Abstract
Purpose of review
We report recently published knowledge regarding gut chemosensory mechanisms focusing on nutrient-sensing G protein-coupled receptors (GPCRs) expressed on gut enteroendocrine cells (EECs), tuft cells, and in afferent nerves in the gastroduodenal mucosa and submucosa.
Recent findings
Gene profiling of EECs and tuft cells have revealed expression of a variety of nutrient-sensing GPCRs. The density of EEC and tuft cells is altered by luminal environmental changes that may occur following bypass surgery or in the presence of mucosal inflammation. Some EECs and tuft cells are directly linked to sensory nerves in the subepithelial space. Vagal afferent neurons that innervate the intestinal villi express nutrient receptors, contributing to the regulation of duodenal anion secretion in response to luminal nutrients. Nutrients are also absorbed via specific epithelial transporters.
Summary
Gastric and duodenal epithelial cells are continually exposed to submolar concentrations of nutrients that activate GPCRs expressed on EECs, tuft cells, and submucosal afferent nerves and are also absorbed through specific transporters, regulating epithelial cell proliferation, gastrointestinal (GI) physiological function, and metabolism. The chemical coding and distribution of EECs and tuft cells are keys to the development of GPCR-targeted therapies.
Keywords: Enteroendocrine cell, tuft cell, afferent nerve, nutrient receptors, gastrointestinal mucosa
Introduction
Gastric and duodenal epithelial cells are continually exposed to mM ranges of nutrients, gastric acid, bacterial metabolites, and bile acids that activate distinct G protein-coupled receptors (GPCRs), including sweet taste/umami receptors (taste receptor type 1, T1R1-3), members of the bitter receptor family (T2R), the bile acid receptor (GPBA), medium- and long-chain fatty acid receptors (FFA1, FFA4, GPR84, GPR119), short-chain fatty acid receptors (FFA2, FFA3), metabolic L-glutamate receptors (mGluRs), the lysophosphatidic acid receptor (LPA5), and the calcium sensing receptor (CaSR). Consistent with human taste concentration thresholds for sweet, umami, bitter, salty, and sour tastes (10 mM sucrose, 2 mM L-glutamate, 0.1 mM propylthiouracil, 17 mM Na+, and 2 mM acetic acid, respectively), most of those GPCRs are activated by ingested nutrients in mM ranges. Mucosal enteroendocrine cells (EECs) and tuft cells are considered to be gastrointestinal (GI) chemosensory cells that monitor luminal nutrients with the aforementioned GPCRs as well as nutrients transported into the submucosa, releasing distinct bioactive molecules in response to GPCR activation. EECs, consisting of ~1% of the epithelial cell population, are essential for chemosensory regulation of physiological digestive functions and systemic metabolism through paracrine, neurocrine, and endocrine pathways. Another secretory epithelial lineage, tuft cells, is also an uncommon but important epithelial cell population also essential for foregut chemosensing. Mutation of the transcription factor neurogenin-3 prevents EEC differentiation with resulting malnutrition in human and mice models [1;2]. Duodenal EEC density is significantly decreased in irritable bowel syndrome (IBS) or in functional dyspepsia (FD) patients [3;4], suggesting the importance of EEC-neuronal signals in GI physiological and afferent functions. Extrinsic afferent nerves that innervate the GI mucosa receive transduction signals from EECs and tuft cells through synapse-like structures and also directly monitor submucosal nutrients via nutrient-sensing GPCRs after absorption through the mucosa (Figure 1).
Figure 1.
