Abstract
N-glycosylation is a common posttranslational modification of secreted and membrane proteins, catalyzed by the two enzymatic isoforms of the oligosaccharyltransferase, STT3A and STT3B. Missense mutations are the most common mutations in inherited diseases; however, missense mutations that generate extra, non-native N-glycosylation sites have not been well characterized. Coagulation factor VIII (FVIII) contains five consensus N-glycosylation sites outside its functionally dispensable B domain. We developed a computer program that identified hemophilia A mutations in FVIII that can potentially create ectopic glycosylation sites. We determined that 18 of these ectopic sites indeed become N-glycosylated. These sites span the domains of FVIII and are primarily associated with a severe disease phenotype. Using STT3A and STT3B knockout cells, we determined that ectopic glycosylation exhibited different degrees of dependence on STT3A and STT3B. By separating the effects of ectopic N-glycosylation from those due to underlying amino acid changes, we showed that ectopic glycans promote the secretion of some mutants, but impair the secretion of others. However, ectopic glycans that enhanced secretion could not functionally replace a native N-glycan in the same domain. Secretion-deficient mutants, but not mutants with elevated secretion levels, show increased association with the endoplasmic reticulum chaperones BiP (immunoglobulin heavy chain-binding protein) and calreticulin. Though secreted to different extents, all studied mutants exhibited lower relative activity than wild-type FVIII. Our results reveal differential impacts of ectopic N-glycosylation on FVIII folding, trafficking and activity, which highlight complex disease-causing mechanisms of FVIII missense mutations. Our findings are relevant to other secreted and membrane proteins with mutations that generate ectopic N-glycans.
Introduction
N-glycosylation is one of the most common posttranslational modifications of secreted and membrane proteins. It involves the covalent attachment of carbohydrate structures to the side-chain amide group of the asparagine (N) residue within a consensus acceptor site N-X-S/T (X≠P), as the substrate protein enters the endoplasmic reticulum (ER) [1]. One of the two isoforms of the oligosaccharyl-transferase (OST) complex, STT3A or STT3B, transfers the core oligosaccharide en bloc to the N residue. The STT3A complex localizes adjacent to the SEC61 translocon and mediates glycosylation by a cotranslational scanning mechanism. In contrast, the STT3B complex acts only after translocation of the glycosylation motif is completed [2]. In the ER of eukaryotic cells, several major chaperone systems that include BiP (immunoglobulin heavy chain-binding protein, also known as GRP78) and calnexin/calreticulin (CNX/CRT) facilitate proper folding of glycoproteins [3–5]. Cycles of terminal glucose trimming and addition, mediated by glucosidase II and UDP-glucose glycoprotein glucosyltransferase, modify N-glycans that serve as critical reporters of glycoprotein folding status [4,5]. Terminally misfolded glycoproteins undergo mannose trimming, thereby committing to the ER-associated degradation (ERAD) pathway. This pathway involves dislocation of the glycoprotein from the ER lumen to the cytosol, followed by proteasomal degradation. Properly folded glycoproteins are exported from the ER, and further trimming and modification of their N-glycans occur in the Golgi, producing fully matured proteins with complex, hybrid or high-mannose glycans.
While many studies have interrogated the relationship between native glycosylation and ER protein homeostasis, few studies have examined the effects of ectopic gain of N-glycosylation on intracellular trafficking, secretion and activities of specific glycoproteins [6–11]. FVIII (factor VIII) is a large glycoprotein of 2351 amino acids (molecular mass of 293 kDa) with an A1-A2-B-A3-C1-C2 domain arrangement, from N- to C-terminus [12,13]. After synthesis in the ER of endothelial cells, FVIII is processed into a non-covalently linked heterodimer of a heavy chain (A1, A2 and B domains) and a light chain (A3, C1 and C2 domains) as it traverses the secretory pathway [14]. Outside of its functionally dispensable B domain, FVIII contains five N-glycosylation sequences (sequons), of which four sites are glycosylated: N41, N239, N1810 and N2118. N-glycosylation appears critical for proper processing of FVIII and blocking N-glycosylation inhibits FVIII secretion [15]. FVIII interacts with many chaperones and lectins, including BiP [16,17], CNX/CRT [18] and the LMAN1–MCFD2 cargo receptor complex [19,20], which mediates the export of FVIII out of the ER [21].
Hemophilia A is a debilitating and even fatal bleeding disorder that results from a broad spectrum of mutations that occur along the entire length of the FVIII gene [22,23]. Missense mutations are the most common type of mutations in hemophilia A, with over 1400 unique mutations identified to date. Although specific FVIII missense mutations correlate with defects including decreased secretion or stability [24–27] and specific functional impairment of FVIII [10,11,28–30], the mechanisms of the majority of missense mutations are poorly understood. In particular, limited knowledge exists on the effects of mutations that introduce an ectopic N-glycan into FVIII [10,11]. In this study, we systematically investigated all hemophilia A missense mutations that introduce a new N-glycan into FVIII. Our analysis revealed differing and mutation site-specific mechanisms through which ectopic N-glycosylation affects FVIII trafficking, secretion and activity.
Materials and methods
Computational identification of FVIII missense mutations that alter N-glycosylation
Missense mutations were gathered from the Center for Disease Control Hemophilia A Mutation Project (CHAMP) F8 Mutation Database and the Factor VIII Variant Database. To identify missense mutations that alter N-glycosylation sites, we created a computer program, nGlycosylChecker (freely available at https://github.com/MVesuviusC/nGlycosylChecker). This program takes the protein sequence and a text file containing information on previously identified missense mutations as input. The program compares these to output information on any sites at which N-glycosylation may be affected by a mutation (addition or removal of an N-glycosylation sequon). The solvent-accessible surface area of each mutation was determined by the method of Fraczkiewicz and Braun [31].
Plasmid construction
Individual domains of wild-type (WT) FVIII, with N-terminal signal sequences and FLAG tags, were cloned into the pED (A1 and A2) or pZ (A3 and C) expression vectors [27]. Missense mutations were introduced into the pED-FLAG-A2, pZ-FLAG-C and the pMT2-FVIII [30] plasmids using the QuikChange Site-Directed Mutagenesis II XL Kit (Agilent) according to the manufacturer’s instructions. Sanger sequencing was used to confirm mutations and rule out unintended sequence changes. Nucleotide numbering of FVIII gene mutations is based on Human Genome Variation Society recommendations, which designate the A of the ATG-translation initiation codon as nucleotide number 1. Amino acid numbering is based on convention, under which the first amino acid after signal peptide cleavage corresponds to amino acid residue number 1.
Reagents
Monoclonal antibodies against FLAG®, CRT and CNX were purchased from Sigma (St. Louis, MO) and monoclonal anti-BiP was from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-human FVIII was a gift from D. Pittman (Bayer). Tunicamycin (TM) was purchased from Sigma. The Peptide-N-Glycosidase F (PNGase F) enzyme was purchased from New England BioLabs (Ipswich, MA). Protein A/G Plus-agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture, Western blotting and immunoprecipitation
Cell culture conditions, methods of transfection, Western blotting and immunoprecipitation protocols were described recently [27]. To control for variations in transfection efficiencies, each experiment was independently performed two to three times. To block N-glycosylation, TM (2 μg/ml) was added to cell culture media and incubated for 12 h. PNGase F digestion of cell lysate was carried out in digestion buffer at 37°C for 1 h as recommended by the manufacturer. Images of chemiluminescent blot signals from horseradish peroxide-conjugated secondary antibodies (Bio-Rad, Hercules, CA) were acquired by exposure to X-ray films or through the Amersham Imager 600 (GE Healthcare, Little Chalfont, U.K.) and quantified using ImageJ software [31].
