Abstract
Lysosomal dysfunction has been implicated in multiple diseases including lysosomal storage disorders such as Gaucher’s disease, in which loss-of-function mutations in the GBA1 gene encoding the lysosomal hydrolase β-glucocerebrosidase (GCase) result in lipid substrate accumulation. In Parkinson’s disease, α-synuclein accumulates in Lewy bodies and neurites contributing to neuronal death. Previous clinical and genetic evidence has demonstrated an important link between Parkinson’s and Gaucher’s disease, as GBA1 mutations and variants increase the risk of Parkinson's, and Parkinson’s patients exhibit decreased GCase activity. Using human midbrain neuron cultures, we have found that loss of GCase activity promotes α-synuclein accumulation and toxicity, while α-synuclein accumulation further contributes to decreased lysosomal GCase activity by disrupting GCase trafficking to lysosomes. Moreover, α-synuclein accumulation disrupts trafficking of additional lysosomal hydrolases, further contributing to lysosomal dysfunction and neuronal dyshomeostasis. Importantly, promoting GCase activity reduces α-synuclein accumulation and rescues lysosomal and neuronal dysfunction, suggesting that GCase may be an important therapeutic target for advancing drug discovery in synucleinopathies including Parkinson’s disease.
Keywords: Parkinson’s disease, Gaucher’s disease, α-synuclein, β-glucocerebrosidase, LIMP-2
Introduction
Studying the cellular mechanisms and pathways disrupted in disease is critical for identifying therapeutic targets to prevent disease onset or slow disease progression. Unfortunately, in multiple neurodegenerative diseases including Alzheimer’s disease, Amyotrophic Lateral Sclerosis (ALS), Huntington’s disease and Parkinson’s disease, our understanding of the cellular pathways which lead to neuronal dysfunction and death is limited, resulting in a lack of validated therapeutic targets for drug discovery.
Recent clinical and genetic evidence has demonstrated an important epidemiological link between Parkinson’s disease and Gaucher’s disease. In addition, results from our lab and others have identified important mechanistic interplays between α-synuclein in Parkinson’s disease and β-glucocerebrosidase (GCase) in Gaucher’s disease, identifying GCase as a potentially effective therapeutic target for Parkinson’s disease drug discovery.
In this Scientific Perspective, we discuss recent work demonstrating the cellular interplay between α-synuclein and GCase in the context of lysosomal trafficking. We first summarize the epidemiological link between Parkinson’s and Gaucher’s disease and discuss previous work on vesicle trafficking defects associated with α-synuclein toxicity. We then discuss work from our lab demonstrating an important bidirectional loop between α-synuclein and the lysosomal hydrolase GCase: namely, that 1) loss of GCase activity promotes α-synuclein accumulation via accumulation of its lipid substrate GlcCer, and 2) α-synuclein accumulation further contributes to decreased GCase activity by disrupting GCase trafficking to lysosomes. Next, we summarize our recent findings that α-synuclein accumulation additionally disrupts trafficking of other lysosomal hydrolases further contributing to overall lysosomal dysfunction. Finally, we address the use of GCase activation as a potential therapeutic target for synucleinopathies including Parkinson’s disease.
The Epidemiological Link: Parkinson’s and Gaucher’s disease
α-Synuclein in Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and is characterized by progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), resulting in the onset of clinical parkinsonian symptoms including bradykinesia, resting tremors, muscular rigidity, and postural instability1. Although the majority of patients do not have a family history of PD, genetic screening of familial PD cases has provided important insight into the pathophysiology of PD.
α-synuclein (SNCA) was the first gene linked to familial PD2, with subsequent studies identifying additional point mutations in the N-terminus of α-synuclein as causative for familial autosomal dominant PD3–7 (Fig. 1A). α-synuclein was also found to accumulate in Lewy bodies and neurites in the brain, spinal cord and peripheral nervous system of sporadic PD patients8, demonstrating a critical role for α-synuclein in both sporadic and familial PD. Both SNCA locus duplication and triplication also cause familial PD9, 10, and importantly, patients with SNCA triplication display earlier PD onset by several decades as compared to patients with SNCA duplication or sporadic PD1, revealing a dose-dependent toxicity from increased α-synuclein levels. Moreover, increased α-synuclein expression due to a risk variant in a non-coding distal enhancer element in SNCA was recently found to be associated with PD, further establishing a role for increased α-synuclein levels in PD pathogenesis11.
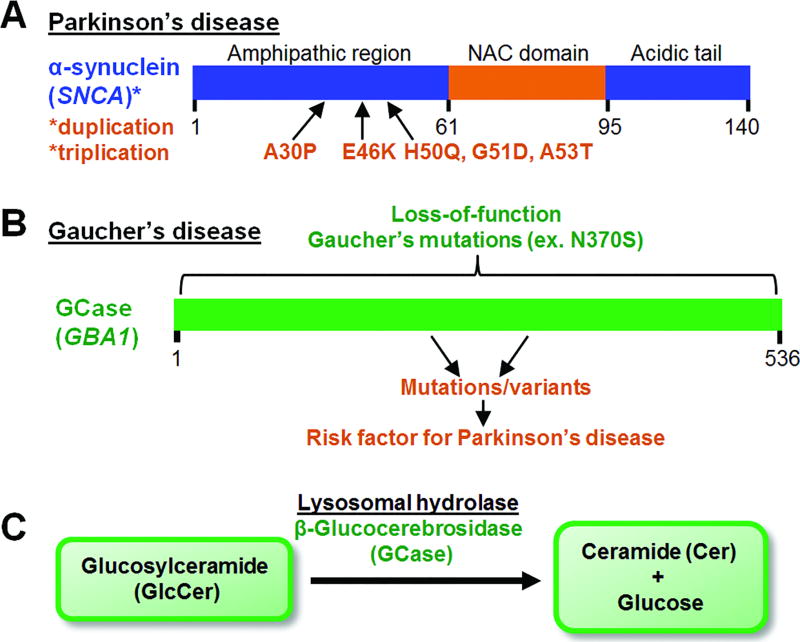
Fig. 1. α-synuclein in Parkinson’s and GCase in Gaucher’s disease.
