Abstract
Sulphonylurea drugs stimulate insulin secretion from pancreatic β-cells primarily by inhibiting ATP sensitive potassium (KATP) channels in the β-cell membrane. The effective sulphonylurea concentration at its site of action is significantly attenuated by binding to serum albumin, which makes it difficult to compare in vitro and in vivo data. We therefore measured the ability of gliclazide and glibenclamide to inhibit KATP channels and stimulate insulin secretion in the presence of serum albumin. We used this data, together with estimates of free drug concentrations from binding studies, to predict the extent of sulphonylurea inhibition of KATP channels at therapeutic concentrations in vivo. KATP currents from mouse pancreatic β-cells and Xenopus oocytes were measured using the patch-clamp technique. Gliclazide and glibenclamide binding to human plasma were determined in spiked plasma samples using an ultrafiltration-mass spectrometry approach. Bovine serum albumin (60g/l) produced a mild, non-significant reduction of gliclazide block of KATP currents in pancreatic β-cells and Xenopus oocytes. In contrast, glibenclamide inhibition of recombinant KATP channels was dramatically suppressed by albumin (predicted free drug concentration <0.1%). Insulin secretion was also reduced. Free concentrations of gliclazide and glibenclamide in the presence of human plasma measured in binding experiments were 15% and 0.05%, respectively. Our data suggest the free concentration of glibenclamide in plasma is too low to account for the drug’s therapeutic effect. In contrast, the free gliclazide concentration in plasma is high enough to close KATP channels and stimulate insulin secretion.
Introduction
Sulphonylureas are oral hypoglycaemic agents that are widely used in the management of type 2 diabetes and certain forms of monogenic diabetes. They reduce blood glucose levels by stimulating insulin secretion from pancreatic β-cells. Their primary target is the sulphonylurea receptor (SUR1) subunit of the ATP-sensitive potassium (KATP) channel in the β-cell plasma membrane [1,2]. The structure of the KATP channel complex (Kir6.2/SUR1) in association with the sulphonylurea glibenclamide has recently been solved at atomic resolution [3]. The sulphonylurea binding pocket lies with the transmembrane domains, towards the inner side of the membrane, and comprises TMs 7, 8, and 11 on one side of the pocket and TMs15 and 17 on the other. Because sulphonylureas are lipid soluble [1], they are effective whether applied from the inside or outside of the membrane [4]. Drug binding induces KATP channel closure, which leads to membrane depolarisation, activation of voltage-dependent Ca2+ channels, and an influx of Ca2+ influx that triggers insulin granule exocytosis [5]. Sulphonylureas also stimulate insulin secretion independently of membrane potential, by interacting with proteins within the exocytotic pathway [6,7].
It is well established that sulphonylureas bind tightly to plasma proteins [8–10] and, as a consequence, their effective (free) concentration in plasma is much less than their total concentration. In order to determine the extent of block of KATP channel activity produced by a sulphonylurea in vivo from the concentration-response relation measured in vitro, it is therefore essential to correct for sulphonylurea binding to plasma proteins. Understanding the extent of drug block is of interest in order to answer a number of questions. For example, why do patients with neonatal diabetes due to activating KATP channel mutations require far higher drug doses than patients with type 2 diabetes? [11]. Why do patients with the more severe KATP channel mutations rarely suffer from hypoglycaemia, despite taking these very large doses? And finally, given the very tight binding of glibenclamide to plasma proteins, are plasma free glibenclamide levels sufficient to quantitatively explain the enhanced insulin secretion as entirely due to KATP channel inhibition? To answer these questions, it is necessary to know the concentration-response relation for sulphonylurea inhibition of KATP channel activity in the absence and presence of plasma proteins.
The total concentration of a drug in a body fluid such as plasma is usually given as the steady-state concentration (Css), which is the drug concentration at the time a dynamic steady state has been achieved, and rates of drug administration and elimination are equal. It is determined in a time-dependent manner by both its rate of metabolism and the apparent distribution volume. Sulphonylureas are predominantly distributed in the extracellular space bound to plasma proteins and are characterized by a low apparent distribution volume. Some sulphonylureas such as glibenclamide have active metabolites that may prolong their duration of action [12]. In addition, the steady-state free concentration of a drug is also determined by the extent to which is bound by plasma proteins.
The most abundant plasma protein is albumin, which is responsible for transporting various fatty acids, hormones and drugs in the circulation [13]. We therefore measured the effect of albumin on KATP channel block by two commonly used sulphonylureas–glibenclamide and gliclazide–which have very different rates of unbinding from SUR1: on the time course of electrophysiological experiments, gliclazide is fast, whereas glibenclamide is slow [14,15]. We also measured the binding affinity of glibenclamide and gliclazide to human plasma. We then estimated the circulating free drug concentration in human patients and in a mouse model of neonatal diabetes and used our data to predict the extent of sulphonylurea inhibition of KATP channels at therapeutic concentrations in vivo.
Methods
Use of animals
Use of animals for this research was approved by Animal Welfare & Ethical Review Board of the University of Oxford and by the UK Animals (Scientific Procedures) Act 1986 UK Home Office license number PPL 30/3198.
Electrophysiology—Isolated mouse pancreatic β-cells
Pancreatic islets were isolated from C57BL/6 mice and dissociated into single cells according to established protocols [16]. Mice were killed by cervical dislocation. Cells were cultured in RPMI medium supplemented with 10% fetal calf serum, 11mmol/l glucose, 100U/ml penicillin and 100μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 in air. Whole-cell KATP currents were recorded from single cells 1–3 days after isolation, using the standard whole-cell configuration. The pipette solution contained (mmol/l): 107 KCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 10 HEPES, 0.3 ATP (pH 7.2 with KOH). The extracellular solution contained (mmol/l) 138 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 10 HEPES (pH 7.4 with NaOH). Gliclazide and bovine serum albumin (BSA) were added as stated in the text. Currents were recorded in response to alternate ±20mV depolarisations from a holding potential of –70mV applied at frequency of 0.3Hz, at 20–22°C. Currents were low-pass filtered at 1kHz, digitised at 5kHz using an Axopatch 200B (Axon Instruments) and analysed using pClamp10 (Axon Instruments) and Origin 8.5 (OriginLab Corporation).
Pancreatic islets are composed mainly of β-cells with much small numbers of α-cells and δ-cells. Given that the KATP channel is composed of Kir6.2 and SUR1 in all three cell types, there should be no difference in glibenclamide block. Indeed, no difference is observed in glibenclamide block of native channels in β-cells or recombinant channels expressed in Xenopus oocytes or mammalian cells [1, 14, 17, 18]. Nevertheless, we selected cells with KATP currents >100pA (whole-cell recordings), >40pA (perforated patch, βV59M β-cells) or >20pA (perforated patch, wild-type cells) to reduce contamination with α- and δ-cells, which have smaller KATP currents [19, 20].
