Abstract
Early caregiving adversity is associated with increased risk for social difficulties. The ventral striatum and associated corticostriatal circuitry, which have demonstrated vulnerability to early exposures to adversity, are implicated in many aspects of social behavior, including social play, aggression, and valuation of social stimuli across development. Here, we used resting state functional magnetic resonance imaging to assess the degree to which early caregiving adversity was associated with altered coritocostriatal resting connectivity in previously institutionalized youth (PI; n=41) relative to youth who were raised with their biological families from birth (n = 47), and the degree to which this connectivity was associated with parent-reported social problems. Using a seed-based approach, we observed increased positive coupling between ventral striatum and anterior regions of medial prefrontal cortex (mPFC) in PI youth. Stronger ventral striatum-mPFC coupling was associated with parent reports of social problems. A moderated-mediation analysis showed that ventral striatal-mPFC connectivity mediated group differences in social problems, and more so with increasing age. These findings show that early institutional care is associated with differences in resting-state connectivity between the ventral striatum and mPFC, and this connectivity seems to play an increasingly important role in social behaviors as youth enter adolescence.
Keywords: ventral striatum, medial prefrontal cortex, resting-state, early adversity, social problems
Introduction
Substantial evidence suggests that development of the ventral striatum is particularly vulnerable to disruptions in early caregiving (reviewed in Fareri & Tottenham, 2016). For example, rodent studies show that early caregiver deprivation leads to decreases in reward-related behaviors that are mediated by the ventral striatum (K. Matthews & Robbins, 2003), in part resulting from corticosterone induced dysregulation of dopaminergic release into the ventral striatum (nucleus accumbens) (Härfstrand et al., 1986; Overton, Tong, Brain, & Clark, 1996). Indeed, many functions linked to the ventral striatum and its structural and functional connections with medial prefrontal cortex (mPFC) in humans have exhibited alterations following caregiving adversity broadly defined (reviewed in Tottenham & Galvan, 2016). To date, the literature has largely focused on psychiatric dimensions associated with corticostriatal loops, such as depressive phenotypes (Goff et al., 2013; Hanson, Hariri, & Williamson, 2015) and increased attention problems and impulsivity (Lovallo et al., 2012; McLaughlin et al., 2014). However, this circuitry has also been widely implicated in social behavior (for reviews see Fareri & Delgado, 2014; Meyer-Lindenberg & Tost, 2012), a behavioral domain also associated with altered development following early adversity. The current paper investigated the association between corticostriatal development and social behavior following early adverse caregiving.
The ventral striatum and mPFC belong to a network of interconnected brain regions that collectively support social behavior (Amodio & Frith, 2006; Burnett, Sebastian, Kadosh, & Blakemore, 2011; Crone & Dahl, 2012; Fareri & Delgado, 2014). The ventral striatum is a key region at the center of neural circuits involved in processing social and affective signals, which shares anatomical connections with mPFC, among other regions such as the amygdala and hippocampus (Groenewegen, Wright, Beijer, & Voorn, 1999; Haber, 2003; Haber & Knutson, 2010; Robbins, Cador, Taylor, & Everitt, 1989). Broadly, connections between the ventral striatum and mPFC support reward-related learning and valuation in both non-social (Bartra, McGuire, & Kable, 2013; Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Knutson, Adams, Fong, & Hommer, 2001; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005; Li, Schiller, Schoenbaum, Phelps, & Daw, 2011; Rutledge, Dean, Caplin, & Glimcher, 2010; Schonberg, Daw, Joel, & O’Doherty, 2007; Wang, Smith, & Delgado, 2016) and social contexts (Fareri, Chang, & Delgado, 2015a; Izuma, Saito, & Sadato, 2008; 2010; Kohls, Perino, Taylor, Madva, & Cayless, 2013; Phan, Sripada, Angstadt, & McCabe, 2010; Smith et al., 2010; Somerville, Heatherton, & Kelley, 2006). These regions often exhibit heightened reactivity during adolescence (Ernst et al., 2005; Fareri, Martin, & Delgado, 2008; Galván & McGlennen, 2012; Galván et al., 2006; Jones et al., 2014; Somerville, Hare, & Casey, 2011) and childhood (Silvers et al., 2014). Resting-state functional connectivity studies have shown strong coupling between ventral striatum and mPFC across development, often with stronger coupling at younger ages (Di Martino et al., 2011; Fareri, Gabard-Durnam, Goff, Flannery, et al., 2015b; Supekar, Musen, & Menon, 2009). Fareri et al. (2015b) also showed that age-related changes in testosterone were associated with the decline in coupling between ventral striatum and ventral regions of mPFC across age. Taken together, these findings indicate strong communication between ventral striatum and mPFC, with dynamic changes across development, particularly in the adolescent years.
Children and adolescents with a history of caregiving adversity are at increased risk for altered development of the ventral striatum and mPFC and associated behaviors. For example, previously-institutionalized (PI) youth, who are raised in the absence of species-typical parenting prior to adoption, exhibit a hypoactive striatal response during processing of reward predictive information (Mehta et al., 2010) and rewarding social cues (Goff et al., 2013). Relatedly, PI youth exhibit alterations in patterns of risk evaluation during reward-based decision-making (Humphreys et al., 2015; Loman, Johnson, Quevedo, Lafavor, & Gunnar, 2014) and impulse control (Lovallo et al., 2012; McLaughlin et al., 2014). Such patterns of altered decision-making are likely also impacted by PI youth exhibiting altered functional interactions between mPFC and both the amygdala and hippocampus during aversive learning situations (Silvers et al., 2016) and in response to social stimuli (Gee, Gabard-Durnam, et al., 2013a).
Difficulties in social behaviors have been noted since some of the earliest reports of PI youth (Hodges & Tizard, 1989), which can range from difficulties maintaining close relationships with peers to attachment difficulties (Bos et al., 2011) to ‘hyper-social’ tendencies such as indiscriminate friendliness (Gleason et al., 2014). Responsive and sensitive caregiving forms the basis for many skills necessary for social competence and engaging in successful social interactions. PI youth therefore may struggle within social relationships (Gunnar & Quevedo, 2007), exhibiting: difficulty establishing close friendships (Roy, Rutter, & Pickles, 2004), poor regulation of social approach behavior (Olsavsky et al., 2013), enhanced feelings of social rejection (Puetz et al., 2014), and attentional avoidance of threatening social stimuli (Tottenham et al., 2011). Though ventral striatal-mPFC loops have been implicated in the reactive and motivational aspects of social approach behavior (i.e., wanting) and the formation of relationships across normative development (Tops, Koole, Ijzerman, & Buisman-Pijlman, 2014; Vrtička & Vuilleumier, 2012), an understanding of how ventral striatal connectivity emerges following early caregiving adversity remains unknown.
Given that previous work has identified alterations in neural circuitry supporting socio-affective processes as a function of caregiving adversity, including the ventral striatum, we sought to examine the nature of ventral striatal rsFC in PI youth. We expected that PI youth would demonstrate significant alterations in ventral striatal rsFC with regions commonly altered by early caregiving adversity (e.g., mPFC, amygdala), and that these alterations would be associated with social problems as reported by parents. Moreover, we anticipated that associations between ventral striatal rsFC and social behavior would be stronger in adolescents.