FVIII activity and antigen detection
We measured FVIII activity in conditioned media using the Coatest SP4 Factor VIII Kit (Chromogenix, Bedford, MA). Pooled WT mouse plasma was used as a standard in activity assays. We used the VisuLize FVIII Antigen Kit (Affinity Biologicals, Ancaster, Canada) to analyze the antigen levels of FVIII in cell lysates and conditioned media.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical significance was calculated using a two-tailed Student’s t-test. P-values <0.05 were considered significant for all assays.
Results
Identification of missense mutations that alter the N-glycosylation pattern in FVIII
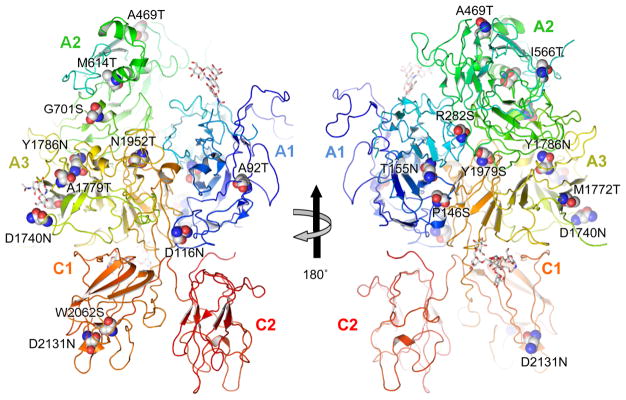
We developed the computer program nGlycosylChecker to scan a list of all known FVIII missense mutations from databases and identify those that disrupt or create an N-X-S/T (X≠P) sequence motif. Mutations that introduce new motifs can be classified into two subgroups: (a) an amino acid located two residues to the N-terminus of a serine or threonine is replaced by asparagine and (b) an amino acid located two residues in the C-terminal direction from an asparagine is replaced by serine or threonine. From two databases, we identified 19 missense mutations at 17 sites that introduce new N-glycosylation sequons (Table 1). These mutations are located in all FVIII domains, except the C2 domain (Figure 1). Five of the 19 mutations belong to the first group, while 14 mutations belong to the second group. Eleven of the mutations are classified as causing severe hemophilia A in the databases, while the rest are classified as mild, moderate or ‘not reported’. Except for I566T and M1772T, no FVIII gain-of-glycosylation mutations have been characterized previously [10,11]. Most of the ectopic glycosylation mutations occur at sites with low surface exposure (Table 1), in contrast with the four native glycosylation sites, which are surface-exposed. In addition, nGlycosylChecker identified eight mutations that abolish an N-glycosylation consensus site. The S241C mutation eliminates the second native glycosylation site in the A1 domain. The other seven mutations all disrupt a previously characterized consensus sequence within the A2 domain, at N582, that is not natively glycosylated in WT FVIII [27].
Table 1.
Missense mutations of FVIII generating potential new N-glycosylation sites in patients
| Mutation | Mutation effect | New sequon | Exon | Domain | Reported severity1 | Surface exposure2 | Domain interface |
|---|---|---|---|---|---|---|---|
| c.331G>A | A92T | NMT | 3 | A1 | Severe | 0% (0%) | |
| c.403G>A | D116N | NQT | 4 | A1 | Severe | 1.5% | A1-C1/C2 |
| c.493C>T | P146S | NGS | 4 | A1 | Severe | 6.7% (2.1%) | A1-A3 |
| c.521C>A | T155N | NYS | 4 | A1 | Severe | 0.8% | A1-A3 |
| c.901C>A | R282S | NHS | 7 | A1 | Moderate | 10% (48%) | A1-A2 |
| c.1462G>A | A469T | NQT | 10 | A2 | Not reported | 0.2% (1.6%) | |
| c.1462G>T | A469S | NQS | 10 | A2 | Moderate | 0.3% (1.6%) | |
| c.1754T>C | I566T | NQT | 12 | A2 | Severe | 62% (44%) | |
| c.1898T>C | M614T | NIT | 12 | A2 | Mild | 0.4% (8%) | |
| c.2158G>A | G701S | NRS | 14 | A2 | Severe | 93% (18.7%) | A2-A3 |
| c.5275G>A | D1740N | NGS | 15 | A3 | Moderate | 77% | |
| c.5372T>C | M1772T | NIT | 15 | A3 | Severe | 31% (26%) | |
| c.5392G>A | A1779T | NQT | 16 | A3 | Severe | 0% (0.8%) | |
| c.5392G>T | A1779S | NQS | 16 | A3 | Moderate | 0.4% (1.3%) | |
| c.5413T>A | Y1786N | NSS | 16 | A3 | Severe | 6.7% | A2-A3 |
| c.5912A>C | N1952T | NET | 18 | A3 | Severe | 0% (15%) | A1/A2-A3 |
| c.5993A>C | Y1979S | NLS | 18 | A3 | Mild | 27% (10%) | A1-A3 |
| c.6242G>C | W2062S | NAS | 21 | C1 | Severe | 12% (8.5%) | |
| c.6448G>A | D2131N | NSS | 23 | C1 | Mild | 72% |
Severity of phenotype is defined by FVIII activity levels relative to normal controls. Severe, <1%; moderate, 1–5%; mild, 5–49%.
Surface exposure refers to SASA of side chain of mutated residue in B-domain-deleted FVIII (in parentheses: SASA of N residue if mutation occurs at the third position of the sequon). SASA shown as a percentage relative to the side chain SASA in a random coil (G-X-G tripeptide).
Figure 1. Overview of mutations in B-domain-deleted FVIII structure.
Individual FVIII domains are labeled. Mutated residues that give rise to potential ectopic N-glycosylation sequons are shown as spheres and labeled. Only oligosaccharides (GlcNAc2Man5) present in the crystallized molecule are shown in stick representation. Structural co-ordinates taken from PDB ID 2R7E.
