Abstract
Introduction
Alcohol misuse is the fifth leading risk factor for premature death and disability worldwide. Fewer than 10% of afflicted Americans receive pharmacological treatment for alcohol use disorder. Gabapentin is a calcium channel GABAergic modulator that is widely used for pain. Studies showing reduced drinking and decreased craving and alcohol-related disturbances in sleep and affect in the months following alcohol cessation suggest therapeutic potential for alcohol use disorder.
Areas covered
Human laboratory and clinical studies assessing gabapentin for alcohol use disorder are reviewed. Data were obtained by searching for English peer-reviewed articles on PubMed, reference lists of identified articles, and trials registered on clinicaltrials.gov. Additionally, the mechanism of action of gabapentin specific to alcohol use disorder, and studies of gabapentin for alcohol withdrawal and non-alcohol substance use disorders are summarized.
Expert opinion
Alcohol use disorder represents a challenge and large, unmet medical need. Evidence from single-site studies lend support to the safety and efficacy of gabapentin as a novel treatment for alcohol use disorder, with unique benefits for alcohol-related insomnia and negative affect, relative to available treatments. Proprietary gabapentin delivery systems may open a path to pivotal trials and registration of gabapentin as a novel treatment for alcohol use disorder.
Keywords: alcoholism, alcohol use disorder, calcium channel/GABA modulator, clinical trial, craving, gabapentin, human laboratory study, insomnia, negative affect, pregabalin, protracted withdrawal
1. INTRODUCTION
Alcohol misuse is the fifth leading risk factor for premature death and disability worldwide.[1] Globally, the World Health Organization estimates alcohol consumption is responsible for 5.9% of deaths (3.3 million people) and 5.1% of disease burden, especially liver cirrhosis, cancers and injuries, accounting for 139 million disability-adjusted life years annually.[2,3]
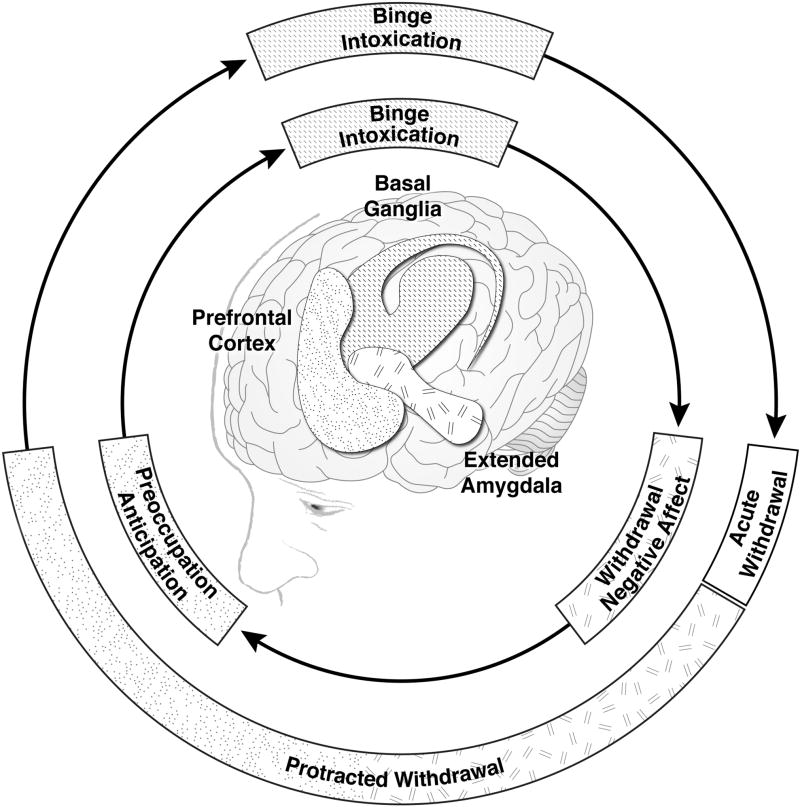
Alcohol use disorder of moderate or greater severity is typically a chronic, relapsing disorder characterized by compulsive heavy alcohol drinking despite harmful consequences.[4] The emergence of an acute (up to 5 days duration) withdrawal syndrome upon marked reduction in or abrupt cessation of drinking is typically followed by a protracted withdrawal phase of indeterminate duration, with a high risk of relapse to pathological drinking; the cyclical nature of the disorder is illustrated in the outer ring of Figure 1. Preventing relapse after acute withdrawal is a leading concern of alcoholism research.[5] The withdrawal-negative affect stage of early abstinence includes symptoms of anxiety, dysphoria and irritability[6] and is associated with activation of brain stress systems, particularly overexpression of corticotropin releasing factor (CRF) in the extended amygdala.[7] Such neuroadaptions extend into protracted withdrawal and the preoccupation-anticipation stage of the addiction cycle (inner ring of Figure 1). Dysregulation of the prefrontal cortex during the preoccupation-anticipation stage drives symptoms of craving, and perpetuates residual negative emotional states and sleep disturbances. All of the above symptoms of protracted withdrawal have been identified as risk factors for drinking relapse.[8–12]
Figure 1.
A conceptual framework for the neurobiology of alcohol use disorder (inner ring), with corresponding clinical states (outer ring). Adapted by permission from Macmillan Publishers Ltd: NEUROPSYCHOPHARMACOLOGY (Koob GF and Volkow ND. Neuropsychopharmacology, 2010, 35(1):217-38), copyright 2010.
The International Classification of Disease 10th Revision (ICD-10)[13], draft ICD-11[14] and the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-4)[15] use the term “alcohol dependence.” DSM-5[4] combines diagnostic criteria for alcohol abuse and dependence under the term “alcohol use disorder,” with severity modifiers of “mild,” “moderate” or “severe,” based on the number of criteria met. DSM-5 alcohol use disorder of moderate or greater severity is essentially equivalent to DSM-4 and ICD-10 criteria for alcohol dependence. Given that DSM-5 was first published in 2013, the admission criteria for the studies reviewed in this paper are based on alcohol dependence, unless otherwise noted.
A comprehensive literature search of PubMed and related databases was performed for this project, using multiple combinations of search terms to yield human laboratory and clinical studies assessing the therapeutic potential of gabapentin for alcohol use disorder. Reference lists of identified publications were searched for additional references, and clinicaltrials.gov was searched for posted results of clinical trials. The search yielded 11 completed prospective studies, including 3 human laboratory studies and 8 treatment studies; all were included in this review.