(A) α-synuclein accumulates in both sporadic and familial forms of Parkinson’s disease. N-terminal mutations in α-synuclein (SNCA) and SNCA duplication/triplication lead to familial PD. The NAC domain of α-synuclein mediates its fibrilization. (B) Loss-of-function mutations in the GBA1 gene encoding β-glucocerebrosidase (GCase) lead to Gaucher’s disease. Variants in GBA1 are one of the most common risk factors for Parkinson’s disease. (C) GCase is a lysosomal hydrolase which converts glucosylceramide (GlcCer) into ceramide and glucose by cleaving the β-glucosyl linkage of GlcCer. Loss of GCase activity results in GlcCer substrate accumulation.
Subsequent studies have identified other genes linked to PD, including LRRK212, 13, VPS3514, 15 and the recently identified TMEM23016 linked to autosomal dominant PD, while parkin17, DJ-118, PINK119 and PARK9/ATP13A220 have been linked to autosomal recessive PD. In addition, various genome-wide associate studies (GWAS) have identified additional risk variants for PD in at least 24 different loci21, 22, including tau (MAPT)23 and α-synuclein (SNCA)24. Interestingly, α-synuclein also accumulates in other synucleinopathies including multiple system atrophy (MSA) and dementia with Lewy bodies (DLB)25, 26.
GCase in Gaucher’s disease
Gaucher’s disease is an autosomal recessive genetic lysosomal storage disorder (LSD) caused by homozygous loss-of-function mutations in GBA1 encoding β-glucocerebrosidase (GCase) (Fig. 1B). More than 300 mutations in GBA1 have now been identified in Gaucher’s disease, with the N370S as the most common mutation within the Ashkenazi Jewish population27. Gaucher’s disease is a systemic disease which commonly results in thrombocytopenia, anemia, hepatosplenomegaly, and bone pain with disease onset at adulthood. However, in some cases, it presents as a more severe, predominantly neuronopathic form involving seizures, cognitive impairment, and oculomotor problems, with death in early childhood27.
GCase is a lysosomal hydrolase which converts glucosylceramide (GlcCer) into ceramide and glucose by cleaving the β-glucosyl linkage of GlcCer28 (Fig. 1C). GCase targeting to lysosomes is mediated via the lysosomal integral membrane protein type-2 (LIMP-2), independently of mannose-6 phosphate29. Although GlcCer accumulates in all forms of Gaucher’s disease, it is still unclear what causes the variability across patients with different forms of Gaucher’s disease.
Epidemiological Links Between Parkinson’s and Gaucher’s Disease
The link between PD and Gaucher’s disease was initially observed when a subset of Gaucher’s patients were found to have parkinsonian symptoms and α-synuclein-positive Lewy body pathology typical of synucleinopathies30–33. In addition, Gaucher patients with parkinsonism often had relatives with parkinsonism who were heterozygous for GBA1 mutations (Gaucher carriers)34. Subsequent genetic studies in large cohorts found that PD patients with GBA1 mutations presented earlier with the disease, and were more likely to have relatives affected with parkinsonism35, 36. GBA1 mutations are now considered one of the most common risk factors for PD, with recent GWAS studies of PD patients further confirming the identification of risk variants within the GBA1 loci21. In addition, mutations in GBA1 have also been linked to patients with the synucleinopathy DLB37 and GBA1 mutations may play an even greater role in DLB etiology than PD38, further suggesting an important link between α-synuclein toxicity and loss of GCase function. Interestingly, variants in the LIMP-2 gene (SCARB2) have also been associated with increased risk for PD39, suggesting that defective lysosomal trafficking of GCase may contribute to PD pathogenesis. More recently, reduced wildtype GCase activity has been observed along with accumulation of GCase substrates in blood and postmortem brains of sporadic PD patients40–42, raising the possibility that α-synuclein accumulation in sporadic PD can also influence GCase lysosomal activity.
Lysosomal Trafficking Defects: Linking α-Synuclein with GCase
Background: Vesicle Trafficking Defects in α -Synuclein Toxicity
α-synuclein was first found to disrupt vesicle trafficking in yeast, as overexpression of either wildtype or the PD-linked mutant A53T α-synuclein perturbed vesicle distribution43. Subsequently, overexpression of wildtype α-synuclein was observed to cause cell death, increase endoplasmic reticulum (ER) stress and impair ER-associated degradation, as well as inhibit ER to Golgi trafficking of a soluble misfolded substrate (CPY) and alkaline phosphatase (ALP)44. A genome-wide screen in yeast to identify suppressors and enhancer of α-synuclein toxicity found that the most effective class of suppressors were involved in vesicle-mediated membrane trafficking including the Rab GTPase Ypt1p/Rab1. Importantly, overexpression of Rab1 was able to rescue dopaminergic neuron death in Drosophila expressing either wildtype or A53T α-synuclein, as well as reduce neurodegeneration in a C. elegans model expressing α-synuclein in dopaminergic neurons44. In addition, expression of Rab1 in rat primary midbrain neurons expressing A53T α-synuclein improved neuronal viability in culture44, demonstrating that rescuing transport of ER-derived vesicles through Rab1 suppressed α-synuclein toxicity.
