Abstract
Production of the iron regulatory peptide hepcidin is tightly controlled by a network of proteins in hepatocytes that sense levels of iron in the circulation (as diferric-transferrin) and in tissues (in ferritin). Human studies show high variability in the normal range of serum hepcidin levels. We have postulated that this may, in part, be related to inter-individual variability in the expression of genes in the iron sensing pathway, potentially governed by epigenetic factors. Here, we have investigated whether genes encoding hepatic iron sensing proteins and hepcidin are regulated by DNA methylation. Experiments were performed on two human hepatoma cell lines, HepG2 cells and Huh7 cells. Basal expression of TFR2 and HAMP was significantly lower in Huh7 cells compared with HepG2 cells. Analysis of bisulphite-converted DNA from Huh7 cells revealed partial methylation of TFR2 (alpha transcript), which could result in gene silencing. Demethylation using 5-aza-2’-deoxycitidine (AZA) increased TFR2 mRNA expression in Huh7. PCR analysis of bisulphite-converted HAMP promoter DNA, using methylation-specific primers, revealed no differences between cell lines. However, HAMP mRNA expression in Huh7 was increased by AZA treatment, suggesting that methylation of one or more iron sensing genes may indirectly influence HAMP expression. Our study provides evidence that DNA methylation might control expression of HAMP and other hepatic iron sensing genes, and indicates that epigenetic influences on iron homeostasis warrant further investigation.
Introduction
Iron homeostasis is maintained by a network of proteins in hepatocytes, which sense changes in circulating and cellular levels of iron. Downstream signalling from these sensors leads to the regulated production of hepcidin (reviewed in [1]). Once released from hepatocytes, serum hepcidin acts as a negative regulator of iron export from a number of cell types, including enterocytes and macrophages, by decreasing expression of the iron transport protein ferroportin [2–5]. In addition, in enterocytes, hepcidin limits iron absorption from diet through down-regulation of DMT1 [5–8].
In hepatocytes, increased cellular iron activates the bone morphogenetic protein (BMP) signalling pathway, which encompasses the ligand BMP6, BMP receptors and the co-receptor hemojuvelin (HJV), and induces the production of hepcidin via the SMAD signalling pathway [9,10]. The protein network which senses changes in serum iron levels (detected as changes in transferrin (Tf) saturation) and translates this information to produce appropriate levels of hepcidin is complex. At low Tf saturation levels, the hereditary haemochromatosis protein HFE and transferrin receptor 1 (TfR1) exist as a complex on the plasma membrane. However, at increased saturation levels, diferric-Tf competes with HFE for binding to TfR1. This causes HFE to dissociate from TfR1 and bind to a second transferrin receptor (TfR2). HFE/TfR2/diferric-Tf binding initiates an iron sensing pathway leading to increased production of hepcidin [11].
The TFR2 gene contains two promoter regions and encodes two receptor isoforms. TFR2-alpha transcript encodes the full length receptor and is expressed on the cell surface of hepatocytes, erythroid progenitors and peripheral blood mononuclear cells [12]. The TFR2-beta transcript is a truncated form which lacks the membrane spanning domain and is highly expressed in spleen, brain and heart [12]. Its physiological role is unclear, but may be involved in regulation of ferroportin in splenic macrophages [13]. The involvement of each of the TFR2 transcripts in iron sensing and the regulation of hepcidin production has not been studied previously and we have addressed this in our current work.
Mutations in TFR2 give rise to type III haemochromatosis [14], which is characterised by liver iron loading and inappropriately low hepcidin levels [15,16]. Furthermore, evidence from knockout studies in mice demonstrates that deletion of TFR2 [17,18], HFE [17,18], HFE2 (HJV) [19,20], or BMP6 [10] leads to inappropriately low HAMP (the gene encoding hepcidin) expression and liver iron loading. Taken together this suggests that each of these iron sensing elements has a specific role to play in the appropriate production of hepcidin. Interestingly, while the HFE/TfR2 and BMP signalling pathways regulating HAMP expression may operate independently of each other [21–23], there is also evidence for interaction between these sensing networks [17,24].
In addition to modulation by iron, HAMP expression is regulated by a variety of other signals including adipokines [25], pro-inflammatory cytokines [26–29], and hypoxia [30–34]. The normal range of serum hepcidin levels in the healthy population is highly heterogeneous [35–37]. While age and gender are significant factors in this distribution, there is still considerable variability (by as much as 50-fold) within population groups. The basis for this is unclear, but it could be related to the non-iron factors identified above (cytokine levels, etc.) or to inter-individual variability in the expression of genes in the iron sensing pathway. Gene expression at an individual level may be controlled by a number of genetic or epigenetic factors. One possible mechanism is through DNA methylation, which occurs at CG sites, resulting in the formation of 5-methylcytosine. CpG-rich regions, known as CpG islands, often occur within the promoter regions of genes, and when methylated result in the repression of gene expression [38]. In this study we have explored ENCODE DNA methylation data to identify putative methylation target sites in the promoter regions of iron sensing genes and HAMP, and assessed the effects of demethylation on mRNA expression of these genes in human hepatoma cell lines.
Materials & methods
Cell culture
HepG2 (ATCC) and Huh7 (gift from Prof. S. Srai, UCL) hepatoma cells were cultured in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 1% non-essential amino acid solution (all purchased from Thermo Fisher Scientific, UK). For experiments, cells were seeded into 6-well plates at a density of 1 x 105 cells/cm2. In some experiments cells were treated with 5-aza-2’-deoxycitidine (AZA, 5 μM) for 72 hours; medium containing AZA was replaced every 24 hours.
Quantitative real-time (qRT)-PCR
RNA isolated from hepatic cells was converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Expression of TFR2 alpha transcript, TFR2 beta transcript, HAMP, HFE, BMP6, HFE2 (HJV) and B2M (reference gene) was analysed using Fast SYBR green Master Mix (Thermo Fisher Scientific) and a ABI Prism 7500 FAST sequence detection system (Applied Biosystems). Primer sequences are given in Table 1. Data were analysed using the 2-ΔΔCt method [39].