2. OVERVIEW OF THE MARKET
There are 3 medications approved for alcohol dependence in the U.S. and throughout much of the world: disulfiram (oral), naltrexone (oral and long-acting intramuscular) and acamprosate (oral). A fourth drug, nalmefene (oral), is approved throughout the European Union, and is to be taken on an “as needed” basis prior to anticipated drinking occasions. Disulfiram was the first drug approved for alcoholism (in 1951) by the U.S. Food and Drug Administration (FDA). It inhibits the enzyme acetaldehyde dehydrogenase, so that if even small amounts of alcohol are consumed, acetaldehyde quickly builds up, with rapid onset of a dilsulfiram-alcohol interaction that includes flushing, nausea and vomiting, changes in mental status and multiple cardiac and respiratory symptoms that could result in death in severe reactions. Disulfiram is used as a deterrent to alcohol use and should never be given to a patient when in a state of alcohol intoxication, or without their full knowledge. The psychological threat of the disulfiram-alcohol interaction may comprise the primary mechanism of disulfiram’s deterrent effect, as opposed to the drug’s pharmacodynamic properties.[16] Medication compliance is a demonstrated problem with disulfiram[17] and outcomes are optimized with supervised administration, e.g. by a spouse, in compliant patients who wish to remain abstinent.[18]
Naltrexone is a pure opioid receptor antagonist that was FDA-approved first for opioid dependence and 10 years later for alcohol dependence (oral 1994; long-acting injectable 2006). Opioid receptor antagonism as a treatment strategy for alcohol dependence was based on the hypothesis that if alcohol consumption was less rewarding, drinking would decrease. Naltrexone should not be given to patients with current prescribed or illicit opiate use, as it will induce acute opioid withdrawal. Problems with patient non-compliance have been noted with oral naltrexone,[17,19] thus the development of a long-acting (30 days) injectable formulation.
Acamprosate was developed in France in the 1980’s and FDA-approved for the maintenance of abstinence in detoxified alcoholics in 2004. Repeated cycles of heavy drinking and withdrawal dysregulate the balance between neuronal excitation (e.g., glutamergic) and inhibition (e.g., GABAergic). The hypothesized mechanism of action of acamprosate ranges from modulation of glutamergic tone to action via its calcium moiety.[20,21] Much of the literature suggests an action in restoring homeostasis in N-methyl-D-aspartate (NMDA)-mediated glutamergic neurotransmission.[22,20] and the calcium mechanism is controversial.[23] Acamprosate is not metabolized in the liver and has been shown to be safe in patients with hepatic impairment. Low bioavailability requires divided dosing taken three times per day.
Recent large-scale meta-analyses show that either acamprosate or naltrexone combined with counseling have superior efficacy for increasing rates of abstinence (acamprosate, NNT=7.5) or no heavy drinking (naltrexone, NNT=8.6) relative to counseling administered in conjunction with placebo, particularly when initiated after detoxification or five days of abstinence.[24,25] Despite these findings for efficacy, results from a nationwide pharmacy survey indicate that fewer than 9% of 8.4 million afflicted Americans filled a prescription for one of the FDA-approved medications for alcohol dependence.[26] The National Institute on Alcohol Abuse and Alcoholism (NIAAA) has identified the development of more effective medications for alcohol use disorder to be a research priority. Frances Collins, Director of the National Institutes of Health (NIH), has encouraged the re-purposing of existing medications approved for other indications, in order to expeditiously meet big, unmet medical needs, such as alcohol use disorder.[22]
3. GABAPENTIN: AN OLD ANTICONVULSANT WITH A NEW POTENTIAL
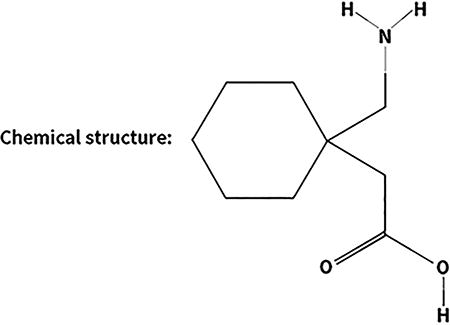
Gabapentin (Box 1) is an oral anticonvulsant that was FDA-approved as an adjunctive treatment for partial epileptic seizures in 1993, and for postherpetic neuralgia in 2004. A Cochrane review (2014) also found evidence supportive of gabapentin as a treatment for diabetic neuropathy. Gabapentin has been available as a generic medication since 2004. Like acamprosate, gabapentin is not metabolized in the liver and has low bioavailability requiring dosing three times per day. Two proprietary, extended release oral formations of gabapentin are currently FDA-approved: Gralise for the once-daily treatment of postherpetic neuralgia (2011), and Horizant gabapentin enacarbil extended release tablets for the treatment of restless leg syndrome (2011) and postherpetic neuralgia (2012).
Box 1. Drug Summary.
Drug name: Gabapentin
Phase: II
Indication under discussion: Alcohol Use Disorder
Pharmacology: Calcium channel γ-aminobutyric acid modulator
Route of administration: Oral
Multi-site Phase II trial:
HORIZANT (Gabapentin Enacarbil Extended-Release Tablets) for the Treatment of Alcohol Use Disorder, NCT 02252536, recruiting completed.
Studies of FDA-approved indications often reported a secondary beneficial effect of gabapentin on measures of sleep.[28–32] Of particular note, administration of alcohol at bedtime to normal subjects was used as a method to induce sleep disruption in a double-blind, randomized, crossover study of gabapentin in a sleep lab setting with polysomnography and subjective measures. Gabapentin improved sleep by significantly decreasing stage 1 sleep and number of awakenings and increasing sleep efficiency relative to placebo. Importantly, gabapentin did not differ from placebo on tests of daytime drowsiness and performance.[33] Off-label clinical studies also reported beneficial effects of gabapentin on sleep,[34–39] as well as on anxiety[40] and mood.[41–49] A medication that decreases drinking in animal models of alcohol dependence[50] and improves symptoms of negative affect and sleep disturbance in clinical samples is of significant interest as a potential treatment for decreasing drinking relapse risk in the protracted withdrawal phase of alcohol dependence.
3.1 PHARMACODYNAMICS AND PHARMACOKINETICS
Gabapentin is an amino acid designed as a structural analog of GABA.[51] Gabapentin binds to the alpha-2-delta type 1 subunit of voltage gated calcium channels.[52,53] Gabapentin selectively inhibits Ca2+ influx through these voltage-operated Ca2+ channels,[54] which results in its ability to reduce postsynaptic excitability and decrease the release of excitatory neurotransmitters.[55] Possibly via its actions to block excitatory neurotransmission, gabapentin increases the concentration of GABA in the brain.[56,57] Gabapentin has been hypothesized to be an agonist for GABA-B receptors,[58] however, when GABA-B receptors were expressed in xenopus laevis oocytes or mammalian cells and assayed by means of electrophysiology or biochemical assays, gabapentin had no direct GABA-B agonist action.[59]
At concentrations up to 100uM, gabapentin does not show affinity to a number of receptor sites, including benzodiazepine, glutamate, NMDA, quisqualate, kainite, strychnine-insensitive or strychnine sensitive glycine, alpha 1, alpha 2, or beta adrenergic, adenosine A1 or A2, cholinergic, muscarinic or nicotinic, dopamine D1 or D2, histamine H1, serotonin S1 or S2, cannabinoid 1,opiate mu, delta or kappa. Gabapentin has not been shown to significantly affect the cellular uptake of dopamine, norepinephrine, or serotonin.
