award to MY (I01 BX000589), as well as an American Heart Association Western States Affiliate Postdoctoral Fellowship (13POST14810019) to JYK. Grants to MY and JYK were administered by the Northern California Institute for Research and Education, and supported by resources provided by the Veterans Affairs Medical Center, San Francisco, California.
Abbreviations
- AP-1
activator protein-1
- BSS
balanced salt solution
- ELISA
enzyme-linked immunosorbent assay
- EMSA
Electrophoretic-Mobility Shift Assay
- Hsp70
70-kDa heat shock protein
- IκB
inhibitor of κB
- IL-1β
interleukin-1 beta
- JAKs
Janus kinases
- JNK
c-Jun N-terminal kinases
- MCAO
middle cerebral artery occlusion
- NF-κB
nuclear factor-κB
- OGD
oxygen and glucose deprivation
- RT-PCR
reverse transcription-polymerase chain reaction
- STAT-1
signal transducers and activator of transcription-1
- TNF-α
tumor necrosis factor alpha
References
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Akaji K, Suga S, Fujino T, Mayanagi K, Inamasu J, Horiguchi T, Sato S, Kawase T. Effect of intra-ischemic hypothermia on the expression of c-Fos and c-Jun, and DNA binding activity of AP-1 after focal cerebral ischemia in rat brain. Brain Res. 2003;975:149–157. doi: 10.1016/s0006-8993(03)02622-2. [DOI] [PubMed] [Google Scholar]
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angstwurm K, Freyer D, Dirnagl U, Hanisch UK, Schumann RR, Einhaupl KM, Weber JR. Tumour necrosis factor alpha induces only minor inflammatory changes in the central nervous system, but augments experimental meningitis. Neuroscience. 1998;86:627–634. doi: 10.1016/s0306-4522(98)00032-3. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Transcription factors and asthma. Eur Respir J. 1998;12:221–234. doi: 10.1183/09031936.98.12010221. [DOI] [PubMed] [Google Scholar]
- Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun. 2010;24:800–811. doi: 10.1016/j.bbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Bode JG, Gatsios P, Ludwig S, Rapp UR, Haussinger D, Heinrich PC, Graeve L. The mitogen-activated protein (MAP) kinase p38 and its upstream activator MAP kinase kinase 6 are involved in the activation of signal transducer and activator of transcription by hyperosmolarity. J Biol Chem. 1999;274:30222–30227. doi: 10.1074/jbc.274.42.30222. [DOI] [PubMed] [Google Scholar]
- Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA, Lee JE. Retroviral expression of arginine decarboxylase attenuates oxidative burden in mouse cortical neural stem cells. Stem Cells Dev. 2011;20:527–537. doi: 10.1089/scd.2010.0312. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Cheng L, Smith DJ, Anderson RL, Nagley P. Human neuroblastoma SH-SY5Y cells show increased resistance to hyperthermic stress after differentiation, associated with elevated levels of Hsp72. Int J Hyperthermia. 2011;27:415–426. doi: 10.3109/02656736.2010.531075. [DOI] [PubMed] [Google Scholar]
- Cho S, Park EM, Kim Y, Liu N, Gal J, Volpe BT, Joh TH. Early c-Fos induction after cerebral ischemia: a possible neuroprotective role. J Cereb Blood Flow Metab. 2001;21:550–556. doi: 10.1097/00004647-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, Hoover DL. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Dong Y, Liu HD, Zhao R, Yang CZ, Chen XQ, Wang XH, Lau LT, Chen J, Yu AC. Ischemia activates JNK/c-Jun/AP-1 pathway to up-regulate 14-3-3gamma in astrocyte. J Neurochem. 2009;109(Suppl 1):182–188. doi: 10.1111/j.1471-4159.2009.05974.x. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Beilharz E, Sirimanne E, Lawlor P, Williams C, Bravo R, Gluckman P. Immediate-early gene protein expression in neurons undergoing delayed death, but not necrosis, following hypoxic-ischaemic injury to the young rat brain. Brain Res Mol Brain Res. 1994;25:19–33. doi: 10.1016/0169-328x(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Xu L, Zhao H, Carrico W, Ouyang Y, Qiao Y, Sapolsky R, Steinberg G, Hu B, Yenari MA. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207:3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Sakai K, Sasaki C, Zhang WR, Warita H, Abe K. C-JUN N-terminal kinase (JNK) and JNK interacting protein response in rat brain after transient middle cerebral artery occlusion. Neurosci Lett. 2000;284:195–199. doi: 10.1016/s0304-3940(00)01024-7. