Abstract
Among various genome editing tools available for functional genomic studies, reagents based on clustered regularly interspersed palindromic repeats (CRISPR) have gained popularity due to ease and versatility. CRISPR reagents consist of ribonucleoprotein (RNP) complexes formed by combining guide RNA (gRNA) that target specific genomics regions and a CRISPR associated nuclease (Cas). The gRNA targeting specific gene sequences may be delivered as a plasmid construct that needs to be transcribed or as a synthetic RNA. The Cas nuclease can be introduced as a plasmid construct, mRNA, or purified protein. The efficiency of target editing is dependent on intrinsic factors specific to each species, the target gene sequence, and the delivery methods of CRISPR gRNA and the Cas nuclease. Although intrinsic factors affecting genome editing may not be altered in most experiments, the delivery method for CRISPR/Cas reagents can be optimized to produce the best results. In this study, the efficiency of genome editing by CRISPR/Cas system in the bollworm, Helicoverpa zea (Boddie), was evaluated using ribonucleoprotein (RNP) complexes assembled by binding synthetic gRNA with purified Cas9 nuclease engineered with nuclear localization signals to target the vermillion (eye color) gene. Mutation rates of adults emerging from embryos microinjected with 1, 2, or 4 μM RNP complexes were compared using replicated experiments. Embryos injected with 2 or 4 μM RNP complexes displayed significantly higher mutation rates (>88%) in surviving adults compared to those injected with 1 μM. The hatch rate in embryos injected with RNP complexes and with injection buffer only (mock injections) was reduced by 19.8(±5.2)% compared to noninjected control embryos, but did not differ significantly between injected embryos. Evaluation of potential off-target sites in H. zea genome did not identify any mutations. This study demonstrates that in vitro assembled synthetic RNP complexes can be used to obtain high genome editing rates in a reproducible manner in functional genomics or genetic manipulation studies.
Introduction
Development of clustered regularly interspersed palindromic repeats (CRISPR) and associated RNA guided Cas nucleases has progressed rapidly since its discovery in 1987 [1–9]. CRISPR is widely used for editing genomes of various organisms ranging from bacteria to mammals. With more than 7,400 manuscripts published from 2005 to 2018, CRISPR has arguably become the most frequently used tool for in vivo site-directed mutagenesis (for an overwiev, see [10]). Recent reviews indicate that CRISPR-mediated genome editing has successfully been conducted in 27 arthropods, of which 23 were insects from five orders including 11 lepidopteran species (e.g. [11–18]).
In insects, genome editing has been achieved by microinjecting freshly laid eggs (embryos) with various combinations of CRISPR reagents. Production of guide RNA (gRNA) targeting the DNA sequences of interest has been carried out using plasmid constructs, in vitro transcription, or chemical synthesis (e.g. [19–24]). Similarly, Cas nucleases have been produced in situ by microinjecting plasmid constructs or mRNA, or delivered into embryos as purified protein [14,19,25–28]. Regardless of the mode or mechanism of gRNA and Cas nuclease delivery, functional ribonucleoprotein (RNP) complex formation by the two components is crucial for successful editing of DNA. In embryo microinjections, success and efficiency of heritable genome editing are dependent on a number of factors including timing of CRISPR/Cas delivery, concentration of the RNP complex, and specificity of gRNA to the target sequence. Formation of the RNP complex from the gRNA and Cas nuclease reagents prior to beginning of embryonic cell division is a critical step in achieving germline editing. Therefore, any delay in transcription of gRNA and Cas from plasmid constructs or translation of Cas mRNA may lead to reduced germline editing efficiency as well as multiple “chimeric” mutations in the embryonic cell population (e.g. [29]). In addition, RNA transcription from plasmids is highly dependent on the promoter sequence; a promoter from one species may not work optimally in a different species. Similarly, mRNA translation efficiency may differ among insects, resulting in highly variable concentrations of functional gRNA/Cas RNP complexes from identical constructs in different species. Microinjecting RNP complexes pre-assembled from synthetic gRNA and purified Cas9 proteins for genome editing is gaining popularity due to the convenience of using commercially prepared high purity reagents and the ability to deliver precise RNP concentrations that facilitate reproducibility of experiments. [30–34]. In addition, quantitative delivery of reagents increase the fidelity of comparative studies between species or between different gene targets in a single species.
Type II CRISPR-Cas systems from bacteria consist of two different RNA molecules: CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) [35–37]. In bacteria, tracrRNA is involved in maturity of crRNA and form functional guide RNA (gRNA) by base pairing of complementary nucleotide sequences present in both molecules. Therefore, the universal tracrRNA molecule can be used to target any number of DNA sequences by simply synthesizing the crRNA molecules specific to each target. These two components can be annealed in vitro and bound with Cas nuclease stoichiometrically to yield RNP complexes of precise concentrations. Furthermore, performance of the Cas nuclease has been improved through the addition of nuclear localization signals (NLS) from various proteins such as SV40 large T-antigen and nucleoplasmin [38,39]. These NLS sequences are recognized by chaperones (e.g. importin α) and are actively transported to the nucleus, facilitating interactions with chromatin. The objective of this study was to identify optimal concentration of RNP complexes for high efficient genome editing in the bollworm, Helicoverpa zea (Boddie). We used synthetic crRNA targeting a morphological marker, the tryptophan 2,3-dioxygenase (TO) gene, which is the first enzyme in the ommochrome synthesis pathway [40]. The loss of function mutations in the TO gene in some insects (e.g. Drosophila melanogaster and Tribolium castaenum) alters wild type eye color to produce a vermillion phenotype. Guide RNA generated by annealing TO crRNA and tracrRNA was combined with a Cas9 nuclease to assemble RNP complexes for quantitative microinjection of embryos to optimize gene editing in H. zea.
Material and methods
Tryptophan 2, 3-dioxygenase (TO) gene sequences
The genome of an H. zea female from the SIMRU laboratory colony was sequenced and assembled by a service provider (Hudson Alpha, Huntsville, AL) using the Chromium Genome Solution platform and Supernova v1.1.5 genome assembly platform (10X Genomics, Pleasanton, CA). The draft genome was used to create a searchable database using BlastStation-Local software v1.5 (TM Software, Inc., Arcadia, CA). The cDNA sequence of H. zea tryptophan oxygenase (accession number MG976796) was used to search the database to identify a scaffold containing the tryptophan oxygenase gene. Exons of the TO gene were identified and annotated using the mRNA nucleotide sequence of H. zea. The National Center for Biotechnology Information (NCBI) peptide database was also searched using a putative H. zea TO peptide sequence translated from the cDNA sequence to identify other insect TO sequences for use in alignments and phylogenetic tree construction. Peptide sequences retrieved from the databases were aligned using the AlignX module of Vector NTI 11.5 Suite and a phylogenetic tree was generated using MEGA 6 [41].