Gabapentin is not metabolized significantly by the liver in humans, thus most pharmacological actions are attributed to the parent compound. A therapeutic range of steady state gabapentin plasma levels has not been established and therapeutic monitoring of gabapentin is not indicated. Bioavailability of gabapentin is not proportional to dosing and gabapentin levels do not correlate directly with ingested doses.[60] Less than 3% of gabapentin circulates bound to protein in plasma. The concentrations of gabapentin in cerebrospinal fluid are approximately 20% of the corresponding plasma concentrations. It is eliminated from the circulation by renal excretion, unchanged. The half-life of gabapentin is 5 to 7 hours, and is not changed based on dosing. The renal clearance is directly proportional to creatinine clearance, thus in patients with impaired renal function, gabapentin clearance is reduced, thereby necessitating dose adjustment. Food has only a small effect on the rate and extent of absorption of the compound. Gabapentin has been designated as pregnancy Category C.[61]
3.2 GABAPENTIN AND ITS ALCOHOL-RELATED MECHANISM OF ACTION
In animal models of alcohol dependence, gabapentin decreased the amplitudes of GABA receptor mediated inhibitory post synaptic currents (IPSCs) in the central nucleus of the amygdala (CeA), and decreased dependence-induced alcohol drinking.[50] Notably, the effects of gabapentin were identical to the effects of a corticotropin releasing factor (CRF) antagonist (decreased IPSCS in dependent rats).[50,62] These results suggest an important GABA-CRF interaction in GABAergic neurotransmission that markedly adapts during development of alcohol dependence, and the ethanol dependence-induced neuroadaptations of GABA-CRF may account for the differential effects of gabapentin observed in CeA.[50] As noted above and in Figure 1, CRF in the CeA is the key brain region associated with negative affect in protracted abstinence.
Additionally, gabapentin blocks the effects of thrombospondin on the alpha-2-delta type 1 subunit.[63] Alpha-2-delta type 1 subunits modulate voltage gated calcium channels but also can perform calcium independent functions. Thrombospondin is an astrocyte-secreted protein that promotes synaptogenesis and gabapentin antagonizes thrombospondin binding to the alpha-2-delta type 1 subunit of voltage gated calcium channel and inhibits excitatory synapse formation, independent of calcium channel activity.[63] Previous work has shown that alpha-2-delta type 1 subunits are upregulated in reward related regions by all major drugs of abuse including alcohol.[64]
Of note, genomic computational methods were used to match the transcriptional signatures from alcohol-dependent samples with gene expression profiles produced by FDA-approved drugs in the Library of Integrated Network-based Cellular Signatures (LINCS) as a strategy to identify available medications that may be repurposed as medications for alcohol use disorder. Gabapentin was identified as having therapeutic potential for alcohol use disorder through this unbiased genomic screening method.[65]
3.3 CLINICAL EFFICACY
3.3.1 HUMAN LABORATORY STUDIES
Drinking relapse is always a safety risk to be considered when prescribing medications for patients with alcohol use disorder. The safety of acute doses of gabapentin (0, 1000 or 2000mg) administered in combination with intoxicating doses of alcohol was evaluated in a PK/PD alcohol-gabapentin interaction study.[66] Alcohol did not affect the pharmacokinetics of alcohol. The combination was well-tolerated and gabapentin did not alter the subjective and performance effects of alcohol, nor did it alter the intoxicating effects of alcohol or alcohol craving. This was a single-dose study in nondependent drinkers who were administered alcohol, and the authors recommended that, given its safety, gabapentin be evaluated for efficacy in abstinent patients with alcohol dependence.
The safety of gabapentin (up to 1200mg/d) administered in combination with alcohol was further supported in a double-blind, placebo-controlled 8-day human laboratory study in non-treatment seeking dependent drinkers.[67] On the seventh day of study drug, subjects were given a priming dose of alcohol followed by a free-choice alcohol self-administration session in a bar-like lab setting. Gabapentin did not significantly affect blood alcohol levels, levels of intoxication, stimulation, sedation, craving or alcohol self-administration, relative to placebo. Again, the safety of the combination of gabapentin and alcohol was supported, but an alcohol administration human laboratory paradigm would be ineffective for inducing the neuroadaptations and subsequent clinical symptoms of early abstinence (e.g., craving) that gabapentin is hypothesized to treat.
The potential efficacy of gabapentin as a treatment for protracted withdrawal symptoms was assessed in a human laboratory model of risk factors for relapse, including induction of emotional states and in vivo exposure to the sight and smell of a subject’s favorite alcoholic beverage without an opportunity for drinking.[68] Non-treatment-seeking volunteers with current alcohol dependence were randomized to one week of treatment with 1200mg/d of gabapentin or matched placebo and were required to abstain from drinking for 3 days prior to alcohol cue reactivity testing. Gabapentin was associated with significantly greater reductions than placebo on several measures of craving in response to alcohol cues, and on several measures of sleep disturbance, with no increase in daytime drowsiness. Gabapentin’s subjective effects were not found to resemble any major classes of abused drugs. Beneficial effects on craving and sleep, with no evidence of abuse potential or significant adverse events in abstinent alcoholics suggested gabapentin may have therapeutic potential for the protracted withdrawal phase in alcohol dependence.
3.3.2 CLINICAL TREATMENT STUDIES
Gabapentin (≤1500mg/d h.s.) showed beneficial effects on insomnia persisting beyond 4 weeks of abstinence in a case series of alcoholic outpatients, with no evidence of tolerance to gabapentin dose.[69] The authors noted that all patients remained totally abstinent during 4–6 weeks of follow-up, except 2 patients who drank 4 drinks on 1 occasion each. A similar clinical population was treated for 4–6 weeks with open label gabapentin (up to 1800mg h.s.) or trazodone, a sedating antidepressant (up to 300mg h.s.).[70] Although both groups reported improved sleep, patients who received gabapentin improved significantly more than those who received trazodone and were less likely to have initial insomnia and daytime fatigue. Two patients from each group reported having at least 1 drink during the study. A 6-week randomized, double-blind, placebo-controlled pilot study (N=21) assessed the efficacy of gabapentin (up to 1500mg/d h.s.) in outpatients with alcohol dependence and insomnia persisting post-withdrawal.[71] Gabapentin significantly delayed the onset to heavy drinking relative to placebo, an effect that persisted through an additional 6 weeks of follow up. Both gabapentin and placebo subjects showed improved sleep from baseline measures.
