Abstract
Objective
To assess dose-response effects of the anti-CD20 monoclonal antibody ofatumumab on efficacy and safety outcomes in a phase 2b double-blind study of relapsing forms of multiple sclerosis (RMS).
Methods
Patients (n = 232) were randomized to ofatumumab 3, 30, or 60 mg every 12 weeks, ofatumumab 60 mg every 4 weeks, or placebo for a 24-week treatment period, with a primary endpoint of cumulative number of new gadolinium-enhancing lesions (per brain MRI) at week 12. Relapses and safety/tolerability were assessed, and CD19+ peripheral blood B-lymphocyte counts measured. Safety monitoring continued weeks 24 to 48 with subsequent individualized follow-up evaluating B-cell repletion.
Results
The cumulative number of new lesions was reduced by 65% for all ofatumumab dose groups vs placebo (p < 0.001). Post hoc analysis (excluding weeks 1–4) estimated a ≥90% lesion reduction vs placebo (week 12) for all cumulative ofatumumab doses ≥30 mg/12 wk. Dose-dependent CD19 B-cell depletion was observed. Notably, complete depletion was not necessary for a robust treatment effect. The most common adverse event was injection-related reactions (52% ofatumumab, 15% placebo), mild to moderate severity in 97%, most commonly associated with the first dose and diminishing on subsequent dosing.
Conclusion
Imaging showed that all subcutaneous ofatumumab doses demonstrated efficacy (most robust: cumulative doses ≥30 mg/12 wk), with a safety profile consistent with existing ofatumumab data. This treatment effect also occurred with dosage regimens that only partially depleted circulating B cells.
Classification of evidence
This study provides Class I evidence that for patients with RMS, ofatumumab decreases the number of new MRI gadolinium-enhancing lesions 12 weeks after treatment initiation.
Selectively targeting B cells with anti-CD20 monoclonal antibodies (mAbs), initially shown with genetically engineered chimeric rituximab1,2 and subsequently with the humanized ocrelizumab,3,4 has proved highly effective at limiting disease activity in patients with relapsing forms of multiple sclerosis (RMS). These studies used intravenous dosing that essentially depletes circulating B cells and substantially reduces the development of new brain lesion activity based on MRI.3,4 However, whether efficacy could be obtained with incomplete peripheral B-cell depletion is of considerable interest, especially considering longer-term treatment in patients with chronic disease. The current study used a range of subcutaneous dose regimens of the human anti-CD20 mAb ofatumumab to identify the minimally effective dose for the treatment of RMS.
Ofatumumab binds to a small-loop epitope of CD20 close to the cell surface, inducing efficient complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity, even when CD20 expression is low.5,6 Intravenous ofatumumab is approved for the treatment of chronic lymphocytic leukemia. A small phase 2 dose-escalation study indicated that intravenous ofatumumab at B-cell–depleting doses (100, 300, and 700 mg) resulted in a robust (≈99%) reduction in new MRI lesion activity in patients with RMS.7 Development of subcutaneous ofatumumab anti-CD20 therapy could simplify administration and has been demonstrated to be well tolerated when used in a small rheumatoid arthritis (RA) study.8
The aim of this study (Ofatumumab Subcutaneous Administration in Subjects With Relapsing-Remitting Multiple Sclerosis [MIRROR]) was to determine whether a range of doses of subcutaneous ofatumumab reduced new brain lesion development in patients with RMS and whether dose-dependent B-cell depletion and repletion kinetics could be demonstrated with anti-CD20 antibody therapy.
Methods
Patients
Eligibility criteria are detailed in the e-supplement, links.lww.com/WNL/A437. Briefly, the trial enrolled patients 18 to 55 years of age with active RMS and an Expanded Disability Status Scale (EDSS) score of 0 to 5.5. Key exclusions were prior use of experimental agents, mAbs (except natalizumab), or immunosuppressive agents. Prior use of other disease-modifying therapies (DMTs) was allowed.
Standard protocol approvals, registrations, and patient consents
The study protocol (clinicaltrials.gov NCT01457924; gsk-clinicalstudyregister.com OMS112831) was approved by all central and local ethics committees. All patients provided written informed consent.