Electrophysiology—Xenopus oocytes
The time course of glibenclamide block is very slow at low drug concentrations, and difficult to distinguish from current rundown [1]. This problem becomes even more severe when the free drug concentration is decreased by binding to albumin. It was therefore not possible to measure glibenclamide inhibition of whole-cell KATP currents in pancreatic β-cells. We therefore used a different approach: we measured the single channel open probability (PO) in cell-attached patches on intact Xenopus oocytes expressing recombinant β-cell KATP channels. Channel activity was increased using the metabolic inhibitor Na-azide (3mmol/l).
Oocytes were surgically removed from Xenopus laevis frogs (European Xenopus Resource Centre, Portsmouth) anesthetized with 5g/l ethyl-m-aminobenzoate methanesulfonate (MP Biomedicals, France); subsequently, euthanasia was performed by decapitation. Macroscopic KATP currents were recorded from cell-attached and excised patches on Xenopus oocytes, heterologously expressing human Kir6.2 (Genbank NMOL/L000525 with E23 and I377) and rat SUR1 (Genbank L40624). Site-directed mutagenesis, preparation of mRNA, and isolation and injection of oocytes with mRNA were as described previously [21]. Currents were recorded at -60mV and 20–22°C, filtered at 5kHz, digitised at 20kHz using an Axopatch 200B and analysed using pClamp10 (Axon Instruments) and Origin 8.5 (OriginLab Corporation). The pipette solution contained (mmol/l): 140 KCl, 1.2 MgCl2, 2.6 CaCl2, 10 HEPES (pH 7.4 with KOH). The bath solution in cell-attached patches (and intracellular in excised patches) contained (mmol/l): 107 KCl, 1 CaCl2, 2 MgCl2, 10 EGTA, 10 HEPES (pH 7.2 with KOH). Oocytes were pre-incubated for 30-60min prior to experiment in bath solution supplemented with 3mmol/l Na-azide, BSA (60mg/ml, or 0.9mmol/l) and glibenclamide as stated in the text.
Electrophysiology—Data analysis
The single-channel open probability (PO) was estimated as
| Eq 1 |
where IMEAN is the mean KATP current in cell-attached configuration, N is the number of active channels in the patch and i is the single-channel current (i = 4pA at -60mV). Following cell-attached recordings, the patch was excised and the number of active channels (N) was estimated from ~1s data stretches obtained once the KATP current had reached its maximum, using noise analysis [22].
The relationship between the sulphonylurea (S) concentration and the macroscopic current (I) or the single-channel open probability (PO) was fit with
| Eq 2 |
where I(S) is the macroscopic current and PO(S) is the cell-attached PO in the presence of the drug, I and PO are the macroscopic current and the cell-attached single channel open probability, respectively, in drug-free solution, IC50 is the drug concentration at which the inhibition is half maximal, h is the Hill coefficient and a is the fraction of KATP current remaining at drug concentrations that saturates the high-affinity binding site on SUR1 (a was set to 0 for glibenclamide). To correct for any rundown of whole-cell currents, control solutions braced each test concentration, and the control current is taken as the mean value of that before and after the test concentration.
Chemicals and biological reagents
A 100mmol/l stock solution of gliclazide or glibenclamide in DMSO was prepared daily and diluted as required. Bovine serum albumin (BSA; Fraction V) was purchased from Roche Diagnostics, Mannheim, Germany. Fatty acid free BSA (Sigma-Aldrich, St Luis, USA) was used for insulin secretion experiments. The BSA concentration (0.9mmol/l; 60g/l) was chosen to approximate that of albumin in plasma (35-55g/l). Human plasma was purchased from Valley Biomedical Inc., Winchester, USA.
Insulin secretion
Pancreatic islets were isolated from C57BL/6 mice using established protocols [16] and cultured overnight in RPMI-1640 (Sigma, UK) supplemented with 10mmol/l glucose, 100U/ml penicillin, 10μg/ml streptomycin and 10% fetal calf serum, at 37°C in a humidified atmosphere of 5% CO2/95% air. Mice were killed by cervical dislocation Insulin secretion was measured from triplicate batches of 10 islets incubated in 0.5 ml of Krebs-Ringer buffer (KRB) containing (mmol/l): 140 NaCl, 3.6 KCl, 1.5 CaCl2, 0.5 MgSO4, 0.5 NaH2PO4, 2 NaHCO3, 10 HEPES, and 0.1% (wt/vol) BSA, pH 7.4. Islets were washed twice and preincubated in 2mmol/l glucose KRB for 1 hour at 37°C, with gentle shaking. The pre-incubation buffer was discarded and islets were stimulated for a further 1 (or 2) hours with the test conditions indicated. An aliquot of the supernatant was collected, in order to quantify secreted insulin, and total insulin was extracted into 0.5 ml of acidified ethanol (75% (vol/vol) ethanol, 1.5% (vol/vol) 1 mmol/l HCl and 0.1% (vol/vol) Triton X-100). Secreted and total insulin concentrations were measured by ELISA (Mercodia, Uppsala, Sweden).
Binding studies
The free plasma concentrations of sulphonylureas were determined in spiked plasma samples using an ultrafiltration approach [23]. For gliclazide, the following total drug concentrations (ng/ml) were used: 0, 1, 5, 20, 50, 200, 500, 1000, 2000, 3000, 4000. For glibenclamide, the total drug concentrations used were (ng/nl): 0; 10000; 50000. Aliquots (500μl) of spiked plasma samples were centrifuged in 50 KDa molecular weight cut-off centrifugal concentrators until >100μl ultrafiltrate was collected. Drug-free plasma ultrafiltrate was produced from blank plasma (10ml) and the obtained blank ultrafiltrate was used to prepare calibration standards and quality control (QC) samples. Sample extraction, analysis by liquid chromatography-mass spectrometry (LC-MS) and data analysis were carried out as described previously for total drug concentrations [24].
The Kd for sulphonylurea binding to albumin in human plasma was calculated according to the equation [25]:
| Eq 3 |
where CT and CF are the total and free concentrations of the sulphonylurea, respectively, [A] is the albumin concentration (0.9mmol/l) and n is the number of sulphonylurea binding sites on albumin (for simplicity and for comparison with previous binding studies on isolated albumin, n = 1).
Statistical analysis
All values are given as mean ± SEM. Statistical significance in electrophysiological experiments was determined using Student’s t-test. Insulin secretion was analysed using a one-way ANOVA followed by Tuckey post-hoc test to compare individual groups.