Methods and Materials
Participants
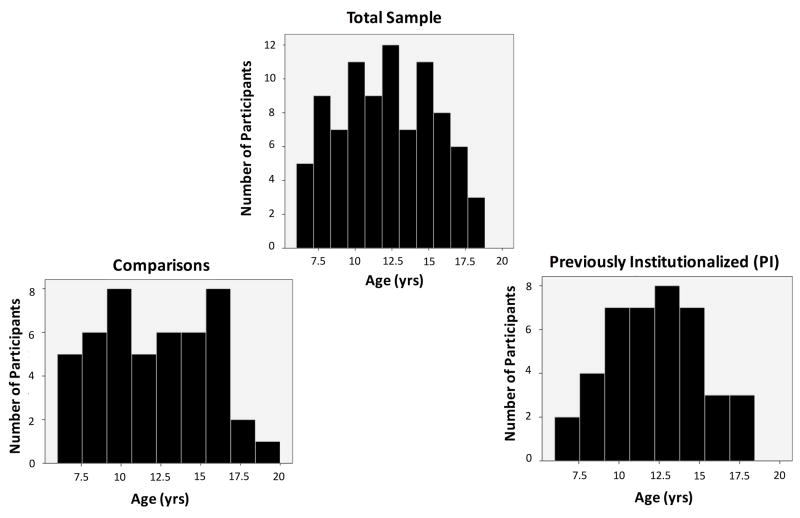
Resting-state fMRI data were acquired for 95 comparison (i.e., raised with biological family from birth) and previously institutionalized (i.e., adopted out of institutional care care; PI) children and adolescents from the greater Los Angeles area. Two participants (1 comparison, 1 PI) were excluded from analyses due to missing parental assessments of child behavior. An additional two comparison participants were excluded due to exhibiting scores in the clinical range on measures of total problems and social problems as measured by the Child Behavior Checklist (Achenbach, 1991). Three PI participants were excluded as a function of the age at which they entered institutionalized care (after age 4). The final sample (see Table 1) included 88 participants between the ages of 6-18 (Comparison: N = 47, 27F/20M, mean age = 12.15, sd = 3.54, Nchildren (6-10 yrs.) = 19, Nadolecents (11-18 yrs.) = 28; PI: N = 41, 28F/13M, mean age = 12.33, sd = 2.95; Nchildren (6-10 yrs.) = 14, Nadolecents (11-18 yrs.) = 27; see Figure 1). Mean age did not differ between comparison and PI groups (t(85.82) = 0.25, p > 0.80).
Table 1.
Participant Demographics
| Comparison | PI | |
|---|---|---|
| Sample size (n) | 47 | 41 |
| Children (6-10 yrs): 19 | Children (6-10 yrs): 14 | |
| Adolescents (11-18): 26 | Adolescents (11-18): 27 | |
| Age at Scan (Years), Mean (SD) | 12.16 (3.54) | 12.33 (2.95) |
| Range | 6.08-18.58 | 6.5-18.33 |
| Sex | 27F, 20M | 28F, 13M |
| Full Scale IQ, Mean (SD) | 113.52 (17.79) | 107.70 (14.00) |
| Age at Adoption, Years (SD) | n/a | 2.08 (1.89) |
| Age Orphaned, Years (SD) | n/a | 0.34 (0.62) |
| Time in Orphanage, Years (SD) | n/a | 1.73 (1.62) |
| Psychotropic Medication (n) | n/a | 9 |
Figure 1.
Distribution of participants’ ages.
Data were collected at the University of California, Los Angeles (UCLA). All procedures were approved by state and local Institutional Review Boards. Comparison participants were recruited for this study via local advertisements and state birth records. PI participants were recruited through family networks in the Greater Los Angeles area. PI participants were placed into institutionalized care in Eastern Europe, Asia, South America and Africa within the first 2 years of life before being adopted into stable families in the United States (mean age placed in orphanage = 0.34 yrs (sd = 0.62); mean age of adoption = 2.08 yrs (sd = 1.89); mean time spent in institutional care = 1.73 yrs (sd = 1.62)). All families of potential study participants—comparisons and PIs—underwent a phone screening to ascertain whether children and adolescents met physical and psychological contraindications for fMRI, including psychotropic medication status, developmental disabilities and neurological disorders. Comparison participants were free of psychotropic medication and clinical diagnoses as per parent report. Nine of the 41 PI participants were taking psychotropic medication. Upon arrival at the laboratory, after obtaining informed consent and assent, parents completed a series of assessments of mental health including the Child Behavior Checklist (CBCL; Achenbach, 1991). All comparison participants were below clinical cutoff scores (T = 64) for measures of CBCL Total Problems (mean = 43.06, sd = 9.91), and below clinical cutoff scores on CBCL Syndrome Scales (T = 70). IQ was assessed prior to the scan session using the Wechsler Abbreviated Scale of Intelligence (Weschsler, 1999); participants in both groups on average were within normal limits (comparisons: mean = 113.52, sd = 17.79; PIs: mean = 107.70, sd = 14.00).
fMRI data acquisition
All participants were acclimated to the fMRI scanner environment through a mock scanning session. Resting-state data were acquired at the end of a forty-five minute scanning session, which also included task-based scanning, to ensure that all participants were fully acclimated to being in the scanner. The resting-state scans were preceded by approximately 15 minutes of structural neuroimaging sequences to minimize influence from task-based scans (Fareri, Gabard-Durnam, Goff, Flannery, et al., 2015b; Gabard-Durnam et al., 2014). We took extensive measures to minimize motion artifact in our data, as motion can have undue influence on measures of functional connectivity, including the use of a vacuum-packed pillow surrounding the head and then placing additional padding around participants’ heads during fMRI scanning. We also employed additional measures to deal with potential motion artifact during data preprocessing (described below). During the resting-state sequence, participants were instructed to remain still with their eyes closed, without falling asleep. A black screen with a white fixation cross was presented during the resting-state scan via MR-compatible video goggles (Resonance Technology, Inc.) to minimize distraction, in case participants did not remain with their eyes closed. Alertness was assessed via direct observation upon completion of the scan session and self-report.
