Abstract
Objective
To examine in a 3-year brain imaging study the effects of higher vs lower adherence to a Mediterranean-style diet (MeDi) on Alzheimer disease (AD) biomarker changes (brain β-amyloid load via 11C-Pittsburgh compound B [PiB] PET and neurodegeneration via 18F-fluorodeoxyglucose [FDG] PET and structural MRI) in midlife.
Methods
Seventy 30- to 60-year-old cognitively normal participants with clinical, neuropsychological, and dietary examinations and imaging biomarkers at least 2 years apart were examined. These included 34 participants with higher (MeDi+) and 36 with lower (MeDi−) MeDi adherence. Statistical parametric mapping and volumes of interest were used to compare AD biomarkers between groups at cross section and longitudinally.
Results
MeDi groups were comparable for clinical and neuropsychological measures. At baseline, compared to the MeDi+ group, the MeDi− group showed reduced FDG-PET glucose metabolism (CMRglc) and higher PiB-PET deposition in AD-affected regions (p < 0.001). Longitudinally, the MeDi−-group showed CMRglc declines and PiB increases in these regions, which were greater than those in the MeDi+ group (pinteraction < 0.001). No effects were observed on MRI. Higher MeDi adherence was estimated to provide 1.5 to 3.5 years of protection against AD.
Conclusion
Lower MeDi adherence was associated with progressive AD biomarker abnormalities in middle-aged adults. These data support further investigation of dietary interventions for protection against brain aging and AD.
Population-attributable risk models estimate that 1 in every 3 cases of Alzheimer disease (AD) may be accounted for by modifiable risk factors and thus may be preventable.1 A large body of epidemiologic evidence2–5 and recent clinical trials6,7 have linked higher adherence to a Mediterranean-style diet (MeDi) to a lower risk of cognitive decline and dementia.
While there is growing interest in implementing dietary recommendations for AD prevention, in vivo AD biomarkers are needed to identify the mechanisms by which the MeDi may be protective against AD. This is crucial during the preclinical stages of AD with no or minimal cognitive deterioration, when the potential for disease prevention is greatest.8
At cross section, cognitively intact elderly with lower MeDi adherence exhibit increased brain AD pathology, atrophy, and glucose hypometabolism compared to those with higher adherence.9–15 However, the effects of the MeDi on AD progression and thus its potential efficacy for AD prevention remain unknown.
This 3-year multimodality brain imaging study characterizes the progression of well-established AD biomarkers, including β-amyloid (Aβ) deposition (11C-Pittsburgh compound B [PiB] PET) and neurodegeneration via glucose metabolism (18F-fluorodeoxyglucose [FDG] PET cerebral metabolic rates of glucose [CMRglc]) and neuronal loss (MRI), as a function of MeDi adherence in middle-aged cognitively normal participants. Our goal was to evaluate how the MeDi influences progression of brain AD biomarkers and to estimate the years of protection it provides against AD.
Methods
Participants
Study participants were derived from longitudinal brain imaging studies conducted at New York University School of Medicine/Weill Cornell Medical College between 2010 and 2016. The studies aimed at examining risk factors for AD among clinically and cognitively normal adults, as described previously.9,16,17 Briefly, participants were derived from multiple community sources, including individuals interested in research participation, family members, and caregivers of impaired participants.
Only participants with clinical, neuropsychological, dietary examinations, and brain imaging, including volumetric MRI, FDG-PET, and PiB-PET at least 2 years apart, were examined. At baseline, all participants had to be 30 to 60 years old with ≥12 years of education, Clinical Dementia Rating score of 0, Geriatric Depression Scale score ≤2, Mini-Mental State Examination score ≥27, Hamilton Depression Scale score <16, and normal cognitive test performance for age and education.18 All participants received neuropsychological evaluations testing memory function, attention, and language at baseline and at follow-up.18 Participants were excluded in case of unstable medical conditions (e.g., unstable heart disease, unmanaged diabetes mellitus, cancer), primary CNS diseases or illnesses, alcohol abuse, and specific medications (e.g., benzodiazepines, cholinesterase inhibitors, psychostimulants, cancer chemotherapy).
A family history of late-onset AD was elicited with standardized questionnaires.16 APOE genotypes were determined with standard quantitative PCR procedures.16
Standard protocol approvals, registrations, and patient consents
All participants provided informed consent to participate in this New York University School of Medicine/Weill Cornell Medical College institutional review board–approved study.