Results
BSA causes a small reduction in gliclazide block of the KATP channel
We first examined the effect of bovine serum albumin (BSA, 0.9mmol/l) on gliclazide block of whole-cell KATP currents in mouse pancreatic β-cells. Following establishment of the whole-cell configuration, the amplitude of the whole-cell KATP current first increases due to washout of intracellular ATP, and then slowly runs down with time. Gliclazide was tested once the maximal current amplitude had been reached, while the current was in a pseudo-steady state.
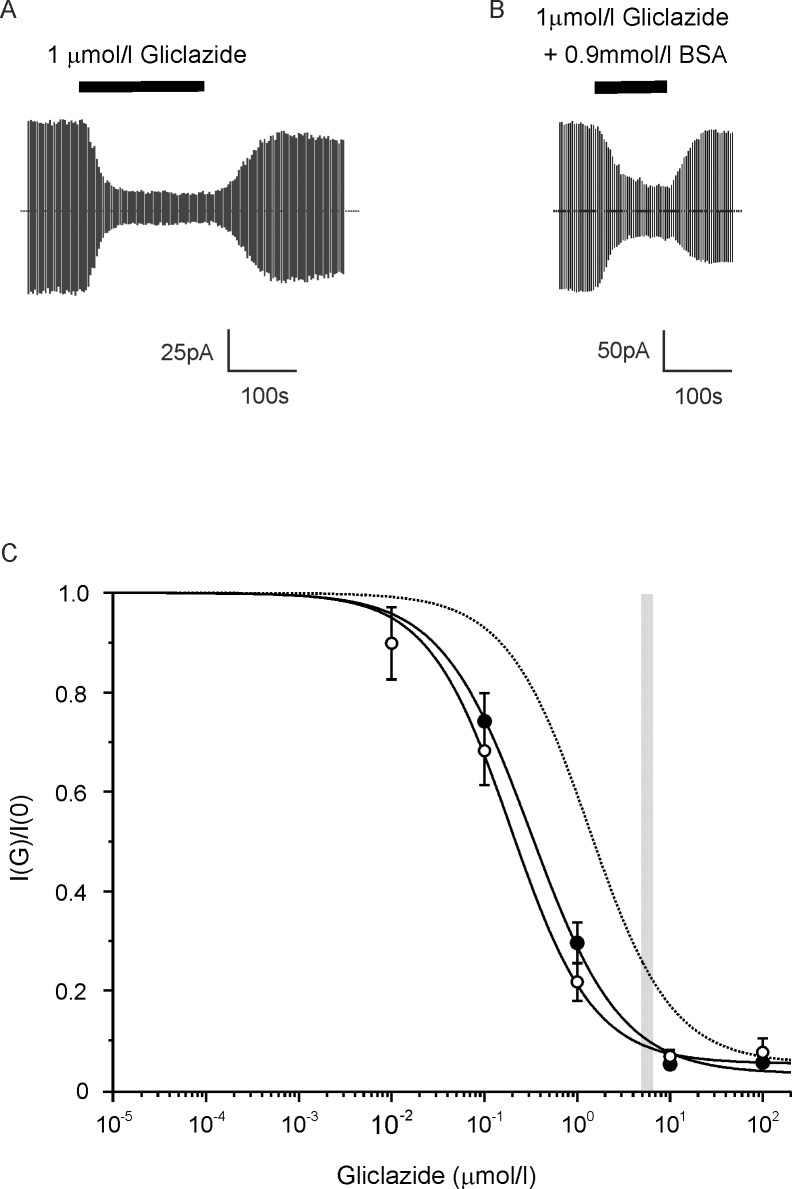
Fig 1A and 1B shows typical current traces recorded in response to 1μmol/l gliclazide in the presence and absence of 0.9mmol/l BSA, respectively. Fig 1C plots the corresponding concentration-inhibition relationships. BSA had a modest effect on gliclazide sensitivity, increasing the half maximal inhibitory concentration (IC50) from 190 to 320nmol/l. However, the effect did not reach significance.
Fig 1. Effect of BSA on gliclazide block of the β-cell KATP current.
(A,B) Representative whole-cell KATP currents recorded from mouse pancreatic β-cells in response to alternating ±20mV steps from a holding potential of -70mV. Gliclazide (1μmol/l) was added (as indicated by the bars) in the absence (A) or presence (B) of 0.9mmol/l BSA. The dotted line indicates the zero current level. (C) Concentration-response relationships for gliclazide inhibition of whole-cell KATP currents in mouse β-cells in the absence (○, n = 6) or presence (●, n = 5) of 0.9mmol/l BSA. Current is expressed relative to that in the absence of gliclazide. The solid lines are the best fit of Eq 2 to the mean data: (○) IC50 = 190nmol/l, h = 0.97, a = 0.05; (●) IC50 = 320nmol/l, h = 0.90, a = 0.03. This shift predicts 60% of drug is bound and a dissociation constant (Kd) for drug binding to BSA of ~1.3mmol/l (Eq 3). The dotted line is the estimated gliclazide block in the presence of human plasma (HP) assuming gliclazide binds to plasma proteins with a Kd of 155μmol/l. The width of the grey bar indicates the mean CSS±SEM (steady-state total plasma concentrations of sulphonylurea drugs) of the total gliclazide concentration in the plasma estimated from a daily dose of 80mg and an AUC (the area under the plasma concentration against time curve) of 44μg.h/ml [26].
BSA dramatically reduces glibenclamide block of the KATP channel
We next investigated the effect of BSA on glibenclamide block of the KATP channel. For reasons outlined in the Methods, it was not possible to measure glibenclamide block of β-cell KATP currents in the same way as for gliclazide. Instead, we measured the single-channel open probability (PO) in cell-attached patches on intact Xenopus oocytes expressing recombinant β-cell KATP channels. Channel activity was increased using the metabolic inhibitor Na-azide (3mmol/l).
