Abstract
Objective
Our aim was to assess risk of Parkinson disease (PD) following traumatic brain injury (TBI), including specifically mild TBI (mTBI), among care recipients in the Veterans Health Administration.
Methods
In this retrospective cohort study, we identified all patients with a TBI diagnosis in Veterans Health Administration databases from October 2002 to September 2014 and age-matched 1:1 to a random sample of patients without TBI. All patients were aged 18 years and older without PD or dementia at baseline. TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident PD more than 1 year post-TBI were identified using ICD-9 codes. Risk of PD after TBI was assessed using Cox proportional hazard models adjusted for demographics and medical/psychiatric comorbidities.
Results
Among 325,870 patients (half with TBI; average age 47.9 ± 17.4 years; average follow-up 4.6 years), 1,462 were diagnosed with PD during follow-up. Compared to no TBI, those with TBI had higher incidence of PD (no TBI 0.31%, all-severity TBI 0.58%, mTBI 0.47%, moderate-severe TBI 0.75%). In adjusted models, all-severity TBI, mTBI, and moderate-severe TBI were associated with increased risk of PD (hazard ratio [95% confidence interval]: all-severity TBI 1.71 [1.53–1.92]; mTBI 1.56 [1.35–1.80]; moderate-severe TBI 1.83 [1.61–2.07]).
Conclusions
Among military veterans, mTBI is associated with 56% increased risk of PD, even after adjusting for demographics and medical/psychiatric comorbidities. This study highlights the importance of TBI prevention, long-term follow-up of TBI-exposed veterans, and the need to determine mechanisms and modifiable risk factors for post-TBI PD.
Mild traumatic brain injury (mTBI), or concussion, affects an estimated 42 million people worldwide each year.1 mTBI is particularly common among athletes2 and military personnel,3 and is a growing epidemic among the elderly.4 There is increasing recognition that mTBI may have long-lasting progressive neurobehavioral consequences5,6 including heightened risk of several psychiatric and neurodegenerative diseases.1,7 The association between mTBI and risk of Parkinson disease (PD), however, remains unclear.
In 2008, the Institute of Medicine reviewed the literature on the association between traumatic brain injury (TBI) and PD, ultimately concluding that while there was “sufficient evidence” for an association between moderate to severe TBI and a clinical diagnosis of PD, there was only “limited/suggestive evidence” for an association between mTBI with loss of consciousness (LOC) and a clinical diagnosis PD.8 Since that time, several studies have assessed risk of PD following mTBI with mixed results.9–11 Thus, it remains unclear whether mTBI is associated with increased risk of PD. Furthermore, only one prior small case-control study has assessed risk of PD following mTBI among military veterans and results were inconclusive.12
To answer this important public health question, we conducted a study of inpatient and outpatient data within the nationwide Veterans Health Administration (VHA) database. Our aim was to determine the risk of PD following any TBI, and specifically following mTBI, among VHA care recipients across the adult age spectrum.
Methods
Standard protocol approvals, registrations, and patient consents
This was a longitudinal cohort study. All procedures were approved by the institutional review boards at the University of California, San Francisco, the San Francisco Veterans Affairs Medical Center, and the US Army Medical Research and Material Command Office of Research Protections and Human Research Protection Office. The need for informed consent was waived because of use of deidentified administrative data.
Data sources and sampling
Data came from 3 nationwide VHA health care system databases: (1) the Comprehensive TBI Evaluation (CTBIE) database, (2) the inpatient and outpatient visits database (National Patient Care Databases [NPCD]), and (3) the Vital Status File database.
The CTBIE is a nationwide database containing detailed information about TBI sustained by veterans of Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) who have enrolled in VHA health care. VHA launched the CTBIE program in 200713 and has since screened almost all qualifying veterans for TBI.14 OEF/OIF veterans in the CTBIE database have been referred by any Veterans Administration provider after screening positive on a brief TBI survey. During the CTBIE visit, a neurologist, or other health care provider supervised by a neurologist, administers a comprehensive TBI exposure survey that gathers detailed TBI exposure information including a determination of TBI status and presence and duration of associated symptoms of posttraumatic amnesia (PTA), alteration of consciousness (AOC), and LOC—all of which are recorded in the CTBIE database. The NPCD captures detailed information, including ICD-9 diagnostic codes, about all inpatient and outpatient VHA encounters nationwide. The Vitas Status File database provides up-to-date information about death of VHA care recipients.