Dietary information
We used the Harvard food frequency questionnaire to obtain dietary data on the average food consumption over the prior year.9,10 We combined food items into 30 food groups based on similarities in food composition and calculated the average intake (grams per day) for each group. After regressing for caloric intake, we assigned a value of 1 for each beneficial food group (fruits, vegetables, legumes, cereals, and fish) with an intake that was equal to or above the sex-specific median, a value of 1 for each detrimental food group (dairy, meat) with a consumption that was below the sex-specific median, a value of 1 for a monounsaturated fat–to–saturated fat ratio above the median, and a value of 1 for mild alcohol intake.9,10 These values were summed to generate an MeDi score, with a greater score indicating greater MeDi adherence, which was used to dichotomize participants into higher vs lower adherence by population median level (i.e., high 5–9 vs low 0–4) and in keeping with previous studies.9,10
Brain imaging
All participants received volumetric 3T MRI, PiB-PET, and FDG-PET scans following standardized procedures.17,19–22 Image analysis was performed with the same fully automated image processing pipeline as described previously.17,19–22 Briefly, for each patient, FDG and PiB scans were coregistered to the corresponding baseline MRI, to each other, and to their follow-up scans with the Normalized Mutual Information routine of Statistical Parametric Mapping (SPM8).23 MRIs were segmented and normalized to the template space by high-dimensional warping (Diffeomorphic Anatomic Registration Through Exponentiated Lie Algebra [DARTEL]) and voxel-based morphometry.23,24 Jacobian modulation was applied to restore gray matter volumes (GMVs) in the images, which were smoothed with an 8-mm full-width half-maximum kernel.23 MRI-coregistered PET scans were spatially normalized with patient-specific transformation matrixes obtained from MRI and smoothed with a 10-mm full-width half-maximum filter.
Statistics
SPSS version 22 (SPPS Inc, Chicago, IL) and SPM12 were used for data analysis. Clinical, demographic, and cognitive measures were examined with χ2 tests and general linear models, univariate and repeated-measures analyses, at p < 0.05.
For SPM analysis, full factorial models with post hoc t contrasts were used to test for regional differences in MRI, PiB, and FDG measures between groups at baseline and longitudinally. The longitudinal model tested for differential group effects over time and for longitudinal changes within groups, controlling for time to follow-up. FDG analyses were adjusted for the global mean by proportional scaling. MRI analyses were corrected for total intracranial volume and PiB analyses for cerebellar uptake.25
Results were examined at p < 0.001, uncorrected (cluster extent ≥20 voxels), within a search volume defined by a set of predefined AD-related regions.17,19–22,25 Anatomic location of brain regions showing significant effects was described with Talairach and Tournoux coordinates.
Results were reexamined controlling for clinical and vascular risk confounds as covariates. These included age, sex, education, and APOE status (APOE ε4 carriers vs noncarriers), and vascular risk factors such as overweight (body mass index [BMI] in kilograms per meter squared), insulin resistance (Quantitative Insulin Sensitivity Check Index, QUICKI scores26), and hypertension.9 All covariates were included in the model for each imaging modality. Second, to avoid overfitting, analyses were repeated with adjustment by clinical confounds first and then separately by vascular confounds. Only covariates showing significant effects were retained in the models.
Linear regressions were used to estimate the number of years before baseline when statistical differentiation across groups became possible. This was done for the brain regions showing baseline and longitudinal group effects by estimating the mean biomarker values for each group every 0.5 years before baseline using the observed baseline measures and annual rates of change (±SEM) within each group.21,27 Every 0.5 years, the estimated biomarker measures were compared between groups with independent-sample t tests at p < 0.05 (2 tailed).
We performed power analyses (G*POWER 3 software)28 on the extracted data to estimate the number of participants needed in a 3-year, randomized, placebo-controlled study to test the efficacy of MeDi interventions to attenuate AD biomarker changes. Specifically, we calculated the number of participants needed in the active treatment and control groups to detect 50%, 33%, and 25% attenuations in AD biomarker changes in the preferentially affected brain regions with 80% power using unpaired t tests and p < 0.001 (1 tailed).
Data availability
All data have been published within the article.