Study design, randomization, and blinding
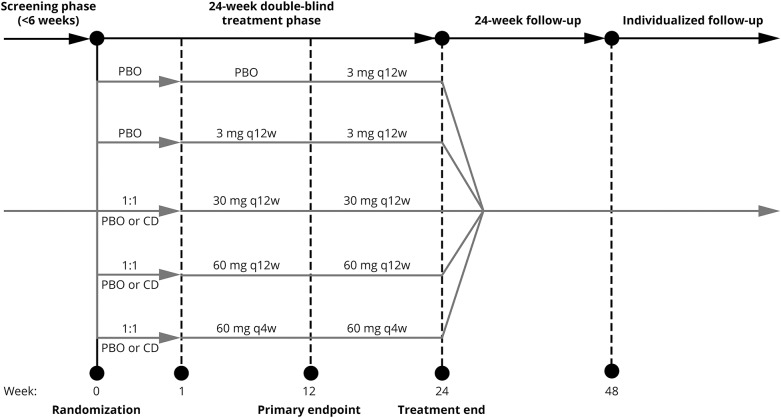
This was a phase 2b, multicenter, randomized, double-blind, placebo-controlled study with 4 phases: screening, 24-week treatment, 24-week follow-up (FU), and individualized FU (IFU) (figure 1). During the first 12 weeks of the treatment phase, eligible patients were randomized (2:1:1:1:2) to placebo or ofatumumab 3-, 30-, or 60-mg doses every 12 weeks or 60 mg every 4 weeks, respectively (dose selection was based on results of an RA single subcutaneous dose study).8 The 12-week placebo-controlled period was considered sufficient to estimate efficacy and dose response of ofatumumab relative to placebo while balancing ethics concerns over prolonged placebo exposure. At week 12, all patients in the placebo group received a single 3-mg ofatumumab dose. The treatment schedule of all groups originally randomized to receive ofatumumab was maintained weeks 12 to 24.
Figure 1. Study design.
Screening was performed up to 6 weeks before randomization. After completion (or premature discontinuation) of the 24-week treatment phase, patients entered the 24-week follow-up, which assessed patient safety and B-cell repletion. Thereafter (week 48 onwards), individual patients whose CD19+ B-lymphocyte counts remained below the LLN and who did not start a DMT, entered the IFU period. CD = conditioning dose; PBO = placebo; q4w = every 4 weeks; q12w = every 12 weeks.
To evaluate whether tolerability to the higher ofatumumab doses could be enhanced by administration of an initial, smaller dose of ofatumumab (which may provide more gradual lysis of B cells and reduce cytokine release reactions), 1 week before their first treatment dose (week 0), patients in the ofatumumab 30 and 60 mg groups were randomized (1:1 ratio) to receive either placebo or a conditioning dose of ofatumumab (3 mg).
Acetaminophen and an antihistamine (cetirizine or equivalent) were administered orally up to 2 hours before each injection. Randomization was computer generated. Patients were administered either placebo or the designated dose of ofatumumab (1.0-mL volume each) by trained site personnel at weeks 0, 1, 4, 8, 12, 16, and 20 to help maintain blinding of patients, neurologists, and study staff. After completion of the treatment phase, the 24-week FU phase monitored patient safety and B-cell repletion. From week 48, individual patients whose CD19+ B-lymphocyte counts remained below the lower limit of normal (LLN) and who did not start a DMT entered the IFU phase to assess B-cell repletion.
Study endpoints
Study endpoints are detailed in the e-supplement, links.lww.com/WNL/A437. The primary efficacy endpoint was defined as the cumulative number of new gadolinium-enhancing (GdE) brain lesions at week 12 (based on T1-weighted MRI scans at weeks 4, 8, and 12). Other MRI endpoints were the cumulative number of new GdE lesions at week 24 and cumulative number and total volume of new and new plus persisting GdE lesions, new and/or newly enlarging T2 lesions, and T1-hypointense lesions at weeks 12 and 24. Double-dose gadolinium (0.2 mmol/kg) contrast was used to enhance detection of MS lesions.9 MRI scans were analyzed by a central vendor blinded to patient treatment (Perceptive Informatics, Billerica, MA). Clinical efficacy was assessed as the proportion of patients who were relapse free from weeks 0 to 12. Other clinical endpoints included the EDSS, Multiple Sclerosis Functional Composite (MSFC), and Modified Fatigue Impact Scale (MFIS) scores.
B-cell depletion and repletion kinetics were assessed by CD19+ peripheral blood B-lymphocyte counts with routine fluorescence-activated cell sorter analysis (lower limit of quantification 5 cells/μL). Ofatumumab trough concentrations were assessed with standard enzyme-linked immunosorbent assay (lower limit of quantification 100 ng/mL) in all patients at weeks 12 and 24 and in a selected pharmacokinetic subpopulation at 1, 2, 3, 4, 7, 14, and 21 days after dose (n = 28).