To build a cohort of patients with and without TBI, we first identified all patients aged 18 years and older with a diagnosis of TBI in the VHA inpatient and outpatient database (October 2002 to September 2014) and the CTBIE database (October 2007 to October 2014) who did not have a diagnosis of PD, secondary parkinsonism, or dementia at baseline (defined as within 2 years before or 1 year after first TBI diagnosis date; n = 182,634). These patients were classified as “TBI.” Next, we identified a 2% random sample of all patients aged 18 years and older with one or more inpatient or outpatient visits in the VHA inpatient and outpatient database (October 2002 to September 2014) who also did not have a diagnosis of TBI,PD, secondary parkinsonism, or dementia at baseline (defined as within 2 years before or 1 year after random selection date; n = 975,277). These patients were classified as “no TBI.” Next, we 1:1 age-matched each patient with TBI to a patient with no TBI based on age ± 1 year and nearest index date (defined as TBI diagnosis date or random selection date, respectively). The final age-matched study population consisted of 162,935 patients with TBI and 162,935 patients without TBI (figure 1).
Figure 1. Data sources and sampling.
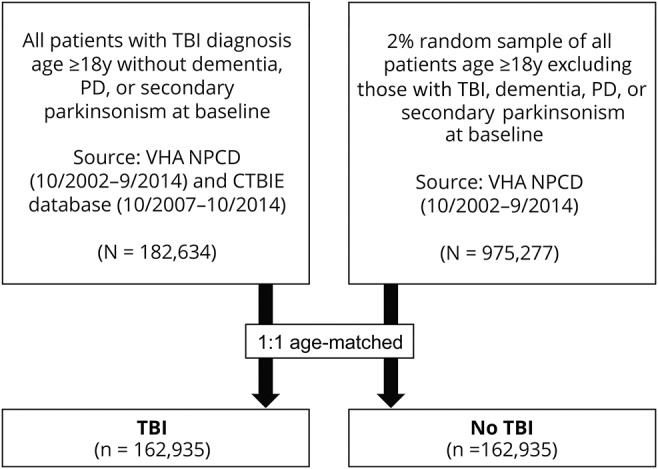
All patients with TBI and a 2% random sample of all patients who met the specified inclusion criteria were identified from the nationwide VHA NPCD and the CTBIE database. A 1:1 age matching of patients with and without TBI was then performed for a final study cohort of 325,870 patients. CTBIE = Comprehensive Traumatic Brain Injury Evaluation; NPCD = National Patient Care Database; PD = Parkinson disease; TBI = traumatic brain injury; VHA = Veterans Health Administration.
Definition of TBI exposure and severity
TBI exposure was defined either by having a diagnosis of TBI after a comprehensive neurologic assessment (CTBIE) or by having at least one inpatient or outpatient TBI diagnosis (NPCD) from a comprehensive list of ICD-9 codes used by the Defense and Veterans Brain Injury Center (DVBIC) and the Armed Forces Health Surveillance Branch for TBI surveillance.15 ICD-9 codes were used as these were available during the study period ending in 2014.
TBI severity was defined as “mild” vs “moderate-severe” according to the 2010 Department of Defense (DoD) clinical criteria or the 2012 DVBIC and Armed Forces Health Surveillance Branch ICD-9 criteria.15 According to the 2010 DoD criteria, mTBI is defined as TBI with LOC of 0 to 30 minutes, AOC of a moment to 24 hours, or PTA of 0 to 24 hours; moderate-severe TBI is defined as TBI with LOC of >30 minutes, AOC of >24 hours, or PTA of >24 hours. ICD-9 codes categorize LOC as none, brief (<1 hour), moderate (1–24 hours), or prolonged (>24 hours). Thus, TBI cases identified through the CTBIE database were classified strictly according to 2010 DoD clinical criteria, while TBI cases identified through the NPCD were classified using DVBIC ICD-9 criteria in which mTBI may include TBI with LOC of 0 to 59 minutes and moderate-severe TBI includes TBI with LOC ≥1 hour. We additionally defined a category of TBI without LOC to be used in the sensitivity analysis described below. In the CTBIE database, TBI without LOC was defined as any TBI with LOC of 0 minutes; in the NPCD, TBI without LOC was coded using only ICD-9 codes for nonpenetrating TBI with specific mention of lack of LOC.
Veterans with more than one TBI-related encounter were classified based on the most severe injury reported during the index year. TBI frequency could not be assessed in this study of nationwide inpatient and outpatient data because multiple TBI-related encounters in the VHA databases may pertain to a single prior TBI event and therefore do not necessarily indicate repeated TBI events. Veterans with undetermined TBI severity were included in the “any TBI” analysis but were not included in the TBI severity analysis.
Definition of PD
Incident PD was defined as any inpatient or outpatient diagnosis of ICD-9 332.0 at least 1 year after TBI or index date. Patients with a diagnosis of PD within 2 years before or 1 year after TBI or index date were considered to have PD at baseline and were excluded from the analysis. Patients with secondary parkinsonism (ICD-9 code 332.1) within 2 years before TBI or index date or at any time during follow-up were also excluded from the analysis.