All data were collected using a Siemens Trio 3T scanner with a 12-channel head coil. Anatomical data were acquired during a T1-weighted MPRAGE sequence prior to the resting-state scan (in-plane resolution: 256 × 256, FOV: 256mm, 192 1 × 1 × 1mm slices). Resting-state data were collected during a 6-minute T2-weighted echoplanar imaging sequence in accordance with the following parameters: 33 oblique-axial slices, voxel size = 3.4 × 3.4 × 4.0 mm, slice thickness = 4mm, FOV = 220mm, 64×64 matrix, TR = 2000ms, TE = 30ms, flip angle = 75 degrees. Functional data were resampled to 3 × 3 × 3 mm space. Previous work indicates that a resting-state sequence of 6-minutes long allows for reliable and stable detection of resting-state functional connectivity (Van Dijk et al., 2010). Oblique axial slices were acquired at an angle of approximately 20-30 degrees in order to maximize functional coverage while minimizing signal drop-out.
fMRI data preprocessing
Processing and analyses of neuroimaging data were conducted using Analysis of Functional Neuroimages (AFNI) software, v. 16.2.01 (Cox, 1996). The first two volumes of the resting state scan were discarded in addition to those discarded by the scanner to account for stabilization of the BOLD signal. Standard preprocessing steps were employed via afni_proc.py including slice-time correction (Fourier interpolation) and six-parameter motion correction (Fourier interpolation). Volumes of data exhibiting greater than 0.5mm frame-to-frame motion and their preceding volume, were excluded from analysis (i.e., censored). The mean amount of volumes censored across participants in our sample was 22.86 (sd = 24.65) (approximately 12.9%). Thus, on average, participants contributed 155.14 (sd = 24.65) usable volumes of resting-state data, and no individual participant had more than 3 sd above the mean amount of volumes censored. Functional data were normalized to percent signal change, and registered to anatomical images and warped to standard Talairach space (Talairach & Tournoux, 1988). Evidence indicates that registration of child and adolescent neuroimaging data to a standard adult template is methodologically appropriate within the age range of this study (Burgund et al., 2002; Kang, Burgund, Lugar, Petersen, & Schlaggar, 2003). Subject specific anatomical data were skull-stripped and segmented into tissue classes to create masks used to subsequently generate subject specific tissue regressors for white matter and CSF, as well as mean global signal across the brain.
Resting-state fMRI data are particularly susceptible to confounding influence from submillimeter motion and physiological noise (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Power, Schlaggar, & Petersen, 2015; Van Dijk, Sabuncu, & Buckner, 2012), particularly in developmental samples (Satterthwaite et al., 2012; Van Dijk et al., 2010; 2012). To address these potential spurious influences on resting state connectivity estimates, we applied a conservative bandpass filter (0.009Hz < f <0.08Hz) to remove high frequency signals that may be more susceptible to motion artifact, thereby exerting undue influence on resting state connectivity estimates (Satterthwaite et al., 2012). We performed simultaneous bandpassing and regression of nuisance signals from the resting-state BOLD data at the single subject level (Hallquist, Hwang, & Luna, 2013). Nuisance regressors included six standard motion parameters (3 translation, 3 rotation) and their first order temporal derivatives for a total of 12 motion regressors (Van Dijk et al., 2010; 2012), separate tissue regressors for white matter and CSF signal across the brain, and a regressor for the mean global signal. There has been extensive debate regarding the inclusion of global signal regression in studies of resting-state connectivity due to the potential to induce spurious negative correlations (Saad et al., 2013; Satterthwaite et al., 2013) and altered distant-dependent functional relationships. However, additional evidence suggests that inclusion of the global signal is an effective method of reducing motion-related artifact in resting-state data (Miranda-Dominguez et al., 2014; Power et al., 2015), particularly in the absence of additional physiological measurements (e.g., heart rate) that could serve as nuisance regressors (Chen et al., 2012).
Gaussian spatial smoothing was performed using the 3dBlurtoFWHM option in AFNI. The 3dBlurtoFWHM program applies an ‘adaptive smoothing’ method such that all participants’ data are smoothed up to an approximate given limit to better ensure consistency in the amount of smoothing applied based on differences in the intrinsic noise of the residuals in single participants’ data. We set an upper limit of smoothing to be 9mm for our data.
We were primarily interested in examining differences between comparison and PI participants in ventral striatal rsFC, and as such, we performed a seed-based analysis. As we had no a priori hypotheses regarding laterality differences, we used a bilateral ventral striatal region of interest mask taken from the Oxford-GSK-Imanova structural striatal atlas (Tziortzi et al., 2011) included in FSL (FMRIB, Oxford, UK), as in our previous work (Fareri, Gabard-Durnam, Goff, Flannery, et al., 2015b). This mask includes the nucleus accumbens, ventral caudate and ventral putamen given difficulties in precisely defining anatomical boundaries between the nucleus accumbens and different ventral striatal subregions in humans, which are best delineated as a function of afferent/efferent projections and differences in neurotransmitter concentrations (Haber & Knutson, 2010; Haber & McFarland, 1999; Haber, Kim, Mailly, & Calzavara, 2006). The ventral striatal ROI was warped to standard Talairach space in AFNI and overlaid on each participant’s anatomical data; the overlap between the ROI and an individual participants’ white matter mask was subtracted out to avoid including white matter voxels within the ventral striatal ROI. Raw timeseries from the ventral striatum was extracted from each participant’s functional data after nuisance regression but prior to spatial smoothing of the data. Single subject regression using 3dREMLFit in AFNI was used to generate single subject, whole-brain resting-state functional connectivity maps; 3dREMLFit uses least squares restricted maximum likelihood estimation via an ARMA(1,1) model to determine the best fit for each voxel in the brain and to address serial autocorrelations in the residuals of the data. Whole-brain maps reflecting parameter estimates from the regression model of ventral striatal connectivity at the single subject level were converted to correlation coefficients (r) to improve stability of the connectivity estimates.
Behavioral Analyses
We conducted linear regression using R statistical language to test for group differences on parent reports of behavior from the CBCL (Total Problems raw scores, Social Problems raw scores), controlling for age and sex. We used raw scores so as not to be redundant in subsequent analyses described below including age and sex as regressors, as T-scores are normed on these variables (Achenbach, 1991).
Group Connectivity Analyses
Our first aim was to characterize the effect of group (PI, comparison) on ventral striatal rsFC. We conducted whole brain analyses in AFNI using 3dttest++. Individual participants’ whole brain connectivity estimates were entered into whole brain linear regression with group, age, and sex as regressors in our model; we also included mean framewise displacement (FD) as a group level motion regressor to further account for potential influence of motion (Fareri, Gabard-Durnam, Goff, Flannery, et al., 2015b; Gabard-Durnam et al., 2014; 2016; Power et al., 2015; Van Dijk et al., 2012).
For whole brain analyses, regressors were mean-centered across groups in AFNI using the ‘–center SAME’ option within 3dttest++. Whole brain results for all analyses were set to an initial height threshold of p<.025 and subsequently corrected for multiple comparisons to a corrected level of p<.05 using the most updated version of the 3dClustSim program in AFNI. This program runs a series of Monte Carlo simulations across the whole brain based on the noise of the residuals of the single subject regression output. Using 3dFWHMx and applying the spatial autocorrelation function (-acf) to account for the non-Gaussian nature of imaging data, we calculated the average smoothness of the residuals of all participants. Across all participants in our sample, the fit of the residual noise in our data to a Gaussian estimation was approximately 45%, and approximately 55% exponential. An effective smoothness was calculated to be 13.76mm when accounting for these factors. (For completeness, we note the average smoothness of the residuals only estimating a Gaussian distribution—i.e., not incorporating the autocorrelation function—was 8.57mm). The noise smoothness estimates were then entered as inputs into 3dClustSim (estimated smoothness = 13.76 mm). Whole brain statistical parametric maps were set to a cluster level threshold of k = 250 voxels (using first nearest neighbor clustering) as determined by 3dClustSim.