Safety was assessed on the basis of adverse event (AE) reporting, the Columbia Suicidality Severity Rating Scale, vital signs, physical and neurologic examinations, laboratory analyses, and immunogenicity (development of human anti-human antibody [HAHA]) with the Meso Scale electrochemiluminescence.
Statistical analysis
The primary research question was whether subcutaneous ofatumumab reduces the development of new GdE brain lesions in patients with RMS (Class I evidence). A sample size of 196 patients was estimated from the Sorman et al.10 placebo estimates (and scaled for the number of MRIs planned in this study) to provide 90% power to detect a 63% reduction between the highest ofatumumab dose group (60 mg every 4 weeks) and placebo and to detect a significant dose response at the 5% significance level for the primary endpoint based on a generalized linear model with underlying negative binomial distribution (e-supplement, links.lww.com/WNL/A437).
The primary efficacy population was the modified intent-to-treat (mITT) population (all patients randomized to treatment who took ≥1 dose of placebo or ofatumumab and who had ≥1 postscreening MRI assessment). The primary dataset for MRI endpoints was all evaluable scans, including all on-treatment MRI scans for each patient in the mITT population, allowing estimates of lesion rates to be calculated across the entire 12-week treatment phase. All MRI endpoint analyses were cumulative counts (intermediate time points were not analyzed separately) and based on preplanned screening MRI stratification for either no GdE lesions or ≥1 GdE lesions. To account for protocol violations, a per-protocol population was also considered in the efficacy analyses (e-supplement, links.lww.com/WNL/A437). For clinical efficacy assessing relapses, the proportion of patients relapsing in each ofatumumab dose group was compared with placebo by the Fisher exact test.
B-cell depletion and repletion were summarized as actual counts and change from baseline counts of CD19+ B lymphocytes. Additional post hoc analyses were conducted with a generalized linear model with negative binomial regression of new lesions used as a linear function of weighted mean CD19+ B cells.
Safety analyses (descriptive statistics) were conducted on the safety population, comprising all patients randomized and receiving study medication. MSFC and MFIS scores were analyzed with an analysis of covariance model adjusting for baseline score and stratum. Safety endpoints were monitored to the end of IFU. Time to B-cell repletion from the last ofatumumab dose to the LLN or baseline (if less than the LLN) was estimated with Kaplan-Meier methods.
Data availability
The results summary for this study (NCT01457924/OMS112831) is available on clinicaltrials.gov, the default register for GlaxoSmithKline Human Subject Research. If the study does not meet the criteria for posting to clinicaltrials.gov, the study will be available on the GlaxoSmithKline Clinical Study Register at gsk-clinicalstudyregister.com. For interventional studies that evaluate our medicines, anonymized patient-level data will be made available to independent researchers, subject to review by an independent panel, at clinicalstudydatarequest.com within 6 months of publication. To protect the privacy of patients and individuals involved in our studies, GlaxoSmithKline does not publicly disclose patient-level data.
Results
Patient disposition
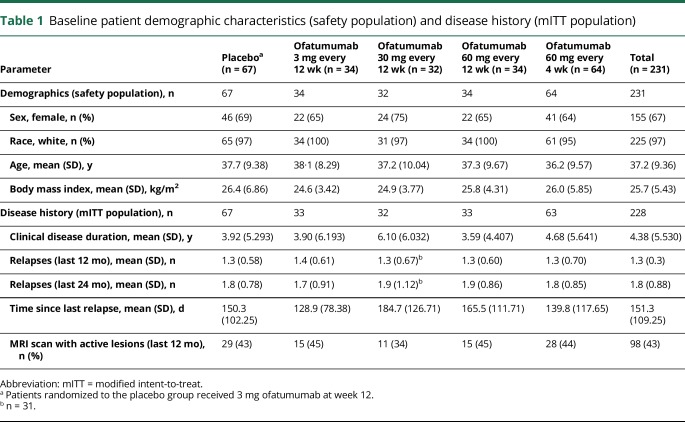
In total, 232 patients were randomized, with 231 receiving ≥1 doses of study drug (analyzed as the safety population) (figure e-1). Of these, 219 (95%) patients completed treatment through week 12, with 214 completing through week 12 to 24. Three of the 231 patients did not have a postbaseline MRI, leaving 228 analyzed for efficacy as the mITT population. At week 24, 221 (96%) patients entered the FU phase, including 7 of 17 patients who withdrew from treatment. A total of 212 (92%) patients completed to week 48. Of the 112 patients entering the IFU phase, 88 completed this phase (e-supplement, links.lww.com/WNL/A437). Baseline characteristics of the safety and mITT populations were generally balanced across treatment groups (table 1).
Table 1.