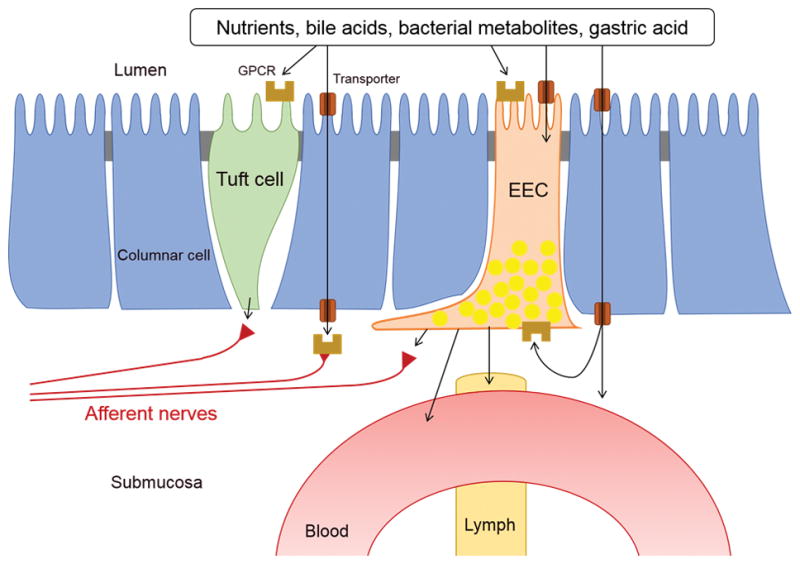
Luminal chemosensing cells in GI, including tuft cell, enteroendocrine cell (EEC), and afferent sensory neurons. A variety of GPCRs are expressed on the sensory cells, activated by luminal solutes before and after absorption through specific transporters. Sub-epithelial afferent nerves may directly receive signals from tuft cells and EECs.
Chemosensory cells in the gastroduodenal mucosa
EECs
EECs release important gut hormones such as 5-hydroxytryptamine (5-HT), gastric inhibitory peptide (GIP), cholecystokinin (CCK), glucagon-like peptides (GLP)-1, and GLP-2 into the portal vein or lymph [5–9]. EEC density is highest in the proximal duodenum, in particular 5-HT-producing enterochromaffin (EC) cell in the human intestine [10]. In 1962, the luminal release of 5-HT from rat duodenum in response to luminal acid was described [11], followed by isolated EC cell responses to a variety of food ingredients associated with nutrient receptor activations [12–14]. Similarly, the observation that luminal nutrients stimulate the release of GIP, CCK, GLP-1 and 2 [5–9] supported the hypothesis that EECs are intestinal ‘taste cells’ or ‘chemosensors’. Recent gene expression profiling studies suggest that EEC characteristics are based on a combination of GPCRs that vary among GI segments [14*,15,16]. In contrast to the classical EEC concept, some gut peptides are present in EECs in unexpected combinations; the mRNA coding for CCK, secretin, GIP, GLP-1, PYY, and neurotensin (NT), but not somatostatin, are co-expressed in a novel grouping of EECs [17]. Indeed, double-immunostaining studies confirm that GIP and GLP-1 are frequently co-expressed in rat duodenal EECs [18], 5-HT and CCK are often co-expressed in mouse duodenal EECs [19]. A subset of EECs co-express xenin-25, a homologue of NT, along with GIP but not 5-HT in canine duodenum [20], whereas xenin-25 is present in a subset of 5-HT/CCK- and GLP-2-expressing EECs rather than in GIP-producing EEC of rat duodenum [21*]. These observations suggest that multiple hormones can be released simultaneously, activating distinct local and metabolic pathways in response to luminal nutrient ligands.
Although isolated cell studies confirmed the predominant receptor profiles in each intestinal segment, in vitro experimental systems are not able to preserve physiological cell polarity. Since the immunoreactivity of many nutrient GPCRs is often detected in the cytosol, it is not always apparent which side (apical or basolateral) of the epithelial cells express nutrient GPCRs. Christensen et al. demonstrated that GLP-1 secretion from an isolated segment representing the proximal third of the small intestine (duodenum and proximal jejunum) was stimulated by intravascularly administered FFA1 synthetic agonists rather than by luminal agonists [22]. Although the duodenal luminal perfusion of FFA2 agonists releases 5-HT into the portal vein [23*], isolated duodenal EC cells are not directly activated by FFA2 ligands [14]. Furthermore, the glucose transporters GLUT2 and SGLT1 are essential to the activation of GIP and GLP-1 release [24;25]. These findings suggest that nutrient absorption prior to nutrient GPCR activation is an alternative pathway for the activation of EECs.