Subsequent work using a reconstituted cell-free assay demonstrated that addition of wildtype or A53T α-synuclein inhibited ER to Golgi transport, and showed that α-synuclein expression led to a time and dosage-dependent accumulation of transport vesicles by electron microscopy (EM) and fluorescence microscopy45. Expression of GFP-tagged α-synuclein showed that it localized to the plasma membrane at early time points, and subsequently localized to cytoplasmic inclusions which were abolished upon Ypt1 overexpression45. In addition, α-synuclein foci were found to colocalize with various Rabs including Ypt145. Indeed, Rab3A and Rab8A overexpression also protected against dopaminergic cell loss in a C. elegans model expressing wildtype α-synuclein in dopaminergic neurons and in rat primary midbrain neurons expressing A53T α-synuclein45, suggesting that multiple Rabs may be involved in α-synuclein toxicity.
Additional studies have found that α-synuclein expression disrupts the ER to Golgi trafficking of other cargo including the dopamine transport (DAT), leading to a reduction in the function and cell surface expression of DAT, and a reduced immobile DAT fraction46. α-synuclein overexpression also disrupts the ER to Golgi trafficking of the autophagy protein Atg9 which may contribute to defective autophagy, and which is rescued by Rab1a overexpression47. One mechanism through which α-synuclein may disrupt ER to Golgi transport is through disruption of ER/Golgi SNAREs, as α-synuclein has previously been shown to interact with the V-SNARE VAMP2 to regulate SNARE complex formation on synaptic vesicles48. Indeed, overexpression of wildtype or A53T α-synuclein disrupted protein trafficking, which was rescued by co-overexpression of ER/Golgi R-SNAREs49. In particular, expression of the ER/Golgi SNARE Ykt6 effectively rescued protein trafficking, while purified A53T α-synuclein inhibited COPII vesicle docking and fusion, and was able to directly bind ER/Golgi SNAREs to inhibit SNARE complex assembly49.
Further screens to identify modifiers of α-synuclein toxicity also identified the compound NAB2 (N-Aryl Benzimidazole) which acts on the E3 ubiquitin ligase Rsp/Nedd4 to promote ubiquitin-mediated endosomal transport50. In yeast, NAB2 treatment rescued α-synuclein’s disruption of bulk endosomal transport from the plasma membrane to the vacuole. Furthermore, NAB2 treatment in cortical neurons generated from induced pluripotent stem (iPS) cells from PD patients harboring α-synuclein mutations prevented defective ER to Golgi trafficking of multiple substrates51, suggesting that defects in vesicle trafficking are a key component of α-synuclein cellular dyshomeostasis, and that targeting vesicle trafficking may be beneficial in PD.
Part 1: GCase Deficiency Promotes α-Synuclein Toxicity
As loss-of-function GCase mutations are associated with α-synuclein accumulation in a subset of Gaucher disease patient brains, and ER to Golgi vesicle trafficking defects have been implicated in α-synuclein toxicity, our lab investigated the cellular interplay between α-synuclein and GCase and whether defective vesicle trafficking due to α-synuclein toxicity might play a role in their cellular interaction (Fig. 2A).
Fig. 2. Lysosomal dysfunction links Parkinson’s and Gaucher’s disease.
(A) Gaucher’s disease and Parkinson’s disease are linked via a bidirectional loop between α-synuclein and the lysosomal hydrolase GCase, in which 1) loss of GCase activity disrupts lysosomal function and also promotes α-synuclein accumulation and fibrilization via accumulation of its lipid substrate GlcCer, while 2) α-synuclein accumulation further contributes to decreased GCase lysosomal activity by disrupting GCase trafficking to lysosomes. α-synuclein accumulation further disrupts the ER/Golgi trafficking of additional lysosomal hydrolases including cathepsin B, β-galactosidase, and hexosaminidase, resulting in reduced lysosomal enzymatic activity and overall lysosomal dysfunction. Additional factors including loss-of-function Gaucher mutations in GBA1 contribute to decreased GCase lysosomal function, while multiple factors including familial Parkinson’s mutations contribute to α-synuclein accumulation and toxicity. (B) Activation of lysosomal GCase activity may be an effective therapeutic for decreasing α-synuclein accumulation and toxicity across synucleinopathies including Parkinson’s disease.
We began by examining the role of GCase depletion on α-synuclein homeostasis, and found that GCase depletion in neurons significantly decreased the rate of overall cellular proteolysis, which was not further inhibited by lysosomal inhibitors, demonstrating that GCase depletion affected a lysosomal-mediated pathway52. Moreover, GCase depletion increased the steady-state protein levels of α-synuclein, but did not affect α-synuclein mRNA levels. These results were further validated in dopaminergic neurons generated from iPS cells derived from skin fibroblasts of a Gaucher’s disease patient harboring a GCase (N370S/84GG insertion) mutation which showed reduced GCase protein levels and activity, concomitant with a dramatic increase in α-synuclein protein levels52. Importantly, huntingtin and tau protein levels showed little or no change, demonstrating that loss of GCase activity from endogenous Gaucher’s mutations affected lysosomal proteolysis resulting in the preferential accumulation of α-synuclein.