Results
Participants
A total of 82 participants were available for analysis. Of these, we excluded 5 participants who did not complete the follow-up scans, 4 with incomplete dietary questionnaires, and 3 who developed medical conditions precluding further participation (baseline age 50 ± 8 years, 75% female, education ≥12 years, Mini-Mental State Examination score 29 ± 1, 77% whites). The remaining 70 participants were examined, including 34 (49%) with higher MeDi adherence (MeDi+) and 36 (51%) with lower adherence (MeDi−). On the MeDi scale, 14% scored <3, 21% scored 3, 14% scored 4, 17% scored 5, 15% scored 6, and 19% scored 7 to 9. The median score was 5 (SD 2.0).
Participants' characteristics are given in table 1. There were no group differences for clinical and neuropsychological measures, frequency of APOE ε4 genotype, and presence of AD family history. Compared to the MeDi+ group, the MeDi− group included a higher frequency of participants with hypertension, which reached significance at follow-up (p < 0.05). The MeDi− group also showed higher BMI and waist-to-hip measurements than the MeDi+ group at both time points (p < 0.05).
Table 1.
Demographic and clinical characteristics by MeDi group
Models for prediction of MRI changes
No group differences in GMV were observed at cross section or longitudinally with and without accounting for age, time to follow-up, and total intracranial volume as covariates.
Models for prediction of FDG changes
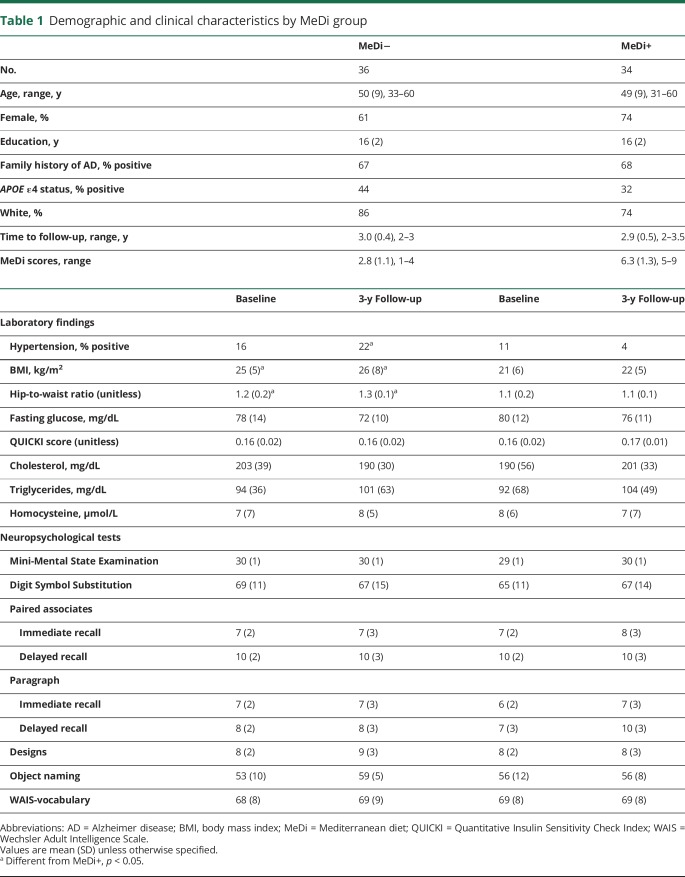
At baseline, the MeDi− group showed reduced CMRglc in temporal cortex bilaterally compared to the MeDi+ group (p < 0.001; figure 1A and table e-1, links.lww.com/WNL/A448). No regions showed reduced CMRglc in MeDi+ vs MeDi−.
Figure 1. Baseline and longitudinal changes in FDG- PET CMRglc as a function of MeDi adherence.
SPMs display (A) reduced baseline CMRglc in participants with lower MeDi adherence (MeDi−) vs participants with higher MeDi adherence (MeDi+), (B) CMRglc declines over 3 years in the MeDi− and MeDi+ groups, and (C) higher rates of CMRglc declines in MeDi− vs MeDi+ participants. Scatterplots show CMRglc changes in the MeDi− and MeDi+ groups. SPMs are represented on a color-coded scale (1 < z < 3, where z > 2 corresponds to p < 0.001) and displayed on a standardized MRI. Two participants did not complete the follow-up scan. Anatomic location and description of brain regions are found in tables e-1 and e-2 (links.lww.com/WNL/A448). CMRglc = cerebral metabolic rate of glucose; FDG = 18F-fluorodeoxyglucose; MeDi = Mediterranean diet; SPM = statistical parametric map; SUVR = standardized uptake value ratios.