Gastric EECs are localized to the lower half of gastric glands, with the acid-producing oxyntic mucosa and acid-sensing antrum having different EEC populations [26]. The gastric corpus has a large population of ‘closed’ type EECs expressing the hunger-stimulating hormone ghrelin. Ghrelin-producing EEC lacks direct access to the lumen, but expresses a variety of nutrient GPCRs such as T1R3, FFA2, FFA4, GPR84, and CaSR [27;28]. Postprandially, the plasma may contain sufficient concentrations of all of these GPCR ligands such as amino acids, acetate, and free fatty acids to inhibit ghrelin release [28]. Gastrin, which stimulates gastric acid secretion through histamine release, is produced by open-type EEC localized exclusively in the antrum. Gastrin-producing cells express the GPCRs LPA5, GPRC6A, and CaSR, which are activated by amino acids, pH elevation, and/or extracellular Ca++ [29;30]. The same GPCRs are expressed by somatostatin-producing EEC [29], and FFA2 is expressed by 5-HT-producing EEC [31]. Both types of EEC are distributed in corpus and antrum, whereas the association of nutrient GPCR activation to somatostatin or 5-HT release is unknown.
Tuft Cells
Tuft cells (also termed as brush, caveolated, or fibrillovesicular cell) are also considered to be intestinal taste cell due to their expression of T1Rs, the Ca++-gated monovalent cation channel transient receptor potential subfamily M (TRPM)5, and gustducin, all molecules important for taste transduction. Differentiation into tuft cells depends on the transcription factor atonal homolog (ATOH)1, similar to other secretory cells (EEC, goblet, and Paneth), but not requiring neurogenin 3, which is essential for EEC differentiation, indicating that tuft cells are distinct from EECs [32]. Electron microscopy identified tuft cells morphologically by long apical microvilli, narrow rootlets, and well-developed tublovesicular structures in the cytoplasm. Tuft cells are present throughout GI tract, including in salivary and bile ducts [33]; they express many bioactive molecules, such as nitric oxide in rat stomach [34], ACh in mouse stomach [35], guanylin in human duodenum, but not in rat duodenum [36*], opioids, uroguanylin, and prostaglandins in mouse duodenum [37;38]. Since tuft cells lack chromogranin-positive granules, they may synthesize transmitters de novo, releasing them predominantly into the lumen. Doublecortin-like kinase 1 protein (DCLK1) and cytokeratin (CK)-18 are well characterized brush cell markers, identified in the rodent fundus, antrum, and duodenum. Duodenal tuft cells express gustducin, T1R1, and T1R3 in mice [39], suggesting the involvement in sweet receptor-dependent glucose transporter upregulation [40].
In the upper half of gastric glands and a few proximal corpus glands beneath the ‘limiting ridge’ in rodent stomach exists a unique population of tuft cells, which express gustducin, T1R3, FFA2, FFA3, and FFA4 [27;41;42]. Gastric tuft cells have an apical membrane projection that reaches the lumen, consistent with their proposed chemosensory functions. Transcription levels of the bitter taste receptor T2R108 and gustducin are relatively higher in the mouse stomach than in other intestinal regions, downregulated by 18-hour-fasting and recovered by only 4-hour-refeeding [43]. Although a T2R-expressing gastric cell type has not been identified, these dynamic changes of chemosensor expression support the nutrient sensing function of specialized cells within the gastric mucosa.
EEC adaptation following bariatric surgery
Bariatric surgery noticeably improves severe obesity and type 2 diabetes with a low risk of relapse [44;45]. Fasting-stimulated increases in the plasma levels of ghrelin are abolished by vertical sleeve gastrectomy in a rodent model and are unaffected in the Roux-en-Y gastric bypass (RYGB) model [46]. Interestingly, ghrelin-deficient mice comparably decrease food intake and body weight in both models, suggesting that bariatric surgery-induced weight loss is independent of the ghrelin-stimulated hunger signal [46]. Bypassing the duodenum without changing gastric volume increases the unique population of EECs that co-produce the incretin hormones GIP and GLP-1, improving pancreatic function in diabetic model rats [18]. The rat RYGB model that has an 80% decrease in stomach volume increases mucosal growth factor expression and crypt cell proliferation associated with circulating GLP-2 increase two weeks following surgery [47], consequently increasing EECs expressing GLP-1, CCK, NT, or 5-HT, associated with mucosal hyperplasia in the distal small intestine after 10–11 months [48]. In human obese patients with and without type 2 diabetes, the density of GIP-, GLP-1- or CCK-producing EECs are increased in the jejunum by RYGB [49]. An increase in postprandial GLP-1 caused by intestinal adaptation is likely to contribute towards improving glucose control and countering obesity. Although GIP stimulates insulin secretion, high-fat diet-induced excess GIP secretion is associated with insulin intolerance, rescued by GIP receptor deficiency [50]. Selective antagonism of the GIP pathway may be a therapeutic target for metabolic disorders.