Moreover, GCase depletion reduced neuronal viability in neurons expressing human wildtype α-synuclein or mutant A53T α-synuclein52. In contrast, no toxicity was observed upon expression of an artificial fibrilization-incompetent mutant (Δ71–82 α-synuclein)53, suggesting that GCase depletion promoted α-synuclein neurotoxicity through a fibrilization-dependent manner. GCase depletion additionally resulted in the formation of high-molecular-weight (HMW) α-synuclein assemblies with a molecular radius of 64–95 Å. Importantly, lysosomal inhibition with leupeptin did not enhance α-synuclein-mediated neurotoxicity or generate soluble HMW α-synuclein species52, demonstrating that alterations in the GCase metabolic pathway, rather than a general lysosomal inhibition, influenced α-synuclein toxicity and stabilization of soluble HMW species.
Inhibition of GCase activity is known to result in the accumulation of its lipid substrate GlcCer. We found that while GlcCer accumulation had no effect on α-synuclein in vitro fibril formation at physiological conditions, increasing the amount of GlcCer to 75% while keeping the total lipid amount constant (PC25/GlcCer75) altered the kinetic profile of α-synuclein fibril formation under acidic conditions which mimicked lysosomal conditions52. Under these conditions, the levels of HMW oligomeric α-synuclein and aggregation-prone conformational α-synuclein intermediates increased, suggesting that GlcCer altered the conformation of α-synuclein to increase solvent-exposed hydrophobic regions. Importantly, other sphingolipids did not alter the levels of soluble α-synuclein oligomers, indicating a specific effect by GlcCer on α-synuclein fibrilization. Misfolded α-synuclein was also observed to localize to GlcCer tubular structures by immuno-EM52. Thus, these results suggested that GlcCer stabilized the formation of a soluble assembly-competent intermediate α-synuclein species during the lag phase of the fibril formation reaction.
The effects of GCase depletion on α-synuclein accumulation and toxicity were further confirmed in multiple in vivo Gaucher’s disease models. In a well-established Gaucher’s mouse model (4L/PS-NA)54, eosinophilic spheroids were observed in multiple brain regions including the substantia nigra and cortex, suggesting the presence of degenerating neurons, as compared to in wildtype mice52. These degenerative changes occurred concomitantly with increased levels of α-synuclein in these regions. Moreover, α-synuclein accumulated in the form of punctate structures in Gaucher’s mice, while wildtype mice showed a normal neuropil α-synuclein staining pattern. Of note, α-synuclein accumulations were also observed in other neural regions including cerebellum, hippocampus, and brainstem in Gaucher’s mice52. Increased levels of Triton-soluble, putative oligomeric forms and aggregated species of α-synuclein were also observed in 4L/PS-NA mice compared to wildtype mice. In a second well-characterized Gaucher’s mouse model with a GCase D409H loss-of-function mutation55, similar increases in α-synuclein punctate structures were observed in addition to higher levels of soluble oligomers and insoluble α-synuclein species. Finally, in a well-established C. elegans model of GCase depletion, α-synuclein was also observed to accumulate, demonstrating that GCase depletion promoted the formation of soluble oligomeric and insoluble α-syn in vivo52.
These observations were further replicated in human brain samples. Brain lysate from a Gaucher’s patient with atypical parkinsonism56 demonstrated a dramatic increase in α-synuclein levels52. In addition, elevated levels of α-synuclein oligomers were observed in patients that were homozygous or heterozygous for GCase mutations with a diagnosis of DLB, and in infants diagnosed with neuronopathic GD, concomitant with lower GCase protein and activity levels52. Finally, both homozygote and heterozygote carriers of GBA1 mutations with a neuronopathic phenotype contained significantly higher levels of pathological α-synuclein oligomers57 compared to controls52, further demonstrating that toxic oligomeric α-synuclein was elevated in patients harboring GBA1 mutations and was preferentially associated with neuronopathic forms of Gaucher’s disease. Thus, these initial observations demonstrated in vitro, in vivo, and in patient brain tissue that loss of GCase activity resulted in the accumulation and stabilization of oligomeric α-synuclein via GlcCer substrate accumulation.
Part 2: α-Synuclein Accumulation Disrupts Trafficking of GCase
We subsequently investigated the effect of α-synuclein accumulation on GCase activity as α-synuclein toxicity has been previously linked to defective ER to Golgi trafficking of various cargo. Indeed, we found that overexpression of human wildtype and A53T mutant α-synuclein in primary cortical neurons disrupted GCase trafficking from the ER, resulting in an altered post-ER/ER GCase ratio with an accumulation of the immature ER form and a decrease in the post-ER forms52. In contrast, the fibrilization-incompetent mutant Δ71–82 α-synuclein did not affect GCase trafficking. Moreover, in lysosomal fractions from primary neuronal cultures, expression of both wildtype and A53T α-synuclein resulted in a significant decrease in GCase lysosomal activity and a concomitant increase in the microsome-enriched fraction activity, while expression of Δ71–82 α-synuclein did not affect GCase lysosomal activity52.