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998;39:106–120. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Hou YC, Liou KT, Chern CM, Wang YH, Liao JF, Chang S, Chou YH, Shen YC. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-kappaB and STAT-1 activation. Phytomedicine. 2010;17:963–973. doi: 10.1016/j.phymed.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Salkowski CA, Zhang F, Aber T, Nagayama M, Vogel SN, Ross ME. The transcription factor interferon regulatory factor 1 is expressed after cerebral ischemia and contributes to ischemic brain injury. J Exp Med. 1999;189:719–727. doi: 10.1084/jem.189.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iademarco MF, Barks JL, Dean DC. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest. 1995;95:264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr, Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson IM, Wester P, Hakova M, Gu W, Seckl JR, Olsson T. Early and delayed induction of immediate early gene expression in a novel focal cerebral ischemia model in the rat. Eur J Neurosci. 2000;12:3615–3625. doi: 10.1046/j.1460-9568.2000.00252.x. [DOI] [PubMed] [Google Scholar]
- Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Suh SW, Berman AE, Hamby AM, Swanson RA. Inhibition of poly (ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab. 2009;29:820–829. doi: 10.1038/jcbfm.2009.9. [DOI] [PubMed] [Google Scholar]
- Kindy MS, Carney JP, Dempsey RJ, Carney JM. Ischemic induction of protooncogene expression in gerbil brain. J Mol Neurosci. 1991;2:217–228. [PubMed] [Google Scholar]
- Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. J Cereb Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim YJ, Kim JY, Lee WT, Yenari MA, Giffard RG. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004;15:499–502. doi: 10.1097/00001756-200403010-00023. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001;170:129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Li L, Han ZY, Li CM, Jiang XQ, Wang GL. Upregulation of heat shock protein 32 in Sertoli cells alleviates the impairments caused by heat shock-induced apoptosis in mouse testis. Cell Stress Chaperones. 2013;18:333–351. doi: 10.1007/s12192-012-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR. Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:786–810. doi: 10.1097/01.WCB.0000062340.80057.06. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hata R, Gillardon F, Hossmann KA. Aggravation of brain injury after transient focal ischemia in p53-deficient mice. Brain Res Mol Brain Res. 2001;88:54–61. doi: 10.1016/s0169-328x(01)00017-1. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Fathali N, Chen H, Suzuki H, Williams S, Zhang JH, Tang J. Heat shock protein 70 upregulation by geldanamycin reduces brain injury in a mouse model of intracerebral hemorrhage. Neurochem Int. 2010;57:844–850. doi: 10.1016/j.neuint.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Holloway J, Salvati VM, Pender SL, Fairclough PD, Croft N, MacDonald TT. Activated STAT4 and a functional role for IL-12 in human Peyer’s patches. J Immunol. 2003;170:300–307. doi: 10.4049/jimmunol.170.1.300. [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Kim HT, Manning WC, Goralski TJ, Krensky AM. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, Bhan A, Autschbach F, Sullivan BM, Szabo SJ, Glimcher LH, Blumberg RS. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Akagi Y, Kinugasa T, Ishibashi N, Iwakuma N, Shiratsuchi I, Shirouzu K. Heat-shock pre-treatment reduces liver injury and aids liver recovery after partial hepatectomy in mice. Anticancer Res. 2013;33:2887–2894. [PubMed] [Google Scholar]
- O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Pavlik A, Aneja IS, Lexa J, Al-Zoabi BA. Identification of cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Brain Res. 2003;973:179–189. doi: 10.1016/s0006-8993(03)02476-4. [DOI] [PubMed] [Google Scholar]
- Pennypacker KR, Kassed CA, Eidizadeh S, O’Callaghan JP. Brain injury: prolonged induction of transcription factors. Acta Neurobiol Exp (Wars) 2000;60:515–530. doi: 10.55782/ane-2000-1373. [DOI] [PubMed] [Google Scholar]