Longitudinally, with correction for baseline age and time to follow-up, both groups showed CMRglc declines in temporal regions (p < 0.001, figure 1B). In addition, group × time interactions were observed in temporal and posterior cingulate cortex/precuneus (pinteraction < 0.001; figure 1C and table e-2, links.lww.com/WNL/A448). On post hoc analysis, these effects were driven by the MeDi− group showing higher rates of CMRglc declines compared to the MeDi+ group (pinteraction < 0.001; figure 1C). In these regions, CMRglc declined by an average of 0.055 standardized uptake value ratio (SUVR) per year (SD 0.087) in the MeDi− group, corresponding to an average CMRglc decline from baseline of 3.33%/y. CMRglc did not show significant declines in the MeDi+ group, with an average change of 0.012 SUVR per year (SD 0.053), corresponding to <1% change per year from baseline.
Results remained significant when age, sex, education, and APOE status were included as covariates (pinteraction < 0.002). Among vascular confounds, BMI and hypertension were not associated with CMRglc changes. QUICKI scores showed borderline associations (p = 0.07), and including them in the model left results unchanged (pinteraction = 0.001).
Assuming a linear progression of CMRglc reductions and the same SEM, CMRglc reductions in the MeDi− group vs the MeDi+ group were estimated to reach significance 1.5 ± 0.5 years before baseline.
Models for prediction of PiB changes
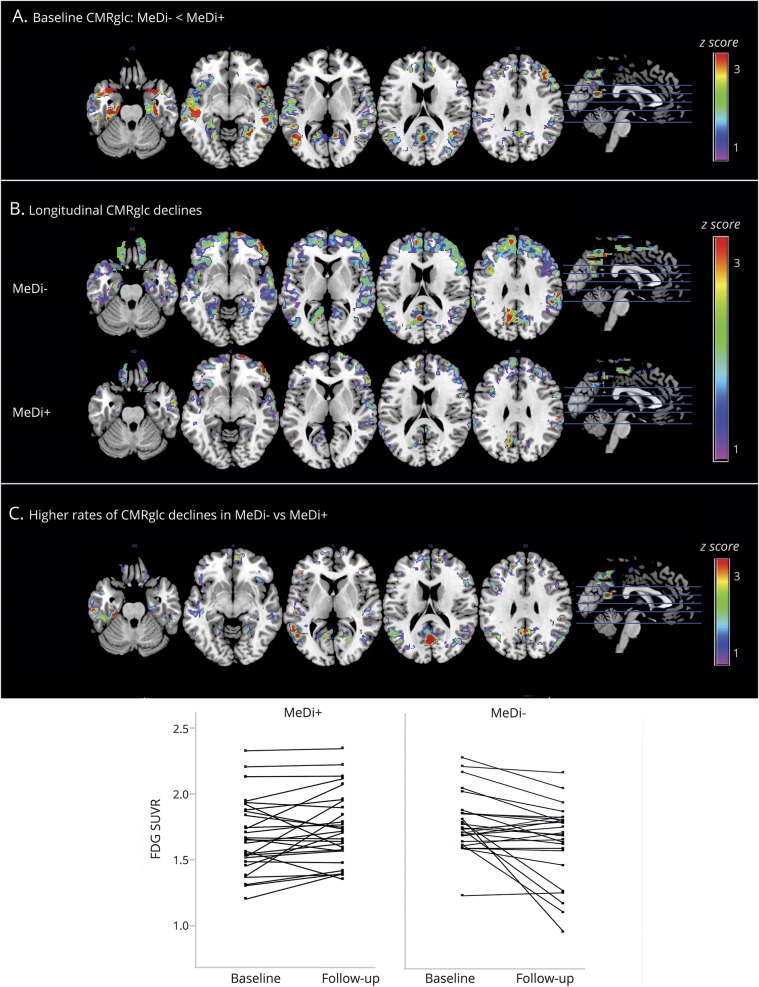
At baseline, the MeDi− group showed higher PiB uptake in the frontal cortex compared to the MeDi+ group (p < 0.001; figure 2A and table e-3, links.lww.com/WNL/A448). No regions showed increased PiB uptake in the MeDi+ vs MeDi− group.