These findings were further confirmed using human brain tissue from elderly healthy controls without common GBA1 mutations. While all samples appeared to have similar levels of post-ER GCase, samples with lower α-synuclein level contained much less of the ER form, while microsome-enriched fractions from samples with higher α-synuclein levels showed increased GCase activity52, suggesting that normal variation of α-synuclein protein levels was able to modulate lysosomal maturation and activity of GCase in vivo. In addition, both total GCase protein levels in Triton-soluble lysates and lysosomal GCase activity from the cingulate cortex of PD brain were dramatically decreased compared to age- and postmortem time-matched controls52, further demonstrating that elevated levels of α-synuclein in PD led to decreased lysosomal activity of normal GCase, which could in turn contribute to further propagation and stabilization of oligomeric α-syn. Thus, our results demonstrate that elevated levels of toxic α-synuclein species lead to depletion of lysosomal GCase and this could be further exacerbated by the stabilization of α-synuclein oligomers by GlcCer accumulation upon GCase depletion (Fig. 2A). Importantly, these results suggest that targeting GCase may be an effective therapeutic target to modulate α-synuclein oligomerization and toxicity.
Part 3: α-Synuclein Disrupts Lysosomal Function via Hydrolase Trafficking Dysfunction
Lysosomal dysfunction has been implicated in multiple diseases including lysosomal storage disorders such as Gaucher’s disease. Our recent data demonstrates that α-synuclein accumulation in synucleinopathies also contributes to lysosomal dysfunction by impairing vesicular trafficking of lysosomal hydrolase during the early secretory pathway, resulting in reduced lysosomal function58 (Fig. 2A). To examine the interaction between α-synuclein, lysosomes, and trafficking components, human midbrain synucleinopathy dopaminergic neurons generated by lentiviral overexpression of α-synuclein in healthy control iPS neuronal lines, or through the generation of patient lines harboring PD-causing α-synuclein (SNCA) triplication were used. Importantly, this stable neuronal model allowed us to culture patient neurons for several hundred days and to validate pathogenic changes in the lysosomal system in the context of naturally occurring mutations leading to α-synuclein accumulation58.
We found that α-synuclein was localized to synapses in control and patient neurons, but began accumulating at the cell body of SNCA triplication neurons at day 6058. Moreover, amyloidogenic α-synuclein inclusions were observed in cell bodies and neurites by day 90, and soluble oligomeric and insoluble α-synuclein species accumulated which persisted till day 33058, demonstrating that this neuronal model recapitulated key pathological features found in the PD brain. When neurons were examined for changes in lysosomal proteolysis, lenti-wildtype-α-synuclein–infected midbrain dopaminergic neurons showed a decline in long-lived proteolysis rates compared to control neurons expressing the fibrilization-incompetent α-synuclein Δ71–82 mutant58, demonstrating a defect in the lysosomal system. In addition, proteolysis rates were decreased in SNCA triplication midbrain neurons at day 180 compared to control lines, while midbrain neurons from Gaucher patients began to show reduced proteolysis at an even earlier stage (day 110). Consistent with lysosomal dysfunction, increased lysosomal mass was observed in both PD and Gaucher patient neurons, and was partially rescued by lenti-shRNA knockdown of α-synuclein in both PD and Gaucher lines58, demonstrating that α-synuclein accumulation contributed to lysosomal dysfunction, but could be partially reversed upon α-synuclein reduction.
Interestingly, the activity of multiple lysosomal hydrolases were defective in both PD and Gaucher patient neurons as compared to control neurons, including lysosomal cathepsin B activity, but were rescued upon reduction of α-synuclein by shRNA knockdown58. In addition, other non-protein-degrading lysosomal enzymes, including GCase, β-galactosidase (β-gal), and hexosaminidase (Hex) showed decreased lysosomal activity in PD and GD neurons, while their total cellular activity were not changed. Idiopathic PD neurons also demonstrated a reduction in GCase activity specifically within acidic subcellular compartments, while neurons expressing Δ71–82 mutant α-synuclein or aggregation-prone poly-Q-huntingtin had a minimal effect on lysosomal GCase activity. Gaucher lines also exhibited reduced GCase activity as expected, but additionally demonstrated decreased activity of other lysosomal hydrolases, including β-gal, Hex, and sulfatase58.
Importantly, the decline in lysosomal enzymatic activity was sufficient to induce substrate accumulation in patient neurons58. Quantitative analysis of GCase substrates, including total hexosylceramide and hexosylsphingosine species revealed a dramatic increase in both PD SNCA triplication and Gaucher neurons. In addition, both glucosyl and galactosylceramide were significantly elevated in PD neurons, whereas ceramide levels were decreased. In contrast, levels of dihydroceramide, a nonlysosomal lipid species, were not altered58, demonstrating that chronic lysosomal hydrolase deficiency in PD midbrain neurons induced lipid accumulation due to α-synuclein accumulation.