The efficacy of gabapentin (600mg/d) for reducing alcohol craving and consumption was evaluated in a 28-day, placebo-controlled trial in 60 outpatients with alcohol dependence who were randomized to double-blind treatment after 7 days of detoxification.[72] Gabapentin was associated with significant reductions in the number of drinks per day, percentage of heavy drinking days, gamma-glutamyl transferase (GGT; elevations in this liver enzyme are considered a biomarker of recent alcohol use), and alcohol craving, as well as a significant increase in the percentage of abstinent days relative to placebo.
A double-blind, placebo-controlled trial randomized 150 outpatients with alcohol dependence to placebo, 900, or 1800mg/d of gabapentin, following ≥3 days of abstinence.[73] The outcomes assessed included measures of alcohol consumption and craving, as well as measures of mood and sleep. Gabapentin was associated with significant linear dose-related reductions in drinking quantity and frequency, GGT, alcohol craving, sleep and negative affect, as well as increased rates of abstinence and no heavy drinking over the 12-week study period. Significant effects on drinking measures typically persisted throughout an additional 12 weeks of post-treatment follow-up, suggesting a return to homeostasis in brain stress systems with gabapentin treatment, particularly at the 1800mg dose. This study included participants at all levels of dependence severity (most in the moderate range), with and without a history of prior treatment and/or detoxification, and had relatively high representation of women (43.3%). Treatment response did not vary across clinical or demographic variables including disease severity and sex.
A 6-month, double-blind, placebo-controlled, multicenter trial of gabapentin enacarbil 1200mg/d in 350 outpatients has recently completed recruitment at 10 sites across the U.S. to assess the efficacy of gabapentin enacarbil for alcohol use disorder (NCT 02252536). Gabapentin enacarbil is a prodrug of gabapentin that was developed to provide more uniform bioavailability, faster time to full therapeutic dose, and less fluctuating gabapentin blood levels with twice daily dosing, relative to generic gabapentin.[74] Results are expected in 2018.
3.3.3 COMBINATION TRIALS IN ALCOHOL DEPENDENCE
Two double-blind randomized clinical trials were conducted that evaluated the efficacy of gabapentin administered in combination with another medication for the treatment of alcohol dependence, relative to placebo.[75,76] In one trial (n=60), outpatients were instructed to not drink the night prior to randomization to flumazenil, a benzodiazepine receptor antagonist (2mg of incremental bolus for 20 minutes for 2 consecutive days), and gabapentin (≤1200mg h.s. for 39 days) or their inactive placebos.[75] Alcohol withdrawal was assessed with Clinical Institute Withdrawal Assessment for Alcohol-Revised (CIWA-Ar)[77] with higher scores indicating greater severity (total score range: 0–67; patients scoring <10 usually do not require medication for withdrawal). Medication effects on drinking outcomes were not obtained; those on active medication reported less daytime sleepiness than those on placebo. The authors further divided the sample based on baseline CIWA-Ar ≥ or <7, and found a significant increase in percent days abstinent and time to first heavy drinking day in those 7 subjects with CIWA-Ar ≥7 treated with active medication relative to those 9 subjects treated with placebo. Conversely, those with CIWA-Ar ≥7 had better sleep if on placebo. Lack of a gabapentin monotherapy group precludes interpretation of gabapentin efficacy, but the safety of the combination of gabapentin and flumazenil is supported.
Gabapentin (≤1200mg/d for first 6 weeks) was also studied in combination with naltrexone (50mg/d) vs naltrexone alone (50mg/d) vs placebo in a 16-week randomized double-blind trial in 150 outpatients who were abstinent ≥4 days prior to randomization.[76] The combination of gabapentin and naltrexone was well tolerated. Again, the absence of a gabapentin alone group in the study design precludes assessment of gabapentin efficacy, but the group that received gabapentin in combination with naltrexone had better outcomes on several measures of drinking, craving and sleep than naltrexone alone or placebo. Benefits tended to fade after the 6-week gabapentin treatment period, suggesting a longer treatment interval may be required to stabilize the central stress systems activated in protracted withdrawal and hypothetically returned to homeostasis by an adequate treatment course with gabapentin. For example, the 12-week trial described above found significant effects of gabapentin on drinking outcomes that were sustained for an additional 12 weeks following titration off gabapentin.[73]
3.3.4 GABAPENTIN FOR THE TREATMENT OF ACUTE ALCOHOL WITHDRAWAL
Abrupt cessation of or significant decrease in alcohol intake in dependent drinkers can precipitate the emergence of an acute withdrawal syndrome within 4 – 12 hours of the last drink that may persist for up to 5 days (DSM-5). Symptoms can range from anxiety, agitation, hand tremor and sympathetic nervous system activation, to delirium tremens and seizures, potentially resulting in death if left untreated.
Benzodiazepines are considered the drugs of choice for treating alcohol withdrawal. Gabapentin has been studied as a potential treatment for acute alcohol withdrawal, based on its modulatory action on brain excitatory (i.e., glutamergic) and inhibitory (i.e., GABAergic) pathways. Gabapentin has some advantages relative to benzodiazepines: it does not interact with alcohol, does not impair cognition or motor coordination, is not metabolized in the liver, has low abuse potential, and has low lethality in overdose.[78] These features are attractive, as alcohol detoxification is increasingly provided on an outpatient basis. However, recent comprehensive reviews of the extant literature conclude that gabapentin should not be considered a stand-alone treatment for severe alcohol withdrawal because of ineffectiveness in suppressing alcohol withdrawal-related seizures.[79,80] Initiating gabapentin dose titration in conjunction with alcohol detoxification may assist in decreasing withdrawal-related anxiety and insomnia, as well as serve as a transition to longer-term relapse prevention treatment during protracted withdrawal. Of note, Myrick and colleagues (2009)[81] followed-up 100 subjects treated with double-blind gabapentin (2 dose conditions: ≤900mg/d and ≤1200mg/d) or lorazepam (a benzodiazepine, ≤6mg/d) in a 4-day alcohol withdrawal study, and found significantly decreased drinking, craving and sedation during the 6-day post-withdrawal follow-up period in patients who had been treated with gabapentin, particularly in the 1200mg/d dose condition, relative to lorazepam. Conversely, in a similar design, Trevisan and colleagues (2008)[82] found no differences between gabapentin (1200mg/d), valproic acid (≤1500mg/d) and placebo for symptoms of acute and protracted alcohol withdrawal; however, this sample was comprised of male veterans with significant comorbidity, primarily post-traumatic stress disorder, depressive and cocaine use disorders, for which gabapentin is not indicated, and the nature and intensity of concomitant treatments was not specified.