Baseline patient demographic characteristics (safety population) and disease history (mITT population)
Efficacy
MRI endpoints
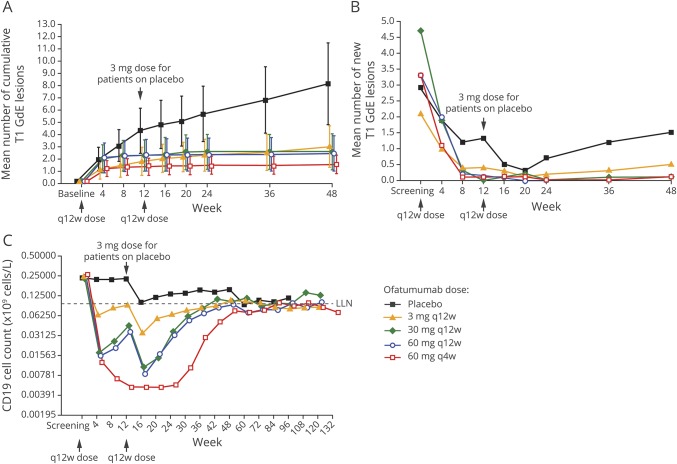
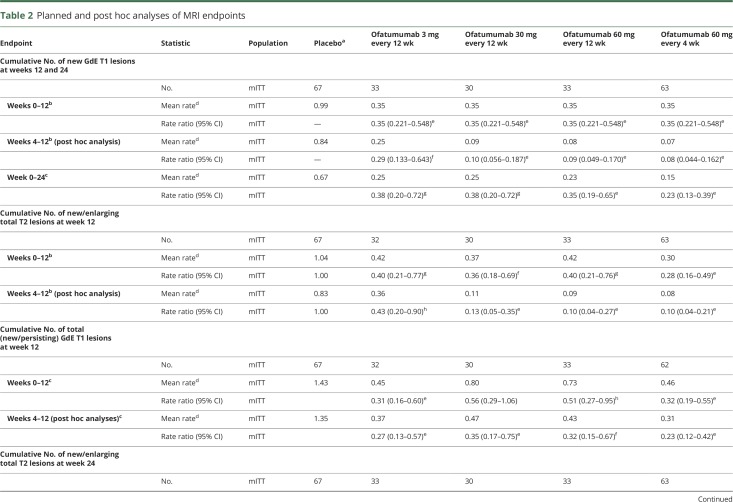
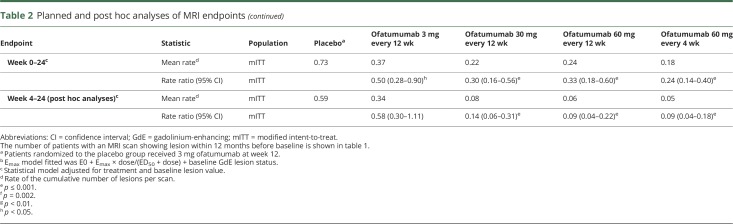
The primary outcome analysis (mITT population) demonstrated a statistically significant 65% reduction in the mean rate of cumulative new GdE lesions for all ofatumumab groups vs placebo between weeks 0 and 12 (rate ratio 0.35, 95% confidence interval [CI] 0.221–0.548, p < 0.001) (figure 2A and table 2). This effect was evident as early as week 4, when the same results are depicted as the mean number of new GdE lesions counted at the same time points (figure 2B). In post hoc analysis, which more closely reflects the approach used in prior studies2,3 (e-supplement, links.lww.com/WNL/A437), the reduction in the mean rate of cumulative new GdE lesions from weeks 4 to 12 ranged from 71% (0.29 [95% CI 0.133–0.643]) to 92% (0.08 [95% CI 0.044–0.162]) across ofatumumab groups vs placebo (p ≤ 0.002), with ≥90% suppression of new lesions at all cumulative doses ≥30 mg over 12 weeks (0.08 [95% CI 0.044–0.162] to 0.10 [95% CI 0.056–0.187]) (table 2 and figure e-2, links.lww.com/WNL/A435). All secondary MRI endpoints, including the cumulative number of T2 lesions (figure e-3, links.lww.com/WNL/A435), as well as all analyses in the per-protocol population, supported the primary analysis (tables e-1 and e-2, links.lww.com/WNL/A436).
Figure 2. Efficacy and pharmacodynamics.