Covariates
Information about demographics and medical and psychiatric comorbidities were obtained from the NPCD. VHA demographic data of age, sex, and race/ethnicity are based on self-report. Education and income strata were derived from zip code at index date coupled to 2000 US Census data. Patients were categorized as living in a zip code in which ≤25% vs >25% of the adult population had completed a college education (bachelor's degree or higher). Patients were also categorized into median income tertiles based on median income for their index zip code calculated separately for patients aged <75 or ≥75 years. Medical and psychiatric comorbidities were considered present if they were coded in an inpatient or outpatient visit on the index date or during the preceding 2 years using standard ICD-9 codes. The following medical and psychiatric comorbidities were included: diabetes mellitus, hypertension, myocardial infarction, cerebrovascular disease, peripheral vascular disease, congestive heart failure, mood disorder (depression, dysthymia, bipolar disorder), anxiety, posttraumatic stress disorder, substance abuse (alcohol, drug), and tobacco use.
Statistical analysis
Baseline characteristics of veterans with and without TBI were compared using t tests for continuous variables and χ2 tests for categorical variables. Missing data was minimal (total with any missing <5%), and thus, imputation was not performed. Cox proportional hazards models were used to estimate risk of PD diagnosis according to TBI exposure (no TBI vs any TBI) and TBI severity (no TBI vs mTBI or moderate-severe TBI) with censoring at death (from Vital Status File database) or last encounter and using years from TBI or index date as the time scale. Models were adjusted for demographics and medical and psychiatric comorbidities. Cumulative incidence of PD as a function of TBI severity (no TBI vs mTBI or moderate-severe TBI) was also examined graphically.
We additionally performed 4 sensitivity analyses. (1) Because clinical criteria of mTBI cap LOC duration at ≤30 minutes,16 but ICD-9–based categories of mTBI may include some cases of TBI with LOC up to 59 minutes, we performed a sensitivity analysis assessing risk of PD after TBI without LOC. (2) To mitigate reverse causation, we imposed a 4-year lag from TBI or index date to PD diagnosis such that any patient with a diagnosis of PD ≤4 years after TBI or index date was considered to have had PD at baseline and was therefore excluded. A 4-year lag was chosen in order to preserve approximately 50% of the original sample size given that average duration of follow-up of the entire cohort was 4.6 years. After excluding patients with a PD diagnosis ≤4 years after index date and excluding patients without at least one visit >4 years from index date, age matching was repeated for a new cohort size of 82,392 patients with no TBI and 82,392 patients with TBI with average age 48.1 ± 16.4 years in each group and average time of follow-up (entire cohort) 6.8 ± 2.24 years. (3) Because male sex is a risk factor for both TBI and for PD, and thus higher incidence of PD in a TBI-exposed cohort might be partially explained by higher prevalence of male sex, we conducted a sensitivity analysis after excluding all women and including age as an additional covariate in adjusted models. Stratification by sex was not performed because there were insufficient numbers of women in this veteran cohort. (4) To improve the specificity of our ICD-9 diagnosis of PD, we additionally performed a sensitivity analysis after excluding all incident PD cases (and each of their age matches) with only 1 or 2 inpatient or outpatient encounters with a diagnosis of PD (ICD-9 332.0). That is, we only included incident PD cases with at least 3 separate inpatient or outpatient encounters in which they received a diagnosis of PD (ICD-9 332.0).
Data availability
Data for this study were obtained from VHA electronic health records and contain protected health information, including age, scrambled social security number, date of birth, date of death, and dates of medical diagnoses. Therefore, we are unable to share our dataset with other investigators or place it in a repository. Investigators wishing to replicate these analyses may contact the authors to discuss the process of obtaining access to VHA data.
Results
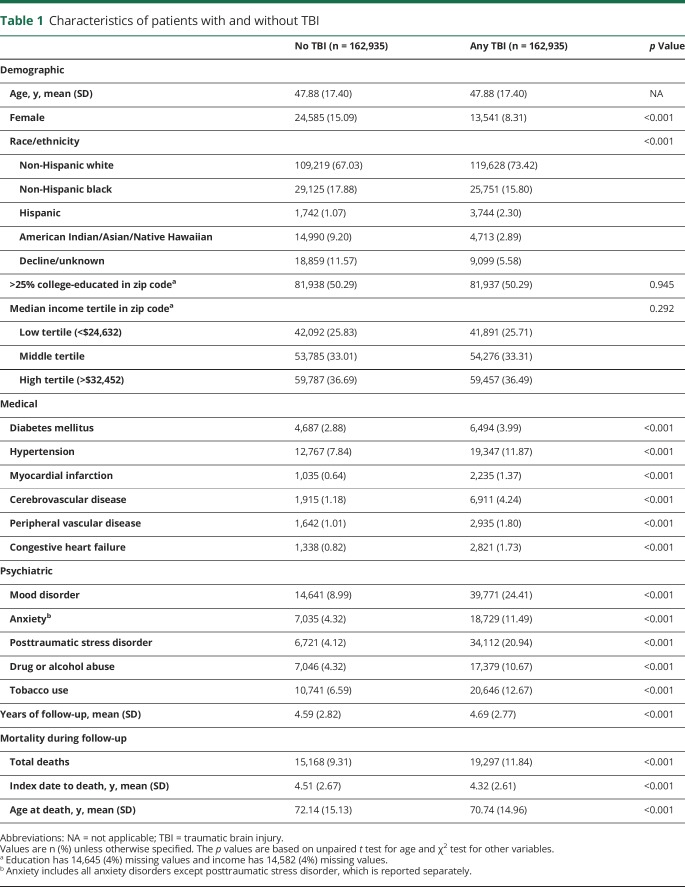
Veterans with and without TBI were well matched for age, with each group having an average age of 47.9 ± 17.4 years. Patients with TBI were more likely to be male, non-Hispanic white, and Hispanic, had higher baseline prevalence of all medical and psychiatric comorbidities assessed, and had higher mortality during follow-up (table 1).