Associations between connectivity and social functioning
Ventral striatal rsFC associations with CBCL Social Problems were assessed. Connectivity estimates were extracted from voxels surviving correction for multiple comparisons (see below) and entered into offline regression analyses in R statistical language, residualized for age, sex and motion. Residual connectivity estimates were then entered into in a linear regression to examine relationships with social problems. We also conducted this analysis controlling for age, sex and measures of CBCL Total Problems (Total Problems was included as a control variable to identify the unique associations for Social Problems).
To assess whether observed group differences in CBCL Social Problems were associated with ventral striatal-mPFC rsFC, and whether age moderated either of these relationships, we conducted a non-parametric bootstrapped moderated mediation analysis (model 15) in SPSS using the PROCESS macro (v. 2.16.3; Hayes, 2012). Moderation and mediation were assessed via bias corrected bootstrapped confidence intervals (Efron, 1987; Hayes & Scharkow, 2013). Confidence intervals not crossing zero indicate significant effects. Age was entered as a moderating variable in this analysis; regressors for the interaction products (i.e., age, group, ventral striatal-mPFC rsFC) were mean centered within PROCESS using the mean center for products option. Mean FD was also entered as an additional mean centered regressor of no interest.
Adoption related analyses
Associations between adoption-related variables (i.e., age of adoption, age placed into institutionalized care, time with family) and clusters exhibiting rsFC with the ventral striatum were assessed. Linear regressions were conducted offline in R statistical language, controlling for age, sex and mean FD.
Results
Group differences in behavioral measures
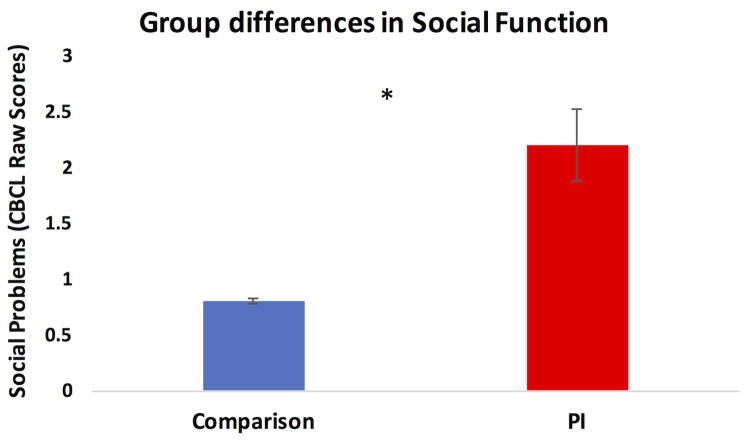
Linear regression revealed significant group differences (β = 1.41, SE = .37, t = 3.81, p<.0005) on parent reported CBCL Social Problems and Total Problems, controlling for age and sex. PI youths demonstrated significantly higher scores on Social Problems (PI: mean = 2.20, sd = 2.18; comparison: mean = 0.81, sd = 1.12; Figure 2) and Total Problems (PI: mean = 35.71, sd = 22.98; comparison: mean = 13.34, sd = 10.71) than comparison youth.
Figure 2.
Group differences in CBCL Social Problems.
Whole Brain Connectivity Analyses: Group Differences
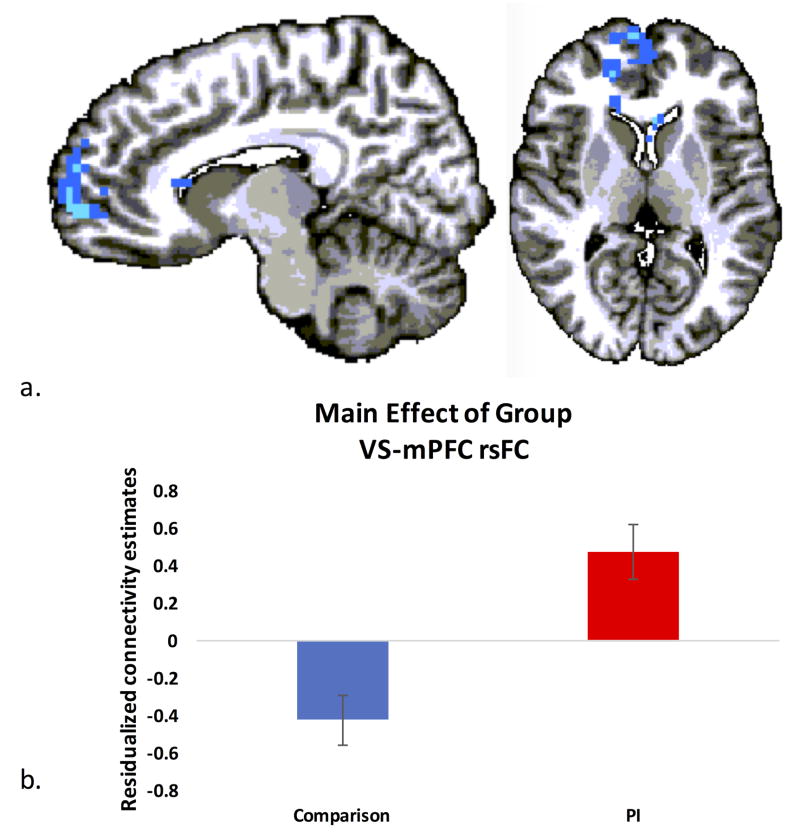
We conducted a whole brain analysis examining a main effect of group, controlling for age, sex and mean FD. Correcting for multiple comparisons, a significant main effect of group emerged in connectivity between the ventral striatum and a large cluster of bilateral medial prefrontal cortex (see Figure 3a, Table 2). This cluster encompassed portions of BA9, BA10, BA24, BA32 and BA47 within mPFC, and extended posteriorly to the head of the caudate bilaterally. Extracting connectivity estimates from voxels in mPFC revealed significantly more positive ventral striatal rsFC with mPFC in PI participants versus comparison participants (Figure 3b). No other regions emerged as showing significant group differences or interactions of group and age, group and gender or main effects of age.
Figure 3. Group differences in ventral striatal rsFC.
a) A whole brain analysis controlling for age, sex and mean framewise displacement revealed significant group differences in ventral striatal rsFC with regions of anterior mPFC (cluster level corrected, p<.05). b) PI youth exhibited significantly more positive rsFC between ventral striatum and mPFC than comparison youth.
Table 2.