Table 1. PCR primers sequences.
| Primer | Forward sequence | Reverse Sequence |
|---|---|---|
| B2M RT | CCA CTG AAA AAG ATG AGT ATG CCT | CCA ATC CAA ATG CGG CAT CTT CA |
| HAMP RT | CTG CAA CCC CAG GAC AGA G | GGA ATA AAT AAG GAA GGG AGG GG |
| TFR2-α RT | GTC AGT GAG GAT GTC AA | CCA CAC GTG GTC CAG CTT CT |
| TFR2-β RT | CCA GAA AAG TCC CCA CCT C | TGC TCT CCG ACC TTC CC |
| HFE RT | AGA ACA GGG CCT ACC TGG AG | TGT GTC ACC TTC ACC AAA GG |
| HFE2 (HJV) RT | GGA GCT TGG CCT CTA CTG GA | ATG GTG AGC TTC CGG GTG |
| BMP6 RT | CCG TGT AGT ATG GGC CTC AGA | TCA CAA CCC ACA GAT TGC TAG T |
| TFR2-α BIS | GGG GGT TGA GGG ATT AGA GAA | CCA AAA CTA TAC CCC CAC CCT TAA AA |
| HAMP Met | TTT TGT TTT CGT TTA TTT TTT TCG T | AAA CTC AAT ACC ATC GTA CCG TC |
| HAMP Unmet | TTT TGT TTT TGT TTA TTT TTT TTG T | AAA AAC TCA ATA CCA TCA TAC CAT |
RT = qRT-PCR primer; BIS = primers for bisulphite-converted DNA; Met = specific primers for methylated bisulphite-converted DNA; Unmet = specific primers for unmethylated bisulphite-converted DNA.
Methylation PCR
DNA from HepG2 and Huh7 cells was subject to bisulphite conversion using EZ DNA Methylation-Gold™ kit (Zymo Research) according to the manufacturer’s instructions. For analysis of the TFR2 alpha promoter, a 246 bp fragment containing the whole of exon 1, plus the 5’ flanking region and intron 1 (base pairs -154 to +92, relative to the translation start site), was amplified using bisulphite-specific primers (Table 1, designed using MethPrimer; www.urogene.org/cgi-bin/methprimer/methprimer.cgi). The amplicon contained a ClaI restriction digest site (ATCGAT) which allowed the PCR product to be cut into two fragments (138 and 108 bp, respectively) if the CpG within the restriction enzyme recognition site was methylated. Aliquots of ClaI-digested and undigested PCR products were resolved on 2% agarose-ethidium bromide gels. Further aliquots of the full amplicon were cloned into E. coli using a TOPO TA Cloning Kit (Invitrogen). Positive colonies were selected and DNA extracted and sequenced (ABI 3730xl DNA Analyser).
For analysis of the HAMP promoter, no suitable bisulphite-specific primers could be designed. Instead we designed methylation-specific primers (Table 1) that would bind to either the predicated methylated or unmethylated DNA sequences within the bisulphite-converted HAMP promoter. Primers targeted identical regions in the HAMP promoter, and differed only at two C/T bases in each of the forward and reverse primers. Analysis was carried out using qRT-PCR.
Statistics
Data are presented as mean ± S.E.M. Data were analysed using Mann-Whitney tests and differences with p < 0.05 considered statistically significant. Linear regression analysis was performed using Sigmaplot (version 13, Systat Software Inc., UK).
Results
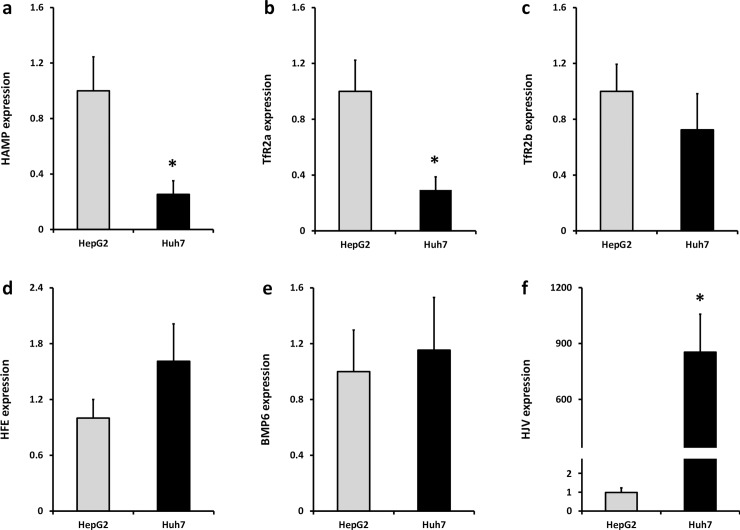
The relative basal RNA expression levels of iron sensing genes were measured in HepG2 and Huh7 cells, respectively (Fig 1). Expression of HAMP and TFR2 alpha was significantly greater in HepG2 cells compared with Huh7 cells (Fig 1A and 1B). There were no significant differences in levels of TFR2 beta (Fig 1C), HFE (Fig 1D) or BMP6 (Fig 1E) between the two cell lines. HFE2 (HJV) levels were significantly higher in Huh7 cells (Fig 1F).
Fig 1. Baseline expression of iron sensing genes in HepG2 and Huh7 cells.
mRNA expression of (a) HAMP, (b) TFR2 alpha, transcript, (c) TFR2 beta transcript, (d) HFE, (e) BMP6 and (f) HFE2 (HJV) were measured by RT-PCR. Data are means ± SEM of 5–8 observations in each group and were normalised to expression in HepG2 cells. * P < 0.02 (Mann-Whitney test).
We hypothesised that differences in expression may be related to cell-specific methylation of the gene promoters. To investigate this possibility, we interrogated data from the ENCODE project [40] on the University of California Santa Cruz Genome Browser. We restricted our search to CpGs within the 5’ promoter for each gene of interest, since methylation in this region has been show to repress gene expression [38]. At least one CpG was identified in the promoter region of each gene of interest and β-values for the degree of methylation are reported in Table 2. Data were available for experiments with human hepatocytes and with HepG2 cells. In the context of the differences observed in basal expression of TFR2 alpha and HAMP in the hepatic cell lines, it was interesting to note that there was some variation in the degree of methylation of CpGs in both the TFR2 alpha and HAMP gene promoters in hepatocytes and HepG2 cells (Table 2).