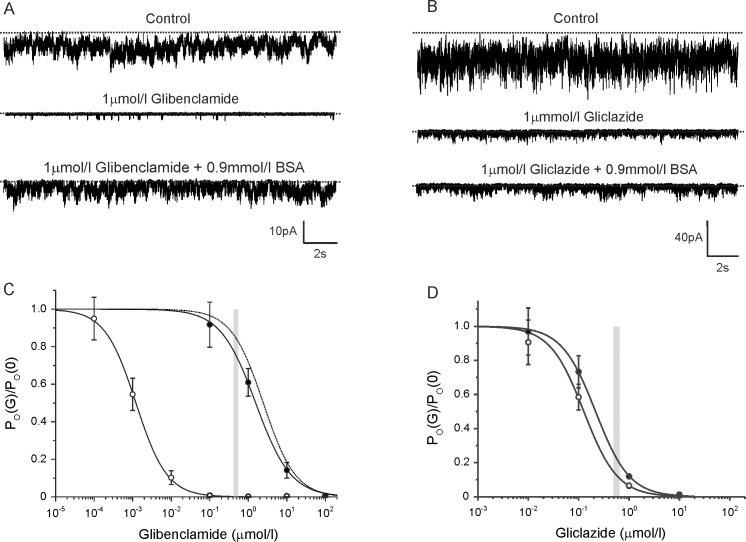
In control solution, the mean PO was 0.32±0.08 (n = 15). Fig 2A shows typical currents recorded from cell-attached patches in control solution (top trace), in the presence of 1μmol/l glibenclamide (middle) and in the presence of 1μmol/l glibenclamide plus 0.9mmol/l BSA (bottom). Fig 2C shows the mean glibenclamide concentration-inhibition curve. In the absence of BSA, the IC50 was 1.2nmol/l, which is close to that reported for inhibition of whole-cell KATP currents in pancreatic β-cells [1,18]. BSA substantially reduced glibenclamide block, the IC50 increasing to 1.6μmol/l. This suggests that BSA reduced the free glibenclamide concentration more than a thousand-fold, giving a predicted Kd of 0.67μmol/l.
Fig 2. Effect of BSA on glibenclamide and gliclazide block of recombinant β-cell KATP channels.
(A,B) Representative Kir6.2/SUR1 currents recorded at -60mV from cell-attached patches on Xenopus oocytes. Currents were recorded in the presence of 3mmol/l Na-azide (top trace), 3mmol/l Na-azide plus 1μmol/l glibenclamide (A, middle) or 3mmol/l Na-azide plus 1μmol/l gliclazide (B, middle), and 3mmol/l Na-azide, 1μmol/l glibenclamide and 0.9mmol/l BSA (A, bottom) or 3mmol/l Na-azide, 1μmol/l gliclazide and 0.9mmol/l BSA (C, bottom). The dotted line indicates the zero current level. (C) Concentration-response relationships for glibenclamide inhibition of Kir6.2/SUR1 currents in the absence (○, n = 10) and presence (●, n = 10) of 0.9mmol/l BSA. Open probability (PO) was recorded in the cell-attached configuration and is expressed relative to that in the absence of glibenclamide. The lines are the best fit of Eq 2 to the mean data: (○) IC50 = 1.2nmol/l, h = 1.1; (●) IC50 = 1.6μmol/l, h = 0.95. a was set at 0 in both cases. The dotted line is the estimated glibenclamide block in the presence of human plasma (HP) assuming the drug binds to plasma proteins with a Kd of 0.44μmol/l. The width of the grey bar indicates the mean CSS±SEM of the total glibenclamide concentration in the plasma of patients with type 2 diabetes [28]. (D) Concentration-response relationships for gliclazide inhibition of Kir6.2/SUR1 currents in the absence (○, n = 10) and presence (●, n = 10) of 0.9mmol/l BSA. Open probability (PO) was recorded in the cell-attached configuration and is expressed relative to that in the absence of gliclazide. The lines are the best fit of Eq 2 to the mean data: IC50 = 128nmol/l, h = 1.3 (○); IC50 = 217nmol/l, h = 1.3 (●). a was set at 0. The IC50 obtained in the absence of BSA (128nmol/l) is similar to that previously reported for whole-cell Kir6.2/SUR1 currents in oocytes (108nmol/l; [27]). The width of the grey bar indicates the mean CSS±SEM of the total gliclazide concentration in the plasma estimated from a daily dose of 80mg and an AUC of 44μg.h/ml [26].
For comparison, we also determined the concentration-response relationship for gliclazide block of β-cell KATP channels expressed in Xenopus oocytes, by measuring PO in cell-attached patches (Fig 2B–2D). In the absence of BSA, the IC50 was 127nmol/l, which is similar to that obtained previously for whole-cell currents in oocytes (108nmol/l; [27], and in pancreatic β-cells (190nmol/l, Fig 1). The presence of BSA produced a small (non-significant) increase in IC50 to 217nmol/l, which is consistent with the data obtained in β-cells (Fig 1).
Insulin secretion
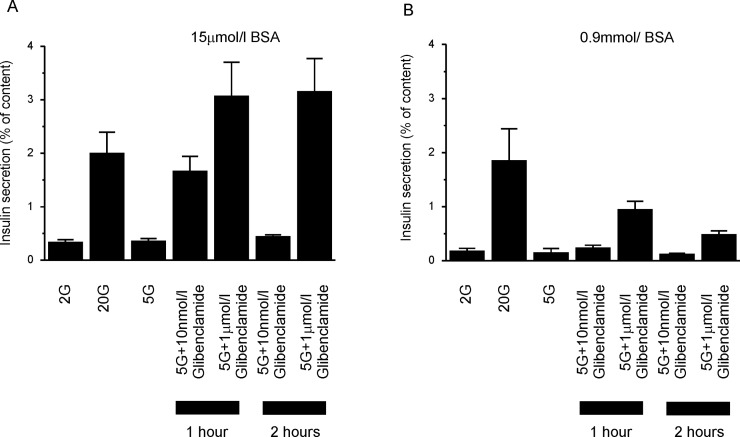
Glucose-stimulated insulin secretion from isolated islets was not significantly affected by 0.9mmol/l BSA (Fig 3), although there was a trend towards reduced basal secretion at 2 and 5mmol/l glucose. We examined the effect of BSA on glibenclamide-stimulated insulin secretion at 5mmol/l glucose, as this is similar to the fasting glucose concentrations in humans. Fig 3 shows that 0.9mmol/l BSA produced a substantial reduction in insulin secretion in response to 1μmol/l glibenclamide, which was ~3-fold lower than in the presence of 15μmol/l BSA. This difference persisted even when the incubation period was extended to 2 hours. Thus BSA markedly reduces the effect of glibenclamide on insulin release as well as on KATP channel activity.
Fig 3. Effect of BSA on glibenclamide stimulated insulin release.
Insulin secretion was evaluated in islets from 8–10 week-old male mice in response to 60–120 minute stimulation with various glucose and glibenclamide concentrations, as indicated, in the presence of (A) 15μmol/l BSA or (B) 0.9mmol/l BSA (n = 3 animals, 3 technical replicates per condition). Insulin secretion is expressed as a percentage of the insulin content. Insulin secretion in the presence of glucose and glibenclamide was significantly affected by the BSA concentration (F(5,12) = 10.77; p = 0.004). For both incubation times and for all drug concentrations tested, insulin secretion was significantly lower in the presence of 0.9mmol/l BSA than 15μM BSA (p<0.05).