(A) Primary efficacy outcome measure: mean (95% confidence interval) cumulative number of GdE T1 lesions over time (all evaluable scans dataset). (B) New lesion evolution (post hoc): mean number of new GdE T1 lesions at different MRI time points. From week 8 through 24, the appearance of new GdE T1 lesions was very low at doses of ≥30 mg every 12 weeks. (C) Pharmacodynamic response showing dose-response depletion of CD19 B cells and repletion kinetics (safety population). The median time to repletion based on Kaplan-Meier estimates was ≈11 months for the ofatumumab 3 and 30 mg every 12 weeks groups and ≈14 months for the ofatumumab 60 mg every 12 and 4 weeks groups. (A) Faster repletion time (of ≈6 months) was noted for the placebo group, who received a single ofatumumab 3 mg dose at week 12 (and in whom 32% did not deplete). Of those patients whose B cells had repleted by the end of the study, the time to repletion appeared to generally be longer in the 60-mg ofatumumab dose groups compared with the other ofatumumab dose groups. There were no signs of B-cell repletion during the 4-week interdosing interval with the every 4 weeks regimen. Some B-cell repletion was seem. GdE = gadolinium-enhancing; LLN = lower limit of normal; q4w = every 4 weeks; q12w = every 12 weeks.
Table 2.
Planned and post hoc analyses of MRI endpoints
Clinical endpoints
Overall, 26 patients relapsed during the first 12 weeks; 11 (42%) relapsed during the first 4 weeks (table e-3, links.lww.com/WNL/A436). Although more relapses occurred in the placebo group, the proportion of patients relapsing during the first 4 weeks (6%–13% across the ofatumumab groups vs 13% placebo) did not differ statistically (p ≥ 0.488). Over the 24-week period, 17 (25%) patients relapsed in the placebo group vs 3 to 10 patients (9%–22%) across the ofatumumab groups. The proportion of relapses remained low throughout the 24-week FU phase across all dose groups (6%–15%). There were no significant differences between ofatumumab and placebo in MSFC and MFIS scores. Most patients (79%) had unchanged EDSS scores at weeks 12 and 24 with no notable differences between groups.
Pharmacodynamics
A dose-dependent depletion of B cells was observed, with greater depletion for the 60-mg dose every 4 weeks (to <2% of baseline levels at maximum depletion) and the 30- and 60-mg dose every 12 weeks (to ≈5% of baseline) than for the 3-mg dose every 12 weeks (to ≈25% of baseline) (figure 2C). While all dose groups appeared to exhibit similar rates of B-cell repopulation, time to onset of repopulation appeared longer for the higher-dose groups. By study end, B-cell repletion was achieved by 64% to 74% of patients across ofatumumab groups (table e-4, links.lww.com/WNL/A436). Post hoc analyses indicated a statistically significant relationship between weighted mean B-cell count and new GdE lesions (slope 0.63, 95% CI 0.41–0.85, p < 0.001) (figure e-4, links.lww.com/WNL/A435).
Safety
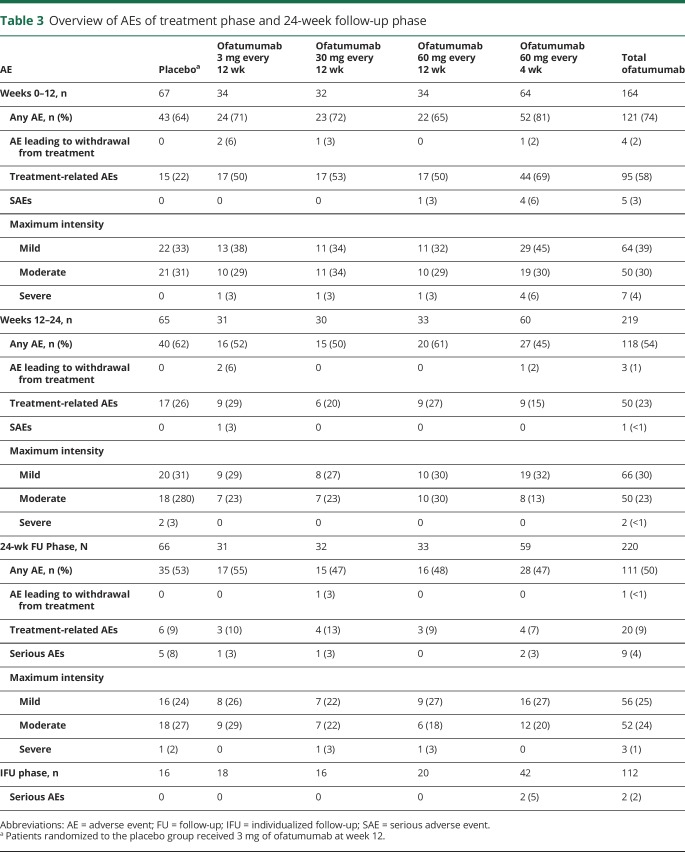
In total, 43 (64%) patients receiving placebo and 121 (74%) receiving ofatumumab (65%–81% across dose groups) experienced AEs during weeks 0 to 12. During weeks 12 to 24 and 24 to 48, the proportions of patients who experienced AEs across treatment groups were 45% to 62% and 47% to 55%, respectively (table 3). AEs were largely mild to moderate in severity, and no patients died. Incidences of serious AEs (SAEs) were 3%, <1%, 4%, and <1% in weeks 0 to 12, 12 to 24, and 24 to 48 and the IFU phase, respectively. The only SAEs to occur in ≥1 patient during the treatment phase were injection-related reactions (IRRs), occurring in 3 patients; all continued in the study, including 1 patient who