Figure 2. Baseline and longitudinal changes in 11C-PiB PET Aβ deposition as a function of MeDi adherence.
SPMs display (A) increased baseline Aβ deposition in participants with lower MeDi adherence (MeDi−) vs participants with higher MeDi adherence (MeDi+), (B) Aβ deposition increases over 3 years in the MeDi− and MeDi+ groups, and (C) higher rates of Aβ deposition in MeDi− vs MeDi+ participants. Scatterplots show PiB changes in the MeDi− and MeDi+ groups. SPMs are represented on a color-coded scale (1 < z < 3, where z > 2 corresponds to p < 0.001) and displayed on a standardized MRI. Two participants did not complete the follow-up scan. Anatomic location and description of brain regions are found in tables e-3 and e-4 (links.lww.com/WNL/A448).Aβ = β-amyloid; FDG = 18F-fluorodeoxyglucose; MeDi = Mediterranean diet; PiB = Pittsburgh compound B; SPM = statistical parametric map; SUVR = standardized uptake value ratios.
Longitudinally, correcting for baseline age and time to follow-up, both groups showed increased PiB uptake in frontal regions (p < 0.001; figure 2B). The MeDi− group showed additional clusters of increasing PiB uptake in the parietal cortex of the right hemisphere (p < 0.001; table e-4, links.lww.com/WNL/A448).
Group × time interactions were observed in frontal cortex and posterior cingulate cortex/precuneus, mostly in the left hemisphere (pinteraction < 0.001; table e-4, links.lww.com/WNL/A448). On post hoc analysis, these effects were driven by the MeDi− group showing higher rates of PiB accumulation compared to the MeDi+ group (pinteraction < 0.001; figure 2C). Quantitatively, PiB uptake increased by an average of 0.028 SUVR per year (SD 0.031) in the MeDi− group, corresponding to an average increase from baseline of 3%/y. PiB uptake did not show significant increases in the MeDi+ group, with an average change of 0.009 SUVR per year (SD 0.020), corresponding to <1% increase from baseline.
Results remained significant when age, sex, education, and APOE status were included as covariates (pinteraction < 0.001). BMI, QUICKI scores, and hypertension were not associated with PiB changes.
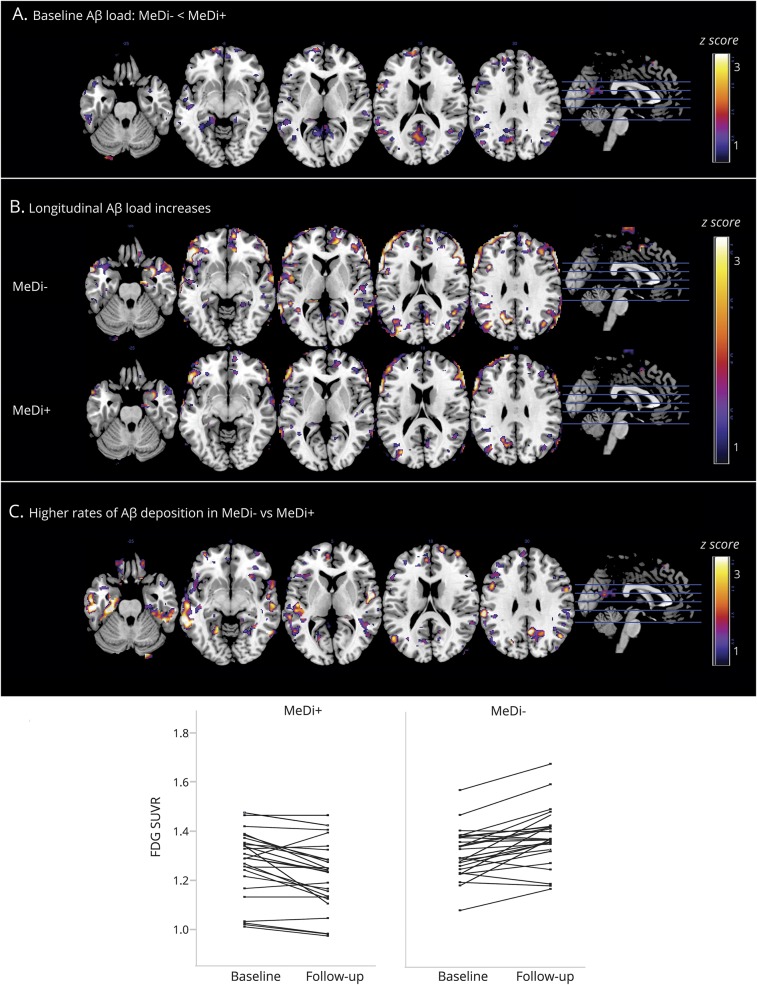
Assuming a linear progression of PiB deposition and the same SEM, PiB increases in the MeDi− group were estimated to reach significance 3.5 ± 0.5 years before baseline compared to the MeDi+ group (figure 3).