Previous studies have shown that albumin acts as a Zn2+ chelator and causes dissociation of the Zn-insulin complex which enables insulin to be measured correctly (Zn-insulin is not detected by the immunoassay) [29,30]. In our experiments increasing albumen from 15μM to 900μM did not affect insulin release measured in response to 20mM glucose in the absence of glibenclamide (Fig 3). This suggests 15μM albumen must be sufficient to dissociate all Zn2+ from insulin. The maximal glibenclamide concentration we used for our experiments was 1μM. This is much less than the albumen concentration (15μM), suggesting glibenclamide should not compromise Zn2+ chelation (or insulin measurements).
If we assume that 1μmol/l glibenclamide is a maximally effective stimulus for KATP channel closure at low BSA levels, as seems reasonable from the concentration-response curves in both oocytes (Fig 2C) and pancreatic β-cells [18], then 10nmol/l glibenclamide produces ~50% stimulation of insulin release (Fig 3A). This is in reasonable agreement with the Kd for glibenclamide binding to SUR1 seen by some authors (4nmol/l, [31]) although others have reported Kd values ~10-fold lower [32]. The IC50 for KATP channel inhibition is also reasonably close, varying from 1-5nmol/l [1, 2, 18], Fig 3A). By contrast, in the presence of 0.9mmol/l BSA, insulin secretion is only ~30% of maximal (Fig 3B). A corresponding 30% reduction in KATP current is produced by ~700nmol/l glibenclamide.
Estimated effect of human plasma on sulphonylurea block of the KATP channel
We next determined the free concentrations of glibenclamide and gliclazide in human plasma. The percentage of free drug was 14.7±1.5% (n = 3) for gliclazide and 0.05±0.02% (n = 3) for glibenclamide. These values predict dissociation constants (Kd) for drug binding to human plasma proteins of 0.44μmol/l for glibenclamide and 155μmol/l for gliclazide (calculated using Eq 3). Thus, the Kd for glibenclamide binding to BSA estimated from electrophysiology (0.67μmol/l) was similar to that for human plasma; in contrast, the Kd for gliclazide binding to BSA estimated from the statistically insignificant shift in the IC50 was very different: 1.3mmol/l compared with 155μmol/l for human plasma.
Using the measured Kd values for human plasma, we estimated the relationship between gliclazide (Fig 1C, dotted line) or glibenclamide (Fig 2C, dotted line) and KATP channel inhibition in presence of human plasma (assuming an albumin concentration in plasma of 60g/l). The predicted glibenclamide sensitivity in the presence of human plasma was similar to that obtained experimentally in the presence of BSA. In contrast, the predicted gliclazide block in the presence of human plasma was significantly lower than that measured in the presence of BSA, presumably because of the difference in the affinity of BSA and human plasma for gliclazide.
Steady-state total plasma concentrations of sulphonylurea drugs (CSS) have been used previously as a proxy for the drug concentration encountered at the site of action [33]. The grey bars indicate the estimated circulating CSS of the drug in patients with type 2 diabetes treated with gliclazide ([26]; Fig 1C, Fig 2D) or glibenclamide ([28]; Fig 2C). This reveals that in the presence of BSA KATP channels are almost fully blocked (>90%) by therapeutic (CSS) levels of gliclazide, whereas they are only blocked by ~75% at CSS levels of glibenclamide.
Discussion
Our binding data confirm that sulphonylureas bind very tightly to plasma proteins, such as BSA and human serum albumin (HSA). We found that 99.9% of glibenclamide is bound, in agreement with earlier studies of drug binding to fatty acid-free HSA [34,35]. Gliclazide also binds to plasma proteins, albeit less tightly than glibenclamide—at the CSS, 85% of gliclazide is bound, when binding to human plasma is measured. Interestingly, considerable variability in the affinity of gliclazide binding to HSA (or BSA) is reported in the literature, with the percentage of free drug ranging from less than 1% [9, 36] to 70% [37]. The most likely explanation for this variability is that gliclazide binding to albumin is strongly affected by the composition of solutions used to measure binding [38]. Our data also suggest that whereas glibenclamide binding to human plasma can be largely attributed to albumin, other plasma proteins may contribute to gliclazide binding.
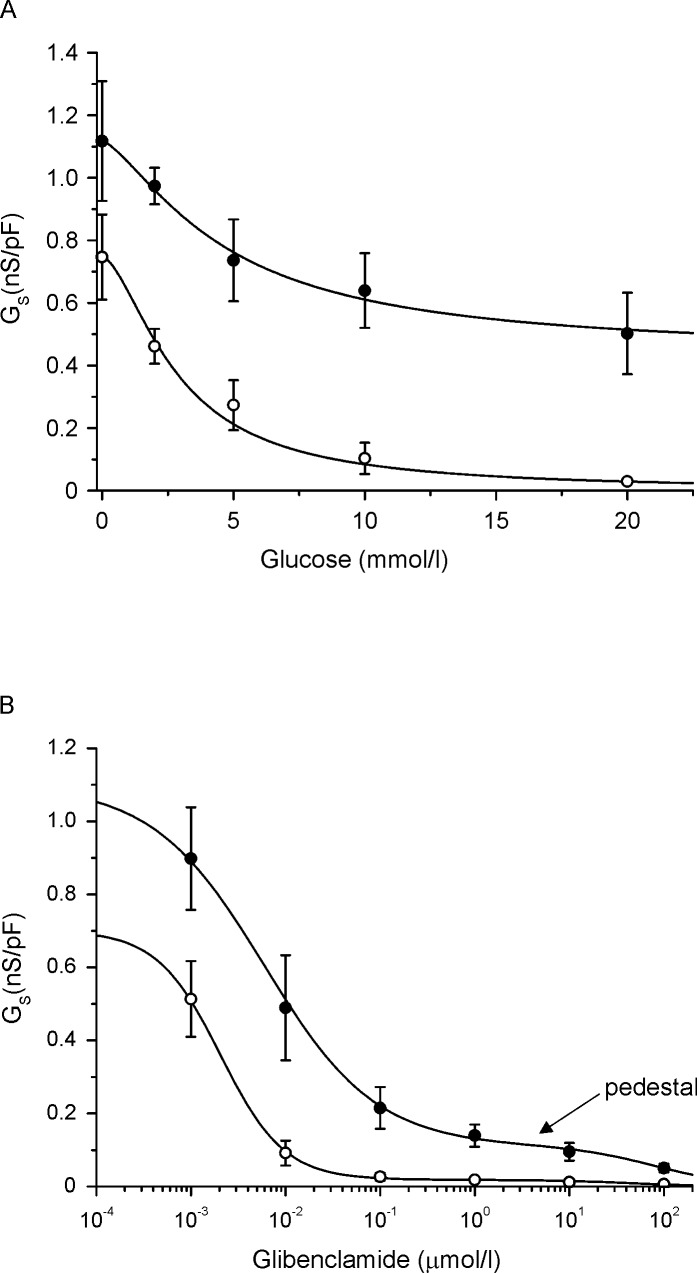
How do these values translate into inhibition of the KATP channel? Sulphonylureas produce a concentration-dependent decrease in the KATP current, which depolarises the β-cell membrane and ultimately (once the threshold is exceeded) stimulates electrical activity. Glucose acts in a similar manner. In both cases, it is the actual magnitude of the current (rather than the percentage block) that determines its impact on the membrane potential, and thus insulin secretion. Fig 4 shows concentration-response curves for glucose (A) and glibenclamide (B) inhibition of the KATP current, measured using the perforated patch configuration to maintain metabolism and retain other regulators of channel activity. The data are expressed as a fraction of the cell capacitance (nS/pF) to compensate for differences in cell size, and shown for both control β-cells and β-cells from mice carrying the Kir6.2-V59M mutation found in patients with neonatal diabetes (βV59M mice). Data have been recalculated from [16] and [18].