Guide RNA design and ribonucleoprotein complex preparation
CRISPR RNA (crRNA) used in the experiments was designed using tools available at http://crispr.mit.edu [42]. Exon sequences of the TO gene were submitted to the design pipeline. The best design for each target location was used to purchase Alt-R™ CRISPR crRNA from IDT DNA (www.idtdna.com) that could be annealed with Alt-R™ CRISPR-Cas9 tracrRNA to form a functional gRNA for each target. Alt-R™ CRISPR-Cas9 tracrRNA and Cas9 nuclease of Streptococcus pyogenes that was engineered to contain one N-terminal nuclear localization sequence (NLS) and two C-terminal NLSs (Alt-R™ S.p. Cas9 Nuclease 3NLS) were purchased from IDT DNA (Madison, WI). Two crRNAs for the TO gene and tracrRNA were resuspended separately in 10 mM Tris-EDTA (TE) buffer to yield a 100 μM final concentration. Each TO crRNA was combined with tracrRNA in nuclease-free tubes to yield a 10 μM final concentration in 1X duplex buffer (30 mM HEPES, pH 7.5; 100 mM potassium acetate; IDT DNA). The mixture was heated to 95°C for 5 min on a heat block and slowly cooled to room temperature to anneal the complementary nucleotide sequences in crRNA and tracrRNA to generate functional gRNA. Annealed gRNA for each target was combined with Alt-R™ S.p. Cas9 Nuclease 3NLS in 1X injection buffer (5 mM KCl; 0.1 M sodium phosphate, pH 6.8) to yield final concentrations of 1, 2, and 4 μM and incubated on ice for 15 min to facilitate the formation of ribonucleoprotein (RNP) complexes.
Insects and eggs
A laboratory colony of H. zea maintained at the USDA-ARS Southern Insect Management Research Unit (SIMRU), Stoneville, MS was used in all experiments. The H. zea colony is routinely maintained using the methods described by Gore et al. [43]. Pupae were obtained from the colony and freshly emerged adults were placed in one gallon paper containers with 3% sugar solution for 3–4 days to facilitate mating followed by transfer of adult females to a 5 x 5 mm wire mesh cage. Wax paper was wrapped around the wire mesh cage, which was subsequently placed inside a laboratory cabinet for 30 min to collect eggs. At the end of 30 min period, the eggs were dislodged from the wax paper using a fine paint brush and mounted on approximately 1 mm wide strips of double-sided tape (3M, St. Paul, MN) attached to the 25 x 25 mm plastic coverslips. Eggs mounted on coverslips were placed in a desiccator containing silica gel and approximately -100 KPa (-30.5 inches of mercury) vacuum was applied for 10 min to dehydrate the eggs.
Microinjections and insect rearing
Injection needles were prepared by pulling siliconized 10 μl quartz micro-capillaries using a Sutter P2000 CO2 laser based horizontal micropipette puller (Sutter Instruments, Novato, CA) and beveled with a BV-10 beveller (Sutter Instruments, Novato, CA). Approximately 3 μl of each injection mix (RNP complex or injection buffer) was backfilled into an injection needle. Eggs were injected using a Narishige micromanipulator model MMN-333 (Narishige International USA, Inc., Amityville, NY) and an Olympus SZ stereo microscope (Olympus Corporation, Waltham, Massachusetts) equipped with a mechanical stage. Each egg was injected with approximately 5 nl of injection mix. Mock injections (negative control) contained only the 1X injection buffer. All microinjections were completed within 30 min of egg collection so that the eggs would be no more than 60 min old at the time of injection. Control hatch rate was calculated using the eggs processed up to the desiccation stage, but not subjected to microinjection. Four replicate injections (n = 100 to 120 eggs) were performed with each experimental and negative control RNP complexes. Coverslips containing injected eggs were placed into 100 mm plastic petri dishes layered with a moist filter paper and covered with a lid. Petri dishes were sealed with plastic tape and placed in a plastic box designated as secondary containment. Eggs were incubated at room temperature for approximately 4 hours prior to transferring to a designated incubator which was set to 26°C and 80% RH. Eggs were observed for larval hatch and neonates were transferred to diet cups containing meridic diet [43]. Eye color of adults was recorded and any insects that had a color different from the wild type eye color were genotyped by sequencing amplicons. Hatch rates of injected eggs were corrected for control hatch rate using Henderson-Tilton's formula [44].
Genotyping and DNA analysis
Eye color mutants, except for those used in crossing experiments, and the wild type insects from injections were frozen for genetic analysis. Genomic DNA was extracted from legs or thorax using Master Pure DNA extraction reagents as described by Perera et al. [45]. Purified DNA from each insect was re-suspended in 20 μl of 10 mM Tris-HCl. Forward and reverse primers were designed to anneal within intron 5 (3788Hz_TO_11500F: 5’-CCATTCTTCTATCTGCCGTCAT-3’) and intron 6 (3789Hz_TO_12084R: 5’-GCCCGATTCGAAAACTTCTT-3’), respectively, of the TO gene. PCR amplifications were performed on a PTC-200 DNA Engine (BioRad, Hercules, CA) with a thermal cycling profile containing 30 second initial denaturation at 95°C, followed by 35 cycles of 10 sec denaturation at 95°C, 10 sec annealing at 52°C, and 45 sec extension at 72°C. Amplification products were visualized by electrophoresis on 0.8% agarose gels (Invitrogen, Carlsbad, CA) using Tris-Acetate-EDTA (TAE) buffer (40 mM Tris-Acetate, 1mM EDTA, pH 8.0). Amplicons were cleaned by binding to AmPure XP paramagnetic beads (Beckman Coulter, Beverly, MA) at DNA:Beads (v/v) ratio of 1:1.8. Nucleotide sequences of the purified amplicons were obtained by direct sequencing with Sanger dideoxy method using the same primers as for PCR amplification (USDA-ARS Genomics and Bioinformatics Research Unit (GBRU), Stoneville, MS). Nucleotide sequence analyses were carried out using Vector NTI Advance v11.5 suite.
Off-target sequence analysis
Seed sequence of the protospacer regions (i.e. 12 nucleotides from the 3’-end of the crRNA molecules, upstream of PAM) were used to search published H. zea genome (GCA_002150865.1) [35,46] using BlastStation-Local software v1.5 software to identify genomic scaffolds with potential off-target sites. Off-target sequences with 0–3 mismatches in the 12-nucleotide seed sequence and contained a 5’-NGG-3’ PAM sequence [35,42,47] were selected for further review. Primer pairs were developed to amplify four of the off-targets with less than 2 mismatches in the seed sequence and the lowest possible total mismatches with the target crRNA (protospacer) sequences. Genomic DNA from 48 eye color mutants resulting from the highest concentration (4μM) of RNP complex injections were amplified with the primer pairs for off-targets and the resulting amplicons were direct sequenced using the primers used for amplification. Nucleotide sequence data were analyzed with Vector NTI 11.5 Advance suite (Invitrogen, Carlsbad, CA).
Insect husbandry
Insects were reared at 28°C, 80% RH, and 14:10 L:D cycles in environmental chambers (Percival Scientific, Perry, IA). Insects with mutations (P0) at the target sites were selected and mated as single pairs with moths of the opposite sex from the SIMRU H. zea laboratory colony. Resulting heterozygotes (F1) from each single-pair mating were inbred to obtain F2 progeny. Fourth instar F2 larvae were genotyped by sequencing the DNA extracted from a drop of hemolymph obtained by clipping the tip of one of the abdominal pseudo-legs. The number of the homozygous mutants, heterozygotes, and homozygous wild type insects were recorded for each cross.