Regions demonstrating a main effect of group in ventral striatal rsFC.
| Talairach Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region of Activation (Peak) | Subpeaks | Brodmann Area | Laterality | X | Y | Z | # Voxels |
| Anterior Cingulate | 32 | R | 14 | 38 | 3 | 348 | |
| Subcallosal Gyrus | 47 | R | 17 | 23 | -9 | ||
| Superior Frontal Gyrus | 9 | L | -5 | 53 | 27 | ||
| Anterior Cingulate | 24/32 | L | 11 | 44 | 6 | ||
| Medial Frontal Gyrus | 10 | R | 14 | 65 | 6 | ||
| Caudate Nucleus | n/a | L | -5 | 17 | 15 | ||
Associations between rsFC and social functioning
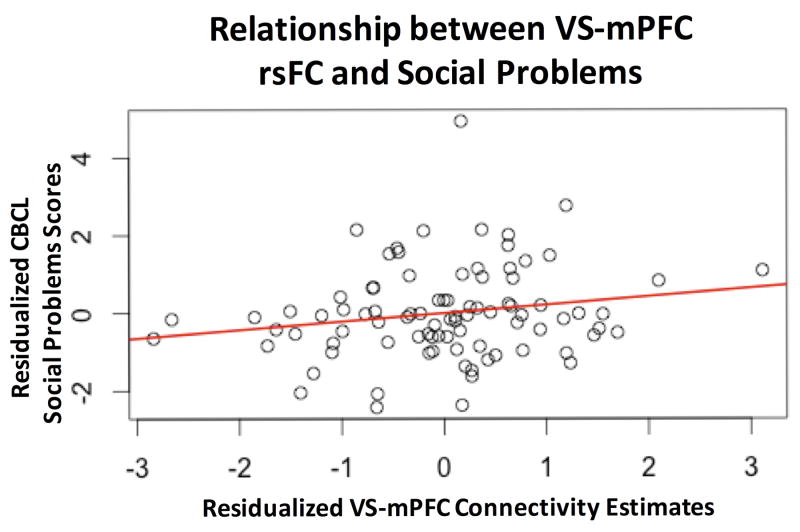
We employed linear regression to examine the relationship between ventral striatal-Mpfc rsFC and CBCL Social Problems. Across all participants in our sample, a significant positive relationship emerged between Social Problems and ventral striatal-mPFC rsFC (Figure 4). Higher Social Problems scores were associated with stronger positive connectivity (β = 0.28, SE = 0.11, t = 2.63, p<.01). This overall pattern held when conducting the analysis controlling for levels of Total Problems, age and sex, albeit we note that the relationship became slightly weaker (β = 0.13, SE = 0.07, t = 1.774, p=0.095).
Figure 4. Relationship between VS-mPFC rsFC and CBCL Social Problems.
A linear regression revealed a positive association between VS-mPFC connectivity and social problems scores across the entire sample (p<.01), which remained a trend when controlling for age, sex and CBCL total problems (p=.095). Plot depicts connectivity estimates residualized for age, motion and sex against CBCL social problems raw scores, residualized for age, sex and CBCL total problems raw scores.
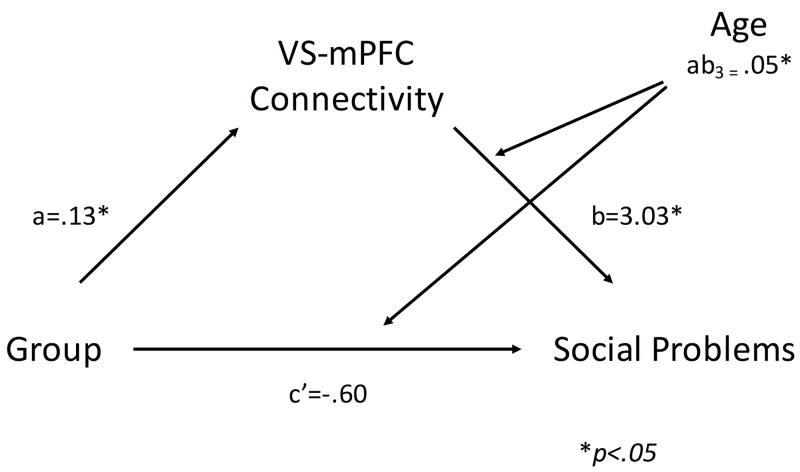
To further examine the relationship between ventral striatal-Mpfc connectivity and social functioning, we conducted a non-parametric bootstrapped moderated mediation analysis to test whether higher levels of social problems in PI youth were significantly mediated by ventral striatal-Mpfc rsFC, examining a moderating role of age. This analysis revealed that the direct effect of group on social problems became non-significant (c’ = .60, BC CI: [-1.27, .07]), with significant indirect effect of group on social problems through ventral striatal-Mpfc rsFC at all values of the moderator (age) (mean centered age value = -3.26: path coefficient = .21, BC CI: [.04, .47]; mean centered age value = 0: path coefficient = .39, BC CI: [.14, .79]; mean centered age value = 3.26: path coefficient = .56, BC CI: [.18, 1.21]; see Figure 5). The positive slope indicated by the path coefficients and confidence intervals, in conjunction with the sign of the index of moderated mediation (ab3 = .05, BC CI: [.002, .14]) suggests that the effect of connectivity on social problems increases with age in our sample.
Figure 5. Age moderates the mediating effect of ventral striatal-mPFC rsFC on group differences in social function.
* denotes p<.05
Adoption-related variables
Separate linear regressions within the PI sample examining associations between ventral striatal-mPFC rsFC and age of adoption, age placed into institutionalized care, and time with family as regressors of interest controlling for age, sex and mean FD revealed no significant associations with adoption-related variables.
Discussion
Early caregiving adversity has been associated with increased risk for socio-affective difficulties and altered development of ventral striatum and mPFC, regions central to social behaviors. The current study identified alterations of the intrinsic functional connectivity between ventral striatum and mPFC and associations with social problems as reported by parents. Our main results showed that ventral striatal rsFC mainly with anterior regions of mPFC differed as a function of early life adversity: on average, PI youth showed significantly more positive connectivity than did comparison youth. Atypical ventral striatal-mPFC rsFC was associated with parental reports of social problems. Ventral striatal-mPFC rsFC significantly mediated the relationship between group differences in early caregiving history and social function. This mediation was moderated by age, such that ventral striatal-mPFC connectivity was more likely to statistically explain social problems in adolescents. These findings suggest that early institutional care increases the risk for social problems, which is mediated by alterations to ventral striatal connectivity with mPFC.
Altered ventral striatal rsFC in PI youth
Our first goal was to examine differences in resting-state connectivity between PI and comparison youth who were raised with their biological families from birth. There is an extensive literature across species suggesting that both the ventral striatum and mPFC are targets of early caregiving adversity (Callaghan & Tottenham, 2015; Fareri & Tottenham, 2016; Gee, Gabard-Durnam, et al., 2013a; K. Matthews & Robbins, 2003; K. Matthews, Dalley, Matthews, Tsai, & Robbins, 2001; K. Matthews, Wilkinson, & Robbins, 1996; Mehta et al., 2010), regions associated with social and affective function (Burnett et al., 2011; Crone & Dahl, 2012; Fareri & Delgado, 2014; Tottenham & Galván, 2016). We found support for our hypothesis of aberrant ventral striatal rsFC, with PI youth exhibiting more positive connectivity between the ventral striatum and mPFC.