- Polla BS, Stubbe H, Kantengwa S, Maridonneau-Parini I, Jacquier-Sarlin MR. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation. 1995;19:363–378. doi: 10.1007/BF01534393. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Bowen KK, Dhodda VK, Song G, Franklin JL, Gavva NR, Dempsey RJ. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J Neurochem. 2002;83:1072–1086. doi: 10.1046/j.1471-4159.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- Raivich G, Behrens A. Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol. 2006;78:347–363. doi: 10.1016/j.pneurobio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Sheppard PW, Sun X, Khammash M, Giffard RG. Overexpression of heat shock protein 72 attenuated NF-kB activation using a combination of regulatory mechanisms in microglia. PLoS Comput Biol. 2014;10:e1003471. doi: 10.1371/journal.pcbi.1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Sinn DI, Chu K, Lee ST, Song EC, Jung KH, Kim EH, Park DK, Kang KM, Kim M, Roh JK. Pharmacological induction of heat shock protein exerts neuroprotective effects in experimental intracerebral hemorrhage. Brain Res. 2007;1135:167–176. doi: 10.1016/j.brainres.2006.11.098. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Stasiolek M, Gavrilyuk V, Sharp A, Horvath P, Selmaj K, Feinstein DL. Inhibitory and stimulatory effects of lactacystin on expression of nitric oxide synthase type 2 in brain glial cells. The role of Ikappa B-beta. J Biol Chem. 2000;275:24847–24856. doi: 10.1074/jbc.M910284199. [DOI] [PubMed] [Google Scholar]
- Stephenson D, Yin T, Smalstig EB, Hsu MA, Panetta J, Little S, Clemens J. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:592–603. doi: 10.1097/00004647-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Strauss M, Rada A, Tejero F, Hermoso T. Heat stress in rat adriamycin cardiomyopathy: heat shock protein 25 and Myosin accumulation. J Toxicol Pathol. 2010;23:235–243. doi: 10.1293/tox.23.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Activated phosphorylation of cyclic AMP response element binding protein is associated with preservation of striatal neurons after focal cerebral ischemia in the rat. Neuroscience. 2000a;100:345–354. doi: 10.1016/s0306-4522(00)00289-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Nagata E, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Exp Neurol. 2000b;161:462–471. doi: 10.1006/exnr.1999.7313. [DOI] [PubMed] [Google Scholar]
- Thomas G, Souil E, Richard MJ, Saunier B, Polla BS, Bachelet M. Hyperthermia assists survival of astrocytes from oxidative-mediated necrotic cell death. Cell Mol Biol (Noisy-le-grand) 2002;48:191–198. [PubMed] [Google Scholar]
- Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Protein kinases in organ ischemia and reperfusion. J Invest Surg. 2008;21:215–226. doi: 10.1080/08941930802130149. [DOI] [PubMed] [Google Scholar]
- Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–695. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stoy C, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab. 2008;28:1009–1016. doi: 10.1038/sj.jcbfm.9600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Wu CY, Sun CC, Yang CM. Oxidized low-density lipoprotein induces matrix metalloproteinase-9 expression via a p42/p44 and JNK-dependent AP-1 pathway in brain astrocytes. Glia. 2009;57:24–38. doi: 10.1002/glia.20732. [DOI] [PubMed] [Google Scholar]
- Wang HY, Fu JC, Lee YC, Lu PJ. Hyperthermia stress activates heat shock protein expression via propyl isomerase 1 regulation with heat shock factor 1. Mol Cell Biol. 2013;33:4889–4899. doi: 10.1128/MCB.00475-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn VL, Hayward NJ, Poat JA, Woodruff GN, Hughes J. The effect of dizocilpine and enadoline on immediate early gene expression in the gerbil global ischaemia model. Neuropharmacology. 1993;32:1047–1059. doi: 10.1016/0028-3908(93)90070-j. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xu L, Dayal M, Ouyang YB, Sun Y, Yang CF, Frydman J, Giffard RG. Chaperonin GroEL and its mutant D87K protect from ischemia in vivo and in vitro. Neurobiol Aging. 2006;27:562–569. doi: 10.1016/j.neurobiolaging.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–591. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]