Results
cDNA and genomic structure of tryptophan 2,3-dioxygenase in H. zea
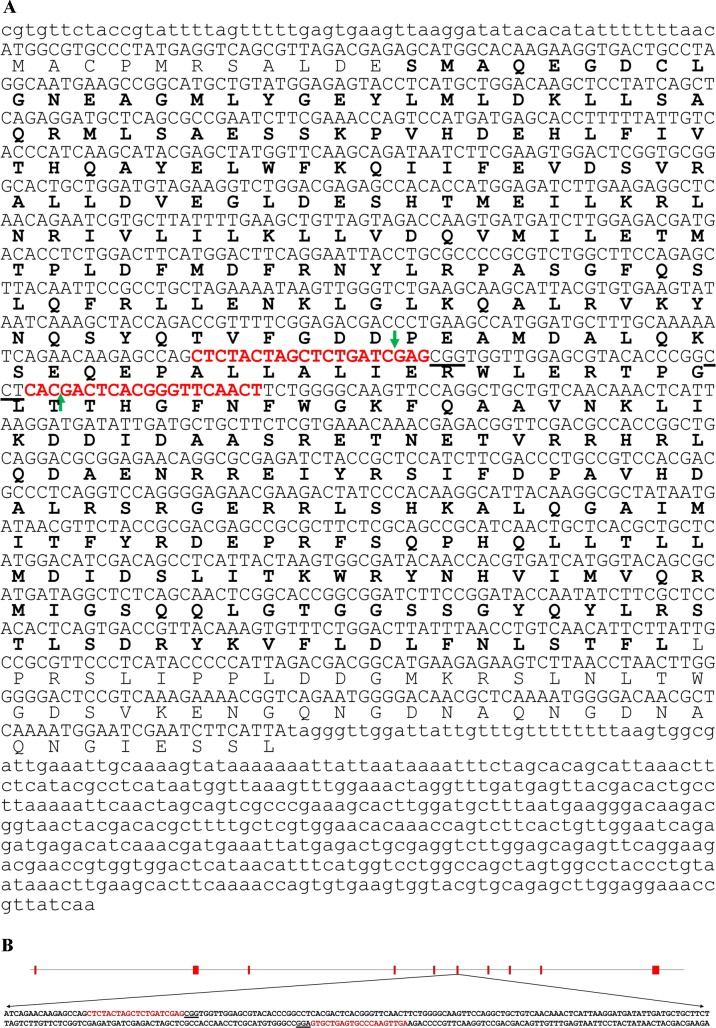
Alignment of the putative amino acid sequence derived from H. zea cDNA sequence with other insect TO peptide sequences and phylogenetic analysis verified the authenticity of the H. zea TO sequence. For a more detailed description please see Supporting information (S1 and S2 Figs). cDNA sequence of H. zea TO and the translated amino acid sequence are shown in Fig 1A. A search of the H. zea genome database with the TO cDNA sequence identified a genomic DNA scaffold (Scaffold 97620) containing the full length TO gene, which was extracted, annotated, and deposited in the GenBank database under the accession number MF598173. The TO gene consisted of ten exons ranging from 73 to 199 bp in size that spanned 17,396 bp of the genome sequence (Fig 1B). The nucleotide sequence of exon 6 generated a number of high quality crRNA designs (http://crispr.mit.edu) and two of the crRNAs with the best scores were selected for editing the TO gene in H. zea (Fig 1B, red text). All crRNA designs generated are given in the Supporting information (S3 Fig).
Fig 1. cDNA sequence, gene organization, and the CRISPR RNA (crRNA) designed for editing targets in the exon 6 of the tryptophan 2,3-dioxygenase (TO) gene in Helicoverpa zea.
(A) Open reading frame of the cDNA sequence is in upper case letters and the amino acids of the tryptophan dioxygenase domain of the TO are indicated in bold text. Target sequences (protospacer) for two crRNA are shown in bold red text. Protospacer adjacent motif (PAM) and double-strand DNA break site for each target site are shown by underlined text and green arrows, respectively (accession number MG976796). (B) The TO gene consists of 10 exons (red boxes) ranging from 73 to 199 bp and introns ranging from 475 to 4323 bp in size, spanning 17,396 bp. The nucleotide sequences of crRNA targets are shown in red text and protospacer adjacent motifs (PAM) are underlined (accession numberMF598173).
Egg hatch and mutation rates
Hatch rates of eggs injected with different concentrations of RNP complexes were adjusted for noninjected control hatch rates using the Henderson-Tilton formula [44] and induced mutation rates of adults emerging from control and injected eggs are shown in Table 1. The H. zea laboratory colony, which is highly inbred, produced low hatch rates ranging from 36.6 to 42.9% with an average (±stdev) of 40.2(±2.6)%. Embryo mortality due to injection, after correction for control hatch rate, was estimated to be 19.8(±5.2)%. Rates of adult emergence ranged from 87.1(±3.5)% in uninjected controls to 77.7(±15.2)% in 4.0 μM RNP injections (Table 1). Mutation recovery rates were positively correlated with the concentration of gRNA (p<0.01). Eggs injected with a standard concentration of 1 μM RNP produced significantly less mutants than the 2 and 4 μM RNP concentrations, and there was no difference between mutation rates of the two highest concentration injections (Table 1). Although a reduction of hatch rate was observed in all injected eggs, differences in hatch rates between mock injections and RNP injected eggs were not significant, indicating that RNP complexes had no deleterious effects on embryo viability.
Table 1. Average number pf eggs injected, average hatch count, corrected hatch count, percentage of adults emerged from larvae, and the percentage of mutants from control, mock injected (with injection buffer), and 1, 2, and 4 μM ribonucleoprotein complexes targeting exon 6 of the tryptophan 2,3-dioxygenase gene in Helicoverpa zea.
| Total # eggs (Avg.) | Avg. Hatch count±SD | Corrected Avg. Hatch count | % adults emerged±SD | % mutants±SD‡ | |
|---|---|---|---|---|---|
| Uninjected Control | 448 (112.0) | 42.3±2.6 | 100.0 | 87.1±3.5 | 0.0 |
| Mock Injected | 560 (116.0) | 32.3±5.2 | 80.5 | 87.6±4.3 | 0.0 |
| 1.0 μM injected | 488 (122.0) | 32.2±3.3 | 87.2 | 79.1±7.5 | 48.1±15.1a |
| 2.0 μM Injected | 570 (142.5) | 30.0±5.0 | 74.7 | 83.3±5.9 | 88.8±3.2b |
| 4.0 μM Injected | 541 (135.3) | 35.9±5.0 | 78.6 | 77.7±15.2 | 93.5±6.3b |
‡ Percentages of mutants from CRISPR/Cas9 injections that were significantly different are indicated by different letters.
Mutant phenotypes and genotypes
Loss of function TO mutants of H. zea yielded mostly yellow eye color, with only a few P0 insects displaying light pink to dark pink eyes (Fig 2). In some insects the pink eye color gradually turned to yellow within a few days after eclosion. Matings between P0 insects with the darkest pink eye color produced insects with yellow eyes in F1 and successive generations and no wild type eye color was observed even after seven successive generations of inbreeding.
Fig 2. Variation in eye color in tryptophan 2,3,-dioxygenase (TO) mutants of Helicoverpa zea.
Yellow eye phenotype of TO mutants inbred for seven generations (A) and different shades of orange color eyes observed in P0 (microinjected) adults (B and C). Various mosaic eye color patterns were also observed in some of the P0 mutants (D-G). Dark black-brown pigmentation (H) and translucent green with a dark middle (I) are common eye color phenotype variations found in the H. zea colony used in the experiments.
The nucleotide sequence (n = 110) of the TO gene was obtained from one non-injected (WT) insect, 86 eye color mutants (including 31 insects used in mating experiments), and 24 injected insects that were classified as having wild type eye color. Alignments of sequence data with a reference identified a diverse array of mutant genotypes that included different combinations of deletions and insertions in one or both target sites (Fig 3 and S4 Fig). The number of various mutant genotypes identified in each category are summarized in Table 2. Mutations at both targets sites were identified in 89.53% of the nucleotide sequences from eye color mutants and remainder (10.47%) were mutations only at the second target site. Mutations at only the first target site were not identified in this analysis indicating a difference in efficacy between two gRNA designs. Surprisingly, all 24 insects originally classified as wild type eye color had mutations at one or both target sites (Fig 3, sequences IW 1 to IW24). Seven of these insects had one codon deletion at the second target site while another had a 12 bp insertion at the first target site in addition to the one codon deletion at the second target site. Other sequences from the phenotypically wild type insects (IW 9, IW 19, and IW 21) had insertion-deletion combinations at both target sites but preserved the open reading frame except for either insertion or deletion of a few amino acids. Careful examination of the eye colors of these insects under a microscope did not reveal any recognizable deviations from wild type eye color. Although the frequencies of yellow eye phenotypes observed in this study for 2 and 4 μM injections were between 88.8 and 93.5%, with the wild type eye color insects that had mutations actual genome editing rate may be close to 100%, (Table 1).