The mechanisms underlying this difference cannot be ascertained from this current study. We did not observe associations with adoption variables, thus leaving open many possibilities as to the reasons for this group difference. For example, it is possible that these neural patterns reflect differences in prenatal or genetic factors to which the PI youth could have been exposed. Alternatively, it is possible that the effect of institutional caregiving is so species-atypical, that there is no dose-response association observable (i.e., even short durations of caregiver deprivation and/or institutional care increases the risk). It is also possible, however, that these brain differences reflect an ontogenetic adaptation to compensate for the lack of expected social input early in life, as has been argued in other neurodevelopmental domains (Callaghan & Richardson, 2011; Gee, Humphreys, et al., 2013b; Tottenham et al., 2011). Viewed through this lens, more positive connectivity between the ventral striatum and mPFC in PI youth may be a neural adaptation to enhance connections between mPFC and a hypoactive ventral striatum (Goff et al., 2013; Mehta et al., 2010). As the more anterior regions of mPFC exhibiting positive connectivity with the ventral striatum in the current sample of PI youth have been associated with positive connectivity in adults (Di Martino et al., 2008), and the comparison youth demonstrated negative ventral striatal-mPFC rsFC, it is possible that PI youth show an accelerated development of ventral striatal-mPFC rsFC. However, the lack of adults in our sample, and lack of a group x age interaction in these voxels makes this purely speculative. Future studies of individuals with a history of previous institutionalization that incorporate adult participants will be important for better evaluating differences in the development of connectivity following early caregiving adversity.
Associations between ventral striatal-mPFC rsFC and social function
A significant positive association was observed between ventral striatal-mPFC rsFC and parental report measures of CBCL Social Problems across the entire sample. This overall pattern of results remained when controlling for parent reported total problems, sex and age, suggesting that ventral striatal-mPFC connectivity at rest is related to social function and cannot be explained completely by general problem behaviors. We also found that the nature of connectivity between the ventral striatum and mPFC significantly mediated group differences in social function. Further, this relationship was moderated by age: the link between ventral striatal-mPFC rsFC and group differences in social problems was a linearly increasing function of age. Because we identified no age-related change in ventral striatal-mPFC rsFC, this finding suggests that atypical ventral striatal-mPFC connectivity is more likely to explain social problems in PI adolescents to a greater degree than it does in PI children (whose social problems might be related to different neural systems). The nature of items making up the social problems scale on the CBCL includes, among other things, parental assessments of their child’s social dependency, loneliness, how well they get along with/are liked by peers, and the degree to which they prefer interacting with same aged and older peers. Thus, higher scores on this composite measure of social function would indicate poorer abilities to relate to and establish normative interactions with same aged peers. Adolescence is a time characterized not only by heightened sensitivity to incentives and increased exploration (Galván, 2010), but a time during which interactions and relationships with peers take on increasing importance (Casey, 2015; Casey, Duhoux, & Malter Cohen, 2010); these behavioral phenomena are often studied within the context of ventral striatal-prefrontal development (Crone & Dahl, 2012; Galván, 2013; Pfeifer et al., 2013; 2011; Somerville, Jones, & Casey, 2010). Placed within this context, the altered patterns of ventral striatal-mPFC connectivity exhibited by PI youth may place them at greater risk for social difficulty during adolescence via instantiation of dysregulated social approach behaviors and a decreased ability to appropriately integrate social signals from others. Future studies should include tasks involving social incentives that incorporating real life social context (i.e., close peer relationships) to better characterize the nature of social function as dependent upon ventral striatal-mPFC connectivity in adolescents with a history of previous institutionalization.
The mechanistic link between ventral striatal-mPFC rsFC and social problems cannot be determined from the current study. There may be many reasons for this link. For example, a robust literature in humans and non-human animals implicates the ventral striatum as critical to reward valuation within social contexts. The ventral striatum is critically involved in social interactions, supporting the learning of others’ reputations (Delgado, Frank, & Phelps, 2005; Fareri, Chang, & Delgado, 2012a), integrating the value of social relationships into the representation of shared experiences (Fareri, Chang, & Delgado, 2015a; Fareri, Niznikiewicz, Lee, & Delgado, 2012b) and representing social actions (Báez-Mendoza, Harris, & Schultz, 2013), the value of reciprocity (Phan et al., 2010) and mutually interdependent outcomes (Rilling et al., 2002). This region may thus serve as a hub to translate social value signals into appropriate social action (Báez-Mendoza & Schultz, 2013). In typically developed humans, the mPFC is implicated in both reward-based (Bartra et al., 2013) and social cognitive processes — i.e., mentalizing, self/other representations, representing the social value of others (Amodio & Frith, 2006; Fareri, Chang, & Delgado, 2015a; C. D. Frith & Frith, 2006; Hampton, Bossaerts, & O’Doherty, 2008; Krienen, Tu, & Buckner, 2010; Mitchell, Banaji, & Macrae, 2005; Smith, Clithero, Boltuck, & Huettel, 2014). Thus the altered ventral striatal-mPFC rsFC observed in PI youth could reflect an altered ability to assess and use socially valued information appropriately. Initial support for this idea can be drawn from related work suggesting altered neural responses in the amygdala and dorsal mPFC in PI youth is related to atypical social evaluation, social approach behaviors and blunted responses to negative social experiences (Olsavsky et al., 2013; Puetz et al., 2014). Alternatively, it is possible that atypical ventral striatal-mPFC connectivity increases impulsivity in social contexts and directs the individual towards more reactive social drives (Tops et al., 2013). The value of the current study is in its identification of a brain-behavior link that necessitates further investigation to identify mechanistic explanations.
Limitations
There are limitations of this study worth noting. First, we used a validated, composite measure of social problem behavior in the CBCL Social Problems scale. However, as noted above, this composite measure makes it difficult to link specific types of social difficulties with ventral striatal-mPFC rsFC. Future work investigating social function in PI samples can employ more specific measures of social domains to examine the functional significance of ventral striatum resting state connectivity (e.g., social approach, social learning, impulsivity, competence) and should explore associations between both resting state measures of connectivity and task-based measures of functional connectivity (Gabard-Durnam et al., 2016) during social processes across development.
We also acknowledge the current state of affairs in the field regarding issues of thresholding and false positives (Eklund, Nichols, & Knutsson, 2016). We note that our cluster formation threshold was lenient, and our findings should be considered in light of that. However, current suggestions to address false positives in neuroimaging data also include more stringent cluster correction procedures, including correctly accounting for the non-Gaussian nature of the intrinsic smoothness of imaging data via spatial autocorrelation. Our findings do survive these stricter algorithms for employing cluster based correction, and therefore we feel confident in our assessment of the differences in ventral striatal-mPFC rsFC between comparison and PI youth.
Conclusions
The current findings identify group differences in resting state connectivity as a function of early care environments and link these to reported social problems in a task-free manner. While there are many more questions that require further study, these data provide a first step towards understanding how early caregiving experiences might influence resting state connectivity and associated behavior.