Glycosylation status of hemophilia A missense mutations that create new N-glycosylation consensus sequences
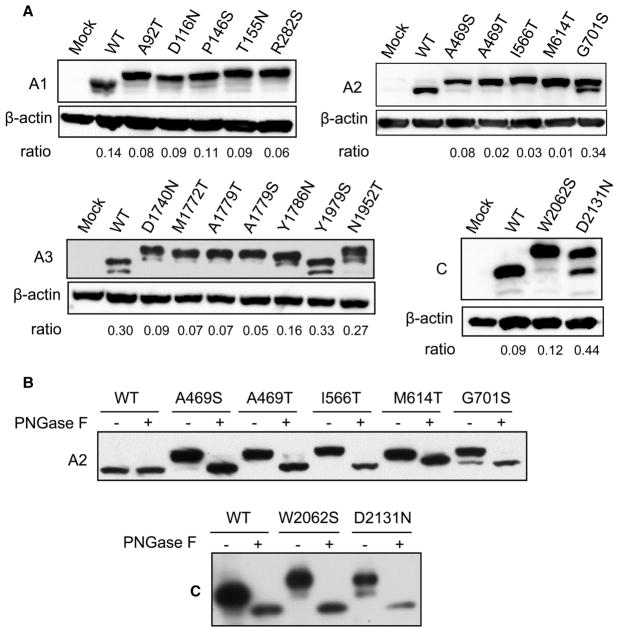
We expressed individual, N-terminally FLAG-tagged domains of FVIII with missense mutations to study the consequences of these mutations for N-glycosylation. Single-domain constructs allow us to readily detect size changes caused by the addition of as little as one glycan. A bioinformatic analysis by Petrescu et al. [32] found that the efficiency of N-glycosylation at a specific sequon is modulated by the local amino acid sequence, but not by more distal sequences. Therefore, N-glycosylation detected in the isolated domains should report accurately on glycosylation patterns found in full-length FVIII. All of the mutants, except Y1979S, exhibited bands of higher apparent molecular mass than WT domains without mutations (Figure 2A). For several mutants, varying levels of a band with similar mobility as the WT domains were also present, suggesting that these mutant domains were incompletely glycosylated (Figure 2A). Incomplete glycosylation is not due to saturation of the translocation/N-glycosylation machinery by the overexpressed proteins (Supplementary Figure S1). To confirm that the slower migration rate of the proteins is due to N-glycosylation, we treated cell lysates of A2 and C domain mutants with PNGase F, which cleaves all N-glycans from proteins. The natively unglycosylated WT A2 domain was unchanged after PNGase treatment, while the WT C domain migrated with a lower apparent molecular mass due to removal of the glycan from its native glycosylation site at residue N2118 (Figure 2B). The sizes of all mutants shifted to those of the corresponding deglycosylated WT species, indicating that slower migration of these mutants (before PNGase F treatment) is due to ectopic N-glycosylation (Figure 2B).
Figure 2.
Hemophilia A-linked missense mutations that create new N-glycosylation sequons typically lead to the addition of an ectopic glycan.(A) 293T cells were transfected with constructs that express FLAG-tagged mutants of individual domains (A1, A2, A3 and C) of FVIII. Thirty-six hours after transfection, cells were lysed and equal amounts of cell extracts were immunoblotted with anti-FLAG and anti-β-actin antibodies. Densitometric ratios of the lower band vs. upper band are shown. For A1 mutants and the N1952T mutation of the A3 domain with multiple lower bands, densitometry values of the first band below the upper band were used. (B) 293T cells were transfected with constructs that express FLAG-tagged mutants of A2 and C domains of FVIII. Thirty-six hours after transfection, cell lysates were digested with or without PNGase F and subsequently immunoblotted with an anti-FLAG antibody.
STT3A and STT3B differentially glycosylate missense mutants at ectopic sites
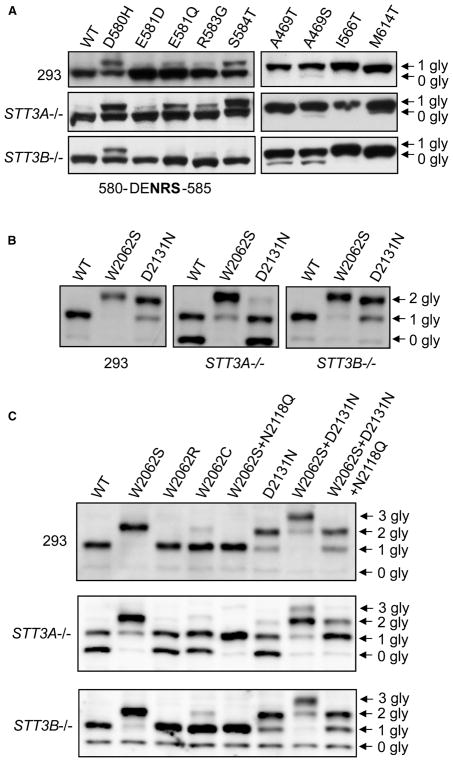
We used STT3A and STT3B knockout HEK293 (human embryonic kidney cells) cell lines [33] to explore the mechanism of ectopic glycosylation of FVIII mutants. We first tested several previously reported missense mutations located near an A2 sequon that is not natively glycosylated in the WT protein [27]. These mutations lead to partial glycosylation of the A2 domain [27]. In STT3A−/− cells, the glycosylation patterns of these A2 mutants were similar to those of WT cells. However, in STT3B−/− cells, ectopic glycosylated bands were diminished or absent (Figure 3A), suggesting that glycosylation of these A2 domain mutants is primarily mediated by STT3B. We next tested ectopic glycosylation mutants located in the A2 and C domains (Supplementary Figure S2). Glycosylation patterns in STT3A−/− cells were similar to those in WT cells, except for the appearance of a minor non-glycosylated band for the M614T mutant. Levels of non-glycosylated A469T and A469S A2 domain mutants were higher in STT3B−/− cells than in WT cells (Figure 3A). Thus, STT3B exclusively glycosylates a subpopulation of the A469T and A469S mutant species. In contrast, the I566T and M614T sites can be glycosylated by either STT3A or STT3B.
Figure 3. STT3A and STT3B contribute differentially to ectopic N-glycosylation of different FVIII mutants.
N-glycosylation patterns of (A) previously reported mutants near the unused A2 domain sequon (sequence of the 310-helical turn containing the sequon is shown below) and A2 domain mutants with ectopic N-glycosylation; (B) C domain single mutants; (C) combinations of single, double and triple mutants of the C domain. WT, STT3A knockout and STT3B knockout cells were transiently transfected with constructs of the indicated mutants. Thirty-six hours after transfection, cells were lysed and equal amounts of cell extracts were immunoblotted with an anti-FLAG antibody. Arrows denote bands with corresponding numbers of N-glycans (gly).
The C1 domain is natively N-glycosylated at N2118. In contrast with A2 mutants, glycosylation patterns of the WT C domain and two C domain mutants, W2062S and D2131N, were very similar in WT and STT3B−/−cells (Figure 3B). However, more than half of the WT C domain and the D2131N C domain were non-glycosylated in STT3A−/− cells, while only a small fraction of the W2062S mutant was present as a singly glycosylated species. These results suggest that both the native (WT) sequon and the ectopic sequon in the D2131N mutant are primarily glycosylated by the STT3A complex, while the W2062S site can be glycosylated by either the STT3A or STT3B complex. Furthermore, efficient glycosylation of the W2062S mutant suggests that introduction of an additional N-glycan at N2060 causes the WT sequon (N2118) to become a better substrate for STT3B.
To further investigate the mechanism of this phenomenon, we first tested two other missense mutations at W2062 (W2062R and W2062C). The glycosylation patterns of both mutants were indistinguishable from that of the WT C domain in WT, STT3A−/− and STT3B−/− cells (Figure 3C). This suggests that amino acid changes at residue 2062 do not by themselves affect glycosylation of the native site at N2118. Next, we studied two double mutations (W2062S + N2118Q or W2062S + D2131N) and a triple mutation (W2062S + D2131N + N2118Q) of the C domain. Abolishing N-glycosylation at N2118 (N2118Q) has no effect on glycosylation of the W2062S sequon. Introduction of the W2062S mutation into the D2131N mutant led to the loss of the three-glycan form in STT3A−/− cells. When the WT sequon was eliminated, half of this triple mutant lost one N-glycan in STT3A−/− cells, which is similar to that of the WT C domain (Figure 3C). Therefore, the W2062S mutation not only introduces an ectopic glycosylation site but also affects how STT3B accesses and acts upon the native sequon (N2118), though not how STT3B affects another ectopic sequon (N2131).