Tuft cell population changes in pathological states
Tuft cell density appears to be a marker of gastrointestinal mucosal pathology. Hyperproliferation of tuft cells is frequently present in mouse and human intestinal adenomata, and in mouse stomach in which inflammation, hyperplasia, and metaplasia is present. Tuft cell hyperplasia is also implicated in GI tumorigenesis linking of excess prostanoid production due to the cyclooxygenase (COX)-2 expression in tuft cells [32;51]. Chemically and genetically induced fundic metaplasia markedly increase the density of DCLK-positive tuft cells in mouse models, and restore the tuft cell population coincident with mucosal restitution [52]. Tuft cells expressing interleukin-25 are increased by small intestinal worm infection [53], further implicating a new function of tuft cells in mucosal immune responses. Recently, Kuga et al. reported that a site- and phosphorylation status–specific antibody against Girdin is a selective tuft cell marker in the mouse and human GI tract [54]. Identifying GI region-specific chemical coding will be useful for further understanding of the function of tuft cells.
Links between epithelial sensory cells and neuronal pathways
Afferent neuronal pathways contribute towards luminal chemosensing that regulates GI function, in addition to transmitting satiety signals and visceral sensations to the central nervous system. Extrinsic afferent nerves originating in the nodose ganglia densely innervate the antrum and duodenal lamina propria [55] and also in the lumen of lymphatic lacteals [56]. Williams et al. recently demonstrated that GLP-1 receptor-expressing nodose neurons were mostly stretch sensors innervating the stomach, whereas the GPR65-expressing population innervated duodenal villi and responded to luminal nutrients [57**]. In addition to specific receptors associated with EEC-released hormones such as 5-HT, CCK, and GLP-2, afferent neurons possess nutrient receptors, including LPA5, mGluRs, and FFA3 [56;58;59]. Sensory nerves in the subepithelial space likely receive EEC signals and directly detect absorbed nutrients.
Similar to taste cells present in the lingual taste buds, EECs and tuft cells may communicate with afferent nerves. Indeed, pre- and post-synaptic markers are expressed in these cells [38;60]. PGP9.5-positive nerves directly contact some duodenal tuft cells, which are labeled by the fluorescent probe TRPM5-GFP [38]. Bohorquez et al. demonstrated the development of EEC-neuronal connections using 3D imaging in vivo, and further documented the formation of connections between isolated CCK-GFP cells and co-cultured sensory neurons in vitro by video capture [60]. Takahashi et al. demonstrated that the epithelial muscarinic ACh receptor antagonist upregulates and its agonist downregulates organoid growth, suggesting that neural and non-neural cholinergic signals modulate epithelial cell differentiation and that epithelial ACh may be a transmitter from epithelial cells to neurons [61].
The TRPV1 activator capsaicin is used to selectively de-afferent experimental models of neural function. Glucose transporter (SGLT1) upregulation induced by a 3-hour jejunal luminal perfusion of glucose is blocked by capsaicin deafferentation [62], suggesting that the TRPV1-expressing afferent neural pathway is required for the acute epithelial adaptation to luminal nutrients.
Duodenal anion secretion is mediated by EECs and by afferent neuronal pathways
Our group has investigated how capsaicin-sensitive afferent nerves contribute to duodenal mucus secretion induced by luminal acid and mGluR4 agonists [63;64]. We recently reported that the rat proximal duodenum possesses an active short-chain fatty acid (SCFA) absorption mechanism and that luminal SCFAs stimulate duodenal bicarbonate secretion via SCFA receptors (FFA2 and FFA3) and SCFA transporter-dependent pathways (Figure 2) [65;66]. Luminal SCFA acetate increases the secretory rate of bicarbonate through FFA2-mediated 5-HT release and FFA3-mediated GLP-2 release, consistent with results of studies in which selective FFA agonists were used singly [23;67*]. Since the response to acetate is significantly reduced by SCFA transporter inhibition or by capsaicin deafferentation [66], afferent nerves may detect SCFA directly after absorption and potentiate EEC transmitter release to increase duodenal mucosal protection.