Table 1.
Characteristics of patients with and without TBI
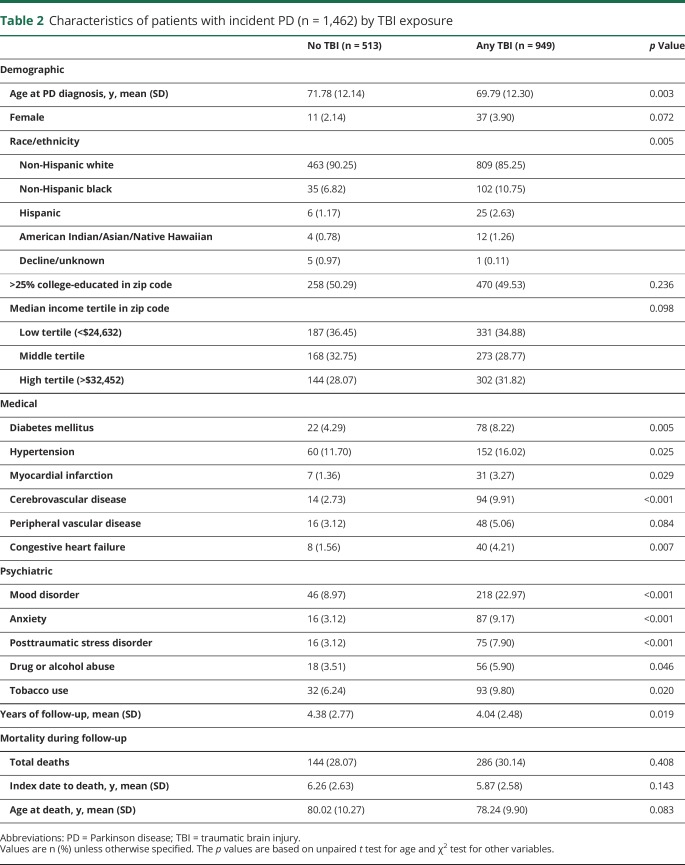
Average follow-up time across the entire cohort was 4.64 ± 2.79 years. During the study period, 1,462 cases of incident PD were diagnosed, of whom 949 (65%) had prior TBI. Among those diagnosed with PD, those with prior TBI did not differ significantly on education, income, or incidence or time to death but were diagnosed with PD at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race/ethnicity, and had significantly higher prevalence of nearly all medical and psychiatric comorbidities assessed compared to those without prior TBI (table 2).
Table 2.
Characteristics of patients with incident PD (n = 1,462) by TBI exposure
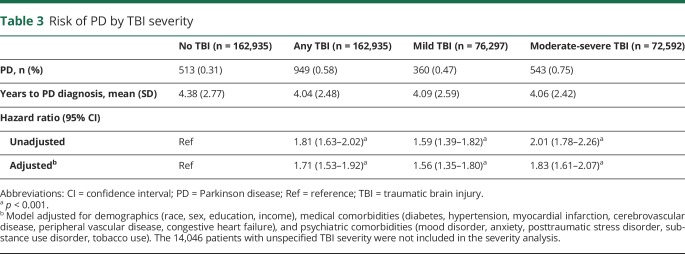
Compared to patients without TBI, those with TBI were significantly more likely to be diagnosed with PD (0.58% vs 0.31%; hazard ratio [HR] [95% confidence interval, CI]: unadjusted 1.81 [1.63–2.02], adjusted 1.71 [1.53–1.92]; table 3). TBI severity analysis revealed that even patients with mTBI were more likely to be diagnosed with PD compared to those without TBI (0.47% vs 0.31%; HR [95% CI]: unadjusted 1.59 [1.39–1.82], adjusted 1.56 [1.35–1.80]; table 3). Cumulative incidence of PD according to TBI severity is shown in figure 2.
Table 3.
Risk of PD by TBI severity
Figure 2. Cumulative incidence of PD by TBI severity.