Table 2. Beta-values from ENCODE database showing degree of methylation of CpG sites in promoters of iron sensing genes.
| Beta-values | |||
|---|---|---|---|
| Gene of Interest | CpG identifier | HepG2 cells | Hepatocytes |
| TfR2α | cg10681065 | 0.09 | 0.28 |
| cg04423314 | 0.09 | 0.28 | |
| TfR2β | cg04499151 | 0.96 | 0.70 |
| HFE | cg06892726 | 0.08 | 0.10 |
| cg05569784 | 0.21 | 0.30 | |
| HJV | cg00953211 | 0.22 | 0.13 |
| cg00987513 | 0.73 | 0.27 | |
| cg06589885 | 0.13 | 0.05 | |
| BMP6 | cg22505205 | 0.50 | 0.13 |
| cg22541378 | 0.24 | 0.03 | |
| cg03447931 | 0.32 | 0.02 | |
| HAMP | cg26283059 | 0.12 | 0.27 |
| cg17907567 | 0.10 | 0.25 | |
| cg23677000 | 0.13 | 0.21 | |
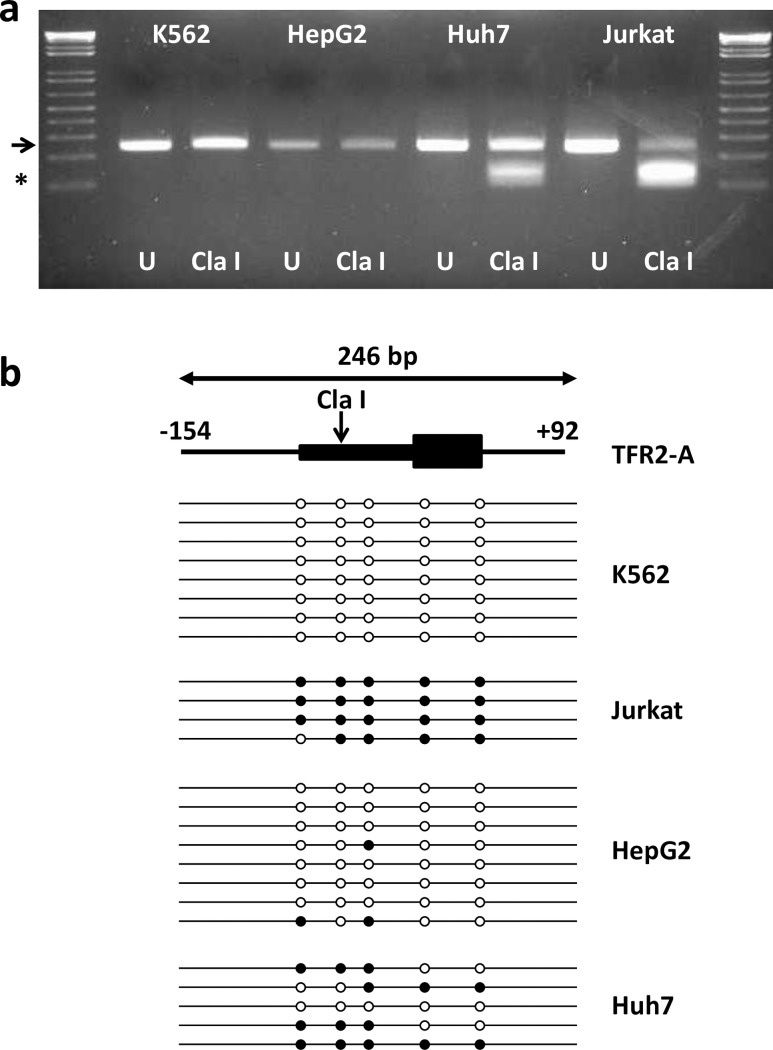
To determine whether the TFR2 alpha and HAMP promoters were methylated in Huh7 hepatoma cells, and to confirm their methylation status in HepG2 cells, we carried out bisulphite conversion of DNA and used specific primers to interrogate the CpGs within each promoter that corresponded to the 450K probe sites (Illumina Infinium HumanMethylation450K BeadChip) from the ENCODE project. For TFR2 alpha, the amplified promoter fragment contained a potential ClaI restriction digest site if the C of the relevant CpG site was retained following bisulphite treatment (i.e., methylated). Following ClaI treatment only the full-length TFR2 alpha PCR product (246 bp) was identified in HepG2 cells, whereas three fragments (246; 138 and 108 bp) were detected in Huh7 cells (Fig 2A) indicating that the TFR2 alpha promoter site was unmethylated in HepG2 cells, but partially methylated in Huh7 cells. K562 cells (ENCODE β-values: cg10681065, 0.04; cg04423314, 0.06) and Jurkat cells (ENCODE β-values: cg10681065, 0.88; cg04423314, 0.69) were used as positive and negative methylation controls, respectively. Selection of these cell lines as controls was based on previously published data showing high expression of TFR2 alpha in K562 cells, but low expression levels in Jurkat cells [12]. Only the full-length TFR2 alpha PCR product (246 bp) was present in K562 cells, whereas the ClaI digested fragments (138 and 108 bp) predominated in Jurkat cells (Fig 2A). To further investigate the cell-specific difference in TFR2 alpha promoter methylation, we subjected amplicons from all cell lines to bisulphite sequencing. Five CpG sites were present in the TFR2 alpha amplicon. All sequenced CpGs in K562 cells and the majority of CpGs in HepG2 cells were unmethylated (Fig 2B). In contrast, 95% of CpG dinucleotides in Jurkat cells and more than 50% of CpGs in Huh7 cells were methylated.
Fig 2. Methylation of TFR2 alpha promoter in human cell lines.
A 246-bp fragment of the human TFR2 alpha promoter was generated by PCR. Amplicons contained a ClaI restriction digest site which will cut the amplicon into 138 & 108 bp fragments if the CpG within the digest site is methylated. Agarose gel (a) shows representative bands from uncut (U; water replacing enzyme) and ClaI-digested bisulphite-converted DNA from K562, HepG2, Huh7 and Jurkat cells. The arrow indicates the position of the full length amplicon (246 bp); * indicates the position of the ClaI digested fragments (138 & 108 bp). The cartoon depicts a representation of TFR2 alpha gene organization and the 246 bp PCR amplicon (b). The 5’ upstream flanking region (from -152 bp relative to the translation start site) and intron 1 (downstream to +92 bp relative to the translation site) are shown as lines; exon 1 containing the promoter and the translated region are shown as boxes. The vertical arrow denotes the ClaI digest site. Horizontal lines below represent bisulphite sequencing of individual amplicons in each cell line. Open circles indicate unmethylated CpGs, filled circles represent methylated CpGs.