3.3.5 PREGABALIN FOR THE TREATMENT OF ACUTE ALCOHOL WITHDRAWAL
Pregabalin is a newer gabapentinoid drug with a mechanism of action and therapeutic indications similar to those of gabapentin, but with greater potency, 3× more rapid absorption and 3× more rapid time to peak plasma concentration (1 vs. 3 hours).[83] Pregabalin also has a longer half-life elimination time than gabapentin, allowing a 2×/d instead of 3×/d dosing schedule. Pregabalin (200–450mg/d) has shown efficacy for treating uncomplicated alcohol withdrawal and related negative affective symptoms in one open-label study (N=40)[84] and one multi-center, randomized, single-blind comparison trial with tiapride and lorazepam (N=111),[85] as well as in a pre-clinical study of alcohol withdrawal.[86] Additionally, a small (N=20), 16-week open-label study of pregabalin (150–450mg/d) in detoxified alcoholics suggested a beneficial effect of treatment on prolonging abstinence and reducing craving and negative affective symptoms during protracted withdrawal.[87] These beneficial effects of pregabalin on abstinence, craving and negative affect were replicated in a 16-week randomized, double-blind comparison trial (N=70) of pregabalin (150–450mg/d) versus naltrexone (50mg/d).[88] The authors of the above studies note that pregabalin was well-tolerated and was not associated with significant safety concerns.
Pregabalin has been designated a Schedule V controlled drug, and the pharmacokinetic improvements over gabapentin, e.g. quicker absorption rates, more rapid time to peak plasma concentration and higher potency, are associated with increased abuse potential.[89] Euphoria was found to be a common adverse event in a systematic review of pregabalin abuse potential.[90] See Section 3.4.1 Abuse Potential for a summary of strategies to identify and monitor risk factors for gabapentinoid abuse.
3.3.6 GABAPENTIN FOR THE TREATMENT OF NON-ALCOHOL SUBSTANCE USE DISORDERS
Alcohol use disorder may occur in conjunction with other substance use disorders, including those for which there are no available pharmacotherapies, such as cocaine, methamphetamine and cannabis use disorders. Gabapentin has been assessed for therapeutic potential across a variety of non-alcohol substance use disorders, with mixed results. Promising results for gabapentin (1200mg/d) in decreasing cocaine craving and use were found in early open-label studies,[91,92] but were not replicated in subsequent controlled trials of gabapentin (≤3200mg/d) treatment of cocaine dependence.[93,94,43,95] Similarly, an open-label study and a 30-day controlled trial of gabapentin (≤1200mg/d) combined with flumazenil and hydroxyzine hydrochloride showed decreased methamphetamine use,[96,97] but these results were not replicated in a 108-day controlled study of the same medication combination relative to placebo.[98]
Interest in gabapentin as an adjunctive treatment for opioid withdrawal and stabilization based on its analgesic, anti-anxiety and anti-craving properties, found support in two small open-label studies (n = 7, gabapentin 1800mg/d;[99] n = 20, gabapentin 1200mg/d[100]). Similarly, a single-blind, 10-day inpatient randomized trial (n = 59) found decreased craving and opioid withdrawal symptoms with gabapentin given in combination with tramadol, relative to methadone combined with tramadol.[101] A double-blind trial of gabapentin 900mg/d in 40 subjects concomitantly treated with methadone did not significantly reduce severity of opiate withdrawal relative to placebo, perhaps as a function of the relatively low dose of gabapentin used (900/mg/d);[46] when gabapentin was given as an add-on to methadone in an open-label study, greater reduction in severity of withdrawal symptoms was obtained with 1600mg/d of gabapentin relative to 900mg/d.[45]
A double-blind, placebo-controlled 12-week study of gabapentin 1200mg/d in 50 outpatients with cannabis dependence found significantly decreased cannabis use, as measured by both quantitative urine drug determinations and self-report, as well as significantly decreased craving, mood and sleep disturbance, relative to placebo.[44] Cannabis withdrawal, like alcohol withdrawal, produces an anxiogenic-like state and increased CRF release in the CeA in rodents.[102] The normalizing effect of gabapentin on these brain stress systems[50] and the decrease in cannabis use and craving, sleep and mood disturbance found in the above clinical sample suggest gabapentin may have therapeutic potential for cannabis use disorder. Of note, gabapentin showed subtle cognitive enhancing effects in the domains of attention, concentration, visual-motor functioning, inhibition and set shifting in normal volunteers in a comparison study with topiramate and placebo,[103] and similar findings were also detected in the above cannabis dependent sample.
3.4 ADVERSE EVENTS, SAFETY, AND TOLERABILITY
Gabapentin (≤1800mg/d) was typically found to be safe and well-tolerated in 11 studies up to 12 weeks in duration involving 655 participants with alcohol use disorder (Table 1). There were no deaths, serious or unexpected drug-related adverse events reported, and side effects were typically mild to moderate in severity and not associated with drug discontinuation. Gabapentin did not differ from placebo on any one adverse event. Common adverse events included headache, insomnia, fatigue, muscle aches and various gastrointestinal complaints in both gabapentin- and placebo-treated patients with alcohol use disorder. Alcohol was not found to interact meaningfully with gabapentin in a PK/PD alcohol-gabapentin drug interaction study.[66] Similarly, gabapentin was not found to increase blood alcohol concentrations nor intoxicating, stimulating or sedating effects of alcohol in a human lab study involving alcohol administration in non-treatment seeking alcoholics.[67] Of note, although somnolence was a common complaint in pain and epilepsy trials (see below), the reverse effects often occurred in alcoholism trials, i.e. improved sleep without daytime drowsiness, fatigue, or reduced performance.[70,33,68,73] However, as with any centrally active drug, patients should be advised not to drive motor vehicles or operate heavy machinery until they have ascertained the drug does not affect their motor performance.
Table 1.