Fig 3. A representative sample of nucleotide sequences of the eye color mutants and wild type eye color insects from CRISPR injection experiments.
Sequences IM 1 through IM 24 and IW 1 through IW 24 are from insects with mutant and wild type eye color phenotype, respectively, from CRISPR/Cas9 injections. Partial nucleotide sequences of exon 6 of the TO gene from the reference sequence (Ref) and uninjected wild type (WT) are also shown. Nucleotides identical to the exon 6 of the reference sequence (accession: MF598173) are indicated by a dot (.) and alignment gaps are shown by a hyphen (-). Nucleotides that differ from the reference are indicated on each sequence. Target (protospacer) sequences for which crRNA were designed are underlined with dotted lines and protospacer adjacent motifs (PAM) are underlined with a solid line.
Table 2. Summary of mutation type combinations identified in tryptophan 2,3-dioxygenase gene target site nucleotide sequences of 86 yellow eye and 24 wild type insects of Helicoverpa zea.
| Combination | Site 1 | Site 2 | # in Yellow Eye | # Wild Type |
|---|---|---|---|---|
| 1 | WT | Deletion | 9 | 17 |
| 2 | Deletion | WT | 0 | 0 |
| 3 | WT | Insertion | 2 | 0 |
| 4 | Insertion | WT | 0 | 0 |
| 5 | Deletion | Insertion | 7 | 0 |
| 6 | Insertion | Deletion | 27 | 4 |
| 7‡ | Deletion | Deletion | 33 | 2 |
| 8 | Insertion | Insertion | 8 | 1 |
| Grand Total | 86 | 24 |
‡ Includes complete deletions of the nucleotide sequences in between the target sites.
Off-target sequence analysis
A search of H. zea genome (GCA_002150865.1) with the protospacer seed sequences identified potential off-targets in scaffolds 522 and 1430 for gRNA 1 and in 40, 180, 185, 1210, 1570, and 2020 for gRNA 2 (Supporting information S1 Table). All identified off-target sites had seven to ten total mismatches with the crRNA sequences and those with less than eight total mismatches and a maximum of two mismatches in the seed sequence regions were identified in scaffolds 185, 522, 1210, 1430, and 1570. Mutations in the Cas9 cleavage sites and the 3’-end of the protospacer region (close to PAM) inhibit Cas9 nuclease activity [35,47]. Therefore, off-target sequences with mismatches within 10 nucleotides from the 3’- end of the protospacer were not considered for analysis. Based on above criteria, primer pairs were developed for PCR amplification and nucleotide sequencing of off-target regions in genomic scaffolds 185, 1210, 1430, and 1570 (Supporting information S1 Table). Off-target sequences in scaffolds 185, 1210, and 1480 did not have any identifiable genes within 2 kbp of flanking regions. The off-target sequence of the scaffold 1570 was located in intron 1 of the transcription factor Sox-8-like gene of H. armigera (accession: XP_021195044.1). Genomic DNA sequences amplified using primers to scaffold 1210 were polymorphic in some insects and were not likely to be off-targets (S5B Fig). Therefore, only some of the insects had off-target sequences matching scaffold 1210 (S5B Fig). Both wild type and yellow eye mutants had significant differences in the off-target sequence compared to scaffold 185 from the published genome sequence (S5B Fig). This is most likely due to genome level differences between insect colonies used for genome sequencing and the TO gene editing experiments. Evaluation of all nucleotide sequences from the selected off-target regions in yellow eye mutants revealed no evidence of non-specific activity, indicating that both gRNA used for mutating the TO gene was specific to the target regions (Supporting information S5 Fig, panels A-D).
Discussion and conclusions
In this study, the efficacy of in vitro assembled riboprotein complexes containing synthetic tracrRNA, crRNA, and a purified Cas9 nuclease in generating mutations in H. zea was studied using different RNP concentrations to target the H. zea TO gene. The microinjection process reduced egg hatch by 19.8%, but there was no significant difference in egg hatching between injections of control solutions or ribonucleoprotein complexes targeting the TO gene. Mutation rates, however, were much higher when injected with 2 and 4 μM concentrations of TO ribonucleoprotein complex compared to eggs injected with the manufacturer recommended 1 μM concentration. In addition, the mutation rates at 2 and 4 μM RNP concentrations were much higher than those obtained by using different combinations of gRNA and Cas9 nuclease delivery methods in other lepidopteran species (e.g [23,24,48,49]). Therefore, we conclude that 2 μM or higher concentrations of ribonucleoprotein complex were efficient in generating desired mutations in H. zea. However, it may be necessary to optimize the RNP complex concentrations in experiments with other species or when using gRNA for different targets to achieve the best results. Furthermore, it was observed that greater than 6 μM concentrations of ribonucleoprotein complexes tend to clog the injection needles quickly during injections, reducing reproducibility and rendering the process much more difficult.
Mutation rates observed in this study with 2 μM or higher RNP concentrations were greater than most of the reported mutation rates produced by injecting various combinations of cDNA, mRNA, and purified Cas9 protein. Injection of DNA constructs (plasmids) coding for gRNA and Cas nuclease has typically produced low heritable mutation rates [19,50] compared to studies that employed transcribed gRNA with Cas protein or mRNA. For example, Markert et al [23] used transcribed gRNA and Cas9 mRNA in the monarch butterfly, Danaus plexippus, and reported that injections of two gRNAs complementary to exons two and three of the cry2 gene at 0.1 μg/μl together with 0.5 μg/μl of mRNA encoding Cas9 resulted in 21.6% larval hatch rate, 61.5% somatic mutation rate, and 100% germline mutation rates in the progeny of mutants tested by crossing (n = 2). However, the exact percentage of germline mutations is not clear because all mutants were not crossed to obtain progeny for testing and the experiments were not replicated. In another study, Li et al. [48] used transcribed gRNA and mRNA for Cas9 at various concentrations to knock out Abdominal-B, ebony, and frizzled genes in Papilio xuthus to achieve morphological mutation rates from 18.3 to 90.9%, in a locus and RNA concentration dependent manner. In most experiments, higher concentrations of dual gRNA and Cas9 mRNA produced higher rates of mutations in P. xuthus. In a similar study on Bombyx mori wnt1 mutations with transcribed gRNA and mRNA for Cas9, high rates of mutations were observed in experiments that used high concentrations of Cas9mRNA and gRNA (300 ng/μl) compared to low concentrations (30 ng/μl) of gRNA [51]. However, egg hatch percentage was 6.6% in 300 ng/μl gRNA injections compared to 28.8% in 30 ng/μl gRNA injections. Again, the experiments were not replicated and statistical analyses were not provided. Varying rates of mutations produced using injections of Cas9 protein and transcribed gRNA have also been reported in the butterfly species Junonia coenia and Vanessa cardui [24] and a species of moth, Spodoptera littoralis [52]. Mutating spalt and distal-less genes in J. coenia and V. cardui using an injection mixture containing 100 ng/μl gRNAs and 200 ng/μl Cas9 protein yielded mutation rates 33.3and 56.0% for spalt and 41.0 and 51.7% for distal-less, respectively. Although J. coenia mutation rates were lower than that of V. cardui in both experiments, it is not known whether high mutation rates could be achieved by using different concentrations of reagents because only one combination of gRNA and Cas9 protein concentrations was reported. In a study targeting half-ATP binding cassette (ABC) transporters in Helicoverpa armigera, Khan et. al. [53] used in vitro transcribed mRNA and sgRNA at 200 and 25 ng/μl concentrations, respectively, to successfully mutate the target genes. However, data pertaining to the efficiency of gene knockouts were not reported. It should be noted that although comparisons between different reagent combinations targeting different genes in a number of insect species showed high levels of variation in mutation efficiency (reviewed by [14]), we have not evaluated the efficacy of different reagent systems (or cannot locate any published research) on a single gene target on the same insect species. Reviews of the literature indicate that depending on the target site, similar gRNA/Cas reagent combinations could produce highly variable mutation rates within a single species (e.g. [26,48]). However, all results reported so far have been obtained by a single set of injections (i.e. no replicates) and the mutation efficiencies were not analyzed statistically. Therefore, having a precisely quantifiable reagent system such as the RNP complexes used in this study may be necessary to obtain reproducible results for comparison of mutation efficiencies between targets or identical targets in different species using replicated experiments. In addition, true comparisons among the efficiencies of different experiments were not possible due to inability to convert of the injected reagent concentrations reported in ng/μl to the concentrations of actual RNP complexes due to unknown efficiency of transcription and translation of plasmids or translation of mRNA in different species.