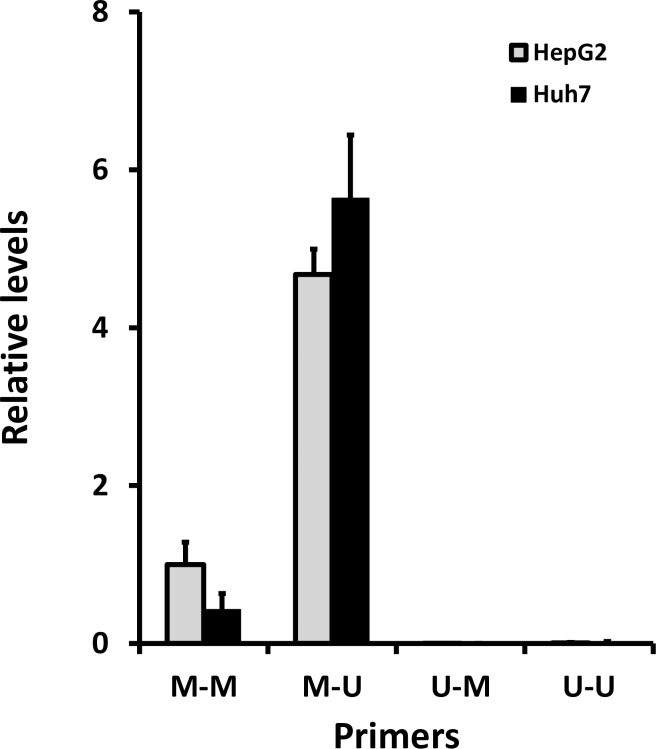
qRT-PCR analysis of the HAMP promoter using combinations of methylated and unmethylated sequence-specific primers revealed no significant differences between HepG2 cells and Huh7 cells for each primer combination (Fig 3). Melt curves indicated only a single PCR product was generated with each of the primer combinations. Lowest Ct values (and therefore highest levels) were observed using the methylated forward: unmethylated reverse primer combination (mean Ct values: 30.6, HepG2; 30.4, Huh7). There was no effect of the demethylating agent 5-deoxy-2’-azacytidine (AZA) on amplicon levels in either cell line using the methylated forward: unmethylated reverse primer combination (HepG2 cells (normalised to control values): control 1.0 ± 0.1; AZA 0.9 ± 0.1; n = 4 for both groups. Huh7 (normalised to control values): control 1.0 ± 0.1; AZA 1.0 ± 0.3; n = 4 for both groups). Since there were no differences in amplicon levels in the presence or absence of AZA in HepG2 and Huh7 cells, we concluded that there was no cell-specific methylation within this region of the HAMP promoter and therefore amplicons were not subjected to full bisulphite sequencing.
Fig 3. Methylation of HAMP promoter.
Bisulphite-converted DNA from HepG2 and Huh7 was subjected to qRT-PCR using a combination of primers specific for methylated and unmethylated sequences. Data are presented as relative levels; ΔCt relative to the methylated forward: methylated reverse primer set (M-M) for HepG2 cells.
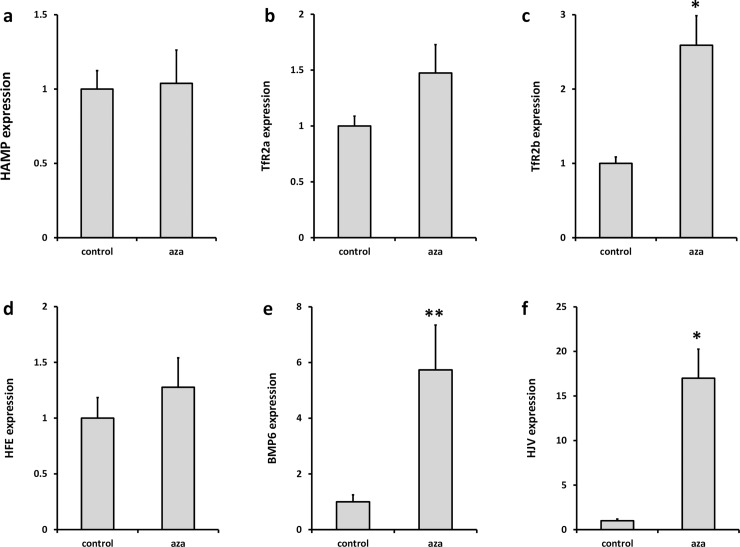
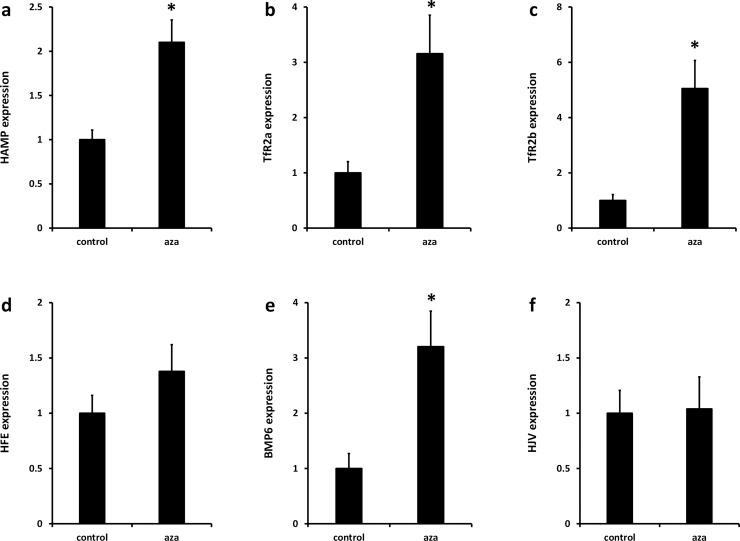
Next we investigated the effects of AZA on the expression of iron sensing genes in HepG2 cells and Huh7 cells. There was no effect of AZA on expression of HAMP and TFR2 alpha in HepG2 cells (Fig 4A and 4B); however, expression of both genes was significantly increased by AZA treatment in Huh7 cells (Fig 5A and 5B). TFR2 beta (Fig 4C; Fig 5C) and BMP6 (Fig 4E; Fig 5E) expression was significantly increased by AZA treatment in both cell lines. HFE2 (HJV) expression was increased in AZA-treated HepG2 cells only (Fig 4F; Fig 5F). There was no effect of AZA on HFE expression in either cell line (Fig 4D; Fig 5D).
Fig 4. Effect of AZA on iron sensing gene expression in HepG2 cells.
Cells were grown in the presence or absence of the global demethylating agent AZA (5 μM, 72 h). Expression of (a) HAMP, (b) TFR2 alpha, transcript, (c) TFR2 beta transcript, (d) HFE, (e) BMP6 and (f) HFE2 (HJV) were measured by RT-PCR. Data have been normalised to the control group and are presented as mean ± SEM of 7–8 observations in each group. Data were analysed using Mann-Whitney tests. * P < 0.005; ** P < 0.03.
Fig 5. Effect of AZA on iron sensing gene expression in Huh7 cells.