Figure 2.
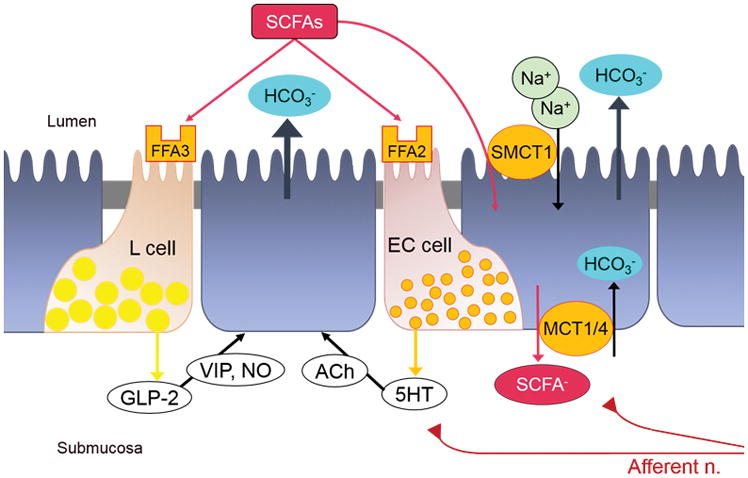
SCFA-activating duodenal bicarbonate secretion (DBS). FFA3 activation stimulates GLP-2 release, followed by vasoactive intestinal peptide (VIP) and nitric oxide (NO) releases, increasing DBS. FFA2 activation stimulates 5-HT release, which activates cholinergic pathway to stimulate DBS. Sodium-dependent and independent monocarboxylate transporters SMCT1, MCT1, and MCT4 mediate SCFA absorption, facilitating DBS via afferent neural pathways.
Xenin, an anorectic hormone, is produced in the brain and in duodenal EECs. Plasma xenin concentrations are increased by meal ingestion or sham feeding in humans, suggesting that vagal reflexes stimulate xenin release from the duodenal mucosa [68]. The infusion of low concentrations (4–12 pmol/kg) of xenin to humans delays gastric emptying, inhibits GLP-1 release, and causes mild diarrhea [69;70]. Since RYGB obesity surgery diminishes exogenous xenin-associated diarrhea and the inhibition of GLP-1 release in response to liquid meal, duodenal xenin signals may significantly activate unknown neural pathways [71*]. Intravenous xenin stimulated duodenal bicarbonate secretion in vivo, and basolateral xenin induced chloride secretion via an afferent neural pathway involving NTS1, NK1, and 5-HT3 receptors in Ussing chambered rat duodenum [21**]. These observations suggest that duodenal xenin activates afferent neural pathways and that the neurotransmitter releases from afferent nerves are likely preserved in isolated intestinal segments. The function of afferent nerves innervating the duodenal mucosa may not be limited to transmitting post-prandial signals to the central nervous system, but may also be involved in the regulation of postprandial mucosal defenses.
Conclusion
Luminal nutrients activate mucosal sensory cells such as EECs and tuft cells, and afferent nerves through specific GPCRs and transporters before and after absorption. The population of EECs and tuft cells are altered by luminal environmental change or mucosal abnormality, suggesting that they have the potential to be disease markers. Understanding GI chemosensing functions opens the possibility that a variety of nutrient GPCRs are potential drug targets for modulating epithelial cell proliferation, GI physiological function, and also metabolic control.
Key points.
A variety of nutrient G protein-coupled receptors (GPCRs) are differentially expressed in enteroendocrine cells (EECs) and in tuft cells of the gastroduodenal mucosa.
The population of EECs and tuft cells are altered by bariatric surgery, functional dyspepsia, or mucosal inflammation.
Luminal nutrients regulate gut hormone releases via GPCR activation and transporter-dependent mechanisms.
Postprandial duodenal anion secretion is stimulated by luminal and absorbed nutrients via EEC and afferent neural activations.