Loss of function mutations in the TO gene in some insect species of Coleoptera and Diptera leads to vermillion eye color [54–56]. RNA interference (RNAi) of the TO gene indicated knock down of eye pigment intensity in Plodia interpunctella larvae, but no definitive color was reported, most likely due to inability to differentiate colors of eye spots in larvae [57]. While most H. zea TO mutants had yellow eye color, others ranged from pink to light red immediately after eclosion, but in some insects, turned yellow within a few days (Fig 2). Knockout of scarlet that dimerizes with white to from the ommochrome precursor transporter in the closely related species Helicoverpa armigera [53] also produced yellow eye color phenotype, which was attributed to the presence of ekapterin, an eye pigment originally described in Ephestia kuehniella [58]. However, pink or light red eye colors observed in TO knockouts of H. zea were not detected in scarlet knockouts of H. armigera. Furthermore, single-pair matings between H. zea TO mutants with reddish eye color produced progeny with yellow eye color only and we postulate that the variations in eye color observed in H. zea were most likely due to differences in expression of genes involved in red and yellow eye pigment production pathways in different insects.
Pteridine and ommochrome eye pigment synthesis pathways include multiple steps in which mutations could result in different eye colors. Various proportions of different eye pigments could produce a range of eye color phenotypes. Mutations in the TO gene effectively terminated the ommochrome pigment xanthommmatin (brown) synthesis leading to unmasking of pigments produced in the pteridine pathway [40,59]. Vermilion eyes are produced in insects that predominantly contain the red pigment drosopterin. Although pteridines may not contribute to eye color in Bombyx mori [60,61], there may be pteridine pigments present in the ommatidia of other lepidopteran species including H. armigera and H. zea. Presence of a red pigment that contributed to pink hues in H. zea may indicate that there are differences in eye color pigment repertoire even in closely related species.
It is possible that wild type eye color in the insects with in-frame mutation events resulted from the unaltered functionality of the TO in mutants with these deletions or insertions. However, lack of detectable eye color change in P0 adults with frame-shift mutations cannot be explained except by assuming that they were chimeras in which RNP complexes were injected into embryos that already had started cell division. In such an event, cells that were not mutated could produce a functional TO enzyme to yield ommochrome pigments. This the most likely scenario in the insects with wild type eye color which had mutations that caused frame shifts that led to truncation of the enzyme. In fact, insect embryonic development begins with a syncytial stage where multiple nuclei are present within the embryo with partially formed cells [18]. In a closely related species H. armigera, a ring of syncytial nuclei cold be seen at 4 hours after oviposition [62] and nuclei division could have started much earlier than 4 hours. Observation of mosaic eye pigmentation patterns that contained patches of wild type eye color in mutants (Fig 2) and identification of mutations that abolish the TO enzyme activity in phenotypically wild type insects (Fig 3) of H. zea in this study clearly indicate the presence multiple nuclei even in embryos injected within 60 minutes of oviposition. The presence of two or more nuclei at the time of injection may be attributed either to start of syncytial nuclei division in H. zea embryos before 60 min or to females holding the fertilized eggs for some time before oviposition. Although a scientifically intriguing matter, this subject was out of the scope of this research and was not further investigated.
Mutations in off-target genomic regions have the potential to generate unexpected complications in genome editing experiments [63]. Undesirable mutations can be significantly reduced by designing high quality gRNA with minimal off-target effects or high fidelity Cas9 nucleases requiring specific target binding by gRNA [64–66]. Although optimal crRNA designs were used in this study, the Cas9 nuclease utilized was not a high fidelity enzyme. Scanning of the H. zea genome for off-target sequences identified several potential sites. However, PCR amplification and nucleotide sequencing of four putative off-target sequence regions in mutant yellow eye insects did not identify any unintended mutations (Supporting information S5 Fig, panels A-D). Therefore, we can confidently state that crRNA designs used in this study were highly specific to the intended targets and mutant phenotypes observed were a direct result of mutations in the TO gene.
In conclusion, this study demonstrates the utility of in vitro assembled RNP complexes made of synthetic guide RNA components and purified Cas9 nuclease containing NLS in a series of replicated experiments using known concentrations of RNP complexes. In H. zea, 2 μM or higher concentrations of RNP complexes targeting the TO gene produced greater than 90% mutants in adults the developed from injected eggs. Results showed that RNP complexes can be introduced to embryos in a quantitative manner to maximize mutation rates in genome editing experiments. Efficiencies in mutating different targets within same species or an identical target between different species could be easily compared by injecting precise concentrations of RNP complexes. In addition, the ability to use precise concentrations of RNP complexes increase the reliability and precision of experimental reproducibility. This research should provide guidelines for standardizing the CRISPR/Cas mediated genome editing in non-model insects.
Supporting information
TO polypeptide sequences from Heliothis virescens (PCG74852.1), Spodoptera litura (XP_022823393.1), Plodia interpunctella (AAR24625.1), Bicyclus anynana (XP_023952864.1), Papilio polytes (XP_013141355.1), Bombyx mori (XP_004922659.1), Anopheles darlingi (ETN63322.1), Anopheles gambiae (XP_312204.2), Aedes aegypti (AAL37360.1), Neodiprion lecontei (XP_015514842.1), and Zootermopsis nevadensis (XP_021924784.1) were downloaded from National Center for Biotechnology Information and aligned using AlignX module of Vector NTI Advance 11.5 suite (Invitrogen). Identical amino acids are indicated by red text with yellow background. A block of similar amino acids are shown in black text with green background, conservative amino acids are shown in dark blue text, and non-similar amino acids are shown in black text. Alignment gaps are indicated by a hyphen (-). Tryptophan dioxygenase domain is underlined.