Fig 4. Glucose and glibenclamide block of the KATP channel.
(A) Concentration-response relationships for glucose inhibition of KATP conductance in pancreatic β-cells from wild-type (○, n = 5) and βV59M (●, n = 8) mice, measured using the perforated patch configuration. Data are the same as those in [16] but are replotted as nS/pF. (B) Concentration-response relationships for glibenclamide inhibition of KATP conductance in pancreatic β-cells from wild-type (○, n = 6) and βV59M (●, n = 6) mice, measured using the perforated patch configuration. Data are the same as those in [18] but are replotted as nS/pF. Note the ‘pedestal’ (arrowed) in the dose-response curve for glibenclamide inhibition. This results because glibenclamide binds to SUR1 with high affinity and acts a partial antagonist of the KATP channel, with a maximal block of ~60–80% [14]. It also produces a low affinity block at Kir6.2. However, like other sulphonylureas [39], glibenclamide also displaces MgADP from NBD2 of SUR1, which prevents MgADP activation and thereby reveals the full extent of ATP block at Kir6.2. Thus, in the presence of intracellular nucleotides, inhibition is the sum of the high-affinity glibenclamide block at SUR1, ATP block at Kir6.2, and a low-affinity block by glibenclamide at Kir6.2.
Measurements of glibenclamide levels in βV59M mice treated with sulphonylureas range from ~230nmol/l (0.25mg/21 day pellet) to 1μmol/l (2.5mg/21 day pellet; [24]). This corresponds to a free glibenclamide concentration of 0.11nmol/l and 0.5nmol/l, respectively (assuming a Kd of 0.44μmol/l), or a whole-cell KATP conductance of 1.05nS/pF or 0.96nS/pF (Fig 4B). In both cases, this is far greater than the threshold level for electrical activity, which occurs when glucose exceeds ~6mmol/l, corresponding to ~0.2nS/pF (Fig 4A). Even if we consider that glucose also produces some reduction in the KATP current of βV59M β-cells, the decrease is not sufficient to initiate electrical activity. This is inconsistent with the fact that glibenclamide produces excellent control of blood glucose in βV59M mice [40].
A similar problem arises when we consider the glibenclamide concentration in human patients. The free glibenclamide level is around 0.2nmol/l in people with type 2 diabetes (calculated from a total concentration of 0.48μmol/l [28], and can be as much as 0.4nmol/l in patients with neonatal diabetes (calculated from a total concentration of 435ng/ml or 880nmol/l; [41]). This corresponds to a block of the KATP current of ~10% (type 2 diabetes) and 16% (neonatal diabetes). Insulin secretion and electrical activity are stimulated at lower glucose levels in human β-cells (3mmol/l; [42,43], probably because they possess very little GLUT2 [44]. Even if we use the mouse data to estimate the KATP current at the free drug concentration found in human plasma (0.67pS/pF and 0.62pS/pF, respectively for type 2 diabetes and neonatal diabetes) this is still too large to quantitatively explain the therapeutic effect of the drug. The situation is even worse when we consider that a child born to a diabetic mother treated with glibenclamide had hypoglycaemia, despite an estimated free plasma glibenclamide level of 10pM (20nmol/l total; [41]). This is expected to reduce the wild-type current by less than 1%.
This suggests the effective glibenclamide concentration must be far greater at its site of action than that measured in plasma. A free concentration of at least 100nmol/l is needed in βV59M mice and 40nmol/l in wild-type mice ([18]; Fig 4B). Two possible explanations for this discrepancy can be excluded. First, recent studies indicated that sulphonylurea binding to albumin is altered by glycation of the protein [45]. However, the effect of glycation on glibenclamide binding to HSA appears to be variable, as both an increase [33] and a decrease [46] have been reported. In addition, no obvious effect of albumin glycation on glibenclamide binding was seen with plasma samples from patients with type 2 diabetes [47]. Secondly, glibenclamide is known to accumulate progressively inside β-cells [48]. This is expected to increase its effective concentration at its binding site, which lies within the intracellular domains of SUR1 [3]. However, this is unlikely to account for the effects we observed as glibenclamide uptake is nearly saturated after 30mins [49], whereas glibenclamide-stimulated insulin secretion was still substantially less after 1 hour in the presence of 0.9mmol/l BSA than 15μmol/l BSA, and increasing the incubation time to 2hrs did not further enhance insulin release (this study). There is thus a discrepancy between the in vitro and in vivo data. A similar discrepancy is seen for other drugs that bind very tightly to plasma proteins, and is hypothesised to result from multiple variables that affect the dynamic free concentration of the drug in vivo [50]. Whatever the explanation for the difference, the data mean it is not possible to deduce the magnitude of the KATP current in vivo from the glibenclamide concentration needed to produce euglycaemia.
The free glibenclamide concentration in βV59M mice must be at least 100nmol/l if it is to stimulate insulin release and control the animal’s diabetes. If we assume this is the case, it is obvious why a 10-fold higher dose does not cause hypoglycaemia. The shape of the dose-response curve is such that only a small reduction in current is produced (to ~0.15nS/pF). Presumably the same explanation accounts for the fact that neonatal diabetes patients with the same mutation rarely suffer from hypoglycaemia, even when taking high doses of glibenclamide. The data also explain why hypoglycaemia is more common in patients with type 2 diabetes, who have wild-type KATP channels, or in neonatal diabetes with less severe KATP channel mutations that do not give rise a pedestal. The threshold for electrical activity (0.2nS/pF) lies on a steep part of the glibenclamide concentration-response curve, and a 10-fold increase in the drug concentration will result in almost total block of the channel.