Acknowledgments
Financial support: Department of Veterans Affairs Merit Review Award
The authors thank Ms. Stacy Jung for her expert administrative assistance.
Footnotes
Conflicts: None
References and recommended reading
- 1.Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martin MG. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- 2.Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010;120:1708–1721. doi: 10.1172/JCI40794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Witte AB, Walker MM, Talley NJ, Aro P, Ronkainen J, Marrazzo V, Agreus L, Schmidt PT. Decreased number of duodenal endocrine cells with unaltered serotonin-containing cells in functional dyspepsia. Am J Gastroenterol. 2016;111:1852–1853. doi: 10.1038/ajg.2016.468. This study quantified the density of chromogranin A-positive cells and serotonin-positive cells in first and second part of duodenal biopsy from 24 FD patients and 12 healthy controls. [DOI] [PubMed] [Google Scholar]
- 4.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Duodenal chromogranin a cell density as a biomarker for the diagnosis of irritable bowel syndrome. Gastroenterol Res Pract. 2014;2014:462856. doi: 10.1155/2014/462856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2163–R2169. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Hokari R, Kurihara C, Sato H, Narimatsu K, Hozumi H, Ueda T, Higashiyama M, Okada Y, Watanabe C, Komoto S, Tomita K, Kawaguchi A, Nagao S, Miura S. Dietary lipids and sweeteners regulate glucagon-like peptide-2 secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G708–G714. doi: 10.1152/ajpgi.00282.2012. [DOI] [PubMed] [Google Scholar]
- 8.Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould HE, Wank SA. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, Vigna SR, Grant AO, Liddle RA. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G528–G537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine-cells in human intestine - an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 11.Resnick RH, Gray SJ. Chemical and histologic demonstration of hydrochloric acid-induced release of serotonin from intestinal mucosa. Gastroenterology. 1962;42:48–55. [PubMed] [Google Scholar]
- 12.Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. 2008;295:G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 13.Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. Regional differences in nutrient-induced secretion of gut serotonin. Physiol Rep. 2017;5:e13199. doi: 10.14814/phy2.13199. The authors isolated serotonin-producing cells from duodenum and colon to compare the responsivity to a variety of sugars and short-chain fatty acids by measurements of intracellular Ca++ and serotonin release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki K, Harada N, Sasaki K, Yamane S, Iida K, Suzuki K, Hamasaki A, Nasteska D, Shibue K, Joo E, Harada T, Hashimoto T, Asakawa Y, Hirasawa A, Inagaki N. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K-cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinol. 2015;156:837–46. doi: 10.1210/en.2014-1653. [DOI] [PubMed] [Google Scholar]
- 16.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM. Overlap of Endocrine Hormone Expression in the Mouse Intestine Revealed by Transcriptional Profiling and Flow Cytometry. Endocrinol. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, Pedersen J, Windelov JA, Fuchtbauer EM, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinol. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. Duodenal-jejunal bypass protects GK rats from β-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923–E932. doi: 10.1152/ajpendo.00422.2010. [DOI] [PubMed] [Google Scholar]
- 19.Cho HJ, Callaghan B, Bron R, Bravo DM, Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 2014;356:77–82. doi: 10.1007/s00441-013-1780-x. [DOI] [PubMed] [Google Scholar]
- 20.Anlauf M, Weihe E, Hartschuh W, Hamscher G, Feurle GE. Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J Histochem Cytochem. 2000;48:1617–1626. doi: 10.1177/002215540004801205. [DOI] [PubMed] [Google Scholar]