Varying effects of additional N-glycans on FVIII domain secretion
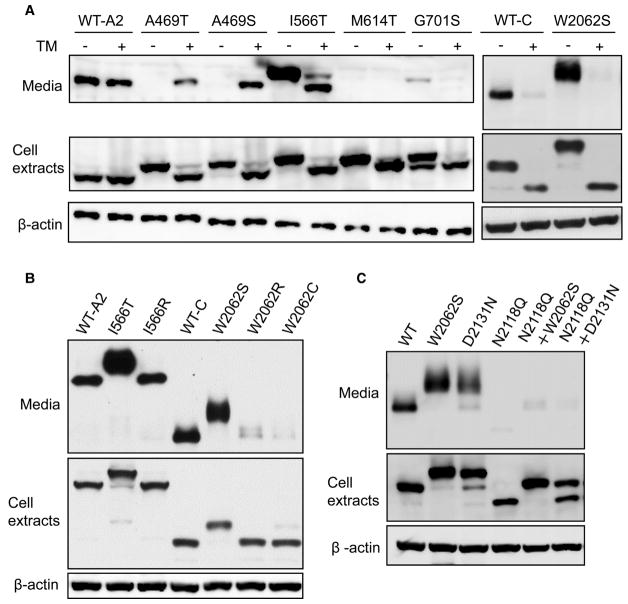
When expressed as individual domains, the A2, C1 and C (C1 + C2) domains were efficiently secreted into conditioned media (Supplementary Figure S3A and Figure 4). Therefore, we investigated how missense mutants in the A2 and C domains affect their secretion. After transfection into 293T cells, I566T and W2062S mutants were detected in conditioned media at levels even higher than the WT domains (Figure 4A). Other mutants were either detected at levels much lower than the WT (A469S and G701S), or were undetectable (A469T and M614T) (Figure 4A and Supplementary Figure S3B). Thus, A2 and C domain mutants with ectopic N-glycosylation exhibit a wide range of mutation-specific secretion phenotypes.
Figure 4. The effects of ectopic N-glycan on FVIII domain secretion.
(A) Secretion of A2 and C domain mutants in the presence or absence of the N-glycosylation inhibitor TM. (B) Mutations at I566 and W2062 that do not lead to ectopic N-glycosylation do not enhance secretion of the A2 or C domain. (C) Ectopic N-glycans caused by W2062S and D2131N mutations do not replace the function of the native N-glycan at N2118. For all experiments, 293T cells were transiently transfected with constructs that express WT A2 and C domains or the indicated mutants. Twenty-four hours after transfection, cells were cultured in fresh medium with or without TM (2 μg/ml) and incubated for 12 h. Conditioned media were collected and immunoprecipitated with a mouse monoclonal anti-FLAG antibody and analyzed by immunoblotting with a rabbit antibody. Cells were lysed and equal amounts of cell extracts were immunoblotted with anti-FLAG and anti-β-actin antibodies.
To distinguish the impact of the ectopic N-glycan from that of the underlying amino acid change caused by a given mutation, we treated cells that expressed WT or mutants with TM to block the initiation of N-glycosylation in the ER. TM treatment markedly decreased secretion of I566T and G701S mutant A2 domains (Figure 4A). In contrast, blocking ectopic glycosylation boosted the secretion of A469S and A469T mutants (Figure 4A). TM treatment had no effect on the M614T mutant. Application of TM to block N-glycosylation of the C domain or its W2062S mutant resulted in non-glycosylated forms of both species. Secretion of the non-glycosylated C domain was barely detectable (Figure 4A).
To further distinguish effects of ectopic N-glycans from amino acid changes, we studied hemophilia mutations of I566 and W2062 that do not create new N-glycosylation sequons (Figure 4B). The I566R mutant of the A2 domain was secreted at a level similar to the WT-A2, rather than at the elevated level of the I566T mutant. W2062R and W2062C mutants of the C domain were secreted at markedly decreased levels compared with WT-C. Therefore, enhanced secretion of the I566T and W2062S A2 domains was not caused by changes of the amino acids but rather by ectopic glycosylation.
The N2118Q mutation, which eliminates the native N-glycan of the C domain, also dramatically decreased secretion (Figure 4C), consistent with suppression of C domain secretion induced by TM treatment (Figure 4A). These results indicate that the N2118 glycan is critical for secretion of the isolated C domain. The W2062S mutation has a net positive effect on C domain secretion (Figure 4C). However, introduction of this mutation into the N2118Q mutant C domain (e.g. an N2118Q + W2062S double mutant) did not rescue the secretion defect induced by the N2118Q mutation alone (Figure 4C). The D2131N mutant is also secretion-competent; however, elimination of the native glycan in this mutant (N2118Q + D2131N double mutant) abolished secretion of the C domain (Figure 4C). Therefore, the ectopic N-glycan resulting from the W2062S or D2131N mutation cannot functionally replace the role played by native glycosylation of N2118 in C-domain secretion.
Increased interactions between FVIII mutants and ER chaperones
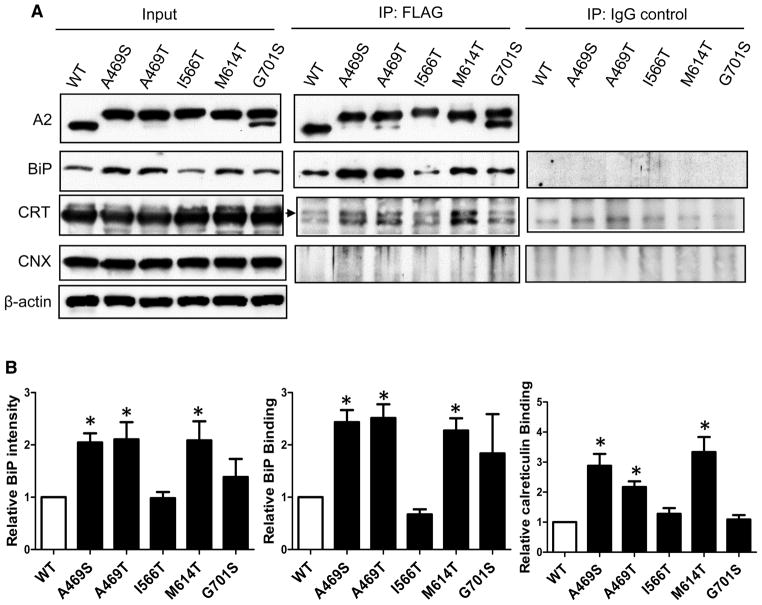
To better understand how ectopic glycosylation mutants influence secretion, we investigated how A2 mutants with differing secretion levels interact with ER chaperones. Expression of all mutants other than I566T increased the intracellular levels of BiP relative to expression of the WT A2 domain. However, expression of mutant A2 domains had no effect on protein levels of CRT or CNX in cell lysates, relative to WT A2 (Figure 5A). Next, we carried out immunoprecipitation to probe the extent to which these ER chaperones associate with the A2 mutants. With the exception of I566T, BiP and CRT co-immunoprecipitated more strongly with A2 mutants than with WT A2 (Figure 5). Overall, interactions of the A2 mutants with BiP and CRT correlated inversely with their secretion levels. Unlike BiP or CRT, we detected no obvious interactions of WT or A2 domain mutants with CNX.
Figure 5. Expression of FVIII missense mutants induces BiP and increases interactions with BiP and CRT.