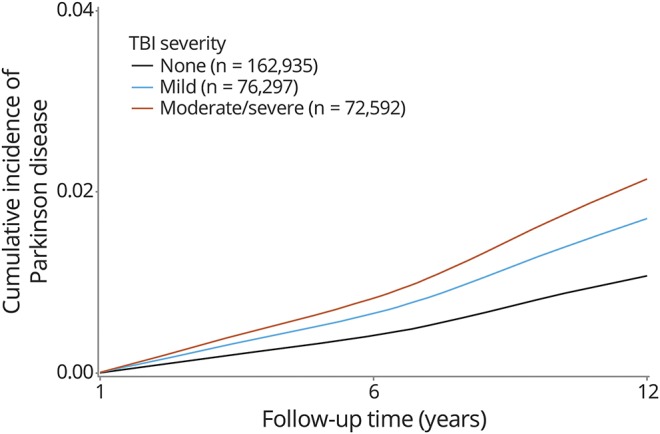
Unadjusted cumulative incidence of PD diagnosis over up to 12 years of follow-up is shown according to TBI severity. Cumulative incidence of PD among patients with mild TBI and moderate-severe TBI is increased compared to patients with no TBI. PD = Parkinson disease; TBI = traumatic brain injury.
Results of the 4 sensitivity analyses were as follows. (1) Stratification of the mTBI group by LOC status revealed that patients with mTBI without LOC (n = 6,497) were more likely to be diagnosed with PD compared to patients without TBI (0.42% vs 0.31%). However, numbers were quite small, with only 27 cases of PD in the mTBI without LOC group, and this finding did not reach statistical significance (HR [95% CI]: unadjusted 1.37 [0.93–2.01], adjusted 1.33 [0.89–1.99]). (2) After imposing a 4-year lag from index date to PD diagnosis, findings remained statistically significant and were similar to the primary analysis (any TBI 0.47% vs no TBI 0.26%, HR [95% CI]: unadjusted 1.78 [1.50–2.10], adjusted 1.67 [1.40–1.99]; mTBI 0.41% vs no TBI 0.26%, HR [95% CI]: unadjusted 1.70 [1.38–2.10], adjusted 1.66 [1.33–2.06]; moderate-severe TBI 0.53% vs no TBI 0.26%, HR [95% CI]: unadjusted 1.81 [1.51–2.18], adjusted 1.68 [1.38–2.04]; all p < 0.001). (3) After excluding all women (n = 38,126), and including age as an additional covariate in adjusted analyses, findings remained statistically significant and were similar to the primary analysis (any TBI 0.61% vs no TBI 0.36%, HR [95% CI]: unadjusted 1.68 [1.51–1.88], adjusted 1.73 [1.54–1.94]; mTBI 0.50% vs no TBI 0.36%, HR [95% CI]: unadjusted 1.49 [1.30–1.71], adjusted 1.65 [1.43–1.91]; moderate-severe TBI 0.78% vs no TBI 0.36%, HR [95% CI]: unadjusted 1.85 [1.64–2.10], adjusted 1.75 [1.54–1.99]; all p < 0.001). (4) After excluding all veterans with PD with fewer than 3 separate encounters with a diagnosis of PD (ICD-9 332.0; n = 715) and any additional age matches (n = 713), findings remained statistically significant and were similar to the primary analysis (any TBI 0.30% vs no TBI 0.16%, HR [95% CI]: unadjusted 1.79 [1.54–2.08], adjusted 1.67 [1.43–1.96]; mTBI 0.23% vs no TBI 0.16%, HR [95% CI]: unadjusted 1.51 [1.24–1.82], adjusted 1.48 [1.21–1.80]; moderate-severe TBI 0.40% vs no TBI 0.16%, HR [95% CI]: unadjusted 2.08 [1.76–2.46], adjusted 1.87 [1.57–2.23]; all p < 0.001).
Discussion
In this longitudinal cohort study of 325,870 military veterans receiving care within the nationwide VHA system, we report that prior TBI is associated with an increased risk of being diagnosed with PD over up to 12 years of follow-up and is also associated with a 2-years-earlier age of diagnosis with PD. The magnitude of risk ranged from 56% increased risk after mTBI to 83% increased risk after moderate to severe TBI, even after accounting for a number of potentially important confounders such as demographics, medical comorbidities, and psychiatric disorders. Imposing a 4-year lag, excluding all women, or excluding patients with PD who had fewer than 3 separate encounters with a PD diagnosis did not substantially change these findings. Patients with TBI without LOC, who represent the mildest form of TBI, had a nonsignificant 33% increased risk of PD, although power was low to detect a significant association.