This deficiency in lysosomal function was subsequently found to be due to dysfunctional trafficking of lysosomal enzymes from the ER. Control midbrain neurons infected with lenti-WT α-synuclein showed reduced ratios of mature to immature hydrolases including Hex A and B, demonstrating a decline in enzyme maturation58. In addition, lysosomal enzymes accumulated in pre-Golgi COPII endoplasmic reticulum (ER)-to-Golgi transport vesicles in cells expressing high levels of α-synuclein, which was reversible upon reduction of α-synuclein expression. Moreover, maturation of GCase, β-gal, iduronate-2-sulfatase, and Hex A were reduced in extracts from patient neurons which was similarly reversible upon lenti-mediated knockdown of α-synuclein. Importantly, hydrolase maturation was not altered by treatment with the lysosomal inhibitor Bafilomycin-A1, indicating a specific trafficking defect induced by α-synuclein58. In addition, patient neurons which showed α-synuclein accumulation in the cell body demonstrated aberrant colocalization of α-synuclein with vesicle-tethering factor GM130 within fragmented, vesicular Golgi structures, which was not observed in control neuron58, suggesting that α-synuclein disrupted hydrolase trafficking at the cis-Golgi level.
The GTPase Rab1a which regulates ER to Golgi trafficking has previously been shown to rescue α-synuclein–induced neurodegeneration44. Although Rab1a levels were not altered between control and patient neurons and α-synuclein did not interact with Rab1a, α-synuclein accumulation in patient lines altered Rab1a localization from its normal perinuclear ER-Golgi localization to a more diffuse pattern58. In addition, lenti-Rab1a expression restored Golgi structure and improved lysosomal hydrolase maturation and activity in both lenti-α-synuclein infected control lines and patient lines at early time points. Furthermore, at later time points, Rab1a overexpression reduced α-synuclein accumulation within cell bodies of SNCA triplication neurons due to lysosomal enhancement, and successfully improved neuronal viability in neurons derived from three distinct synucleinopathy patients (SNCA triplication, Gaucher’s disease, and idiopathic PD)58. Thus, these results demonstrate that α-synuclein accumulation disrupts overall lysosomal function by perturbing lysosomal hydrolase trafficking at the early secretory pathway in multiple patient neuronal models of synucleinopathies, further contributing to neuronal dyshomeostasis (Fig. 2A).
As Rab1a promotes vesicle tethering at the cis-Golgi, Rab1a overexpression may rescue neuronal viability by increasing vesicle-fusing opportunities at acceptor membranes, thus contributing to enhanced lysosomal hydrolase trafficking in patient neurons. In addition, this increased trafficking of lysosomal enzymes to lysosomes may help restore lysosomal function, and further help to reduce α-synuclein accumulation through chaperone mediated autophagy or macroautophagy-mediated degradation of α-synuclein59–61. Moreover, Rab1a may help decrease GlcCer substrate levels by restoring GCase activity, resulting in the reduced ability for GlcCer to stabilize α-synuclein oligomers52. Importantly, therapies which enhance the trafficking machinery of lysosomal hydrolases may thus prove beneficial across synucleinopathies in which α-synuclein accumulates.
Therapeutic Implications: Targeting GCase Activity in Synucleinopathies
Multiple studies from our group and others have now demonstrated that α-synuclein accumulation contributes to decreased lysosomal activity and trafficking of wildtype GCase40, 51, 52, 58, 62. Indeed, midbrain neurons from PD SNCA triplication patients expressing wildtype GBA1 show reduced lysosomal GCase activity, which is reversible upon shRNA-mediated α-synuclein knock-down58. Moreover, wildtype GCase activity is decreased in blood samples and postmortem brains of sporadic PD patients40, 41 and GCase substrates accumulate in certain regions of synucleinopathy brain expressing wildtype GBA142. Thus, GCase substrates can accumulate in neurons with either mutant or wildtype GCase, and reducing these substrates may be beneficial to both idiopathic and familial PD patients regardless of whether they harbor GBA1 mutations.
In addition, numerous studies have convincingly demonstrated that GCase depletion or loss-of-function GBA1 mutations lead to α-synuclein accumulation52, 58, 63–67, although toxic gain-of-function mechanisms may additionally contribute to α-synuclein toxicity68. Of note, reducing GCase trafficking by loss of LIMP-2 also results in α-synuclein accumulation and neurotoxicity in dopaminergic neurons69, while LIMP-2 derived peptides which activate endogenous lysosomal GCase are able to reduce α-synuclein levels70. Various studies in both cell and animal models have also shown that reducing GCase activity promotes α-synuclein aggregation63, 67, 71–74. Most importantly, multiple epidemiological studies have now demonstrated a link between loss-of-function GBA1 mutations and PD and DLB75.
Thus, promoting GCase lysosomal activity offers an obvious therapeutic target for decreasing α-synuclein accumulation and toxicity across synucleinopathies (Fig. 2B). Indeed, recent studies in synucleinopathy mouse models have demonstrated that increasing GCase activity by virus-mediated overexpression of GCase reduces α-synuclein levels, improves cognitive behavior and is protective from loss of striatal dopaminergic markers76–79.
We recently demonstrated that a non-inhibitory small molecule modulator of GCase NCGC00188758 (758)80 was capable of increasing GCase activity and decreasing α-synuclein accumulation and toxicity in human midbrain neuron cultures81. Treatment with 758 preferentially activated GCase lysosomal activity, but had no effect on its non-lysosomal activity in human synucleinopathy midbrain dopaminergic neurons from patients with GBA1 gene mutations or PD SNCA triplication81. Moreover, 758 treatment was able to reduce lysosomal GluCer levels and whole-cell hexosylsphingosine levels in SNCA triplication neurons. Importantly, treatment with 758 reduced the accumulation of α-synuclein across multiple synucleinopathy lines, including SNCA triplication lines, GBA1 mutant lines, idiopathic PD lines, a PD-linked PARK9/ATP13A2 mutant line82 and a familial PD A53T α-synuclein mutant line83. Treatment with 758 also reduced the accumulation of amyloidogenic Thioflavin S-positive α-synuclein in cell bodies and neurites, and rescued α-synuclein’s proper synaptic localization in human dopaminergic neurons81.