Clinical studies of gabapentin in alcohol use disorder.
| Reference | Research Design |
Sample Size |
Gabapentin Dose |
Treatment Duration |
Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Drinking | Craving | Mood | Sleep | ||||||
| Human laboratory studies in alcohol use disorder | Mason et al., 2009 |
|
33 | 1200 mg/d | 1 week | N.R. | + | — | + |
| Myrick et al., 2007 |
|
35 | ≤1200 mg/d | 8 days | — | — | — | N.R. | |
| Bisaga & Evans, 2005 |
|
17 | 1000, 2000 mg/d | single dose | N.R. | — | — | N.R. | |
|
| |||||||||
| Treatment studies in alcohol dependence | Mason et al., 2014 |
|
150 | 900, 1800 mg/d | 12 weeks | + | + | + | + |
| Brower et al., 2008 |
|
21 | 1500 mg/d h.s. | 6 weeks | + | N.R. | N.R. | — | |
| Trevisan et al., 2008 |
|
57 | 1200 mg/d | 4 weeks | — | — | — | — | |
| Furieri et al., 2007 |
|
60 | 600 mg/d | 4 weeks | + | + | N.R. | N.R. | |
| Karem-Hage et al., 2003 |
|
55 | ≤1800 mg/d h.s. | 4–6 weeks | N.R.* | N.R. | N.R. | + | |
| Karem-Hage & Brower, 2000 |
|
17 | ≤1500 mg/d h.s. | 4–6 weeks | N.R.* | N.R. | N.R. | + | |
| Combination treatment studies in alcohol dependence | Anton et al., 2011 |
|
150 | ≤1200 mg/d for first 6 weeks + naltrexone 50 mg/d vs. naltrexone 50 mg/d vs. placebo | 16 weeks | + | — | — | + |
| Anton et al., 2009 |
|
60 | ≤1200 mg/d h.s. for 39d + flumazenil 2mg bolus (20m) for 2d vs. placebo | 39 days | ±** | N.R. | N.R. | + | |
+ drug showed a significant (p<0.05) effect relative to a comparator group or baseline value
— drug did not show a significant (p>0.05) effective relative to a comparator group or baseline value
N.R. not reported
h.s. hour of sleep dosing
Authors note only 2 subjects drank while on active medication, but statistical comparisons with pretreatment values were not provided.
Results in the randomized sample were not significant; however, the authors reported a significant drinking reduction in a subgroup of seven subjects with pretreatment alcohol withdrawal scores (CIWA) >7 who were randomized to active treatments.
Italicized items in the Research Design column indicate methodological details that may have contributed to negative outcomes.
Based on significant clinical and post-marketing experience for approved pain and epilepsy indications, gabapentin is considered to have a good safety and tolerability profile. The most commonly reported adverse events with gabapentin in comparison to placebo-treated patients in pivotal trials of post-herpetic neuralgia (not alcohol use disorder) include dizziness, somnolence, and peripheral edema. The most commonly reported adverse events in pivotal epilepsy trials were somnolence, dizziness, ataxia, fatigue and nystagmus. Antiepileptic drugs including gabapentin increase the risk of suicidal thoughts or behavior in about 1 in 500 patients taking these drugs for any indication. Abrupt withdrawal from gabapentin has precipitated seizures and status epilepticus; on- and off-titration of gabapentin is indicated. The overall incidence of adverse events and types of adverse events are similar in men and women across the pivotal trials for approved indications. Refer to the package insert for detailed prescribing information.[61]
3.4.1 ABUSE POTENTIAL
Reports of abuse of gabapentinoids, such as gabapentin and pregabalin, are increasingly being documented in high-risk populations, notably opioid and prescription drug abusers.[104] Gabapentin is not a controlled or scheduled substance. There was no evidence of tolerance to gabapentin dose, rebound with titration off drug, nor evidence of abuse potential in studies of alcohol dependence (Table 1). However, patients undergoing opioid withdrawal, those who misuse prescriptions recreationally, and prison populations may be at increased risk to misuse gabapentin, with self-administered doses often far exceeding (3–25×) the therapeutic range.[105–107, 89, 108] Pregabalin has more rapid absorption and faster onset of action than gabapentin, and thus is associated with greater abuse.[89] It has been classified as a schedule V (lowest potential for abuse) controlled substance by the U.S. Drug Enforcement Agency, comparable to the benzodiazepine, diazepam. Risk factors for pregabalin abuse, often at doses 3–20× the highest approved dose, include a history of substance abuse, especially opioid abuse.[90] Hence, patients with risk histories should be monitored for potential gabapentinoid misuse or diversion, e.g. self-dose escalation or a “need” for unusually high doses, repeated requests to replace “lost” medication, and other drug-seeking behaviors.
3.5 CONCLUSION
All FDA-approved medications for alcohol use disorder have a history of both negative and positive clinical trial outcomes. The potential efficacy of gabapentin in alcohol use disorder is supported by 5 of the 6 single-site treatment studies reporting drinking outcomes.[72,71,75,76,73] These study designs were double-blind, placebo-controlled with random assignment, and were conducted across multiple geographic regions, including California, Michigan and South Carolina, U.S.A. in North America, and Espírito Santo, Brazil in South America. Studies evaluating two doses of gabapentin found greater efficacy associated with the higher dose (1200mg/d, 1600mg/d, 1800mg/d) relative to the lower dose studied (900mg/d),[81,45,73] lending further support to the likelihood of a true pharmacological effect of gabapentin on study outcomes. The only treatment study not showing a comparative benefit of gabapentin on drinking outcomes was comprised of a male veteran sample with multiple clinically significant co-occurring disorders, and the type and intensity of any treatments for these comorbid disorders is unknown and may have impacted drinking outcomes.[82] All the treatment studies included in this review were conducted in subjects who concomitantly received the behavioral support typically provided in their setting for alcohol use disorder,[69,70,82] or manual-guided counseling.[72,71,75,76,73] Thus, the evidence presented is generally a comparison of gabapentin with behavioral support relative to placebo with behavioral support. Results indicate that combining gabapentin with behavioral support typically provides an incremental advantage in drinking outcomes relative to placebo with behavioral support.
Studies requiring ≥3 days of abstinence at treatment initiation showed the most robust treatment effects.[72,71,68,76,73] Conversely, studies that did not require abstinence at treatment initiation or that involved alcohol administration were unlikely to show gabapentin efficacy for craving and/or drinking outcomes.[66,67,82,75] These results are consistent with the hypothesized mechanism of action of gabapentin in alcohol dependence, i.e., modulating and stabilizing central stress systems dysregulated by drinking cessation. Thus, future studies and clinical applications in alcohol use disorder should stipulate an abstinence baseline.
Six of eight studies with sleep measures reported a significant beneficial effect of gabapentin on alcohol-related sleep disturbance, a key precipitant of relapse in protracted withdrawal; these beneficial effects on sleep were not associated with daytime drowsiness or dysfunction.[69,70,75,68,76,73] Results showing gabapentin-related decreases in negative affect are found embedded in alcohol withdrawal measures and in weekly Beck Depression Inventory scores over a 12-week randomized clinical trial.[73] The potential benefits of gabapentin for reducing alcohol consumption, craving and related disturbances in sleep and affect are validated by results obtained with pregabalin,[84,85,88] a similar gabapentinoid drug discussed above.
Gabapentin has shown no safety concerns among the 655 outpatients with alcohol use disorder who participated in the 11 studies included in this review. Adverse events tended to be mild to moderate, and to not differ from placebo. The lack of appreciable hepatic metabolism is a pharmacokinetic advantage of gabapentin, as chronic heavy drinking is often associated with liver injury. Importantly, gabapentin treatment outcomes and adverse effects do not appear to differ between men and women with alcohol use disorder.