- 21**.Kaji I, Akiba Y, Kato I, Maruta K, Kuwahara A, Kaunitz JD. Xenin augments duodenal anion secretion via activation of afferent neural pathways. J Pharmacol Exp Ther. 2017;361:151–161. doi: 10.1124/jpet.116.238485. In this study, a duodenal specific gut hormone xenin-25 was identified in serotonin-, GLP-2-, and CCK-positive EEC in rat duodenum. Xenin-induced duodenal anion secretion was mediated by afferent neural pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen LW, Kuhre RE, Janus C, Svendsen B, Holst JJ. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol Rep. 2015;3:e12551. doi: 10.14814/phy2.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Akiba Y, Maruta K, Narimatsu K, Said H, Kaji I, Kuri A, Iwamoto KI, Kuwahara A, Kaunitz JD. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. Am J Physiol Gastrointest Liver Physiol. 2017 doi: 10.1152/ajpgi.00041.2017. In Press. The authors monitored serotonin concentration in portal vein, mucosal blood flow, and duodenal bicarbonate secretion under duodenal loop perfusion with FFA2 agonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhre RE, Frost CR, Svendsen B, Holst JJ. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64:370–382. doi: 10.2337/db14-0807. [DOI] [PubMed] [Google Scholar]
- 26.Choi E, Roland JT, Barlow BJ, O’Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–1720. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010;339:493–504. doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- 28.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2:376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haid DC, Jordan-Biegger C, Widmayer P, Breer H. Receptors responsive to protein breakdown products in g-cells and d-cells of mouse, swine and human. Front Physiol. 2012;3:65. doi: 10.3389/fphys.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci U S A. 2010;107:17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaji I, Akiba Y, Kaunitz JD, Karaki Si, Kuwahara A. Differential expression of short-chain fatty acid receptor FFA2 and FFA3 in foregut. Gastroenterology. 2012;142:S494. [Google Scholar]
- 32.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato A. Tuft cells. Anat Sci Int. 2007;82:187–199. doi: 10.1111/j.1447-073X.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 34.Kugler P, Hofer D, Mayer B, Drenckhahn D. Nitric oxide synthase and NADP-linked glucose-6-phosphate dehydrogenase are co-localized in brush cells of rat stomach and pancreas. J Histochem Cytochem. 1994;42:1317–1321. doi: 10.1177/42.10.7523487. [DOI] [PubMed] [Google Scholar]
- 35.Schutz B, Jurastow I, Bader S, Ringer C, von EJ, Chubanov V, Gudermann T, Diener M, Kummer W, Krasteva-Christ G, Weihe E. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Brenna O, Furnes MW, Munkvold B, Kidd M, Sandvik AK, Gustafsson BI. Cellular localization of guanylin and uroguanylin mRNAs in human and rat duodenal and colonic mucosa. Cell Tissue Res. 2016;365:331–341. doi: 10.1007/s00441-016-2393-y. Using in-situ hybridization with immunohistochemistry, this study identified the epithelial cell types producing guanylin or uroguanylin in human and rat colon and duodenum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology. 2009;137:598–606. doi: 10.1053/j.gastro.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, le CJ, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 39.Bezencon C, le CJ, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 40.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen S, Laermans J, Iwakura H, Tack J, Depoortere I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. Plos One. 2012;7:e40168. doi: 10.1371/journal.pone.0040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberle JA, Widmayer P, Breer H. Receptors for short-chain fatty acids in brush cells at the “gastric groove”. Front Physiol. 2014;5:152. doi: 10.3389/fphys.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H, Sternini C. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. Plos One. 2014;9:e107732. doi: 10.1371/journal.pone.0107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mingrone G, Panunzi S, De GA, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 46.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschop MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144:50–52. doi: 10.1053/j.gastro.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taqi E, Wallace LE, de HE, Chelikani PK, Zheng H, Berthoud HR, Holst JJ, Sigalet DL. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45:987–995. doi: 10.1016/j.jpedsurg.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 48.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25:e70–e79. doi: 10.1111/nmo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee NA, Wahlgren CD, Pedersen J, Mortensen B, Langholz E, Wandall EP, Friis SU, Vilmann P, Paulsen SJ, Kristiansen VB, Jelsing J, Dalboge LS, Poulsen SS, Holst JJ, Vilsboll T, Knop FK. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia. 2015;58:2254–2258. doi: 10.1007/s00125-015-3696-3. [DOI] [PubMed] [Google Scholar]