(A) Thirty-six hours after transfection of the indicated mutants, expression levels in input cell extracts were analyzed by immunoblotting with the indicated antibodies. Interactions between A2 mutants and ER chaperones were analyzed by co-immunoprecipitation (IP). Cell extracts were immunoprecipitated with a mouse monoclonal anti-FLAG antibody or normal mouse IgG. The immunoprecipitates from anti-FLAG IP and IgG control IP were analyzed by immunoblotting with the indicated antibodies. CRT: calreticulin; CNX: calnexin. Arrow points to the CRT band in the IP data. The lower band is non-specific as it also appears in IgG controls. (B) The amounts of BiP in cell lysates, BiP and CRT that co-immunoprecipitated with A2 domain mutants were densitometrically quantified and expressed as fold changes relative to WT. Data are mean ± SEM, n = 3, *P < 0.05.
Secretion and activity levels of full-length FVIII mutants
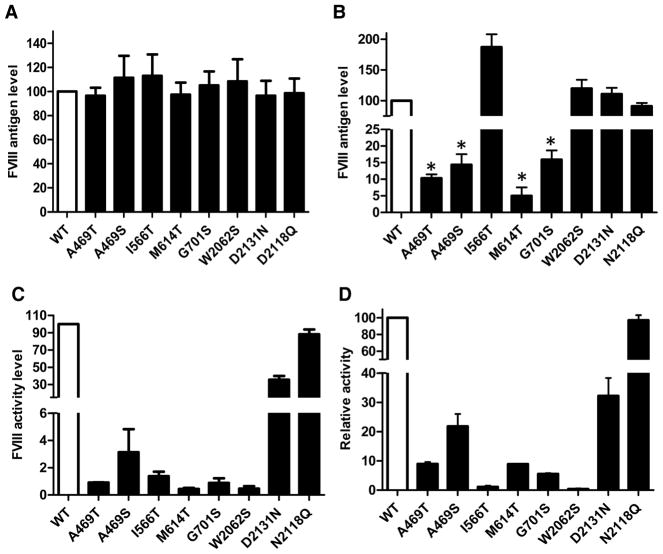
We introduced missense mutations that were first tested in individual A2 or C domains into a full-length FVIII expression plasmid. After transfection into 293T cells, there were no significant differences in protein levels between the FVIII mutants and WT FVIII in cell extracts (Figure 6A). In contrast, the level of I566T FVIII mutant in conditioned media was nearly two-fold greater than the level of WT FVIII. Levels of W2062S and D2131N FVIII mutants in conditioned media were comparable to those of WT FVIII. Antigen levels of all other FVIII mutants in conditioned media were less than 20% of the WT FVIII level (Figure 6B). Therefore, secretion levels of these FVIII mutants were largely consistent with the results obtained from individual domains, with the exception of the W2062S mutant, which was not secreted at a level significantly higher than WT. Surprisingly, secretion of the N2118Q mutant was comparable to that of WT FVIII. This contrasted strongly to the greatly reduced secretion of the isolated N2118Q C domain (Figure 4C).
Figure 6. Levels of intracellular and secreted full-length FVIII mutants and their relative activities.
(A) and (B) Forty-eight hours after transfection into 293T cells with constructs of WT and the indicated mutants of full-length FVIII, cell extracts (A) and conditioned media (B) were collected. FVIII antigen levels measured by ELISA were plotted as percentages of WT FVIII (data are mean ± SEM, n = 3, *P < 0.05). (C) Forty-eight hours after transfection, FVIII activity levels in conditioned media were analyzed by a chromogenic assay. Average activities of mutants were plotted as percentages of WT FVIII activity (n = 3). Activities of all the mutants were significantly reduced compared with WT FVIII (P < 0.05). (D) Relative activity for a given mutant was calculated as the ratio of the activity level and the antigen level in conditioned media. Relative activities are shown as percentages of the WT FVIII relative activity. Relative activities of all mutants were significantly reduced compared with WT FVIII (P < 0.05).
We next measured the activity of FVIII mutants in conditioned media of transiently transfected 293T cells. With the exception of M614T, the activity levels of FVIII mutants were consistent with the mutant phenotypes in hemophilia patients (Figure 6C). M614T reportedly leads to mild phenotype of hemophilia, but its activity level was below 1% in our study. The D2131N FVIII mutant had an activity of ~30% of WT. The activity level of the N2118Q mutant FVIII was comparable to that of WT. By normalizing the activities by the corresponding antigen levels, we determined relative activities for each mutant secreted into conditioned media. Relative activities of all mutants, with the exception of N2118Q, were less than those of WT FVIII. The A469S and D2131N exhibited 20–30% of WT activity, while all other FVIII mutants showed less than 10% of WT activity. Of note, two mutants with higher secretion levels, I566T and W2062S, actually showed the lowest relative activities (Figure 6D).
Discussion
In the present study, we systematically explored hemophilia A missense mutations that potentially lead to ectopic N-glycosylation of FVIII. Our computer program, nGlycosylChecker, is a useful tool for detection of mutations that alter N-glycosylation sites in other secreted and membrane proteins, including other coagulation factors [8,34]. We found that all mutations, except Y1979S, resulted in additional N-glycan, though incomplete N-glycosylation was observed for several mutants. The Y1979S mutation creates a poor N-X-S sequon that is immediately followed by a proline, which is strongly associated with sequon skipping [32,35]. In contrast with the mostly mild-to-moderate phenotypes generally associated with missense mutations in FVIII, 11 of the 19 ectopic glycosylation mutations lead to severe hemophilia A. This suggests that ectopic N-glycosylation has more negative effect on the level and activity of FVIII in patients than do single amino acid changes alone. N-glycosylation patterns observed in isolated domains likely also occur in the full-length FVIII mutants. We recently showed that N-glycosylation patterns of isolated A2 domain mutants, including I566T, D580H, N582D and S584T, are identical with those in full-length FVIII mutants [27]. In addition, incomplete glycosylation of N1810 in the isolated A3 domain is also observed in recombinant FVIII [36].
Our recent studies found that certain mutations lead to partial glycosylation of an unused sequon at N582 located in a highly stable 310-helical turn of the A2 domain [27]. Here, we further demonstrated that glycosylation of these mutants is primarily carried out by STT3B, while mutations that add ectopic glycans are mostly STT3A-dependent. These results are likely relevant to full-length FVIII. NXT/S sites in nascent polypeptides become accessible to the STT3A active site when they are ~65 residues from the peptidyltransferase site on the ribosome [37,38]. Therefore, N- or C-terminal domains of FVIII will have no impact upon cotranslational glycosylation by STT3A. In addition, unless a glycosylation site is located within the C-terminal 50 residues of a protein domain, it is not expected to be STT3B-dependent in the context of the protein domain, but STT3A-dependent within the context of the whole protein [39]. The partial STT3B dependence of A2 domain sites is not explained by an extreme C-terminal location. The STT3B dependence of mutants like S584T is more likely explained by a protein-folding defect that allows posttranslational glycosylation of the cryptic sequon [40]. On the other hand, with the exception of the D2131N mutant, the ectopic glycosylation events that we investigated in our study are primarily mediated by STT3A. Interestingly, an ectopic glycan generated by W2062S enhances STT3B-mediated glycosylation of an endogenous sequon 56 amino acid residues to the C-terminus (N2118), but not another mutant sequon just 13 amino acid residues away (generated by D2131N). These effects may be caused by a localized structural perturbation due to the ectopic glycosylation of a normally buried side chain, rendering the WT sequon more accessible to STT3B (Supplementary Figure S1). We also note that mutations preceding the native N1810 sequon in the A3 domain appear to increase glycosylation efficiency of N1810 (Figure 1A), which could be the result of increased access to STT3B due to localized structural perturbation caused by the ectopic glycan. Interdependency of distant glycosylation sites has been observed in β-glucuronidase [41]. However, to our knowledge, our study is the first report that the addition of one glycan changes the glycosylating enzyme preference or compatibility of a distant glycosylation site.