Acknowledgments
This work was supported by the National Institute of Mental Health (R01MH091864 to N.T.) and the Dana Foundation.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. http://doi.org/10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. http://doi.org/10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Frontiers in Neuroscience. 2013;7:233. doi: 10.3389/fnins.2013.00233. http://doi.org/10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Harris CJ, Schultz W. Activity of striatal neurons reflects social action and own reward. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1211342110. http://doi.org/10.1073/pnas.1211342110. [DOI] [PMC free article] [PubMed]
- Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA. Psychiatric Outcomes in Young Children with a History of Institutionalization. Harvard Review of Psychiatry. 2011;19(1):15–24. doi: 10.3109/10673229.2011.549773. http://doi.org/10.3109/10673229.2011.549773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. http://dx.doi.org/10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Kadosh KC, Blakemore S-J. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews. 2011;35(8):1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. http://doi.org/10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral Neuroscience. 2011;125(1):20–28. doi: 10.1037/a0022008. http://doi.org/10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2015;41(1):163–176. doi: 10.1038/npp.2015.204. http://doi.org/10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annual Review of Psychology. 2015;66(1):295–319. doi: 10.1146/annurev-psych-010814-015156. http://doi.org/10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–760. doi: 10.1016/j.neuron.2010.08.033. http://doi.org/10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Ward BD, Li W, Antuono P, Li S-J. A method to determine the necessity for global signal regression in resting-state fMRI studies. Magnetic Resonance in Medicine. 2012;68(6):1828–1835. doi: 10.1002/mrm.24201. http://doi.org/10.1002/mrm.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magentic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. http://doi.org/10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Frank R, Phelps EA. Perceptions of moral character modulate the neural systmes of reward during the trust game. Nature Neuroscience. 2005;8:1611–1618. doi: 10.1038/nn1575. http://doi.org/doi:10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll D, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo X-N, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biological Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. http://doi.org/10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, et al. Functional Connectivity of Human Striatum: A Resting State fMRI Study. Cerebral Cortex (New York, NY : 1991) 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. http://doi.org/10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Efron B. Better bootstrap confidence intervals. Journal of the American Statistical Association. 1987;82(397):171–185. http://doi.org/doi:10.1080/01621459.1987.10478410. [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Correction for Eklund et al., Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(33):E4929–E4929. doi: 10.1073/pnas.1602413113. http://doi.org/10.1073/pnas.1612033113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. http://doi.org/10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Delgado MR. The Importance of Social Rewards and Social Networks in the Human Brain. The Neuroscientist. 2014 doi: 10.1177/1073858414521869. http://doi.org/10.1177/1073858414521869. [DOI] [PubMed]
- Fareri DS, Tottenham N. Effects of early life stress on amygdala and striatal development. Developmental Cognitive Neuroscience. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. http://doi.org/10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Chang LJ, Delgado MR. Effects of direct social experience on trust decisions and neural reward circuitry. Frontiers in Neuroscience. 2012a;6:148–17. doi: 10.3389/fnins.2012.00148. http://doi.org/10.3389/fnins.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Chang LJ, Delgado MR. Computational substrates of social value in interpersonal collaboration. Journal of Neuroscience. 2015a;35(21):8170–8180. doi: 10.1523/JNEUROSCI.4775-14.2015. http://doi.org/10.1523/JNEUROSCI.4775-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, et al. Normative development of ventral striatal resting state connectivity in humans. NeuroImage. 2015b;118(C):422–437. doi: 10.1016/j.neuroimage.2015.06.022. http://doi.org/10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, Delgado MR. Reward-related processing in the human brain: developmental considerations. Development and Psychopathology. 2008;20(4):1191–1211. doi: 10.1017/S0954579408000576. http://doi.org/10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social Network Modulation of Reward-Related Signals. Journal of Neuroscience. 2012b;32(26):9045–9052. doi: 10.1523/JNEUROSCI.0610-12.2012. http://doi.org/10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–534. doi: 10.1016/j.neuron.2006.05.001. http://doi.org/10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. http://doi.org/10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Gee DG, Goff B, Flannery J, Telzer E, Humphreys KL, et al. Stimulus-Elicited Connectivity Influences Resting-State Connectivity Years Later in Human Development: A Prospective Study. Journal of Neuroscience. 2016;36(17):4771–4784. doi: 10.1523/JNEUROSCI.0598-16.2016. http://doi.org/10.1523/JNEUROSCI.0598-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. http://doi.org/10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. Neural Systems Underlying Reward and Approach Behaviors in Childhood and Adolescence. Current Topics in Behavioral Neurosciences. 2013 doi: 10.1007/7854_2013_240. http://doi.org/10.1007/7854_2013_240. [DOI] [PubMed]
- Galván A, McGlennen KM. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience. 2012;25(2):284–296. doi: 10.1162/jocn_a_00326. http://doi.org/10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. http://doi.org/10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110(39):15638–15643. doi: 10.1073/pnas.1307893110. http://doi.org/10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013b;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. http://doi.org/10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MM, Fox NA, Drury SS, Smyke AT, Nelson CA, Zeanah CH. Indiscriminate Behaviors in Previously Institutionalized Young Children. Pediatrics. 2014;133(3):e657–e665. doi: 10.1542/peds.2013-0212. http://doi.org/10.1542/peds.2013-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. http://doi.org/10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P. Convergence and Segregation of Ventral Striatal Inputs and Outputs. Annals of the New York Academy of Sciences. 1999;877(1 ADVANCING FRO):49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. http://doi.org/10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. http://doi.org/10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. http://doi.org/10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Annals of the New York Academy of Sciences. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. http://doi.org/doi:10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim K-S, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. http://doi.org/10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82C:208–225. doi: 10.1016/j.neuroimage.2013.05.116. http://doi.org/10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging. 2010;28(8):1051–1057. doi: 10.1016/j.mri.2010.03.021. http://doi.org/10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O’Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(18):6741–6746. doi: 10.1073/pnas.0711099105. http://doi.org/10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Archival Report. Biological Psychiatry. 2015;78(9):598–605. doi: 10.1016/j.biopsych.2015.05.010. http://doi.org/10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation and conditional process modeling [White paper] 2012 Retrieved July 28, 2014, from http://www.afhayes.com/public/process2012pdf.