Gabapentin was not found to interact with alcohol, i.e. did not increase blood alcohol concentration, feelings of sedation or motor impairment. Although considered to have low abuse potential in the general population, patients in opioid withdrawal, or those who abuse prescription drugs, may be at increased risk for gabapentinoid abuse or diversion.
Conclusions must be drawn with caution, as pivotal trials of gabapentin have yet to be conducted. Overall, the extant literature suggests that gabapentin shows promise as a relatively safe and effective treatment for alcohol use disorder, with unique benefits for alcohol-induced insomnia and negative affect compared to available treatments.
4. EXPERT OPINION
Alcohol use disorder is associated with significant mortality and morbidity, ranking fifth among risk factors for death and disability worldwide.[1] Remarkably, a nationwide pharmacy survey suggests fewer than 9% of Americans with alcohol use disorder received a prescription for any of the FDA-approved pharmacotherapies for alcohol dependence.[26] Thus the development of more effective, better-tolerated and more widely-utilized medications for alcohol use disorder has high public health significance.
Gabapentin (1800mg/d) effect sizes for rates of complete abstinence and no heavy drinking[73] are comparable or superior to those found in a recent meta-analysis of acamprosate’s effect on abstinence and naltrexone’s effect on no heavy drinking.[24] However, relative to approved treatments, gabapentin is uniquely associated with significant improvements in alcohol-related sleep disturbance and sub-syndromal negative affective states, which are both leading precipitants of drinking relapse in protracted withdrawal. Additionally, unlike disulfiram and naltrexone, gabapentin is not appreciably metabolized in the liver and has no association with hepatotoxicity. Also from a safety perspective, gabapentin will not induce opiate withdrawal in contrast to naltrexone, and does not have potentially life-threatening interactions with alcohol, as does disulfiram. Rather, gabapentin is hypothesized to restore homeostasis in key brain stress systems that become dysregulated in acute and protracted withdrawal, simultaneously reducing symptoms of insomnia, craving and negative affect that drive drinking relapse. Thus, unlike treatment with available medications for alcohol dependence, patients treated with gabapentin are likely to experience improved sleep and less negative affect when cutting down or quitting drinking. These beneficial effects may reduce problems with medication adherence associated with disulfiram and naltrexone.
Gabapentin is a widely-prescribed medication across many medical specialties, primarily as a comparatively safe and well-tolerated treatment for neuropathic pain, with generally low abuse potential. Thus, a broad range of physicians are familiar with gabapentin’s side effects, dosing and PK/PD profile, and may be more inclined to use it than available treatments for alcohol use disorder. As noted above, fewer than 9% of afflicted Americans have ever received a prescription for an FDA-approved medication for alcohol use disorder; these prescriptions are provided primarily by psychiatrists and not by a broad range of medical providers, although alcoholics often interact with the medical system in urgent care and general medicine settings.
Gabapentin is currently available as an inexpensive generic drug produced by multiple manufacturers. There is no financial incentive for one generic manufacturer to obtain FDA-approval for a new indication (alcohol use disorder), which typically involves funding at least two positive multicenter pivotal trials. Thus, gabapentin as studied in this review is unlikely to be marketed as a new medication for alcohol use disorder. However, gabapentin enacarbil (Horizant), a proprietary prodrug of gabapentin that produces extended release of gabapentin and is FDA-approved for pain and restless leg syndrome, is currently being evaluated as a treatment for alcohol use disorder in a Phase II multi-center trial (1200mg/d) conducted by the National Institute on Alcohol Abuse and Alcoholism in collaboration with the manufacturer, Arbor Pharmaceuticals. Proprietary formulations of gabapentin may provide a path forward for drug registration and marketing of gabapentin as a new treatment for alcohol use disorder. However, different forms of gabapentin, including immediate release, sustained release, and enacarbil sustained release, are absorbed in the body differently. Thus, studies with different formulations of gabapentin should heed the multiple studies of generic gabapentin that report dose response effects up to 1800mg/d, and ensure sustained pharmacokinetic dose equivalence to achieve comparable treatment outcomes. Finally, all the treatment studies included in this review were conducted in subjects who concomitantly received some form of behavioral support, as is standard for clinical trials in alcohol use disorder. Thus, the evidence presented is generally a comparison of gabapentin with behavioral support relative to placebo with behavioral support. The preponderance of evidence suggests that combining gabapentin with behavioral support provides an incremental advantage in drinking outcomes relative to behavioral support alone.
Although pivotal trials have not been conducted and conclusions must be drawn with caution, results from the single-site studies included in this review suggest that gabapentin could provide a new and potentially widely-used treatment for alcohol use disorder.
Acknowledgments
The authors wish to thank Sam Reed from The Scripps Research Institute for his assistance in manuscript preparation.
Funding
Support for this work was provided by the Pearson Center for Alcoholism and Addiction Research and grants R01AA023152 and U01AA025476 from the National Institute on Alcohol Abuse and Alcoholism to B. Mason.
Footnotes
Declaration of Interest
B. Mason has served as a scientific consultant to DepoMed and Mitsubishi Tanabe. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Barbara J. Mason, Pearson Center for Alcoholism and Addiction Research, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037, (858) 784-7324, (858) 784-7340 (fax), mason@scripps.edu.
Susan Quello, Pearson Center for Alcoholism and Addiction Research, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037, (858) 784-7327, (858) 784-7340 (fax), squello@scripps.edu.