Our results show that TM treatment decreased secretion of surface exposed I566T and G701S A2 domain mutants, suggesting that detrimental effects of missense mutations are corrected by the ectopic N-glycosylation. Ectopic glycosylation likely also enhanced secretion of the W2062S C domain mutant. In contrast, blocking N-glycosylation restores secretion of A469S and A469T A2 domain mutants to the WT level, suggesting that the ectopic N-glycans destabilize these mutants and perturb proper protein folding, or act directly as non-native recognition signals for ER chaperones. Both mechanisms may be relevant, since both BiP (which binds to disordered protein segments) and CRT (which binds primarily to glycans) associate more strongly with these mutants than with WT-A2. In the case of the M614T mutant, the amino acid substitution alone appears sufficient to completely disrupt folding in the ER. A469 and M614 sequons are not surface-exposed, and their glycosylation may lead to severe perturbation of the A2 domain structure. Full-length FVIII with I566T, W2062S and D2131N mutations secrets at levels that are comparable or significantly higher than WT FVIII, suggesting that patients carrying these mutations have cross-reacting material-positive hemophilia A. Increased secretion of the I566T mutant is in agreement with the reported FVIII antigen level (154–200% of normal) for a patient with this mutation [10,11]. All other mutants are secreted into conditioned media at less than 20% of the level of WT FVIII. These results suggest that the effects of these mutations on full-length FVIII may be primarily attributed to their impact upon folding and secretability of the individual domains, although additional perturbation of adjacent domains cannot be ruled out, particularly for ectopic glycans that are located at domain interfaces [42,43]. Variations in mutant FVIII secretion suggest that the effects of ectopic N-glycans depend on their location, solvent accessibility of the asparagine side chain and the underlying amino acid change responsible for the ectopic glycosylation site.
Previous studies determined that elevated binding of BiP to FVIII may contribute to the lower secretion of FVIII compared with FV [16,17]. We found that expression of poorly secreted A2 domain mutants results in higher BiP expression, potentially due to induction of the unfolded protein response pathway. In turn, such mutants also interact with BiP more extensively than the WT A2 domain. CRT interactions with secretion defective mutants are also increased markedly, suggesting that the CRT cycle is a major quality control system for these hyperglycosylated mutants. These results are consistent with our recent report that partially glycosylated FVIII A2 mutants robustly engage the CRT cycle [27]. However, A2 mutants that are secretion competent, including I566T and to a lesser extent G701S, do not significantly induce BiP and do not associate more extensively with CRT. Thus, the secretion and misfolding severity of A2 mutants correlate inversely with the association of ER chaperones. These results suggest that non-secreted FVIII mutants are more strongly retained in the ER compared with secretion-competent FVIII variants. We anticipate that such ER-retained FVIII mutants are eventually degraded by the ERAD pathway.
Curiously, the N2118Q mutation, which abolishes secretion of the isolated C domain, has little effect on full-length FVIII secretion and activity. The latter observation is surprising, given that the N2118 sequon is conserved from mammals to birds, reptiles and fish (Supplementary Figure S4). Mutation of another highly conserved sequon, N239, reportedly reduced FVIII secretion and activity, while mutations of less conserved N41 and N1810 sequons had only mild effects [44]. Evolutionary conservation suggests that the N2118 glycan may play a yet undefined role in FVIII biology. Analysis of the FVIII structure potentially explains the observed difference between the secretion of the isolated N2118Q mutant C domain and the corresponding full-length protein. Although the N2118 glycan helps stabilize the packing between the C1 and A3 domains by interacting with both, these domains also engage in direct interactions with each other that likely remain in the absence of the glycan (Supplementary Figure S5A). On the other hand, this glycan also helps stabilize the orientations of two loops (residues 2110–2125 and 2135–2142) and a connecting segment (residues 2017–2022) from the C1 domain itself (Supplementary Figure S5B). Without the rest of FVIII and the glycan, these loops may present as particularly labile structure and subject the N2118Q C domain mutant to retention by the quality control machinery. Ectopic N-glycans introduced by W2062S and D2131N mutations would not be in a position to replace the stabilizing role of the native N-glycan at N2118.
All mutations studied decreased FVIII activity. Impaired FVIII function could be due to overall perturbation of FVIII structure or due to specific defects in FVIII functions. Removal of surface glycans by N-glycanase digestion increases the activities of FVIII I566T and M1772T mutants [10,11], supporting the notion that ectopic N-glycans can directly inhibit FVIII activity. The I566T mutation is located just outside an FIXa (activated factor IX) interaction site (558–565) [45,46] and results in decreased FIXa binding [11]. Therefore, even though the ectopic glycan promotes the secretion of I566T mutant, the bulky oligosaccharide chain may interfere with its function. The C1 domain is involved in FIXa binding [42,47,48] and phospholipid binding [42,49–51]. Although not located in currently identified functional regions, the W2062S and D2131N mutations could disturb the C1 domain structure and disrupt FVIII interaction with FIXa and/or phospholipids, leading to reduced activity of the secreted FVIII mutants. Several mutations are localized at interfaces between different domains of FVIII (Table 1). Ectopic N-glycans and/or the underlying amino acid changes could cause additional disruption of domain packing and alter the stability of FVIII [24,26].
In conclusion, we uncovered that a range of hemophilia mutations in FVIII result in ectopic N-glycosylation. The different ectopic glycans have varied effects on FVIII folding, stability, trafficking/secretion and activity, overall resulting in deficient forms of FVIII compared with the WT protein. These mutations all have biochemically similar local consequences, primarily the addition of a glycan to a normally unglycosylated position. However, the different underlying amino acid changes and different structural contexts of glycosylation mediate very different functional effects, and highlight the complex disease-causing mechanisms of FVIII missense mutations. Our results are also relevant to other secreted and membrane proteins with mutations that generate ectopic N-glycans.
Supplementary Material
Acknowledgments
Funding
The present study was supported by the National Institutes of Health [R01HL094505 to B.Z. and R01GM043768 to R.G.], the National Natural Science Foundation of China [81428002], the Natural Science Foundation of Xinjiang Uygur Autonomous Region [2016D01A020] and the 1000 Talent Plan (to M.Z.).
We thank Joy Nyaanga and Shixuan Wang for assistance in mutant plasmid construction, and Jason Zhang for software assistance.