- 50.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 51.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, Samuelson LC, Merchant JL. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol. 2011;136:191–204. doi: 10.1007/s00418-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi E, Petersen CP, Lapierre LA, Williams JA, Weis VG, Goldenring JR, Nam KT. Dynamic expansion of gastric mucosal doublecortin-like kinase 1-expressing cells in response to parietal cell loss is regulated by gastrin. Am J Pathol. 2015;185:2219–2231. doi: 10.1016/j.ajpath.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von MJ, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuga D, Ushida K, Mii S, Enomoto A, Asai N, Nagino M, Takahashi M, Asai M. Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut. J Histochem Cytochem. 2017 doi: 10.1369/0022155417702586. 22155417702586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poole DP, Lee M, Tso P, Bunnett NW, Yo SJ, Lieu T, Shiu A, Wang JC, Nomura DK, Aponte GW. Feeding-dependent activation of enteric cells and sensory neurons by lymphatic fluid: evidence for a neurolymphocrine system. Am J Physiol Gastrointest Liver Physiol. 2014;306:G686–G698. doi: 10.1152/ajpgi.00433.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. Using in vivo Ca++ imaging techniques in mouse nodose ganglia, the authors visualized two different populations of afferent sensory neurons, which differently innervate stomach and duodenum and respond to mechanical and chemical stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Moller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 59.Page AJ, Young RL, Martin CM, Umaerus M, O’Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 60.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi T, Ohnishi H, Sugiura Y, Honda K, Suematsu M, Kawasaki T, Deguchi T, Fujii T, Orihashi K, Hippo Y, Watanabe T, Yamagaki T, Yuba S. Non-neuronal acetylcholine as an endogenous regulator of proliferation and differentiation of Lgr5-positive stem cells in mice. FEBS J. 2014;281:4672–4690. doi: 10.1111/febs.12974. [DOI] [PubMed] [Google Scholar]
- 62.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg. 2010;251:865–871. doi: 10.1097/SLA.0b013e3181d96e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akiba Y, Watanabe C, Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multi glutamate receptors in rat. Gastroenterology. 2009;136:A689. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akiba Y, Kaunitz JD. Luminal chemosensing and upper gastrointestinal mucosal defenses. Am J Clin Nutr. 2009;90:826S–831S. doi: 10.3945/ajcn.2009.27462U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A, Kaunitz JD. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015;593:585–599. doi: 10.1113/jphysiol.2014.280792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, Akiba Y. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2015;308:G188–G197. doi: 10.1152/ajpgi.00298.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67*.Said H, Akiba Y, Narimatsu K, Maruta K, Kuri A, Iwamoto KI, Kuwahara A, Kaunitz JD. FFA3 activation atimulates duodenal bicarbonate secretion and prevents NSAID-induced enteropathy via the GLP-2 pathway in rats. Dig Dis Sci. 2017;62:1944–52. doi: 10.1007/s10620-017-4600-4. This study reported that duodenal luminal perfusion with FFA3 selective agonist stimulate GLP-2 release into the portal vein possessing mucosal protective effects in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feurle GE, Ikonomu S, Partoulas G, Stoschus B, Hamscher G. Xenin plasma concentrations during modified sham feeding and during meals of different composition demonstrated by radioimmunoassay and chromatography. Regul Pept. 2003;111:153–159. doi: 10.1016/s0167-0115(02)00281-1. [DOI] [PubMed] [Google Scholar]
- 69.Gault VA, Martin CM, Flatt PR, Parthsarathy V, Irwin N. Xenin-25[Lys13PAL]: a novel long-acting acylated analogue of xenin-25 with promising antidiabetic potential. Acta Diabetol. 2015;52:461–471. doi: 10.1007/s00592-014-0681-0. [DOI] [PubMed] [Google Scholar]
- 70.Chowdhury S, Reeds DN, Crimmins DL, Patterson BW, Laciny E, Wang S, Tran HD, Griest TA, Rometo DA, Dunai J, Wallendorf MJ, Ladenson JH, Polonsky KS, Wice BM. Xenin-25 delays gastric emptying and reduces postprandial glucose levels in humans with and without type 2 diabetes. Am J Physiol Gastrointest Liver Physiol. 2014;306:G301–G309. doi: 10.1152/ajpgi.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Sterl K, Wang S, Oestricker L, Wallendorf MJ, Patterson BW, Reeds DN, Wice BM. Metabolic responses to xenin-25 are altered in humans with Roux-en-Y gastric bypass surgery. Peptides. 2016;82:76–84. doi: 10.1016/j.peptides.2016.06.001. The authors investigated the effects of xenin-25 on gut peptide releases and glucose homeostasis after the bariatric surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]