Finally, as α-synuclein disrupts lysosomal function due to defective lysosomal hydrolase trafficking58, 758 treatment was also able to improve hydrolase trafficking of both GCase and lysosomal Hex B, and restored the lysosomal activity of Hex and β-gal in synucleinopathy patient neurons, but had no effect in control neurons81. Importantly, 758 treatment improved proteolysis and rescued neuronal viability in SNCA triplication neurons81, further demonstrating that activation of GCase by non-inhibitory small molecules can rescue pathological α-synuclein toxicity in human neurons. Indeed, another non-inhibitory GCase chaperone NCGC607 also restores GCase activity and reduces α-synuclein levels in human iPS neurons84. Ultimately, the work from our lab and others strongly suggests that activating GCase in human patients will be an effective therapeutic target for decreasing α-synuclein toxicity and may be greatly beneficial for accelerating drug discoveries in synucleinopathies including Parkinson’s disease.
Acknowledgments
Funding sources: This work was supported by NIH/NINDS R01 NS076054 (D.K.) and NIH Training Grant T32NSO41234 (Y.C.W.).
Footnotes
Conflict of Interest: D.K. is a founder and chair of SAB at Lysosomal Therapeutics, Inc
Author Contributions: Y.C.W. and D.K. wrote and reviewed the manuscript.
References
- 1.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 3.Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nature genetics. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 4.Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Annals of neurology. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 5.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2013;28(6):811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 6.Proukakis C, Dudzik CG, Brier T, et al. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology. 2013;80(11):1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesage S, Anheim M, Letournel F, et al. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Annals of neurology. 2013;73(4):459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 8.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 9.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 10.Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 11.Soldner F, Stelzer Y, Shivalila CS, et al. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature. 2016;533(7601):95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paisan-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44(4):595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Zimprich A, Benet-Pages A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. American journal of human genetics. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilarino-Guell C, Wider C, Ross OA, et al. VPS35 mutations in Parkinson disease. American journal of human genetics. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng HX, Shi Y, Yang Y, et al. Identification of TMEM230 mutations in familial Parkinson's disease. Nature genetics. 2016 doi: 10.1038/ng.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 18.Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 19.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez A, Heimbach A, Grundemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nature genetics. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 21.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nature genetics. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nature genetics. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin ER, Scott WK, Nance MA, et al. Association of single-nucleotide polymorphisms of the tau gene with late-onset Parkinson disease. Jama. 2001;286(18):2245–2250. doi: 10.1001/jama.286.18.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruger R, Vieira-Saecker AM, Kuhn W, et al. Increased susceptibility to sporadic Parkinson's disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Annals of neurology. 1999;45(5):611–617. doi: 10.1002/1531-8249(199905)45:5<611::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neuroscience letters. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 26.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 28.Brady RO, Kanfer J, Shapiro D. The Metabolism of Glucocerebrosides. I. Purification and Properties of a Glucocerebroside-Cleaving Enzyme from Spleen Tissue. The Journal of biological chemistry. 1965;240:39–43. [PubMed] [Google Scholar]
- 29.Reczek D, Schwake M, Schroder J, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM : monthly journal of the Association of Physicians. 1996;89(9):691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 31.Sidransky E. Gaucher disease and parkinsonism. Molecular genetics and metabolism. 2005;84(4):302–304. doi: 10.1016/j.ymgme.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Molecular genetics and metabolism. 2003;79(2):104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 33.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Molecular genetics and metabolism. 2004;82(3):192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. Journal of medical genetics. 2004;41(12):937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. The New England journal of medicine. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. The New England journal of medicine. 2004;351(19):1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 37.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67(5):908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 38.Nalls MA, Duran R, Lopez G, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA neurology. 2013;70(6):727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopfner F, Schulte EC, Mollenhauer B, et al. The role of SCARB2 as susceptibility factor in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2013;28(4):538–540. doi: 10.1002/mds.25349. [DOI] [PubMed] [Google Scholar]
- 40.Gegg ME, Burke D, Heales SJ, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Annals of neurology. 2012;72(3):455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alcalay RN, Levy OA, Waters CC, et al. Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations. Brain : a journal of neurology. 2015;138(Pt 9):2648–2658. doi: 10.1093/brain/awv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha EM, Smith GA, Park E, et al. Progressive decline of glucocerebrosidase in aging and Parkinson's disease. Annals of clinical and translational neurology. 2015;2(4):433–438. doi: 10.1002/acn3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302(5651):1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313(5785):324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gitler AD, Bevis BJ, Shorter J, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oaks AW, Marsh-Armstrong N, Jones JM, Credle JJ, Sidhu A. Synucleins antagonize endoplasmic reticulum function to modulate dopamine transporter trafficking. PloS one. 2013;8(8):e70872. doi: 10.1371/journal.pone.0070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winslow AR, Chen CW, Corrochano S, et al. alpha-Synuclein impairs macroautophagy: implications for Parkinson's disease. The Journal of cell biology. 