reportedly experienced a cytokine-release syndrome within hours of the first ofatumumab (60 mg) dose. Other SAEs occurring in single patients were cholelithiasis and hypokalemia (both with 60 mg ofatumumab every 4 weeks) and angioedema and urticaria (both in the same patient receiving 3 mg ofatumumab). There was no pattern of SAEs in the 24-week FU phase. During the IFU, 2 (2%) patients, both in the ofatumumab 60 mg every 4 weeks group, reported a total of 2 SAEs: head injury and malignant melanoma stage IV. The latter was considered treatment related, and the patient recovered (as noted by the investigator).
Table 3.
Overview of AEs of treatment phase and 24-week follow-up phase
The incidence of AEs was highest in the ofatumumab 60 mg every 4 weeks group during weeks 0 to 12 of treatment (81%) and was lowest in this group during weeks 12 to 24 (45%). The most common week 0 to 12 AEs in the ofatumumab groups were IRRs (41%–66% vs 15% for placebo, table e-5, links.lww.com/WNL/A436, and figure e-5, links.lww.com/WNL/A435). Their incidence was similar for each regimen regardless of preconditioning dose (table e-6, links.lww.com/WNL/A436). Most IRRs were of mild to moderate severity, resolved the same or following day, and were associated primarily with the first ofatumumab dose (29%–50%); their incidence diminished with subsequent ofatumumab dosing (1%–18% at week 12).
Overall rates of any infection-related AEs were similar across treatment groups (table e-5, links.lww.com/WNL/A436), with no cases of opportunistic infections (including progressive multifocal leukoencephalopathy) or hepatitis B reactivation. There were no clinically meaningful changes in mood, vital signs, or laboratory parameters, including cytopenias. Four patients (3 at 3 mg and 1 at 30 mg ofatumumab) had a single positive result for HAHAs during the treatment phase (all titers ≤32), and 1 patient (3 mg) also had a positive titer during FU phase week 36 (negative at week 48); B-cell depletion was as expected in all.
No new or unexpected safety findings occurred in either the week 24 to 48 FU or IFU periods. AEs leading to withdrawal were reported in ≤2% of patients in each phase. In total, 8 patients discontinued because of AEs, mostly IRRs (2 patients) and decreased immunoglobulin G (2 patients). Further pharmacokinetic results are presented in figure e-6, links.lww.com/WNL/A435.
Discussion
This phase 2b study of subcutaneously administered ofatumumab used a wide range of doses to explore a minimally effective dose as a potential treatment for relapsing-remitting MS (RRMS). In the primary (week 0–12) efficacy analysis, ofatumumab treatment across dosing regimens significantly reduced new GdE lesions by 65% vs placebo. Early MRI time points are often excluded in a priori efficacy analyses2,3,7 because disease activity initiated before the onset of action of therapy could potentially dilute actual treatment effect. In keeping with this, post hoc analysis excluding week 0 to 4 MRI data revealed an even greater (≥90%) reduction of new GdE lesions vs placebo for ofatumumab at all doses ≥30 mg every 12 weeks. Similar patterns were seen with multiple secondary imaging outcome measures and with the number of patients relapsing (weeks 4–12 less than weeks 0–4). Week 4 was used as the baseline in post hoc analyses regardless of whether a conditioning dose of 3 mg ofatumumab was administered. While it is possible that the conditioning dose may have confounded the data, this study focused on the steady-state outcome, and we do not believe the conditioning dose would have had a significant effect on efficacy in the context of the overall exposure to the higher doses at steady state.