Abbreviations
- BiP
immunoglobulin heavy chain binding protein
- CNX
calnexin
- CRT
calreticulin
- ER
endoplasmic reticulum
- ELISA
enzyme-linked immunosorbent assay
- ERAD
ER-associated degradation
- FIXa
activated factor IX
- FVIII
factor VIII
- IP
immunoprecipitation
- PNGase F
Peptide-N-Glycosidase F
- SEM
standard error of the mean
- TM
tunicamycin
- WT
wild type
Footnotes
Author Contribution
W.W., M.Z. and B.Z. conceived and designed the research, and wrote the manuscript. M.V.C. wrote the software code. W.W. and M.Z. performed experiments and analyzed data. R.G. provided knockout cell lines and analyzed data. S.M. performed protein structural analysis. R.G., S.M., M.Z., X.Z. and R.Y. analyzed data and revised the manuscript.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. https://doi.org/10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136:272–283. doi: 10.1016/j.cell.2008.11.047. https://doi.org/10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caramelo JJ, Parodi AJ. A sweet code for glycoprotein folding. FEBS Lett. 2015;589:3379–3387. doi: 10.1016/j.febslet.2015.07.021. https://doi.org/10.1016/j.febslet.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Cherepanova N, Shrimal S, Gilmore R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol. 2016;41:57–65. doi: 10.1016/j.ceb.2016.03.021. https://doi.org/10.1016/j.ceb.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin Cell Dev Biol. 2015;41:79–89. doi: 10.1016/j.semcdb.2014.12.001. https://doi.org/10.1016/j.semcdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolaou N, Margadant C, Kevelam SH, Lilien MR, Oosterveld MJ, Kreft M, et al. Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J Clin Invest. 2012;122:4375–4387. doi: 10.1172/JCI64100. https://doi.org/10.1172/JCI64100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prada V, Passalacqua M, Bono M, Luzzi P, Scazzola S, Nobbio LA, et al. Gain of glycosylation: a new pathomechanism of myelin protein zero mutations. Ann Neurol. 2012;71:427–431. doi: 10.1002/ana.22695. https://doi.org/10.1002/ana.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, et al. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet. 2005;37:692–700. doi: 10.1038/ng1581. https://doi.org/10.1038/ng1581. [DOI] [PubMed] [Google Scholar]
- 9.Waite A, De Rosa MC, Brancaccio A, Blake DJ. A gain-of-glycosylation mutation associated with myoclonus-dystonia syndrome affects trafficking and processing of mouse ε-sarcoglycan in the late secretory pathway. Hum Mutat. 2011;32:1246–1258. doi: 10.1002/humu.21561. https://doi.org/10.1002/humu.21561. [DOI] [PubMed] [Google Scholar]
- 10.Aly AM, Higuchi M, Kasper CK, Kazazian HH, Jr, Antonarakis SE, Hoyer LW. Hemophilia A due to mutations that create new N-glycosylation sites. Proc Natl Acad Sci USA. 1992;89:4933–4937. doi: 10.1073/pnas.89.11.4933. https://doi.org/10.1073/pnas.89.11.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amano K, Sarkar R, Pemberton S, Kemball-Cook G, Kazazian HH, Jr, Kaufman RJ. The molecular basis for cross-reacting material-positive hemophilia A due to missense mutations within the A2-domain of factor VIII. Blood. 1998;91:538–548. [PubMed] [Google Scholar]
- 12.Toole JJ, Knopf JL, Wozney JM, Sultzman LA, Buecker JL, Pittman DD, et al. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984;312:342–347. doi: 10.1038/312342a0. https://doi.org/10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- 13.Vehar GA, Keyt B, Eaton D, Rodriguez H, O’Brien DP, Rotblat F, et al. Structure of human factor VIII. Nature. 1984;312:337–342. doi: 10.1038/312337a0. https://doi.org/10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman RJ, Fay PJ, Popolo L, Ortel TL. Factor V and Factor VIII. In: Marder VJ, Aird WC, Bennet JS, Schulman S, White GC, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 6. Lippincott William & Wilkins; Philadelphia: 2013. pp. 179–196. [Google Scholar]
- 15.Dorner AJ, Bole DG, Kaufman RJ. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. https://doi.org/10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquette KA, Pittman DD, Kaufman RJ. A 110-amino acid region within the A1-domain of coagulation factor VIII inhibits secretion from mammalian cells. J Biol Chem. 1995;270:10297–10303. doi: 10.1074/jbc.270.17.10297. https://doi.org/10.1074/jbc.270.17.10297. [DOI] [PubMed] [Google Scholar]
- 17.Swaroop M, Moussalli M, Pipe SW, Kaufman RJ. Mutagenesis of a potential immunoglobulin-binding protein-binding site enhances secretion of coagulation factor VIII. J Biol Chem. 1997;272:24121–24124. doi: 10.1074/jbc.272.39.24121. https://doi.org/10.1074/jbc.272.39.24121. [DOI] [PubMed] [Google Scholar]
- 18.Pipe SW, Morris JA, Shah J, Kaufman RJ. Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J Biol Chem. 1998;273:8537–8544. doi: 10.1074/jbc.273.14.8537. https://doi.org/10.1074/jbc.273.14.8537. [DOI] [PubMed] [Google Scholar]
- 19.Zheng C, Liu HH, Yuan S, Zhou J, Zhang B. Molecular basis of LMAN1 in coordinating LMAN1-MCFD2 cargo receptor formation and ER-to-Golgi transport of FV/FVIII. Blood. 2010;116:5698–5706. doi: 10.1182/blood-2010-04-278325. https://doi.org/10.1182/blood-2010-04-278325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C, Page RC, Das V, Nix JC, Wigren E, Misra S, et al. Structural characterization of carbohydrate binding by LMAN1 protein provides new insight into the endoplasmic reticulum export of factors V (FV) and VIII (FVIII) J Biol Chem. 2013;288:20499–20509. doi: 10.1074/jbc.M113.461434. https://doi.org/10.1074/jbc.M113.461434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Kaufman RJ, Ginsburg D. LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J Biol Chem. 2005;280:25881–25886. doi: 10.1074/jbc.M502160200. https://doi.org/10.1074/jbc.M502160200. [DOI] [PubMed] [Google Scholar]
- 22.Mannucci PM, Tuddenham EG. The hemophilias — from royal genes to gene therapy. N Engl J Med. 2001;344:1773–1779. doi: 10.1056/NEJM200106073442307. https://doi.org/10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 23.Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187–197. doi: 10.1016/S0140-6736(15)01123-X. https://doi.org/10.1016/S0140-6736(15)01123-X. [DOI] [PubMed] [Google Scholar]
- 24.Hakeos WH, Miao H, Sirachainan N, Kemball-Cook G, Saenko EL, Kaufman RJ, et al. Hemophilia A mutations within the factor VIII A2-A3 subunit interface destabilize factor VIIIa and cause one-stage/two-stage activity discrepancy. Thromb Haemost. 2002;88:781–787. [PubMed] [Google Scholar]
- 25.Pipe SW, Kaufman RJ. Factor VIII C2 domain missense mutations exhibit defective trafficking of biologically functional proteins. J Biol Chem. 1996;271:25671–25676. doi: 10.1074/jbc.271.41.25671. https://doi.org/10.1074/jbc.271.41.25671. [DOI] [PubMed] [Google Scholar]