2010;190(6):1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC. Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Molecular biology of the cell. 2010;21(11):1850–1863. doi: 10.1091/mbc.E09-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tardiff DF, Jui NT, Khurana V, et al. Yeast reveal a "druggable" Rsp5/Nedd4 network that ameliorates alpha-synuclein toxicity in neurons. Science. 2013;342(6161):979–983. doi: 10.1126/science.1245321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung CY, Khurana V, Auluck PK, et al. Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342(6161):983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzulli JR, Xu YH, Sun Y, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. The Journal of biological chemistry. 2001;276(4):2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y, Quinn B, Witte DP, Grabowski GA. Gaucher disease mouse models: point mutations at the acid beta-glucosidase locus combined with low-level prosaposin expression lead to disease variants. Journal of lipid research. 2005;46(10):2102–2113. doi: 10.1194/jlr.M500202-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. The American journal of pathology. 2003;163(5):2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Molecular genetics and metabolism. 2001;73(4):313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 57.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Annals of neurology. 2002;52(2):205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 58.Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(7):1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 60.McGlinchey RP, Lee JC. Cysteine cathepsins are essential in lysosomal degradation of alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(30):9322–9327. doi: 10.1073/pnas.1500937112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. The Journal of biological chemistry. 2003;278(27):25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 62.Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound alpha-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Molecular genetics and metabolism. 2013;108(1):56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schondorf DC, Aureli M, McAllister FE, et al. iPSC-derived neurons from GBA1-associated Parkinson's disease patients show autophagic defects and impaired calcium homeostasis. Nature communications. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- 64.Bae EJ, Yang NY, Lee C, et al. Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and alpha-synuclein aggregation. Experimental & molecular medicine. 2015;47:e188. doi: 10.1038/emm.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y, Florer J, Mayhew CN, et al. Properties of neurons derived from induced pluripotent stem cells of Gaucher disease type 2 patient fibroblasts: potential role in neuropathology. PloS one. 2015;10(3):e0118771. doi: 10.1371/journal.pone.0118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen-Plotkin AS, Martinez-Lage M, Sleiman PM, et al. Genetic and clinical features of progranulin-associated frontotemporal lobar degeneration. Archives of neurology. 2011;68(4):488–497. doi: 10.1001/archneurol.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu YH, Sun Y, Ran H, Quinn B, Witte D, Grabowski GA. Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Molecular genetics and metabolism. 2011;102(4):436–447. doi: 10.1016/j.ymgme.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cullen V, Sardi SP, Ng J, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Annals of neurology. 2011;69(6):940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- 69.Rothaug M, Zunke F, Mazzulli JR, et al. LIMP-2 expression is critical for beta-glucocerebrosidase activity and alpha-synuclein clearance. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(43):15573–15578. doi: 10.1073/pnas.1405700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zunke F, Andresen L, Wesseler S, et al. Characterization of the complex formed by beta-glucocerebrosidase and the lysosomal integral membrane protein type-2. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(14):3791–3796. doi: 10.1073/pnas.1514005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osellame LD, Rahim AA, Hargreaves IP, et al. Mitochondria and quality control defects in a mouse model of Gaucher disease--links to Parkinson's disease. Cell metabolism. 2013;17(6):941–953. doi: 10.1016/j.cmet.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30(6):1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Rocha EM, Smith GA, Park E, et al. Sustained Systemic Glucocerebrosidase Inhibition Induces Brain alpha-Synuclein Aggregation, Microglia and Complement C1q Activation in Mice. Antioxidants & redox signaling. 2015;23(6):550–564. doi: 10.1089/ars.2015.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cleeter MW, Chau KY, Gluck C, et al. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochemistry international. 2013;62(1):1–7. doi: 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain : a journal of neurology. 2014;137(Pt 5):1304–1322. doi: 10.1093/brain/awu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sardi SP, Clarke J, Viel C, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sardi SP, Clarke J, Kinnecom C, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(29):12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rocha EM, Smith GA, Park E, et al. Glucocerebrosidase gene therapy prevents alpha-synucleinopathy of midbrain dopamine neurons. Neurobiology of disease. 2015;82:495–503. doi: 10.1016/j.nbd.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Rockenstein E, Clarke J, Viel C, et al. Glucocerebrosidase modulates cognitive and motor activities in murine models of Parkinson's disease. Human molecular genetics. 2016 doi: 10.1093/hmg/ddw124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patnaik S, Zheng W, Choi JH, et al. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. Journal of medicinal chemistry. 2012;55(12):5734–5748. doi: 10.1021/jm300063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzulli J, Zunke F, Tsunemi T, et al. Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. Journal of Neuroscience. 2016 doi: 10.1523/JNEUROSCI.0628-16.2016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park JS, Mehta P, Cooper AA, et al. Pathogenic effects of novel mutations in the P-type ATPase ATP13A2 (PARK9) causing Kufor-Rakeb syndrome, a form of early-onset parkinsonism. Human mutation. 2011;32(8):956–964. doi: 10.1002/humu.21527. [DOI] [PubMed] [Google Scholar]
- 83.Soldner F, Laganiere J, Cheng AW, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146(2):318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aflaki E, Borger DK, Moaven N, et al. A New Glucocerebrosidase Chaperone Reduces alpha-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36(28):7441–7452. doi: 10.1523/JNEUROSCI.0636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]