(PDF)
A maximum likelihood evolutionary tree was constructed using TO sequences from H. zea (MG976796), Heliothis virescens (PCG74852.1), Spodoptera litura (XP_022823393.1), Plodia interpunctella (AAR24625.1), Bicyclus anynana (XP_023952864.1), Papilio polytes (XP_013141355.1), Bombyx mori (XP_004922659.1), Anopheles darlingi (ETN63322.1), Anopheles gambiae (XP_312204.2), Aedes aegypti (AAL37360.1), Neodiprion lecontei (XP_015514842.1), and Zootermopsis nevadensis (XP_021924784.1). Using the MEGA 6.0 we identified the Jones-Taylor-Thornton model with a discrete gamma distribution and some evolutionarily invariable sites (JTT+G+I) as the best model for gene phylogeny reconstruction. JTT+G+I model was run with 10,000 bootstrap replicates was used to generate the evolutionary tree. Lepidopteran and dipteran TO sequences were grouped in two distinct evolutionary branches with 100% bootstrap support. Hymenoptera (N. lecontei) and isopteran (Z. nevadensis) TO sequences were basal to the evolutionary tree with weak bootstrap support.
(PDF)
Nucleotide sequences of the crRNA used in the experiments are shown in bold red text followed by protospacer adjacent sequence (PAM) in bold black text.
(PDF)
Nucleotides identical to the exon 6 of the reference sequence (accession: MF598173) are indicated by a dot (.) and alignment gaps are shown by a hyphen (-). Nucleotide that differ from the reference are indicated on each sequence. CRISPR RNA (crRNA) target sequences and protospacer adjascent motifs (PAM) are underlined with dotted lines and solid lines, respectively.
(PDF)
One hundred nucleotides from each scaffold, including 38–40 flanking the off-target sequence obtained from wild type insects (WT) and yellow eye (IM) mutants were aligned with H. zea DNA sequence from corresponding scaffold. Off-target sequence is marked in red text and the PAM sequence is underlined. Nucleotides identical to wild type sequence are indicated by a dot (.) and alignment gaps are indicated by a hyphen (-). Nucleotides that are different from the genomic scaffold are shown on each sequence. (A) Alignment of nucleotide sequences corresponding to the off-target site in the scaffold 185 (S185). Nucleotide sequence of the off-target site from the published genome (S185) was significantly different from those obtained from wild type and yellow eye mutants. (B) Alignment of nucleotide sequences corresponding to the off-target site in the scaffold 1210 (S1210). Two different nucleotide variants, one identical to the genomic scaffold 1210 sequence (S2010a) and the other with a number of polymorphisms (S2010b), were identified in the yellow eye mutants. Alignment of S2010a and S1210b (a) indicates that S2010b variants have additional mutations in the protospacer seed sequence and significantly different from the crRNA design for TO. Alignment of the nucleotide sequences similar to S2010a off-target region from wild type and yellow eye mutants (b) indicates that there were no off-target mutations in the insects with edited TO gene. (C) Alignment of nucleotide sequences corresponding to the off-target site in H. zea genomic scaffold 1430 (S1430). Nucleotide sequences from yellow eye mutants indicate that there were no off-target effects in the edited TO gene. (D) Alignment of nucleotide sequences corresponding to the off-target site in H. zea genomic scaffold 1570 (S1570). Nucleotide sequences from yellow eye mutants indicate that there were no off-target effects in the edited TO gene.
(PDF)
Seed sequence (12 nucleotides upstream of the protospacer adjacent motif) of the target sequences of the TO gene was used to search genome of H. zea to identify potential off-targets that had three or less mismatches with the seed sequences of the targets and ten or less total mismatches. Primer pairs designed to PCR amplify and sequence off-target sequences are shown next to the selected sequences. Seed sequence region is shown in blue text and nucleotide mismatches are shown in lowercase text. PAM sequences are shown in bold black text. A lower case “c” at the end of the start position indicates an off-target sequence on the complementary strand of a scaffold. N/A: primers were not designed to these off-targets.
(PDF)
Acknowledgments
We thank Priya Chatakondi for assistance with insect husbandry, Fanny Liu (USDA ARS Genomics and Bioinformatics Research Unit, Stoneville, MS) for assistance with DNA sequencing, Dr. Kent Shelby (USDA ARS Biological Control of Insects Research Laboratory, Columbia, MO), Dr. Brad Coates (USDA ARS Corn Insects and Genetics Research Unit, Ames IA), and Dr. Juan Luis Jurat-Fuentes (Department of Entomology and Plant Pathology, University of Tennessee, Knoxville, TN) for critically reading an earlier version of this manuscript, and Dr. Margaret Allen (USDA ARS Biological Control of Pests Research Unit, Stoneville, MS) for assistance in preparing quartz microinjection needles. The use or mention of a trademark or proprietary product does not constitute an endorsement, guarantee, or warranty of the product and does not imply its approval to the exclusion of other suitable products by the U.S. Department of Agriculture, an equal opportunity employer. This research was funded by USDA Agricultural Research Service project 6066-22000-085-00D.
Data Availability
All relevant data are within the paper and its Supporting Information files. Nucleotide sequence data were submitted to GenBank under Accession numbers MG976796 and MF598173.
Funding Statement
This research was funded by USDA Agricultural Research Service project number 6066-22000-085-00D. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. Journal of Bacteriology 169: 5429–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mojica FJ, Diez-Villasenor C, Soria E, Juez G (2000) Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol 36: 244–246. [DOI] [PubMed] [Google Scholar]
- 3.Jansen R, Embden JD, Gaastra W, Schouls LM (2002) Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43: 1565–1575. [DOI] [PubMed] [Google Scholar]
- 4.Barrangou R, Doudna JA (2016) Applications of CRISPR technologies in research and beyond. Nat Biotechnol 34: 933–941. doi: 10.1038/nbt.3659 [DOI] [PubMed] [Google Scholar]
- 5.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315: 1709–1712. doi: 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- 6.Horvath P, Barrangou R (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327: 167–170. doi: 10.1126/science.1179555 [DOI] [PubMed] [Google Scholar]
- 7.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, et al. (2008) Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321: 960–964. doi: 10.1126/science.1159689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marraffini LA, Sontheimer EJ (2008) CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322: 1843–1845. doi: 10.1126/science.1165771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, et al. (2009) RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139: 945–956. doi: 10.1016/j.cell.2009.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundersen-Rindal DE, Adrianos SL, Allen ML, Becnel JJ, Chen YP, Choi M-Y, et al. (2017) Arthropod genomics research in the United States Department of Agriculture, Agricultural Research Service: Applications of RNA interference and CRISPR gene-editing technologies in pest control. Trends in Entomology 13: 109–137. [Google Scholar]
- 11.Reid W, O'Brochta DA (2016) Applications of genome editing in insects. Curr Opin Insect Sci 13: 43–54. doi: 10.1016/j.cois.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Beumer KJ, Carroll D (2014) Targeted genome engineering techniques in Drosophila. Methods 68: 29–37. doi: 10.1016/j.ymeth.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venken KJ, Sarrion-Perdigones A, Vandeventer PJ, Abel NS, Christiansen AE, Hoffman KL (2016) Genome engineering: Drosophila melanogaster and beyond. Wiley Interdiscip Rev Dev Biol 5: 233–267. doi: 10.1002/wdev.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taning CN, Van Eynde B, Yu N, Ma S, Smagghe G (2017) CRISPR/Cas9 in insects: Applications, best practices and biosafety concerns. J Insect Physiol 98: 245–257. doi: 10.1016/j.jinsphys.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Sun J-L, Yu L (2017) Application of the CRISPR gene-editing technique in insect functional genome studies–a review. Entomologia Experimentalis et Applicata 162: 124–132. [Google Scholar]