At least 27 prior case-control studies7,10,17–20 and only 3 prior cohort studies9,21,22 have investigated the association between all-severity TBI and risk of PD. Most of these studies were captured in 2 recent meta-analyses that both reported statistically significant pooled odds ratio of approximately 1.57 to 1.6,17 which is similar in magnitude to our findings. Most,9,18–20 but not all,10 studies investigating risk of PD following TBI that were published since these 2 meta-analyses have confirmed a positive association. Some have suggested that risk of PD may be particularly increased when TBI is sustained either during very early18,20 or very late life.9
Few prior studies—including only one cohort study9 and only one study in veterans12—have assessed risk of PD following specifically mTBI.10–12,23–25 Findings from these studies were mixed likely because of differences in study design, such as heterogeneity in TBI definition,10 choice of controls,23 lag between TBI exposure and PD diagnosis,11 and variable sample size. The prior veteran study was a small case-control study of 93 discordant World War II veteran twin pairs that was interpreted as positive by the authors,12 negative by this meta-analysis,26 and was limited by substantial missing LOC duration data, making definitive interpretation difficult. The only prior cohort study to investigate risk of PD following mTBI—our California-wide longitudinal cohort study—identified a 24% increased risk of PD among older adults with mTBI compared to those with limb fracture.9 Our prior study, however, was limited by lack of outpatient data, comparison to fracture patients (leading to potential underestimate of magnitude of risk), and restriction to a single US state. Thus, our current study is a rare nationwide cohort study in a combined inpatient and outpatient sample investigating the association between mTBI and risk of PD. Our current study is also the first cohort study to investigate this question in military veterans.
There are several human studies that together provide a compelling biological basis for a causal association between TBI and PD. The neuropathologic hallmark of PD is abnormal accumulation of α-synuclein into Lewy bodies and Lewy neurites.27 Following severe TBI, α-synuclein may be elevated in the CSF28 and within injured axons of patients who died of TBI.29 Severe TBI may also occasionally lead to acute posttraumatic parkinsonism.30,31 The combination of TBI and paraquat exposure or certain PD genetic risk alleles synergistically increase risk of PD in a more than additive fashion, providing additional support for a biological mechanism.32,33 At least 2 prior studies have reported an association between a history of earlier-life TBI and mild parkinsonian signs on examination later in life that did not necessarily meet criteria for a clinical diagnosis of PD.34,35 Lastly, a large autopsy study that pooled data from 3 large longitudinal cohort studies of aging (n = 1,682 brains) has recently identified an association between earlier-life TBI with LOC and later-life Lewy body neuropathology.34
Consistent with prior cohort studies in veterans and civilians,9,35 we identified significantly higher prevalence of medical and psychiatric comorbidities in those with TBI vs those without TBI. In our novel comparison of baseline demographic, medical, and psychiatric characteristics of incident PD cases with and without prior TBI (shown in table 2), we additionally identified several potential risk factors for post-TBI PD that deserve further study, including race/ethnicity and medical/psychiatric comorbidities. The relationships among comorbidities, TBI exposure, and PD diagnosis are complex. Some comorbidities, such as substance abuse and depression, may increase risk of sustaining a TBI36 or be a direct consequence of TBI.37,38 Others may be a consequence of unmeasured behavioral risk factors that may increase risk of both development of chronic diseases and TBI exposure. Still others, such as depression and smoking, may be risk or protective factors for PD39,40 or reflect prodromal symptoms of PD.41,42 Thus, our models that adjust for all of these comorbidities likely mitigate many important confounders but may also underestimate the full magnitude of risk by adjusting for prodromal features of PD or direct consequences of TBI. The higher mortality we identified among TBI-exposed veterans deserves further study and may additionally lead to underestimation of the magnitude of risk of PD if TBI-exposed veterans are more likely to die before they can be diagnosed with PD. Given the growing evidence for several potentially modifiable risk factors for PD,43 an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent PD.
This study has many strengths, including the use of physician diagnosis of TBI and PD, longitudinal cohort design, and large sample size. Our study was sufficiently powered to allow for adjustment for many potential confounders and also to examine the association of TBI and PD stratified by TBI severity of mild vs moderate-severe, though we were underpowered to examine the association between mTBI without LOC and PD. Limitations of this study include the use of ICD-9 codes for a diagnosis of TBI and PD, which may miss some cases, such as TBI with polytrauma or mTBI sustained in the combat theatre, in which TBI is frequently underreported.44,45 While ICD-9 diagnosis of PD may be less sensitive and specific than expert consensus clinical diagnosis, we mitigated these limitations by including both inpatient and outpatient data (thereby improving sensitivity) and by demonstrating similar results in our sensitivity analysis requiring at least 3 separate encounters with a PD diagnosis (thereby improving specificity). While this study is limited by an average of 4.6 years of follow-up for the entire cohort, thus limiting our ability to impose an extended lag between TBI exposure and PD diagnosis,11 the fact that our findings were virtually unchanged after imposing a 4-year lag argues against reverse causation. Furthermore, the CTBIE screening process by definition records historical, rather than incident, TBIs that were sustained during OEF (2001–2014) and OIF (2003–2011). Thus, the actual lag time between TBI exposure and PD diagnosis in this study is likely substantially longer than the lag time between diagnosis dates.