- Hayes AF, Scharkow M. The Relative Trustworthiness of Inferential Tests of the Indirect Effect in Statistical Mediation Analysis: Does Method Really Matter? Psychological Science : a Journal of the American Psychological Society / APS. 2013;24(10):1918–1927. doi: 10.1177/0956797613480187. http://doi.org/10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(24):9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J, Tizard B. Social and Family Relationships of Ex-Institutional Adolescents. Journal of Child Psychology and Psychiatry. 1989;30(1):77–97. doi: 10.1111/j.1469-7610.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Lee SS, Telzer EH, Gabard-Durnam LJ, Goff B, Flannery J, Tottenham N. Exploration-exploitation strategy is dependent on early experience. Developmental Psychobiology. 2015;57(3):313–321. doi: 10.1002/dev.21293. http://doi.org/10.1002/dev.21293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–294. doi: 10.1016/j.neuron.2008.03.020. http://doi.org/10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2010;22(4):621–631. doi: 10.1162/jocn.2009.21228. http://doi.org/10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, et al. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, & Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0257-z. http://doi.org/10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. http://doi.org/doi:10.1016/S1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams C, Fong G, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. http://doi.org/20015472[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. http://doi.org/10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51(11):2062–2069. doi: 10.1016/j.neuropsychologia.2013.07.020. http://doi.org/10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan Mentality: Evidence That the Medial Prefrontal Cortex Responds to Close Others. Journal of Neuroscience. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. http://doi.org/10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nature Neuroscience. 2011;14(10):1250–1252. doi: 10.1038/nn.2904. http://doi.org/10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Quevedo K, Lafavor TL, Gunnar MR. Risk-taking and sensation-seeking propensity in postinstitutionalized early adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2014;55(10):1145–1152. doi: 10.1111/jcpp.12208. http://doi.org/10.1111/jcpp.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early Life Adversity Contributes to Impaired Cognition and Impulsive Behavior: Studies from the Oklahoma Family Health Patterns Project. Alcoholism, Clinical and Experimental Research. 2012;37(4):616–623. doi: 10.1111/acer.12016. http://doi.org/10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neuroscience and Biobehavioral Reviews. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. http://doi.org/10.1016/S0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic Maternal Separation of Neonatal Rats Produces Region- and Gender- Specific Effects on Biogenic Amine Content in Postmortem Adult Brain. Synapse. 2001;40:1–10. doi: 10.1002/1098-2396(200104)40:1<1::AID-SYN1020>3.0.CO;2-E. http://doi.org/10.1002/1098-2396(200104)40:1. [DOI] [PubMed] [Google Scholar]
- Matthews K, Wilkinson LS, Robbins TW. Repeated Maternal Separation of Preweanling Rats Attenuates Behavioral Responses to Primary and Conditioned Incentives in Adulthood. Physiology and Behavior. 1996;59(1):99–107. doi: 10.1016/0031-9384(95)02069-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry. 2014;76(8):629–638. doi: 10.1016/j.biopsych.2013.08.016. http://doi.org/10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. http://doi.org/10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience. 2012;15(5):663–668. doi: 10.1038/nn.3083. http://doi.org/10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. Bridging the Gap between the Human and Macaque Connectome: A Quantitative Comparison of Global Interspecies Structure-Function Relationships and Network Topology. Journal of Neuroscience. 2014;34(16):5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. http://doi.org/10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The Link between Social Cognition and Self-referential Thought in the Medial Prefrontal Cortex. Dx Doi.org. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. http://doi.org/10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Olsavsky AK, Telzer EH, Shapiro M, Humphreys KL, Flannery J, Goff B, Tottenham N. Indiscriminate amygdala response to mothers and strangers after early maternal deprivation. Biological Psychiatry. 2013;74(11):853–860. doi: 10.1016/j.biopsych.2013.05.025. http://doi.org/10.1016/j.biopsych.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Tong ZY, Brain PF, Clark D. Preferential occupation of mineralocorticoid receptors by corticosterone enhances glutamate-induced burst firing in rat midbrain dopaminergic neurons. Brain Research. 1996;737(1-2):146–154. doi: 10.1016/0006-8993(96)00722-6. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, et al. Longitudinal Change in the Neural Bases of Adolescent Social Self-Evaluations: Effects of Age and Pubertal Development. Journal of Neuroscience. 2013;33(17):7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. http://doi.org/10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, III, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering Adolescence: Resistance to Peer Influence, Risky Behavior, and Neural Changes in Emotion Reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. http://doi.org/10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Sripada CS, Angstadt M, McCabe K. Reputation for reciprocity engages the brain reward center. Proceedings of the National Academy of Sciences of the United States of America. 2010:1–6. doi: 10.1073/pnas.1008137107. http://doi.org/10.1073/pnas.1008137107. [DOI] [PMC free article] [PubMed]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. http://doi.org/10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105(C):536–551. doi: 10.1016/j.neuroimage.2014.10.044. http://doi.org/10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, Kohn N, MSc, D B, PhD, Z MM, Inform, S AD, PhD, S RT, et al. Neural Response to Social Rejection in Children With Early Separation Experiences. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(12):1328–1337.e8. doi: 10.1016/j.jaac.2014.09.004. http://doi.org/10.1016/j.jaac.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/s0896-6273(02)00755-9. http://doi.org/http://dx.doi.org/10.1016/S0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Current Opinion in Neurobiology. 1989;13(2-3):155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Roy P, Rutter M, Pickles A. Institutional care: associations between overactivity and lack of selectivity in social relationships. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(4):866–873. doi: 10.1111/j.1469-7610.2004.00278.x. http://doi.org/10.1111/j.1469-7610.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Rutledge RB, Dean M, Caplin A, Glimcher PW. Testing the reward prediction error hypothesis with an axiomatic model. Journal of Neuroscience. 2010;30(40):13525–13536. doi: 10.1523/JNEUROSCI.1747-10.2010. http://doi.org/10.1523/JNEUROSCI.1747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z, Reynolds RC, Jo HJ, Gotts SJ, Chen G, Martin A, Cox R. Correcting Brain-Wide Correlation Differences in Resting-State FMRI. Brain Connectivity. 2013 doi: 10.1089/brain.2013.0156. http://doi.org/10.1089/brain.2013.0156. [DOI] [PMC free article] [PubMed]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. http://doi.org/10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. http://doi.org/10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Daw N, Joel D, O’Doherty J. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. Journal of Neurocscience. 2007;27(47):12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. http://doi.org/27/47/12860[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Weber J, Mischel W, et al. Curbing Craving: Behavioral and Brain Evidence That Children Regulate Craving When Instructed to Do So but Have Higher Baseline Craving Than Adults. Psychological Science : a Journal of the American Psychological Society / APS. 2014;25(10):1932–1942. doi: 10.1177/0956797614546001. http://doi.org/10.1177/0956797614546001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, et al. Previous Institutionalization Is Followed by Broader Amygdala-Hippocampal-PFC Network Connectivity during Aversive Learning in Human Development. Journal of Neuroscience. 2016;36(24):6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. http://doi.org/10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Clithero JA, Boltuck SE, Huettel SA. Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Social Cognitive and Affective Neuroscience. 2014;9(12):2017–2025. doi: 10.1093/scan/nsu005. http://doi.org/10.1093/scan/nsu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong T-K, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. Journal of Neuroscience. 2010;30(7):2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. http://doi.org/10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. http://doi.org/10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–1008. doi: 10.1038/nn1728. http://doi.org/10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. http://doi.org/10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biology. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. http://doi.org/10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. George Thieme Verlag 1988 [Google Scholar]
- Tops M, Koole SL, Ijzerman H, Buisman-Pijlman FTA. Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry, and Behavior. 2014;119:39–48. doi: 10.1016/j.pbb.2013.07.015. http://doi.org/10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Galván A. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience and Biobehavioral Reviews. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. http://doi.org/10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. http://doi.org/10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. http://doi.org/10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. http://doi.org/10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. http://doi.org/10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P, Vuilleumier P. Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience. 2012;6:212. doi: 10.3389/fnhum.2012.00212. http://doi.org/10.3389/fnhum.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, Smith DV, Delgado MR. Using fMRI to study reward processing in humans: past, present, and future. Journal of Neurophysiology. 2016;115(3):1664–1678. doi: 10.1152/jn.00333.2015. http://doi.org/10.1152/jn.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschsler D. Wechsler Abbreviated Scale of Intelligence. New York: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]