Cells were grown in the presence or absence of the global demethylating agent AZA (5 μM, 72 h). Expression of (a) HAMP, (b) TFR2 alpha, transcript, (c) TFR2 beta transcript, (d) HFE, (e) BMP6 and (f) HFE2 (HJV) were measured by RT-PCR. Data have been normalised to the control group and are presented as mean ± SEM of 9–14 observations in each group. Data were analysed using Mann-Whitney tests. * P < 0.01.
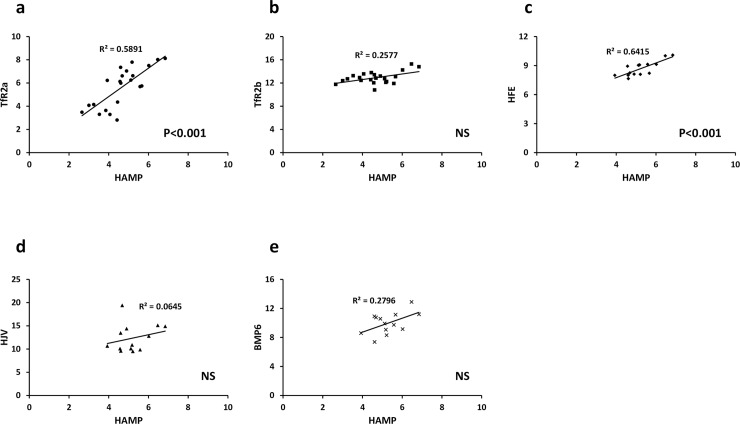
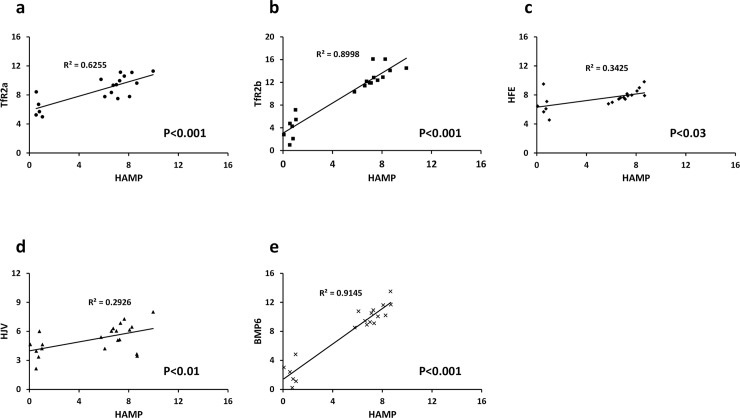
Our data indicated that cellular expression and regulation of HAMP and TFR2 alpha followed a similar pattern; i.e., highest expression in HepG2 cells (Fig 1A and 1B) and up-regulation by AZA-treatment only in Huh7 cells (Fig 5A and 5B). However, while there were clear differences in methylation of TFR2 alpha in HepG2 and Huh7 cells (Fig 2), there was no evidence for cell-specific differences in HAMP promoter methylation at documented CpG sites (Fig 3, Table 2). We therefore investigated the possibility that changes in expression of one or more of the iron sensing genes might indirectly influence HAMP expression. In HepG2 cells there was significant positive correlation between HAMP and TFR2 alpha, and HFE (Fig 6). We carried out multiple linear regression to identify the variables which most significantly predict HAMP expression and found that HFE was the only significant predictor of HAMP mRNA levels (P<0.005). In contrast, in Huh7 cells there was significant correlation between HAMP and all iron sensing genes (i.e., TFR2 alpha, TFR2 beta, HFE, BMP6 and HFE2 (HJV); Fig 7). Multivariate analysis revealed that BMP6 (P<0.001) and HFE2 (HJV) (P<0.03) were the only significant predictors of HAMP expression in Huh7 cells.
Fig 6. Correlation between iron sensing genes and HAMP in HepG2 cells.
ΔCt values (using B2M as the reference gene) from control and AZA-treated cells were plotted for each gene of interest vs HAMP. Data were analysed using linear regression.
Fig 7. Correlation between iron sensing genes and HAMP in Huh7 cells.
ΔCt values (using B2M as the reference gene) from control and AZA-treated cells were plotted for each gene of interest vs HAMP. Data were analysed using linear regression.
Discussion
In this study we employed two well-characterised human hepatoma cell lines, HepG2 and Huh7 cells, to investigate the putative effects of methylation on expression of hepatic iron sensing genes. HepG2 cells were originally derived from a well-differentiated hepatocellular carcinoma [41], which displays a high level of morphological and functional differentiation in vitro. Huh7 hepatoma cells were derived from a Japanese patient [42] and it has been shown that these cells contain a mutated form of HFE [43]. Given that HFE is required for iron sensing and appropriate control of HAMP expression [17,18], we hypothesized that iron sensing and HAMP expression would differ in the two model cell lines.
Basal expression of TFR2 alpha and HAMP was significantly greater in HepG2 cells than Huh7 cells. Differential tissue expression of TFR2 isoforms has been well documented [12]. TFR2 alpha is the predominant isoform in liver and blood mononuclear cells, and is highly expressed in associated tumour cell lines, e.g. HepG2 hepatoma cells and K562 erythroleukaemia cells. In contrast, highest expression of TFR2 beta was observed in spleen and T-cell leukaemia cell lines, e.g., Jurkat cells; these cells also had low expression of TFR2 alpha [12]. One explanation for the differential expression patterns of the TFR2 transcripts might be regulation at the level of DNA methylation. To investigate this possibility, we used data from the ENCODE project [40] including the methylation of specific CpG dinucleotides from experiments using the Illumina Infinium HumanMethylation450K BeadChip array platform. We focussed on CpGs in the 5’ promoter of TFR2, since methylation of CpGs in gene promoters is associated with silencing of gene expression [38]. For TFR2 alpha, the methylation pattern from ENCODE (Table 2) and our bisulphite sequencing analysis (Fig 2) showed that CpGs within the TFR2 alpha promoter were unmethylated in K562 cells and HepG2 cells, but methylated in Jurkat cells. These findings are consistent with previously published mRNA expression data showing high expression of the TFR2 alpha transcript in K562 and HepG2 cells, but low expression in Jurkat cells [12]. Interestingly, our data demonstrated that the TFR2 alpha promoter was partially methylated in Huh7, and this was associated with significantly lower basal TFR2 alpha mRNA expression in Huh7 cells compared with HepG2 (Fig 1B). ENCODE data for HepG2 cells and hepatocytes (Table 2) and our own analysis in Huh7 cells (56% methylation of promoter CpG dinucleotides) found significant methylation of the TFR2 beta promoter, which may account for low expression of this transcript in both HepG2 and Huh7 cells. Together, we have evidence that differences in DNA methylation are strongly associated with tissue-specific expression of the TFR2 isoforms.