Figure 3. Predicted origin of divergence and differentiation in (A) FDG-PET and (B) PiB-PET uptake within Alzheimer disease–related regions between MeDi groups.
Asterisks mark the estimated time at which biomarker differences reached statistical significance in participants with lower MeDi adherence (MeDi−) vs participants with higher MeDi adherence (MeDi+) (p < 0.05). This corresponded to 1.5 ± 0.5 years before baseline for FDG-PET and 3.5 ± 0.5 years for PiB-PET. Annual rates of biomarker changes are reported next to each group. FDG = 18F-fluorodeoxyglucose; MeDi = Mediterranean diet; PiB = Pittsburgh compound B; SUVR = standardized uptake value ratios.
Testing the efficacy of MeDi interventions
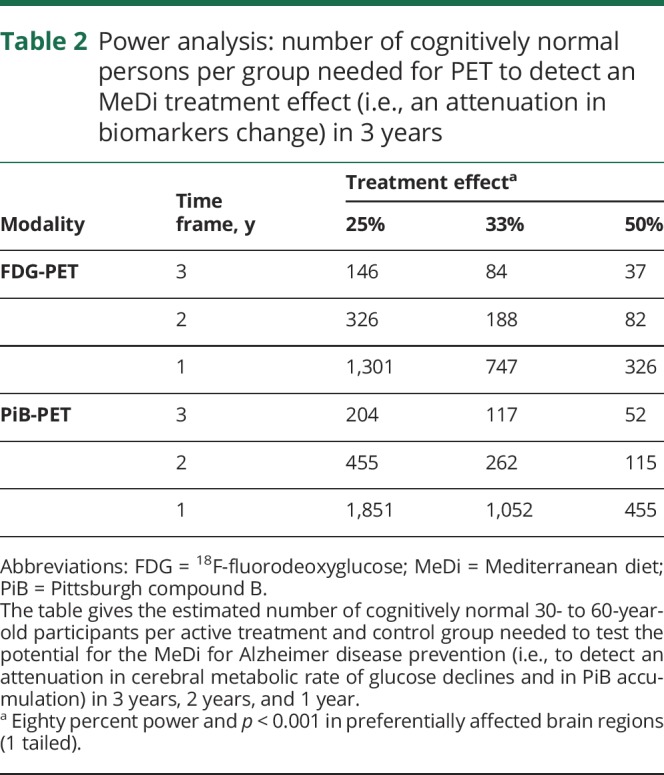
We estimate that between 146 and 204 participants would be needed per active MeDi intervention and control group to detect a 25% attenuation in biomarker changes with 80% power and p < 0.001 in the preferentially affected brain regions in 3 years (table 2).
Table 2.
Power analysis: number of cognitively normal persons per group needed for PET to detect an MeDi treatment effect (i.e., an attenuation in biomarkers change) in 3 years
Discussion
The main conclusions from this 3-year multimodality brain imaging study of young to late middle-aged adults are as follows: (1) at baseline, MeDi− participants exhibit reduced CMRglc and increased Aβ deposition compared to MeDi+ participants; (2) longitudinally, hypometabolism and Aβ deposition progress at higher rates in MeDi− than MeDi+ participants; (3) AD biomarker abnormalities in MeDi− participants were estimated to originate at least 1.5 years before baseline; and (4) no effects were found on structural MRI.
The present findings show that lower MeDi adherence is associated with the emergence and longitudinal progression of Aβ deposition and hypometabolism in midlife. Longitudinal studies in elderly without dementia showed that, with a mean increase of 0.043 SUVR per year, Aβ deposition would take ≈19 years to reach the levels observed in patients with AD.29 In our middle-aged MeDi− participants, PiB uptake increased at a slightly lower annual rate of 0.028 SUVR, supporting previous findings that Aβ deposition likely extends for >2 decades. Our MeDi− participants also exhibited CMRglc declines, which are reflective of neuronal dysfunction.30 These changes preceded any evidence of cognitive deterioration and were independent of possible risk factors for late-onset AD such as age, sex, education, and APOE ε4 genotype, as well as vascular comorbidity.