- 16.Sun D, Guo Z, Liu Y, Zhang Y (2017) Progress and Prospects of CRISPR/Cas Systems in Insects and Other Arthropods. Front Physiol 8: 608 doi: 10.3389/fphys.2017.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo A, Crisp P, Saint R, O'Keefe LV, Baxter SW (2018) CRISPR/Cas9‐mediated mutagenesis of the white gene in the tephritid pest Bactrocera tryoni. Journal of Applied Entomology 142: 52–58. [Google Scholar]
- 18.Livraghi L, Martin A, Gibbs M, Braak N, Arif S, Breuker CJ (2017) CRISPR/Cas9 as the Key to Unlocking the Secrets of Butterfly Wing Pattern Development and Its Evolution. Advances in Insect Physiology: Academic Press. [Google Scholar]
- 19.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, et al. (2013) Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194: 1029–1035. doi: 10.1534/genetics.113.152710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baena-Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent JP (2013) Accelerated homologous recombination and subsequent genome modification in Drosophila. Development 140: 4818–4825. doi: 10.1242/dev.100933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, et al. (2016) A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol 34: 78–83. doi: 10.1038/nbt.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng B, Zhan S, Wang Y, Huang Y, Xu J, Liu Q, et al. (2016) Expansion of CRISPR targeting sites in Bombyx mori. Insect Biochem Mol Biol 72: 31–40. doi: 10.1016/j.ibmb.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Markert MJ, Zhang Y, Enuameh MS, Reppert SM, Wolfe SA, Merlin C (2016) Genomic Access to Monarch Migration Using TALEN and CRISPR/Cas9-Mediated Targeted Mutagenesis. G3 (Bethesda) 6: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Reed RD (2016) Genome editing in butterflies reveals that spalt promotes and Distal-less represses eyespot colour patterns. Nature Communications 7: 11769 doi: 10.1038/ncomms11769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gokcezade J, Sienski G, Duchek P (2014) Efficient CRISPR/Cas9 plasmids for rapid and versatile genome editing in Drosophila. G3 (Bethesda) 4: 2279–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassett AR, Tibbit C, Ponting CP, Liu JL (2013) Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep 4: 220–228. doi: 10.1016/j.celrep.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu S, Aryan A, Overcash JM, Samuel GH, Anderson MA, Dahlem TJ, et al. (2015) Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in Aedes aegypti. Proc Natl Acad Sci U S A 112: 4038–4043. doi: 10.1073/pnas.1502370112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S, Ewen-Campen B, Ni X, Housden BE, Perrimon N (2015) In Vivo Transcriptional Activation Using CRISPR/Cas9 in Drosophila. Genetics 201: 433–442. doi: 10.1534/genetics.115.181065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Li Z, Xu J, Zeng B, Ling L, You L, et al. (2013) The CRISPR/Cas System mediates efficient genome engineering in Bombyx mori. Cell research 23: 1414–1416. doi: 10.1038/cr.2013.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mircetic J, Steinebrunner I, Ding L, Fei J-F, Bogdanova A, Drechsel D, et al. (2017) Purified Cas9 Fusion Proteins for Advanced Genome Manipulation. Small Methods 1: 1600052. [Google Scholar]
- 31.Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, et al. (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8: 14261 doi: 10.1038/ncomms14261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suresh B, Ramakrishna S, Kim H (2017) Cell-Penetrating Peptide-Mediated Delivery of Cas9 Protein and Guide RNA for Genome Editing. Methods Mol Biol 1507: 81–94. doi: 10.1007/978-1-4939-6518-2_7 [DOI] [PubMed] [Google Scholar]
- 33.Kim K, Park SW, Kim JH, Lee SH, Kim D, Koo T, et al. (2017) Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res 27: 419–426. doi: 10.1101/gr.219089.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meccariello A, Monti SM, Romanelli A, Colonna R, Primo P, Inghilterra MG, et al. (2017) Highly efficient DNA-free gene disruption in the agricultural pest Ceratitis capitata by CRISPR-Cas9 ribonucleoprotein complexes. Sci Rep 7: 10061 doi: 10.1038/s41598-017-10347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. doi: 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A 109: E2579–2586. doi: 10.1073/pnas.1208507109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. (2011) CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471: 602 doi: 10.1038/nature09886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalderon D, Roberts BL, Richardson WD, Smith AE (1984) A short amino acid sequence able to specify nuclear location. Cell 39: 499–509. [DOI] [PubMed] [Google Scholar]
- 39.Dingwall C, Robbins J, Dilworth SM, Roberts B, Richardson WD (1988) The nucleoplasmin nuclear location sequence is larger and more complex than that of SV-40 large T antigen. The Journal of Cell Biology 107: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryall RL, Howells A (1974) Ommochrome biosynthetic pathway of Drosophila melanogaster: Variations in levels of enzyme activities and intermediates during adult development. Insect Biochemistry 4: 47–61. [Google Scholar]
- 41.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31: 827–832. doi: 10.1038/nbt.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gore J, Adamczyk JJ, Blanco CA (2005) Selective feeding of tobacco budworm and bollworm (Lepidoptera: Noctuidae) on meridic diet with different concentrations of Bacillus thuringiensis proteins. Journal of Economic Entomology 98: 88–94. [DOI] [PubMed] [Google Scholar]
- 44.Henderson CF, Tilton EW (1955) Tests with Acaricides against the Brown Wheat Mite. J Econ Entomol 48: 157–161. [Google Scholar]
- 45.Perera OP, Allen KC, Jain D, Purcell M, Little NS, Luttrell RG (2015) Rapid identification of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) using ribosomal RNA internal transcribed spacer 1. Journal of Insect Science 15: 155 doi: 10.1093/jisesa/iev137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce SL, Clarke DF, East PD, Elfekih S, Gordon KHJ, Jermiin LS, et al. (2017) Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biology 15: 63 doi: 10.1186/s12915-017-0402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, et al. (2013) CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31: 833–838. doi: 10.1038/nbt.2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Fan D, Zhang W, Liu G, Zhang L, Zhao L, et al. (2015) Outbred genome sequencing and CRISPR/Cas9 gene editing in butterflies. Nat Commun 6: 8212 doi: 10.1038/ncomms9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Zhang H, Wang H, Zhao S, Zuo Y, Yang Y, et al. (2016) Functional validation of cadherin as a receptor of Bt toxin Cry1Ac in Helicoverpa armigera utilizing the CRISPR/Cas9 system. Insect Biochem Mol Biol 76: 11–17. doi: 10.1016/j.ibmb.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 50.Li F, Scott MJ (2016) CRISPR/Cas9-mediated mutagenesis of the white and Sex lethal loci in the invasive pest, Drosophila suzukii. Biochem Biophys Res Commun 469: 911–916. doi: 10.1016/j.bbrc.2015.12.081 [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Aslam AF, Liu X, Li M, Huang Y, Tan A (2015) Functional analysis of Bombyx Wnt1 during embryogenesis using the CRISPR/Cas9 system. J Insect Physiol 79: 73–79. doi: 10.1016/j.jinsphys.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 52.Koutroumpa FA, Monsempes C, Francois MC, de Cian A, Royer C, Concordet JP, et al. (2016) Heritable genome editing with CRISPR/Cas9 induces anosmia in a crop pest moth. Sci Rep 6: 29620 doi: 10.1038/srep29620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan SA, Reichelt M, Heckel DG (2017) Functional analysis of the ABCs of eye color in Helicoverpa armigera with CRISPR/Cas9-induced mutations. Sci Rep 7: 40025 doi: 10.1038/srep40025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Summers KM, Howells AJ, Pyliotis NA (1982) Biology of Eye Pigmentation in Insects In: Berridge MJ, Treherne JE, Wigglesworth VB, editors. Advances in Insect Physiology: Academic Press; pp. 119–166. [Google Scholar]