Currently approved anti-CD20 treatment generally results in complete/near-complete depletion of circulating B cells, although it is not clear that this is necessary to achieve a high level of efficacy.4 Here, ofatumumab treatment resulted in rapid dose-dependent B-cell depletion, which correlated with efficacy outcomes. A cumulative dose of 60 mg ofatumumab administered over 12 weeks provided maximal benefit, with no additional suppression of lesions at higher cumulative doses. Distinct from other studies of B-cell depletion in RRMS, ofatumumab dosage regimens that did not completely deplete circulating B cells could achieve robust treatment effects. Indeed, while the ofatumumab 3-mg dose every 12 weeks reduced circulating B-cell levels to ≈25% of baseline (clearly less than the 5% of baseline achieved with 30- and 60-mg dose every 12 weeks), it was surprisingly effective in significantly reducing new T1 GdE lesions, with a 71% reduction in mean rate of cumulative new GdE lesions from weeks 4 to 12. On the basis of post hoc analyses, ≥90% suppression of GdE MRI lesion activity appeared to be achievable in this study when B cells were depleted to a level of ≈32 cells/µL (although the meaning and utility of such a measure require further study). B-cell repletion between dosing was seen with the less frequent administration of ofatumumab (i.e., every 12 weeks) but not with the more frequent dosing (i.e., every 4 weeks), and the time to repletion was longer for the higher-dose groups. Repletion in all ofatumumab doses occurred faster than previously reported with anti-CD20 therapy.4,11 A dose response in the kinetics of depletion-repletion suggested that higher-dose/higher-frequency regimens result in greater depth of B-cell depletion in tissues. This insight from patients is consistent with anti-CD20 animal studies in which higher doses led to greater depth of B-cell depletion in different lymphoid tissues.12,13 Indeed, the kinetics of repletion may be more informative than the degree of initial depletion, especially with regimens that result in near-complete depletion, where the nadir of circulating B cells becomes an insensitive measure of both the depth of initial B-cell depletion and the onset of repletion.
Prior ofatumumab exposure (in oncology, MS, and other autoimmune disorders)7,14–17 has largely been intravenous, with the exception of a limited single subcutaneous dose study in RA.8 We report overall good tolerability and no new/unexpected safety findings with subcutaneous ofatumumab. As expected, IRRs were the most common AEs; most were associated with the first dose of ofatumumab and resolved within 1 day of onset. We found no benefit of adding a (3 mg) conditioning dose. Subcutaneous injection may have greater practicality compared with intravenous administration requiring repeat access to health care providers or infusion facilities. For some patients, more control over the exact timing and circumstance of administration would also be an advantage. As a fully human (compared with chimeric or humanized18) antibody, ofatumumab would be expected to exhibit very low immunogenicity, and indeed, no HAHAs were reported in the ofatumumab intravenous study in MS.7 In the current study, very low-titer HAHAs were reported in 4 patients. Further investigation into the incidence of HAHAs with ofatumumab is warranted.
Overall, this study demonstrates that ofatumumab has a high capacity to suppress new brain MRI lesions with subcutaneous administration at considerably lower (and incompletely B-cell depleting) doses compared with those previously studied in patients with MS. The ≥90% suppression of new T1 GdE and T2 lesions with ofatumumab is consistent with the effects demonstrated by other anti-CD20 mAbs at doses resulting in maximal peripheral B-cell depletion.2–4 The prospect of an efficacious subcutaneous B-cell–targeting therapy raises the possibility of self-administration and therefore improvement over intravenous administration in terms of both convenience of use and the use of health care resources. It remains to be seen whether the less profound depletion and faster repletion of B cells achieved with ofatumumab will also translate into a more favorable safety profile. Our findings thus support investigation of low-dose subcutaneous ofatumumab in longer-term efficacy studies in RRMS.
Acknowledgment
The sponsors and authors thank the patients who volunteered to participate in this study and acknowledge Ken Wiesen, PhD, Francesca Balordi, PhD (Medicus International New York), and Joyce Willetts, PhD (consultant for PharmaWrite), for writing assistance funded by GlaxoSmithKline. The sponsors and authors also thank each of the 52 investigators and their participating sites, as well as the Independent Data Monitoring Committee (Chris Polman, Aaron Miller, Gary Cutter) and Progressive Multifocal Leukoencephalopathy Adjudication Committee (David Clifford, Eugene Major, Avindra Nath).