According to our estimates, higher MeDi adherence may provide up to 3.5 years of protection against brain aging and AD. These data indicate that diet may indeed influence AD progression during the normal stages of cognition and provide a possible pathophysiologic substrate to clinical studies showing a favorable relation of the MeDi with reduced risk of progression to dementia.2–5
The biological mechanisms by which the MeDi may confer protection against AD are under investigation. From a nutritional perspective, the MeDi is characterized by high consumption of plant-based foods and minimal consumption of red meat, sweets, and processed foods.31 Besides being linked to lower risk of dementia, this dietary pattern has been shown to support insulin regulation and cardiovascular health,32 whereas diets rich in refined carbohydrates and saturated fat are known to increase the risk of cardiovascular disease, insulin resistance, and inflammation, possibly contributing to accelerated brain aging and neuronal loss.33 The present imaging findings are consistent with the medical literature thus far and support investigation of dietary interventions for protection against brain aging and AD.
Using the maximal rate of biomarker changes in the preferentially affected brain regions, we estimate that between 146 and 204 participants 30 to 60 years of age are needed per active treatment and control group to test the efficacy of the MeDi to detect a 25% attenuation in the progression of AD biomarkers in 3 years. In comparison, on the basis of clinical trials in participants 55 to 80 years of age,6,7 >2,000 participants per group would be needed to detect a 25% attenuation in cognitive decline in ≈4 years. These estimates might be even higher in a younger, cognitively intact cohort like ours.
In contrast to previous studies in the elderly, we did not observe MRI effects. A 3-year longitudinal MRI regions-of-interest study of elderly without dementia reported higher atrophy rates in MeDi− compared to MeDi+ participants.11 Although the voxel-based morphology procedure we used is reportedly as sensitive as region-of-interest sampling in normal aging,23 it is possible that volumetric analysis of specific regions might have yielded different results. Alternatively, because our participants were substantially younger than those in previous studies (mean age 50 vs ≥ 73 years10,11), our findings suggest that the effects of diet on MRI-based atrophy become measurable at older ages, in keeping with the proposed model of biomarker trajectories in AD.30 In addition, there is evidence for associations between MeDi adherence and preserved white matter microstructure in the absence of GMV reductions, which suggests that the MeDi may protect against dementia by supporting vasculature health.15
Several issues remain to be addressed. At baseline, >90% of the surveyed participants reported stability of their dietary patterns for ≥5 years (most since childhood), and 8% reported stability over the past 2 to 5 years. Only a few MeDi− participants reported their nutritional behavior starting within the last 1 to 2 years. While it is difficult to ascertain the accuracy of retrospective, self-reported data, erroneous grouping would have conservatively included MeDi− participants in the MeDi+ group and vice versa, thus reducing power to detect significant differences. However, because of the synchronous timing of dietary and brain imaging assessments, we cannot exclude that MeDi adherence may be a recent lifestyle choice in our cohort. More studies are needed to determine whether AD biomarker changes emerge after long-term exposure to the MeDi or whether short-term exposure is sufficient to ward off the emergence of AD. Furthermore, while we accounted for several clinical and vascular risk factors for AD, we did not have complete data on smoking status. Other studies are needed to examine whether baseline and interim smoking would affect the results.
We cannot exclude that our screening criteria may have biased effect estimates.34 First, participation was limited to carefully screened, healthy, highly educated, middle-aged research participants without severe cardiovascular or cerebrovascular disease. While our goal was to examine the effects of the MeDi before severe disease and at a young enough age for potential interventions to be impactful, this limits our ability to generalize the results to the rest of the population. Second, although the method we used to construct MeDi adherence scores is the most widely adopted, it is based on the characteristics of the sample, which also limits the generalizability of the results.35 In a supplemental sensitivity analysis (appendix e-1, links.lww.com/WNL/A449), we showed that the results remained substantially unchanged when the analysis was restricted to those who were on the lower vs upper end of the MeDi score distribution and when an alternative, literature-based MeDi scoring system was used.35
Finally, random measurement error can introduce bias to an estimate of the association between a risk factor and a disease or disease marker.36 While a regression dilution bias correction may be made with data from a validity study or a reliability study, we currently do not have access to an independent sample to replicate the results.