The tissue-specific epigenetic control of TFR2 observed here points to tissue-specific functions of the isoforms in the regulation of iron homeostasis. In addition to its well-documented role in hepatic iron sensing, TfR2 is a component of the erythropoietin receptor signalling complex and is required for efficient erythropoiesis [44]. Haematopoietic deletion of Tfr2 in mice results in impaired erythroid differentiation [45,46]. High expression of TFR2 in erythroid cells [47] and its role in erythroid differentiation have led to speculation of a clinically relevant role in haematological disorders such as myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML). In support of this, a recent retrospective analysis of samples from patients with MDS found that expression of TFR2 alpha and TFR2 beta was significantly more variable in MDS samples than in non-malignant bone marrow samples. Furthermore, low expression of both TFR2 transcripts was associated with poorer survival rates in patients with myelodysplastic syndrome with excess blasts than for those with normal to high TFR2 levels [48]. Similarly, a study in patients with AML found higher expression of both TFR2 transcripts was associated with significantly longer survival rates [49]. Interestingly, aberrant DNA methylation is the dominant epigenetic alteration in MDS [50] and DNA methyltransferase inhibitors such as azacitidine are approved as treatment strategies in patients with MDS [51].
HAMP expression is also known to be tissue specific with highest expression in liver [52] and lower levels present in other tissues including adipose and peripheral blood mononuclear cells [53,54]. ENCODE data suggest that HAMP promoter CpG sequences adjacent to the translation start site are unmethylated in HepG2 cells (Table 2), but are partially methylated in both K562 and Jurkat cells (β-scores: cg26283059, 0.31—K562, 0.72—Jurkat; cg17907567, 0.31—K562, 0.45—Jurkat). This is consistent with relatively low expression of HAMP in peripheral blood mononuclear cells compared to hepatic cells [54], and may indicate a role for methylation in determining cell- and tissue-specific expression of HAMP. To determine whether methylation accounted for the observed differences in basal expression of HAMP between HepG2 and Huh7 cells we analysed the region of the promoter containing the CpG dinucleotides cg17907567, cg23677000 and cg26283059. These CpGs are unmethylated in HepG2 cells (Table 2) and our studies suggest that there is no difference in methylation between HepG2 and Huh7 cells. In summary, our analysis does not support the hypothesis that differential methylation of this region of the HAMP promoter explains the differences in basal expression of HAMP between HepG2 and Huh7 cells.
To assess the effects of methylation on HAMP and TFR2 alpha mRNA expression we treated cells with the 5-aza-2’deoxycitidine (AZA), which inhibits DNA methyltransferase activity and results in global DNA demethylation [55]. AZA treatment increased TFR2 alpha mRNA expression in Huh7 cells, but not in HepG2 cells. Taken together, our data and those from ENCODE suggest that differential expression of TFR2 alpha between HepG2 cells and Huh7 cells is mediated by methylation of promoter CpG dinucleotides. Furthermore, this is also a likely explanation for tissue-specific expression of TfR2 isoforms demonstrated previously [12]. Expression of TFR2 beta and BMP6 was increased in both HepG2 and Huh7 cells, and HFE2 (HJV) was elevated in HepG2 cells following AZA treatment indicating that methylation plays an important role in regulating the expression of a number of iron sensing genes.
AZA treatment significantly increased HAMP expression in Huh7 cells, but did not alter HAMP levels in HepG2 cells. Interestingly, a recent report has shown that HAMP expression is supressed in hepatocellular carcinoma through hypermethylation of CpGs within the gene promoter [56]. These findings are in contrast to the data presented here. However, it is important to note that the HAMP promoter region analysed by Udali et al. (-940 to -398 bp relative to the translation start site) [56], did not overlap with the promoter amplicon studied in our analysis (-186 to +14 bp relative to the translation start site). It is possible therefore that hypermethylation within the distal promoter modulates HAMP expression. Moreover, it is possible that epigenetic silencing of elements within one or more of the iron signalling pathways may contribute to decreased HAMP expression. For example, SOSTDC1, an inhibitor of BMP 2, 4 and 7 activity, decreases SMAD-signalling and subsequent hepcidin secretion in prostate epithelial cells [57]. Interestingly in prostate cancer the SOSTDC1 gene promoter is highly methylated leading to suppression of gene transcription, and this is associated with increased HAMP expression and poorer prognosis in patients with prostate tumours [57].
A further possibility is that differential methylation of one or more of the hepatic iron sensing genes could have an indirect effect on HAMP expression. Our previous work shows that TFR2 mRNA correlates significantly with HAMP expression levels in human primary hepatocytes [58], while others have shown strong correlation between HFE and HAMP in HepG2 cells [59]. Here we found a significant correlation between levels of both TFR2 alpha and HFE, and HAMP in HepG2 cells, with HFE being the most significant predictor of HAMP expression. Interestingly, while there is significant positive correlation between HAMP and each individual iron sensing gene in Huh7 cells, multivariate analysis showed HFE2 (HJV) and BMP6 to be the only significant predictors of HAMP. This divergence between the iron sensing pathways that correlate with HAMP expression in HepG2 and Huh7 cells is perhaps not surprising given that there is a mutation in the HFE gene in Huh7 cells [43], which alters the conformation of the α3 domain of the mature protein. TfR2 interacts with the HFE α3 domain [60], and therefore this mutation might silence downstream signalling pathways in Huh7 cells which regulate HAMP expression. Similarly, the very low basal expression of HFE2 (HJV) in HepG2 cells, due at least in part to DNA methylation, is likely to dampen BMP/SMAD signalling in these cells. Interestingly, in patients with MDS, the HFE2 promoter in bone marrow cells was found to be hypermethylated. Treatment with AZA increased HFE2 expression and this was associated with elevated serum hepcidin levels [61]. Taken together, our data suggest that HFE/TfR2 signalling predominates as the iron sensing pathway in HepG2 cells, while the BMP/HJV/SMAD pathway is dominant in Huh7 cells. Key iron sensing genes in both cell lines may be silenced by DNA methylation and this may have an important bearing on HAMP expression.