- 55.Green MM (1952) Mutant isoalleles at the vermilion locus in Drosophila melanogaster. Proceedings of the National Academy of Sciences 38: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenzen MD, Brown SJ, Denell RE, Beeman RW (2002) Cloning and characterization of the Tribolium castaneum eye-color genes encoding tryptophan oxygenase and kynurenine 3-monooxygenase. Genetics 160: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabrick JA, Kanost MR, Baker JE (2004) RNAi-induced silencing of embryonic tryptophan oxygenase in the Pyralid moth, Plodia interpunctella. Journal of Insect Science 4: 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viscontini M, Stierlin H (1961) Isolation, structure, and synthesis of pterins from Ephestia kuhnellia Zeller. Preliminary communication. Helvetica Chimica Acta 44: 1783–1785. [Google Scholar]
- 59.Ferré J, Silva FJ, Real MD, Ménsua JL (1986) Pigment patterns in mutants affecting the biosynthesis of pteridines and xanthommatin in Drosophila melanogaster. Biochemical Genetics 24: 545–569. [DOI] [PubMed] [Google Scholar]
- 60.Ki Tatematsu, Yamamoto K, Uchino K, Narukawa J, Iizuka T, Banno Y, et al. (2011) Positional cloning of silkworm white egg 2 (w‐2) locus shows functional conservation and diversification of ABC transporters for pigmentation in insects. Genes to Cells 16: 331–342. doi: 10.1111/j.1365-2443.2011.01490.x [DOI] [PubMed] [Google Scholar]
- 61.Kômoto N, Quan G-X, Sezutsu H, Tamura T (2009) A single-base deletion in an ABC transporter gene causes white eyes, white eggs, and translucent larval skin in the silkworm w-3oe mutant. Insect biochemistry and molecular biology 39: 152–156. doi: 10.1016/j.ibmb.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 62.Jarjees EA, Merritt DJ (2004) The effect of parasitization by Trichogramma australicum on Helicoverpa armigera host eggs and embryos. Journal of Invertebrate Pathology 85: 1–8. doi: 10.1016/j.jip.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 63.Zischewski J, Fischer R, Bortesi L (2017) Detection of on-target and off-target mutations generated by CRISPR/Cas9 and other sequence-specific nucleases. Biotechnol Adv 35: 95–104. doi: 10.1016/j.biotechadv.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 64.Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. (2016) High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529: 490–495. doi: 10.1038/nature16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee CM, Cradick TJ, Fine EJ, Bao G (2016) Nuclease Target Site Selection for Maximizing On-target Activity and Minimizing Off-target Effects in Genome Editing. Mol Ther 24: 475–487. doi: 10.1038/mt.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naito Y, Hino K, Bono H, Ui-Tei K (2015) CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31: 1120–1123. doi: 10.1093/bioinformatics/btu743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TO polypeptide sequences from Heliothis virescens (PCG74852.1), Spodoptera litura (XP_022823393.1), Plodia interpunctella (AAR24625.1), Bicyclus anynana (XP_023952864.1), Papilio polytes (XP_013141355.1), Bombyx mori (XP_004922659.1), Anopheles darlingi (ETN63322.1), Anopheles gambiae (XP_312204.2), Aedes aegypti (AAL37360.1), Neodiprion lecontei (XP_015514842.1), and Zootermopsis nevadensis (XP_021924784.1) were downloaded from National Center for Biotechnology Information and aligned using AlignX module of Vector NTI Advance 11.5 suite (Invitrogen). Identical amino acids are indicated by red text with yellow background. A block of similar amino acids are shown in black text with green background, conservative amino acids are shown in dark blue text, and non-similar amino acids are shown in black text. Alignment gaps are indicated by a hyphen (-). Tryptophan dioxygenase domain is underlined.
(PDF)
A maximum likelihood evolutionary tree was constructed using TO sequences from H. zea (MG976796), Heliothis virescens (PCG74852.1), Spodoptera litura (XP_022823393.1), Plodia interpunctella (AAR24625.1), Bicyclus anynana (XP_023952864.1), Papilio polytes (XP_013141355.1), Bombyx mori (XP_004922659.1), Anopheles darlingi (ETN63322.1), Anopheles gambiae (XP_312204.2), Aedes aegypti (AAL37360.1), Neodiprion lecontei (XP_015514842.1), and Zootermopsis nevadensis (XP_021924784.1). Using the MEGA 6.0 we identified the Jones-Taylor-Thornton model with a discrete gamma distribution and some evolutionarily invariable sites (JTT+G+I) as the best model for gene phylogeny reconstruction. JTT+G+I model was run with 10,000 bootstrap replicates was used to generate the evolutionary tree. Lepidopteran and dipteran TO sequences were grouped in two distinct evolutionary branches with 100% bootstrap support. Hymenoptera (N. lecontei) and isopteran (Z. nevadensis) TO sequences were basal to the evolutionary tree with weak bootstrap support.
(PDF)
Nucleotide sequences of the crRNA used in the experiments are shown in bold red text followed by protospacer adjacent sequence (PAM) in bold black text.
(PDF)
Nucleotides identical to the exon 6 of the reference sequence (accession: MF598173) are indicated by a dot (.) and alignment gaps are shown by a hyphen (-). Nucleotide that differ from the reference are indicated on each sequence. CRISPR RNA (crRNA) target sequences and protospacer adjascent motifs (PAM) are underlined with dotted lines and solid lines, respectively.
(PDF)
One hundred nucleotides from each scaffold, including 38–40 flanking the off-target sequence obtained from wild type insects (WT) and yellow eye (IM) mutants were aligned with H. zea DNA sequence from corresponding scaffold. Off-target sequence is marked in red text and the PAM sequence is underlined. Nucleotides identical to wild type sequence are indicated by a dot (.) and alignment gaps are indicated by a hyphen (-). Nucleotides that are different from the genomic scaffold are shown on each sequence. (A) Alignment of nucleotide sequences corresponding to the off-target site in the scaffold 185 (S185). Nucleotide sequence of the off-target site from the published genome (S185) was significantly different from those obtained from wild type and yellow eye mutants. (B) Alignment of nucleotide sequences corresponding to the off-target site in the scaffold 1210 (S1210). Two different nucleotide variants, one identical to the genomic scaffold 1210 sequence (S2010a) and the other with a number of polymorphisms (S2010b), were identified in the yellow eye mutants. Alignment of S2010a and S1210b (a) indicates that S2010b variants have additional mutations in the protospacer seed sequence and significantly different from the crRNA design for TO. Alignment of the nucleotide sequences similar to S2010a off-target region from wild type and yellow eye mutants (b) indicates that there were no off-target mutations in the insects with edited TO gene. (C) Alignment of nucleotide sequences corresponding to the off-target site in H. zea genomic scaffold 1430 (S1430). Nucleotide sequences from yellow eye mutants indicate that there were no off-target effects in the edited TO gene. (D) Alignment of nucleotide sequences corresponding to the off-target site in H. zea genomic scaffold 1570 (S1570). Nucleotide sequences from yellow eye mutants indicate that there were no off-target effects in the edited TO gene.
(PDF)
Seed sequence (12 nucleotides upstream of the protospacer adjacent motif) of the target sequences of the TO gene was used to search genome of H. zea to identify potential off-targets that had three or less mismatches with the seed sequences of the targets and ten or less total mismatches. Primer pairs designed to PCR amplify and sequence off-target sequences are shown next to the selected sequences. Seed sequence region is shown in blue text and nucleotide mismatches are shown in lowercase text. PAM sequences are shown in bold black text. A lower case “c” at the end of the start position indicates an off-target sequence on the complementary strand of a scaffold. N/A: primers were not designed to these off-targets.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Nucleotide sequence data were submitted to GenBank under Accession numbers MG976796 and MF598173.