Since 2000, more than 361,000 active duty military personnel have been diagnosed with TBI, of whom 82% sustained mTBI.46 According to population-based estimates of lifetime exposure to TBI in civilians47 and veterans,48 upwards of 40% of the 20 million veterans (e.g., >8 million veterans) alive today likely experienced a TBI at some point in their lives. In this study, we have identified a significant association between TBI, including mTBI, and development of PD in a nationwide cohort of military veterans. This finding has substantial public health implications for our active duty military and veteran populations. Furthermore, because the majority of TBIs sustained by military veterans occur during civilian life either before or after military service,35 the results of this study may additionally have important implications for civilian and athlete populations. Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and PD to inform treatment and prevention of post-TBI PD.
Glossary
- AOC
alteration of consciousness
- CI
confidence interval
- CTBIE
Comprehensive Traumatic Brain Injury Evaluation
- DoD
Department of Defense
- DVBIC
Defense and Veterans Brain Injury Center
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- LOC
loss of consciousness
- mTBI
mild traumatic brain injury
- NPCD
National Patient Care Database
- OEF
Operation Enduring Freedom
- OIF
Operation Iraqi Freedom
- PD
Parkinson disease
- PTA
posttraumatic amnesia
- TBI
traumatic brain injury
- VHA
Veterans Health Administration
Author contributions
Raquel C. Gardner: design of the study, interpretation of the data, drafting the manuscript. Amy L. Byers and Deborah E. Barnes: design of the study, interpretation of the data, revising the manuscript for intellectual content. Yixia Li: analysis of the data. John Boscardin: design of the study, analysis and interpretation of the data. Kristine Yaffe: obtained funding, design and conceptualization of the study, interpretation of the data, revising the manuscript for intellectual content.
Study funding
This study was funded by the US Army Medical Research and Material Command and by the US Department of Veterans Affairs [Chronic Effects of Neurotrauma Consortium] under awards W81XWH-13-2-0095 and 1I01CX001246. The US Army Medical Research Acquisition Activity, Fort Detrick, MD, is the awarding and administering acquisition office. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the US Government, or the US Department of Veterans Affairs, and no official endorsement should be inferred. This work was also funded by the National Institute of Neurological Disorders and Stroke (Beeson K23 NS095755 to R.C.G.), National Institute on Aging (K24 AG031155 to K.Y.), and the American Federation for Aging Research (to R.C.G.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015;66:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailes JE, Cantu RC. Head injury in athletes. Neurosurgery 2001;48:26–45; discussion 45–46. [DOI] [PubMed] [Google Scholar]
- 3.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med 2008;358:453–463. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht JS, Hirshon JM, McCunn M, et al. Increased rates of mild traumatic brain injury among older adults in US emergency departments, 2009–2010. J Head Trauma Rehabil 2016;31:E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Risk of negative outcomes after traumatic brain injury: a statewide population-based survey. J Head Trauma Rehabil 2016;31:E43–E54. [DOI] [PubMed] [Google Scholar]
- 6.Mac Donald CL, Barber J, Jordan M, et al. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol 2017;74:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016;124:511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine (US) Committee on Gulf War and Health: Brain Injury in Veterans and Long-Term Health Outcomes. Gulf War and Health: Volume 7: Long-term Consequences of Traumatic Brain Injury. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 9.Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 2015;77:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenborg L, Rugbjerg K, Lee PC, et al. Head injury and risk for Parkinson disease: results from a Danish case-control study. Neurology 2015;84:1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ 2008;337:a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson's disease risk in twins. Ann Neurol 2006;60:65–72. [DOI] [PubMed] [Google Scholar]
- 13.Department of Veterans Affairs. Screening and Evaluation of Possible Traumatic Brain Injury in Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) Veterans: VHA Directive 2010–2012. Washington, DC: Veterans Health Administration; 2010. [Google Scholar]
- 14.Sayer NA. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annu Rev Med 2012;63:405–419. [DOI] [PubMed] [Google Scholar]
- 15.AFHSC. Traumatic brain injury: DoD standard surveillance case definition for TBI adapted for AFHSB use [online]. Available at: health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Epidemiology-and-Analysis/Surveillance-Case-Definitions. Accessed June 28, 2017.