- 26.Summers RJ, Meeks SL, Healey JF, Brown HC, Parker ET, Kempton CL, et al. Factor VIII A3 domain substitution N1922S results in hemophilia A due to domain-specific misfolding and hyposecretion of functional protein. Blood. 2011;117:3190–3198. doi: 10.1182/blood-2010-09-307074. https://doi.org/10.1182/blood-2010-09-307074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei W, Zheng C, Zhu M, Zhu X, Yang R, Misra S, et al. Missense mutations near the N-glycosylation site of the A2 domain lead to various intracellular trafficking defects in coagulation factor VIII. Sci Rep. 2017;7:45033. doi: 10.1038/srep45033. https://doi.org/10.1038/srep45033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert GE, Novakovic VA, Kaufman RJ, Miao H, Pipe SW. Conservative mutations in the C2 domains of factor VIII and factor V alter phospholipid binding and cofactor activity. Blood. 2012;120:1923–1932. doi: 10.1182/blood-2012-01-408245. https://doi.org/10.1182/blood-2012-01-408245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacquemin M, Lavend’homme R, Benhida A, Vanzieleghem B, d’Oiron R, Lavergne JM, et al. A novel cause of mild/moderate hemophilia A: mutations scattered in the factor VIII C1 domain reduce factor VIII binding to von Willebrand factor. Blood. 2000;96:958–965. [PubMed] [Google Scholar]
- 30.Pipe SW, Eickhorst AN, McKinley SH, Saenko EL, Kaufman RJ. Mild hemophilia A caused by increased rate of factor VIII A2 subunit dissociation: evidence for nonproteolytic inactivation of factor VIIIa in vivo. Blood. 1999;93:176–183. [PubMed] [Google Scholar]
- 31.Fraczkiewicz R, Braun W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J Comput Chem. 1998;19:319–333. https://doi.org/10.1002/(SICI)1096-987X(199802)19:3<319::AID-JCC6>3.0.CO;2-W. [Google Scholar]
- 32.Petrescu AJ, Milac AL, Petrescu SM, Dwek RA, Wormald MR. Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structure, and folding. Glycobiology. 2003;14:103–114. doi: 10.1093/glycob/cwh008. https://doi.org/10.1093/glycob/cwh008. [DOI] [PubMed] [Google Scholar]
- 33.Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci Rep. 2016;6:2430. doi: 10.1038/srep20946. https://doi.org/10.1038/srep20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazumder R, Morampudi KS, Motwani M, Vasudevan S, Goldman R. Proteome-wide analysis of single-nucleotide variations in the N-glycosylation sequon of human genes. PLoS ONE. 2012;7:e36212. doi: 10.1371/journal.pone.0036212. https://doi.org/10.1371/journal.pone.0036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellquist JL, Kasturi L, Spitalnik SL, Shakin-Eshleman SH. The amino acid following an asn-X-Ser/Thr sequon is an important determinant of N-linked core glycosylation efficiency. Biochemistry. 1998;37:6833–6837. doi: 10.1021/bi972217k. https://doi.org/10.1021/bi972217k. [DOI] [PubMed] [Google Scholar]
- 36.Shestopal SA, Hao JJ, Karnaukhova E, Liang Y, Ovanesov MV, Lin M, et al. Expression and characterization of a codon-optimized blood coagulation factor VIII. J Thromb Haemost. 2017;15:709–720. doi: 10.1111/jth.13632. https://doi.org/10.1111/jth.13632. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson I, Kelleher DJ, Miao Y, Shao Y, Kreibich G, Gilmore R, et al. Photocross-linking of nascent chains to the STT3 subunit of the oligosaccharyltransferase complex. J Cell Biol. 2003;161:715–725. doi: 10.1083/jcb.200301043. https://doi.org/10.1083/jcb.200301043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitley P, Nilsson IM, von Heijne G. A nascent secretory protein 5 traverse the ribosome/endoplasmic reticulum translocase complex as an extended chain. J Biol Chem. 1996;271:6241–6244. doi: 10.1074/jbc.271.11.6241. https://doi.org/10.1074/jbc.271.11.6241. [DOI] [PubMed] [Google Scholar]
- 39.Shrimal S, Trueman SF, Gilmore R. Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J Cell Biol. 2013;201:81–95. doi: 10.1083/jcb.201301031. https://doi.org/10.1083/jcb.201301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T, Sako Y, Sho M, Momohara M, Suico MA, Shuto T, et al. STT3B-dependent posttranslational N-glycosylation as a surveillance system for secretory protein. Mol Cell. 2012;47:99–110. doi: 10.1016/j.molcel.2012.04.015. https://doi.org/10.1016/j.molcel.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Shipley JM, Grubb JH, Sly WS. The role of glycosylation and phosphorylation in the expression of active human beta-glucuronidase. J Biol Chem. 1993;268:12193–12198. [PubMed] [Google Scholar]
- 42.Ngo JC, Huang M, Roth DA, Furie BC, Furie B. Crystal structure of human factor VIII: implications for the formation of the factor IXa-Factor VIIIa complex. Structure. 2008;16:597–606. doi: 10.1016/j.str.2008.03.001. https://doi.org/10.1016/j.str.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Shen BW, Spiegel PC, Chang CH, Huh JW, Lee JS, Kim J, et al. The tertiary structure and domain organization of coagulation factor VIII. Blood. 2007;111:1240–1247. doi: 10.1182/blood-2007-08-109918. https://doi.org/10.1182/blood-2007-08-109918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selvaraj SR, Miao HSP. Elucidation of the roles of individual asparagine-linked glycans outside of the B domain on factor VIII secretion. Blood. 2011;118:2238. [Google Scholar]
- 45.Bajaj SP, Schmidt AE, Mathur A, Padmanabhan K, Zhong D, Mastri M, et al. Factor IXa:factor VIIIa interaction. helix 330–338 of factor ixa interacts with residues 558–565 and spatially adjacent regions of the a2 subunit of factor VIIIa. J Biol Chem. 2001;276:16302–16309. doi: 10.1074/jbc.M011680200. https://doi.org/10.1074/jbc.M011680200. [DOI] [PubMed] [Google Scholar]
- 46.Fay PJ, Beattie T, Huggins CF, Regan LM. Factor VIIIa A2 subunit residues 558–565 represent a factor IXa interactive site. J Biol Chem. 1994;269:20522–20527. [PubMed] [Google Scholar]
- 47.Ebberink EH, Bouwens EA, Bloem E, Boon-Spijker M, van den Biggelaar M, Voorberg J, et al. Factor VIII/V C-domain swaps reveal discrete C-domain roles in factor VIII function and intracellular trafficking. Haematologica. 2017;102:686–694. doi: 10.3324/haematol.2016.153163. https://doi.org/10.3324/haematol.2016.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakabayashi H, Fay PJ. Replacing the factor VIII C1 domain with a second C2 domain reduces factor VIII stability and affinity for factor IXa. J Biol Chem. 2013;288:31289–31297. doi: 10.1074/jbc.M113.497289. https://doi.org/10.1074/jbc.M113.497289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J, Pipe SW, Miao H, Jacquemin M, Gilbert GE. A membrane-interactive surface on the factor VIII C1 domain cooperates with the C2 domain for cofactor function. Blood. 2011;117:3181–3189. doi: 10.1182/blood-2010-08-301663. https://doi.org/10.1182/blood-2010-08-301663. [DOI] [PubMed] [Google Scholar]
- 50.Meems H, Meijer AB, Cullinan DB, Mertens K, Gilbert GE. Factor VIII C1 domain residues Lys 2092 and Phe 2093 contribute to membrane binding and cofactor activity. Blood. 2009;114:3938–3946. doi: 10.1182/blood-2009-01-197707. https://doi.org/10.1182/blood-2009-01-197707. [DOI] [PubMed] [Google Scholar]
- 51.Bloem E, van den Biggelaar M, Wroblewska A, Voorberg J, Faber JH, Kjalke M, et al. Factor VIII C1 domain spikes 2092–2093 and 2158–2159 comprise regions that modulate cofactor function and cellular uptake. J Biol Chem. 2013;288:29670–29679. doi: 10.1074/jbc.M113.473116. https://doi.org/10.1074/jbc.M113.473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.