Glossary
- AE
adverse event
- CI
confidence interval
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- FU
follow-up
- GdE
gadolinium-enhancing
- HAHA
human anti-human antibody
- IFU
individualized follow-up
- IRR
injection-related reaction
- LLN
lower limit of normal
- mAb
monoclonal antibodies
- MFIS
Modified Fatigue Impact Scale
- MIRROR
Ofatumumab Subcutaneous Administration in Subjects With Relapsing-Remitting Multiple Sclerosis
- mITT
modified intent-to-treat
- MSFC
Multiple Sclerosis Functional Composite
- RA
rheumatoid arthritis
- RMS
relapsing multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- SAE
serious adverse event
Footnotes
Class of Evidence: NPub.org/coe
Author contributions
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Amit Bar-Or, MD, designed the study, was a study investigator, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Richard A. Grove, MSc, designed the study, did statistical analyses, was involved in the conduct of the study, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Daren J. Austin, PhD, designed the study, was responsible for pharmacokinetic and pharmacodynamic analyses, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Jerry M. Tolson, PhD, designed the study, was involved in the conduct of the study, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Susan A. VanMeter, MD, and Eric W. Lewis, MD, were involved in the conduct of the study, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Frederick J. Derosier, DO, designed the study, was involved in the conduct of the study, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Monica C. Lopez was involved in the conduct of the study, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Sarah T. Kavanagh, MPH, did statistical analyses, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Aaron E. Miller, MD, served on the Independent Data Monitoring Committee, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published. Per S. Sorenson, MD, designed the study, was a study investigator, reviewed and interpreted the data, participated in writing the initial manuscript draft, and approved the final version of the manuscript to be published.
Study funding
This study (ClinicalTrials.gov NCT01457924) was sponsored by GlaxoSmithKline (GlaxoSmithKline study number OMS112831). Editorial support in the form of collating author comments, grammatical editing, and submission support was provided by Alex Lowe, PhD, of Fishawack Indicia Ltd, funded by GlaxoSmithKline.
Disclosure
A. Bar-Or, R. Grove, D. Austin, J. Tolson, S. VanMeter, E. Lewis, F. Derosier, M. Lopez, and S. Kavanagh were employees of/stockholders in GlaxoSmithKline at the time of this study. A. Miller has served as a consultant or a member of the scientific advisory board for GlaxoSmithKline. P. Sorenson has served on scientific advisory boards for Genmab (a codeveloper of ofatumumab) and GlaxoSmithKline; has served on steering committees or independent data monitoring boards in clinical trials sponsored by Genmab and GlaxoSmithKline and has received funding of travel for these activities; and has served as editor-in-chief of the European Journal of Neurology. Go to Neurology.org/N for full disclosures.
References
- 1.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008;63:395–400. [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 3.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. [DOI] [PubMed] [Google Scholar]
- 4.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. [DOI] [PubMed] [Google Scholar]
- 5.Bleeker WK, Munk ME, Mackus WJ, et al. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol 2008;140:303–312. [DOI] [PubMed] [Google Scholar]
- 6.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 2006;177:362–371. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 2014;82:573–581. [DOI] [PubMed] [Google Scholar]
- 8.Kurrasch R, Brown JC, Chu M, et al. Subcutaneously administered ofatumumab in rheumatoid arthritis: a phase I/II study of safety, tolerability, pharmacokinetics, and pharmacodynamics. J Rheumatol 2013;40:1089–1096. [DOI] [PubMed] [Google Scholar]
- 9.Stecco A, Migazzo E, Saponaro A, et al. Gadolinium dose optimisation in patients with multiple sclerosis: intra- and inter-individual comparisons. Eur J Radiol 2006;57:37–42. [DOI] [PubMed] [Google Scholar]
- 10.Sorman MP, Bruzzi P, Rovaris M, et al. Modelling new enhancing MRI lesion counts in multiple sclerosis. Mult Scler 2001;7:298–304. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Kaine JL, Lowenstein MB, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 2008;58:2652–2661. [DOI] [PubMed] [Google Scholar]
- 12.Chan AC. B cell immunotherapy in autoimmunity: 2010 update. Mol Immunol 2011;48:1344–1347. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann-Horn K, Kronsbein HC, Weber MS. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther Adv Neurol Disord 2013;6:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010;28:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011;118:5126–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostergaard M, Baslund B, Rigby W, et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum 2010;62:2227–2238. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis 2011;70:2119–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004;104:1793–1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The results summary for this study (NCT01457924/OMS112831) is available on clinicaltrials.gov, the default register for GlaxoSmithKline Human Subject Research. If the study does not meet the criteria for posting to clinicaltrials.gov, the study will be available on the GlaxoSmithKline Clinical Study Register at gsk-clinicalstudyregister.com. For interventional studies that evaluate our medicines, anonymized patient-level data will be made available to independent researchers, subject to review by an independent panel, at clinicalstudydatarequest.com within 6 months of publication. To protect the privacy of patients and individuals involved in our studies, GlaxoSmithKline does not publicly disclose patient-level data.