- 16.Kristman VL, Borg J, Godbolt AK, et al. Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014;95:S265–S277. [DOI] [PubMed] [Google Scholar]
- 17.Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013;28:1222–1229. [DOI] [PubMed] [Google Scholar]
- 18.Taylor KM, Saint-Hilaire MH, Sudarsky L, et al. Head injury at early ages is associated with risk of Parkinson's disease. Parkinsonism Relat Disord 2016;23:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PC, Bordelon Y, Bronstein J, Sinsheimer JS, Farrer M, Ritz B. Head injury, alpha-synuclein genetic variability and Parkinson's disease. Eur J Neurol 2015;22:874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Liu R, Zhao E, et al. Head injury, potential interaction with genes, and risk for Parkinson's disease. Parkinsonism Relat Disord 2015;21:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams DB, Annegers JF, Kokmen E, O'Brien PC, Kurland LT. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology 1991;41:1554–1557. [DOI] [PubMed] [Google Scholar]
- 22.Spangenberg S, Hannerz H, Tuchsen F, Mikkelsen KL. A nationwide population study of severe head injury and Parkinson's disease. Parkinsonism Relat Disord 2009;15:12–14. [DOI] [PubMed] [Google Scholar]
- 23.Seidler A, Hellenbrand W, Robra BP, et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology 1996;46:1275–1284. [DOI] [PubMed] [Google Scholar]
- 24.Kuopio AM, Marttila RJ, Helenius H, Rinne UK. Environmental risk factors in Parkinson's disease. Mov Disord 1999;14:928–939. [DOI] [PubMed] [Google Scholar]
- 25.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: a case-control study. Neurology 2003;60:1610–1615. [DOI] [PubMed] [Google Scholar]
- 26.Marras C, Hincapié CA, Kristman VL, et al. Systematic review of the risk of Parkinson's disease after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil 2014;95:S238–S244. [DOI] [PubMed] [Google Scholar]
- 27.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA 1998;95:6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mondello S, Buki A, Italiano D, Jeromin A. α-Synuclein in CSF of patients with severe traumatic brain injury. Neurology 2013;80:1662–1668. [DOI] [PubMed] [Google Scholar]
- 29.Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol 2007;208:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formisano R, Zasler ND. Posttraumatic parkinsonism. J Head Trauma Rehabil 2014;29:387–390. [DOI] [PubMed] [Google Scholar]
- 31.Peran P, Catani S, Falletta Caravasso C, Nemmi F, Sabatini U, Formisano R. Supplementary motor area activation is impaired in severe traumatic brain injury parkinsonism. J Neurotrauma 2014;31:642–648. [DOI] [PubMed] [Google Scholar]
- 32.Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology 2012;79:2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman SM, Kamel F, Ross GW, et al. Head injury, α-synuclein Rep1, and Parkinson's disease. Ann Neurol 2012;71:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crane PK, Gibbons LE, Dams-O'Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016;73:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner RC, Peltz CB, Kenney K, Covinsky KE, Diaz-Arrastia R, Yaffe K. Remote traumatic brain injury is associated with motor dysfunction in older military veterans. J Gerontol A Biol Sci Med Sci 2017;72:1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHugo GJ, Krassenbaum S, Donley S, Corrigan JD, Bogner J, Drake RE. The prevalence of traumatic brain injury among people with co-occurring mental health and substance use disorders. J Head Trauma Rehabil 2017;32:E65–E74. [DOI] [PubMed] [Google Scholar]
- 37.Corrigan JD, Rust E, Lamb-Hart GL. The nature and extent of substance abuse problems in persons with traumatic brain injury. J Head Trauma Rehabil 1995;10:29–46. [Google Scholar]
- 38.Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma 2017;34:2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology 2010;74:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson H, Nordstrom A, Nordstrom P. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology 2015;84:2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritz B, Lee PC, Lassen CF, Arah OA. Parkinson disease and smoking revisited: ease of quitting is an early sign of the disease. Neurology 2014;83:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacob EL, Gatto NM, Thompson A, Bordelon Y, Ritz B. Occurrence of depression and anxiety prior to Parkinson's disease. Parkinsonism Relat Disord 2010;16:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotagal V, Albin RL, Muller ML, Koeppe RA, Frey KA, Bohnen NI. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology 2014;82:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson NW, Anderson CR, Thuras P, et al. Factors associated with inconsistency in self-reported mild traumatic brain injury over time among military personnel in Iraq. Br J Psychiatry 2015;206:237–244. [DOI] [PubMed] [Google Scholar]
- 45.Chapman JC, Diaz-Arrastia R. Military traumatic brain injury: a review. Alzheimers Dement 2014;10:S97–S104. [DOI] [PubMed] [Google Scholar]
- 46.DoD Worldwide Numbers for TBI. Falls Church, VA: Defense and Veterans Brain Injury Center; 2017. [Google Scholar]
- 47.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: a statewide population-based survey. J Head Trauma Rehabil 2016;31:E55–E62. [DOI] [PubMed] [Google Scholar]
- 48.Veitch DP, Friedl KE, Weiner MW. Military risk factors for cognitive decline, dementia and Alzheimer's disease. Curr Alzheimer Res 2013;10:907–930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study were obtained from VHA electronic health records and contain protected health information, including age, scrambled social security number, date of birth, date of death, and dates of medical diagnoses. Therefore, we are unable to share our dataset with other investigators or place it in a repository. Investigators wishing to replicate these analyses may contact the authors to discuss the process of obtaining access to VHA data.