Electrical activity in mouse β-cells is initiated when the glucose concentration exceeds 6mmol/l glucose [51], where the KATP conductance is approximately 0.2 pS/pF (Fig 4A). A similar current magnitude can be expected at the threshold potential in human β-cells. Assuming a daily dose of 80mg and an AUC of 44μg.h/ml [26], the estimated free gliclazide concentration in the plasma of patients with type 2 diabetes is around 855nmol/l (assuming 85% is bound). This will reduce the wild-type KATP current in pancreatic β-cells by ~75% [52] to 0.19nS/pF, even in the absence of glucose. This is very close to the threshold for electrical activity (0.2nS/pF). Thus it seems that, even in the presence of plasma protein, gliclazide block is quantitatively sufficient to stimulate insulin secretion and the functional data are in reasonable agreement with the free CSS concentration of drug in the plasma of patients with type 2 diabetes. Interestingly, this calculation does not include any potential inhibition of the KATP current by glucose. This is consistent with the fact that ATP levels do not increase in response to glucose in βV59M isolated from patients with type 2 diabetes [53], which presumably means glucose produces little reduction in KATP channel activity.
Conclusions
Our results indicate that free plasma concentration of gliclazide in humans is sufficient to quantitatively explain the enhanced insulin secretion as primarily due to KATP channel inhibition. However, a substantially higher concentration of glibenclamide at its site of action is required. Our data also stress the importance of unknown factor(s) that influence the free concentration of gliclazide in human plasma and thus its ability to inhibit KATP channels.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Wellcome Trust (https://wellcome.ac.uk/): grant numbers 884655 and 089795 - to FMA; European Union: ERC Advanced grant (https://erc.europa.eu/funding/advanced-grants): 322620 - to FMA; ERC Advanced Investigator award (https://erc.europa.eu/funding/advanced-grants) - to FMA; and Royal Society Research Wolfson Merit Award (https://royalsociety.org/grants-schemes-awards/grants/wolfson-research-merit/) - to FMA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zünkler BJ, Lenzen S, Männer K, Panten U, Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988;337: 225–230. [DOI] [PubMed] [Google Scholar]
- 2.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46: 875–891. doi: 10.1007/s00125-003-1143-3 [DOI] [PubMed] [Google Scholar]
- 3.Martin GM, Kandasamy B, DiMaio F, Yoshioka C, Shyng S. Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM. Elife. 2017; 6: e31054 doi: 10.7554/eLife.31054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwanstecher M, Schwanstecher C, Dickel C, Chudziak F, Moshiri A, Panten U. Location of the sulphonylurea receptor at the cytoplasmic face of the beta cell membrane. Br J Pharmacol. 1994;113: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta cell. Prog Biophys Mol Biol. 1989;54: 87–143. [DOI] [PubMed] [Google Scholar]
- 6.Renström E, Barg S, Thévenod F, Rorsman P. Sulfonylurea-mediated stimulation of insulin exocytosis via an ATP-sensitive K+ channel-independent action. Diabetes. 2002;51 Suppl 1: S33–S36. [DOI] [PubMed] [Google Scholar]
- 7.Zhang CL, Katoh M, Shibasaki T. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325: 607–610. doi: 10.1126/science.1172256 [DOI] [PubMed] [Google Scholar]
- 8.Hsu P, Ma JKH, Luzzi LA. Interactions of sulfonylureas with plasma proteins. J Pharm Sci. 1974;63: 570–573. [DOI] [PubMed] [Google Scholar]
- 9.Seedher N, Kanojia M. Reversible binding of antidiabetic drugs, repaglinide and gliclazide, with human serum albumin. Chem Biol Drug Des. 2008;72: 290–296. doi: 10.1111/j.1747-0285.2008.00704.x [DOI] [PubMed] [Google Scholar]
- 10.Seedher N, Kanojia M. Mechanism of interaction of hypoglycemic agents glimepiride and glipizide with human serum albumin. Cent Eur J Chem. 2009;7: 96–104. [Google Scholar]
- 11.Pearson ER, Flechtner I, Njølståd PR, Malecki MT, Flanagan SE, Larkin B et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. New Engl J Med. 2006;355: 467–477. doi: 10.1056/NEJMoa061759 [DOI] [PubMed] [Google Scholar]
- 12.Rydberg T, Jönsson A, Røder M, Melander A (1994) Hypoglycemic activity of glyburide (glibenclamide) metabolites in humans. Diabetes Care 17:1026–1030 [DOI] [PubMed] [Google Scholar]
- 13.Peters T Jr. All about Albumin. Biochemistry, Genetics, and Medical Applications. Academic Press, San Diego, CA; 1996. [Google Scholar]
- 14.Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta cell KATP channels. Diabetes. 1998;47: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 15.Russ U, Kühner P, Prager R, Stephan D, Bryan J, Quast U. Incomplete dissociation of glibenclamide from wild-type and mutant pancreatic KATP channels limits their recovery from inhibition. Br J Pharmacol. 2009;156: 354–361. doi: 10.1111/j.1476-5381.2008.00005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119: 80–90. doi: 10.1172/JCI35772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopalakrishnan M, Molinari EJ, Shieh CC, Monteggia LM, Roch JM, Whiteaker KL et al. Pharmacology of human sulphonylurea receptor SUR1 and inward rectifier K+ channel Kir6.2 combination expressed in HEK-293 cells. Br J Pharmacol. 2000; 129:1323–1332. doi: 10.1038/sj.bjp.0703181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proks P, de Wet H, Ashcroft FM. Molecular mechanism of sulphonylurea block of KATP channels carrying mutations that impair ATP inhibition and cause neonatal diabetes. Diabetes. 2013;62: 3909–3919. doi: 10.2337/db13-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Göpel SO, Kanno T, Barg S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting d-cells in intact mouse pancreatic islets. J Physiol. 2000. 528:497–507. doi: 10.1111/j.1469-7793.2000.00497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Göpel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol. 2000; 528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proks P, Girard C, Ashcroft FM. Functional effects of KCNJ11 mutations causing neonatal diabetes: enhanced activation by MgATP. Hum Mol Genet. 2005;14: 2717–2726. doi: 10.1093/hmg/ddi305 [DOI] [PubMed] [Google Scholar]
- 22.Proks P, de Wet H, Ashcroft FM. Activation of the KATP channel by Mg-nucleotide interaction with SUR1. J Gen Physiol. 2010;136: 389–405. doi: 10.1085/jgp.201010475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong TC. Free drug measurements: methodology and clinical significance. Clin Chim Acta. 1985;151: 193–216. [DOI] [PubMed] [Google Scholar]