We caution that clinical application is not yet justified. Other studies with larger samples and longer longitudinal follow-ups are needed to replicate and assess the generalizability of these research findings in community-based samples with more diverse economic, medical, and social backgrounds and to determine whether AD biomarker changes are predictive of future AD in relation to MeDi adherence.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- BMI
body mass index
- CMRglc
cerebral metabolic rate of glucose
- FDG
18F-fluorodeoxyglucose
- GMV
gray matter volume
- MeDi
Mediterranean-style diet
- PiB
Pittsburgh compound B
- QUICKI
Quantitative Insulin Sensitivity Check Index
- SUVR
standardized uptake value ratio
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Dr. Berti: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Walters and Dr. Sterling: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Quinn: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Ms. Logue: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Andrews: analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Matthews and Dr. Osorio: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Pupi: study concept and design, critical revision of the manuscript for important intellectual content. Dr. Vallabhajosula: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Isaacson: study concept and design, critical revision of the manuscript for important intellectual content. Dr. de Leon: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Mosconi: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Study funding
Study funding from the NIH/National Institute on Aging (AG035137, AG13616, 2PAG026572); funding from the Department of Neurology at Weill Cornell Medical College; and philanthropic support of the Alzheimer's Prevention Clinic, Weill Cornell Memory Disorders Program.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009;66:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol 2006;59:912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 2013;84:1318–1325. [DOI] [PubMed] [Google Scholar]
- 7.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 2015;175:1094–1103. [DOI] [PubMed] [Google Scholar]
- 8.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease: the challenges ahead. Nat Rev Neurol 2013;9:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosconi L, Murray J, Tsui WH, et al. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer's disease. J Prev Alzheimers Dis 2014;1:23–32. [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y, Brickman AM, Stern Y, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015;85:1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luciano M, Corley J, Cox SR, et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology 2017;88:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill DA, Siddarth P, Raji CA, et al. Modifiable risk factors and brain positron emission tomography measures of amyloid and tau in nondemented adults with memory complaints. J Am Assoc Geriatr Psychiatry 2016;24:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DC, Davies M, Murray J, et al. Physical activity, Mediterranean diet and biomarkers-assessed risk of Alzheimer's: a multi-modality brain imaging study. Adv J Mol Imaging 2014;4:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staubo SC, Aakre JA, Vemuri P, et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement 2017;13:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelletier A, Barul C, Feart C, et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimers Dement 2015;11:1023–1031. [DOI] [PubMed] [Google Scholar]
- 16.Mosconi L, Mistur R, Switalski R, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology 2009;72:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosconi L, Rinne JO, Tsui WH, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer's. Proc Natl Acad Sci USA 2010;107:5949–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Santi S, Pirraglia E, Barr W, et al. Robust and conventional neuropsychological norms: diagnosis and prediction of age-related cognitive decline. Neuropsychology 2008;22:469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berti V, Mosconi L, Glodzik L, et al. Structural brain changes in normal individuals with a maternal history of Alzheimer's. Neurobiol Aging 2011;32:2325.e17–2325.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Leon MJ, Mosconi L, Blennow K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann NY Acad Sci 2007;1097:114–145. [DOI] [PubMed] [Google Scholar]
- 21.Mistur R, Mosconi L, Santi SD, et al. Current challenges for the early detection of Alzheimer's disease: brain imaging and CSF studies. J Clin Neurol 2009;5:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosconi L, Rinne JO, Tsui W, et al. Amyloid and metabolic PET imaging of cognitively normal adults with Alzheimer's parents. Neurobiol Aging 2012;34:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. NeuroImage 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 25.Mosconi L, Murray J, Tsui WH, et al. Brain imaging of cognitively normal individuals with 2 parents affected by late-onset AD. Neurology 2014;82:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 27.Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging 2008;29:676–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 29.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–367. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61:1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 32.Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open 2015;5:e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging 2005;26(suppl 1):31–35. [DOI] [PubMed] [Google Scholar]
- 34.Weuve J, Proust-Lima C, Power MC, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement 2015;11:1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014;17:2769–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data have been published within the article.