In summary, our study provides evidence for a role of DNA methylation in controlling hepatic iron sensing and the production of hepcidin. Serum hepcidin levels in the healthy population are highly heterogeneous [35–37] and differential patterns of DNA methylation may be important in determining the variability in iron status and hepcidin production at a population level. This possibility remains to be explored. It has been demonstrated recently that there is widespread inter-individual epigenetic variation, including DNA methylation, in human neutrophils from healthy individuals, which might relate to phenotypic differences and potentially susceptibility to a range of diseases [62]. The epigenetic influences on iron homeostasis and the risk of developing iron metabolism disorders may be similarly linked and this possibility warrants further investigation.
Data Availability
All relevant data are included within the paper.
Funding Statement
This work was funded by the Dunedin School of Medicine. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013. October;93(4):1721–41. doi: 10.1152/physrev.00008.2013 [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004. December 17;306(5704):2090–3 doi: 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 3.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005. September 15;106(6):2196–9. doi: 10.1182/blood-2005-04-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaston T, Chung B, Mascarenhas M, Marks J, Patel B, Srai SK, et al. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008. March;57(3):374–82. doi: 10.1136/gut.2007.131722 [DOI] [PubMed] [Google Scholar]
- 5.Chung B, Chaston T, Marks J, Srai SK, Sharp PA. Hepcidin decreases iron transporter expression in vivo in mouse duodenum and spleen and in vitro in THP-1 macrophages and intestinal Caco-2 cells. J Nutr. 2009. August;139(8):1457–62. doi: 10.3945/jn.108.102905 [DOI] [PubMed] [Google Scholar]
- 6.Yamaji S, Sharp P, Ramesh B, Srai SK. Inhibition of iron transport across human intestinal epithelial cells by hepcidin. Blood. 2004. October 1;104(7):2178–80. doi: 10.1182/blood-2004-03-0829 [DOI] [PubMed] [Google Scholar]
- 7.Mena NP, Esparza A, Tapia V, Valdés P, Núñez MT. Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gastrointest Liver Physiol. 2008. January;294(1):G192–8. doi: 10.1152/ajpgi.00122.2007 [DOI] [PubMed] [Google Scholar]
- 8.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011. April;140(4):1261–1271. doi: 10.1053/j.gastro.2010.12.037 [DOI] [PubMed] [Google Scholar]
- 9.Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009. April;41(4):482–7. doi: 10.1038/ng.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009. April;41(4):478–81. doi: 10.1038/ng.320 [DOI] [PubMed] [Google Scholar]
- 11.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006. September 29;281(39):28494–8. doi: 10.1074/jbc.C600197200 [DOI] [PubMed] [Google Scholar]
- 12.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999. July 23;274(30):20826–32. [DOI] [PubMed] [Google Scholar]
- 13.Roetto A, Di Cunto F, Pellegrino RM, Hirsch E, Azzolino O, Bondi A, et al. Comparison of 3 Tfr2-deficient murine models suggests distinct functions for Tfr2-alpha and Tfr2-beta isoforms in different tissues. Blood. 2010. April 22;115(16):3382–9. doi: 10.1182/blood-2009-09-240960 [DOI] [PubMed] [Google Scholar]
- 14.Camaschella C, Roetto A, Calì A, De Gobbi M, Garozzo G, Carella M, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000. May;25(1):14–5. doi: 10.1038/75534 [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005. February 15;105(4):1803–6. doi: 10.1182/blood-2004-08-3042 [DOI] [PubMed] [Google Scholar]
- 16.Pelucchi S, Mariani R, Trombini P, Coletti S, Pozzi M, Paolini V, et al. Expression of hepcidin and other iron-related genes in type 3 hemochromatosis due to a novel mutation in transferrin receptor-2. Haematologica. 2009. February;94(2):276–9. doi: 10.3324/haematol.13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009. December;50(6):1992–2000. doi: 10.1002/hep.23198 [DOI] [PubMed] [Google Scholar]
- 18.Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, et al. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. 2011. November;141(5):1907–14. doi: 10.1053/j.gastro.2011.06.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005. August;115(8):2180–6. doi: 10.1172/JCI25683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gkouvatsos K, Fillebeen C, Daba A, Wagner J, Sebastiani G, Pantopoulos K. Iron-dependent regulation of hepcidin in Hjv-/- mice: evidence that hemojuvelin is dispensable for sensing body iron levels. PLoS One. 2014. January 7;9(1):e85530 doi: 10.1371/journal.pone.0085530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011. July;54(1):273–84. doi: 10.1002/hep.24359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011. April;53(4):1333–41. doi: 10.1002/hep.24178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Q, Migas MC, Waheed A, Britton RS, Fleming RE. Ferritin upregulates hepatic expression of bone morphogenetic protein 6 and hepcidin in mice. Am J Physiol Gastrointest Liver Physiol. 2012. June 15;302(12):G1397–404. doi: 10.1152/ajpgi.00020.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009. June;94(6):765–72. doi: 10.3324/haematol.2008.003541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007. November;137(11):2366–70. doi: 10.1093/jn/137.11.2366 [DOI] [PubMed] [Google Scholar]
- 26.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003. April 1;101(7):2461–3. doi: 10.1182/blood-2002-10-3235 [DOI] [PubMed] [Google Scholar]
- 27.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004. May;113(9):1271–6. doi: 10.1172/JCI20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007. January 1;109(1):353–8. doi: 10.1182/blood-2006-07-033969 [DOI] [PubMed] [Google Scholar]
- 29.Matak P, Chaston TB, Chung B, Srai SK, McKie AT, Sharp PA. Activated macrophages induce hepcidin expression in HuH7 hepatoma cells. Haematologica. 2009. June;94(6):773–80. doi: 10.3324/haematol.2008.003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi SO, Cho YS, Kim HL, Park JW. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPalpha and STAT-3. Biochem Biophys Res Commun. 2007. April 27;356(1):312–7. doi: 10.1016/j.bbrc.2007.02.137 [DOI] [PubMed] [Google Scholar]
- 31.Braliou GG, Verga Falzacappa MV, Chachami G, Casanovas G, Muckenthaler MU, Simos G. 2-Oxoglutarate-dependent oxygenases control hepcidin gene expression. J Hepatol. 2008. May;48(5):801–10. doi: 10.1016/j.jhep.2007.12.021 [DOI] [PubMed] [Google Scholar]