- 24.Lahmann C, Kramer HB, Ashcroft FM. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLOS One. 2015;10: e0134476 doi: 10.1371/journal.pone.0134476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seibert H, Morchel S, Gulden M. Factors influencing nominal effective concentrations of chemical compounds in vitro: medium protein concentration. Toxicol In Vitro. 2002;16: 289–297. [DOI] [PubMed] [Google Scholar]
- 26.Park JY, Kim KA, Park PW, Park CW, Shin JG. Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther. 2003; 74: 334–340. doi: 10.1016/S0009-9236(03)00221-2 [DOI] [PubMed] [Google Scholar]
- 27.Proks P, Reimann FR, Green N, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51: S368–S376. [DOI] [PubMed] [Google Scholar]
- 28.Sartor G, Melander A, Schersten B, Wahlin-Boll. Serum glibenclamide in diabetic patients, and influence of food on the kinetics and effects of glibenclamide. Diabetologia. 1980;18: 17–22. [DOI] [PubMed] [Google Scholar]
- 29.Pertusa JAG, León-Quinto T, Berná G, Tejedo JR, Hmadcha A, Bedoya FJ et al. Zn2+ chelation by serum albumin improves hexameric Zn2+-insulin dissociation into monomers after exocytosis. PLoS One. 2017;12:e0187547 doi: 10.1371/journal.pone.0187547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrer R, Soria B, Dawson CM, Atwater I, Rojas E. Effects of Zn2+ on glucose-induced electrical activity and insulin release from mouse pancreatic islets. Am J Physiol. 1984;246:C520–C527. doi: 10.1152/ajpcell.1984.246.5.C520 [DOI] [PubMed] [Google Scholar]
- 31.Ammälä C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA et al. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996; 379: 545–548. doi: 10.1038/379545a0 [DOI] [PubMed] [Google Scholar]
- 32.Schwanstecher M, Löser S, Rietze I, Panten U. Phosphate and thiophosphate group donating adenine and guanine nucleotides inhibit glibenclamide binding to membranes from pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol. 1991;343: 83–89. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmoneim AS, Hasenbank SE, Seubert JM, Brocks DR, Light PE, Simpson SH. Variations in tissue selectivity amongst insulin secretagogues: A systematic review. Diabet Obes Metab. 2012;14: 130–138. [DOI] [PubMed] [Google Scholar]
- 34.Crooks MJ, Brown KF. Potentiating effects of drugs on the binding of glibenclamide to pancreatic beta cells. Metabolism. 1974;23: 839–846. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda R, Anguizola J, Joseph KS, Hage DS. Analysis of drug interactions with modified proteins by high-performance affinity chromatography: binding of glibenclamide to normal and glycated human serum albumin. J Chromatogr A. 2012;1265: 114–122. doi: 10.1016/j.chroma.2012.09.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuda R, Anguizola J, Joseph KS, Hage DS. High-performance affinity chromatography and the analysis of drug interactions with modified proteins: binding of gliclazide with glycated human serum albumin. Anal Bioanal Chem. 2011;401: 2811–2819. doi: 10.1007/s00216-011-5382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi K, Igaki A, Kimura M, Sakoguchi T, Shimosawa M, Matsuoka A. Influence of blood proteins on biochemical analysis. IX. Protective effects of human serum proteins on anion-induced degradation of gliclazide. Chem Pharm Bull. 1986;34: 2957–2962. [DOI] [PubMed] [Google Scholar]
- 38.Brown KF, Crooks MJ. Binding of sulfonylureas to serum albumin. II The influence of salt and buffer compositions on tolbutamide and glyburide. Can J Pharm Sci. 1974;9: 75–77. [Google Scholar]
- 39.Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol. 1997;504: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brereton MF, Iberl M, Shimomura K Zhang Q, Adriaenssens AE, Proks P et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nature Com. 2014;5: 4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myngheer N, Allegaert K, Hattersley A, McDonald T, Kramer H, Ashcroft FM et al. Fetal Macrosomia and Neonatal Hyperinsulinemic Hypoglycemia Associated with Transplacental Transfer of Sulfonylurea in a Mother with KCNJ11-Related Neonatal Diabetes. Diabetes Care. 2014;37: 3333–3335. doi: 10.2337/dc14-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doliba NM, Qin W, Najafi H, Liu C, Buettger CW, Sotiris J et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab. 2011;302: E87–E102. doi: 10.1152/ajpendo.00218.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2005;55: 3470–3477. [DOI] [PubMed] [Google Scholar]
- 44.McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab. 2011;104: 648–653. doi: 10.1016/j.ymgme.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 45.Anguizola J, Matsuda R, Barnaby OS Hoy KS, Wa C, DeBolt E et al. Glycation of human serum albumin. Clin Chem Acta. 2013;425: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuchiya S, Sakurai T, Sekiguchi S. Nonenzymatic glucosylation of human serum albumin and its influence on binding capacity of sulfonylureas. Biochem Pharmacol. 1984;33: 2967–2971. [DOI] [PubMed] [Google Scholar]
- 47.Olsen KM, Kearns GL, Kemp SF. Glyburide protein binding and the effect of albumin glycation in children, young adults, and older adults with diabetes. J Clin Pharmacol. 1995;35: 739–45. [DOI] [PubMed] [Google Scholar]
- 48.Hellman B, Sehlin J, Täljedal IB. Glibenclamide is exceptional among hypoglycaemic sulphonylureas in accumulating progressively in beta cell-rich pancreatic islets. Acta Endocrinol. 1984;105: 385–390. [DOI] [PubMed] [Google Scholar]
- 49.Carpentier JL, Sawano F, Ravazzola M, Malaisse WJ. Internalization of 3H-glibenclamide in pancreatic islet cells. Diabetologia. 1986;29: 259–261. [DOI] [PubMed] [Google Scholar]
- 50.Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010;9: 929–939. doi: 10.1038/nrd3287 [DOI] [PubMed] [Google Scholar]
- 51.Gomis A, Sanchez-Andres JV, Valdeolmillos M. Oscillatory patterns of electrical activity in mouse pancreatic islets of Langerhans recorded in vivo. Pflugers Arch. 1996;432: 510–515. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence CL, Proks P, Rodrigo GC, Jones P, Hayabuchi Y, Standen NB et al. Gliclazide produces high-affinity block of KATP channels in mouse isolated pancreatic β-cells but not rat heart or arterial smooth muscle cells. Diabetologia. 2001;44: 1019–1025. doi: 10.1007/s001250100595 [DOI] [PubMed] [Google Scholar]
- 53.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48: 282–2879. doi: 10.1007/s00125-004-1627-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.