- 32.Chaston TB, Matak P, Pourvali K, Srai SK, McKie AT, Sharp PA. Hypoxia inhibits hepcidin expression in HuH7 hepatoma cells via decreased SMAD4 signaling. Am J Physiol Cell Physiol. 2011. April;300(4):C888–95. doi: 10.1152/ajpcell.00121.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakhal S, Schödel J, Townsend AR, Pugh CW, Ratcliffe PJ, Mole DR. Regulation of type II transmembrane serine proteinase TMPRSS6 by hypoxia-inducible factors: new link between hypoxia signaling and iron homeostasis. J Biol Chem. 2011. February 11;286(6):4090–7. doi: 10.1074/jbc.M110.173096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnweber T, Nachbaur D, Schroll A, Nairz M, Seifert M, Demetz E, et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut. 2014. December;63(12):1951–9. doi: 10.1136/gutjnl-2013-305317 [DOI] [PubMed] [Google Scholar]
- 35.Kroot JJ, Hendriks JC, Laarakkers CM, Klaver SM, Kemna EH, Tjalsma H, et al. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies. Anal Biochem. 2009. June 15;389(2):124–9. doi: 10.1016/j.ab.2009.03.039 [DOI] [PubMed] [Google Scholar]
- 36.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, van Tienoven D, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011. June 23;117(25):e218–25. doi: 10.1182/blood-2011-02-337907 [DOI] [PubMed] [Google Scholar]
- 37.Handley S, Couchman L, Sharp P, Macdougall I, Moniz C. Measurement of hepcidin isoforms in human serum by liquid chromatography with high resolution mass spectrometry. Bioanalysis. 2017. March;9(6):541–553. doi: 10.4155/bio-2016-0286 [DOI] [PubMed] [Google Scholar]
- 38.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002. January 1;16(1):6–21. doi: 10.1101/gad.947102 [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001. December;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 40.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012. September 6;489(7414):57–74. doi: 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980. July 25;209(4455):497–9. [DOI] [PubMed] [Google Scholar]
- 42.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982. September;42(9):3858–63. [PubMed] [Google Scholar]
- 43.Vecchi C, Montosi G, Pietrangelo A. Huh-7: a human "hemochromatotic" cell line. Hepatology. 2010. February;51(2):654–9. doi: 10.1002/hep.23410 [DOI] [PubMed] [Google Scholar]
- 44.Forejtnikovà H, Vieillevoye M, Zermati Y, Lambert M, Pellegrino RM, Guihard S, et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood. 2010. December 9;116(24):5357–67. doi: 10.1182/blood-2010-04-281360 [DOI] [PubMed] [Google Scholar]
- 45.Nai A, Lidonnici MR, Rausa M, Mandelli G, Pagani A, Silvestri L, et al. The second transferrin receptor regulates red blood cell production in mice. Blood. 2015. February 12;125(7):1170–9. doi: 10.1182/blood-2014-08-596254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rishi G, Secondes ES, Wallace DF, Subramaniam VN. Hematopoietic deletion of transferrin receptor 2 in mice leads to a block in erythroid differentiation during iron-deficient anemia. Am J Hematol. 2016. August;91(8):812–8. doi: 10.1002/ajh.24417 [DOI] [PubMed] [Google Scholar]
- 47.Kawabata H, Nakamaki T, Ikonomi P, Smith RD, Germain RS, Koeffler HP. Expression of transferrin receptor 2 in normal and neoplastic hematopoietic cells. Blood. 2001. November 1;98(9):2714–9. [DOI] [PubMed] [Google Scholar]
- 48.Di Savino A, Gaidano V, Palmieri A, Crasto F, Volpengo A, Lorenzatti R, et al. Clinical significance of TFR2 and EPOR expression in bone marrow cells in myelodysplastic syndromes. Br J Haematol. 2017. February;176(3):491–495. doi: 10.1111/bjh.13968 [DOI] [PubMed] [Google Scholar]
- 49.Nakamaki T, Kawabata H, Saito B, Matsunawa M, Suzuki J, Adachi D, et al. Elevated levels of transferrin receptor 2 mRNA, not transferrin receptor 1 mRNA, are associated with increased survival in acute myeloid leukaemia. Br J Haematol. 2004. April;125(1):42–9. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O'Keefe C,et al. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009. February 5;113(6):1315–25. doi: 10.1182/blood-2008-06-163246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan C, Pathe N, Fazal S, Lister J, Rossetti JM. Azacitidine in the management of patients with myelodysplastic syndromes. Ther Adv Hematol. 2012. December; 3(6): 355–373. doi: 10.1177/2040620712464882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001. March 16;276(11):7811–9. doi: 10.1074/jbc.M008923200 [DOI] [PubMed] [Google Scholar]
- 53.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006. September;131(3):788–96. doi: 10.1053/j.gastro.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 54.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011. October 13;118(15):4129–39. doi: 10.1182/blood-2011-04-351957 [DOI] [PubMed] [Google Scholar]
- 55.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008. July 1;123(1):8–13. doi: 10.1002/ijc.23607 [DOI] [PubMed] [Google Scholar]
- 56.Udali S, Castagna A, Corbella M, Ruzzenente A, Moruzzi S, Mazzi F, et al. Hepcidin and DNA promoter methylation in hepatocellular carcinoma. Eur J Clin Invest. 2017. December 13 doi: 10.1111/eci.12870 [DOI] [PubMed] [Google Scholar]
- 57.Tesfay L, Clausen KA, Kim JW, Hegde P, Wang X, Miller LD, et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015. June 1;75(11):2254–63. doi: 10.1158/0008-5472.CAN-14-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rapisarda C, Puppi J, Hughes RD, Dhawan A, Farnaud S, Evans RW, et al. Transferrin receptor 2 is crucial for iron sensing in human hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2010. September;299(3):G778–83. doi: 10.1152/ajpgi.00157.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009. March;9(3):217–27. doi: 10.1016/j.cmet.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Chloupková M, Gao J, Chapman-Arvedson TL, Enns CA. HFE modulates transferrin receptor 2 levels in hepatoma cells via interactions that differ from transferrin receptor 1-HFE interactions. J Biol Chem. 2007. December 21;282(51):36862–70. doi: 10.1074/jbc.M706720200 [DOI] [PubMed] [Google Scholar]
- 61.Shucheng G, Chunkang C, Youshan Z, Juan G, Chengming F, Xi Z, et al. Decitabine treatment could ameliorate primary iron-overload in myelodysplastic syndrome patients. Cancer Invest. 2015. April;33(4):98–106. doi: 10.3109/07357907.2014.1001895 [DOI] [PubMed] [Google Scholar]
- 62.Chatterjee A, Stockwell PA, Rodger EJ, Duncan EJ, Parry MF, Weeks RJ, et al. Genome-wide DNA methylation map of human neutrophils reveals widespread inter-individual epigenetic variation. Sci Rep. 2015. November 27;5:17328 doi: 10.1038/srep17328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included within the paper.