Farhad Shadan, Head, Hospitalist Medicine, Scripps Clinic and Scripps Green Hospital, 10666 N. Torrey Pines Road, La Jolla, CA 92037, (858) 455-9100, (858) 784-7340 (fax), shadan.farhad@scrippshealth.org.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global status report on alcohol and health – p. XIV, 2014 ed. Geneva: World Health Organization; 2014. [Last accessed 6 December 2017]. Available at: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf. [Google Scholar]
- 3.Global status report on alcohol and health – p. XIII, 2014 ed. Geneva: World Health Organization; 2014. [Last accessed 6 December 2017]. Available at: http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Press; 2013. • Contains the diagnostic criteria for alcohol use disorder with severity modifiers. [Google Scholar]
- 5.Gordis E. Relapse and craving: a commentary. Alcohol Alert. 1989;6:3. [Google Scholar]
- 6.Geller A. Protracted abstinence. In: Miller NS, editor. Comprehensive Handbook of Drugs and Alcohol Addiction. New York, NY: Marcel Dekker; 1991. pp. 905–13. [Google Scholar]
- 7.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. • Describes neuroadaptations associated with chronic heavy alcohol use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowman C, Allen J, Stout RL. Replication and extension of Marlatt’s taxonomy of relapse precipitants: overview of procedures and results. The relapse research group. Addiction. 1996;91(S51):71. [PubMed] [Google Scholar]
- 9.Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcoholism Clin Exp Res. 1998;22(8):1864–71. [PubMed] [Google Scholar]
- 10.Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcoholism Clin Exp Res. 1999;23(6):1044–51. [PubMed] [Google Scholar]
- 11.Clark CP, Gillin JC, Golshan S, et al. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;43:601–07. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- 12.Gillin JC, Smith TL, Irwin M, et al. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51:189–97. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- 13.The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Heath Organization; 1992. [Google Scholar]
- 14.ICD-11 Beta Draft. Geneva: World Health Organization; 2016. [Last accessed 6 December 2017]. Available at: https://icd.who.int/dev11/l-m/en. [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 16.Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA. 1986;256(11):1449–55. [PubMed] [Google Scholar]
- 18.Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749–58. doi: 10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 19.Volpicelli JR, Rhines KC, Volpicelli LA, et al. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54(8):737–42. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- 20.Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J Addict Med. 2007;1(3):115–25. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- 21.Spanagel R, Vengeliene V, Jandeleit B, et al. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology. 2014;39(4):783–91. doi: 10.1038/npp.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason BJ, Heyser CJ. Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets. 2010;9(1):23–32. doi: 10.2174/187152710790966641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann K, Hoffmann S, Pawlak CR. Does acamprosate really produce its anti-relapse effects via calcium? No support from the PREDICT study in human alcoholics. Neuropsychopharmacology. 2016;41(3):659–60. doi: 10.1038/npp.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2012;108:275–93. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- 26.Mark TL, Kassed CA, Vandivort-Warren R, et al. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99(1–3):345–9. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins FS. Mining for therapeutic gold. Nature Rev Drug Discov. 2011;10:397. doi: 10.1038/nrd3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Placidi F, Diomedi M, Scalise A, et al. Effect of anticonvulsants on nocturnal sleep in epilepsy. Neurology. 2000;54(5 Suppl 1):S25–32. [PubMed] [Google Scholar]
- 29.Ehrenberg B. Importance of sleep restoration in co-morbid disease: effect of anticonvulsants. Neurology. 2000;54(5 Suppl 1):S33–7. [PubMed] [Google Scholar]
- 30.Mehta N, Bucior I, Bujanover S, et al. Relationship between pain relief, reduction in pain-associated sleep interference, and overall impression of improvement in patients with postherpetic neuralgia treated with extended-release gabapentin. Health Qual Life Outcomes. 2016;14:54. doi: 10.1186/s12955-016-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogan RK, Lee DO, Buchfuhrer MJ, et al. Treatment response to sleep, pain, and mood disturbance and their correlation with sleep disturbance in adult patients with moderate-to-severe primary restless legs syndrome: Pooled analyses from 3 trials of gabapentin enacarbil. Annals of Medicine. 2015;47(3):269–77. doi: 10.3109/07853890.2015.1025825. [DOI] [PubMed] [Google Scholar]
- 32.Canafax DM, Bhanegaonkar A, Bharmal M, Calloway M. Validation of the post sleep questionnaire for assessing subjects with restless legs sydrome: results from two double-blind, multicenter, placebo-controlled clinical trials. BMC Neurology. 2011;11:48. doi: 10.1186/1471-2377-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bazil CW, Battista J, Basner RC. Gabapentin improves sleep in the presence of alcohol. J Clin Sleep Med. 2005;1(3):284–7. • Polysomnography study of gabapentin effects on alcohol-induced sleep disturbance. [PubMed] [Google Scholar]
- 34.Mowla A, Ahmadzadeh L, Jahromi LR, Dastgheib SA. Comparing gabapentin with clonazepam for residual sleeping problems following antidepressant therapy in patients with major depressive disorder: a randomized clinical trial. Clin Drug Investig. 2015;35:513–7. doi: 10.1007/s40261-015-0304-8. [DOI] [PubMed] [Google Scholar]
- 35.Furey SA, Hull SG, Leibowitz MT, et al. A randomized, double-blind, placebo-controlled, multicenter, 28-day, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1101–09. doi: 10.5664/jcsm.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093–1100. doi: 10.5664/jcsm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo H-S, Yang C-M, Lo HG, et al. Treatment effects of gabapentin for primary insomnia. Clin Neuropharm. 2010;33:84–90. doi: 10.1097/WNF.0b013e3181cda242. [DOI] [PubMed] [Google Scholar]
- 38.Yurchesen ME, Guttuso T, McDermott M, et al. Effects of gabapentin on sleep in menopausal women with hot flashes as measured by a Pittsburgh Sleep Quality Index factor scoring model. J Women’s Health. 2009;18(9):1355–60. doi: 10.1089/jwh.2008.1257. [DOI] [PubMed] [Google Scholar]
- 39.Hamner MB, Brodrick PS, Labbate LA. Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psych. 2001;13(3):141–6. doi: 10.1023/a:1012281424057. [DOI] [PubMed] [Google Scholar]
- 40.Lavigne JE, Mustian K, Mathew JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300mg versus 900mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):479–86. doi: 10.1007/s10549-012-2251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasmin S, Carpenter LL, Leon Z, et al. Adjunctive gabapentin in treatment-resistant depression: a retrospective chart review. J Affect Disord. 2001;63(1):243–7. doi: 10.1016/s0165-0327(00)00187-7. [DOI] [PubMed] [Google Scholar]
- 42.Harden CL, Lazar LM, Pick LH, et al. A beneficial effect on mood in partial epilepsy patients treated with gabapentin. Epilepsia. 1999;40(8):1129–34. doi: 10.1111/j.1528-1157.1999.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 43.Mancino MJ, McGaugh J, Chopra MP, et al. Clinical efficacy of sertraline alone and augmented with gabapentin in recently abstinent cocaine-dependent patients with depressive symptoms. J Clin Psychopharmacol. 2014;34(2):234–9. doi: 10.1097/JCP.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacol. 2012;37:1689–98. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salehi M, Kheirabadi GR, Maracy MR, Ranjkesh M. Importance of gabapentin dose in treatment of opioid withdrawal. J Clin Psychopharmacol. 2011;31(5):593–6. doi: 10.1097/JCP.0b013e31822bb378. [DOI] [PubMed] [Google Scholar]
- 46.Kheirabadi GR, Ranjkesh M, Maracy MR, Salehi M. Effect of add-on gabapentin on opioid withdrawal symptoms in opium-dependent patients. Addiction. 2008;103:1495–9. doi: 10.1111/j.1360-0443.2008.02248.x. [DOI] [PubMed] [Google Scholar]
- 47.Perugi G, Toni C, Frare F, et al. Effectiveness of adjunctive gabapentin in resistant bipolar disorder: is it due to anxious-alcohol abuse comorbidity? J Clin Psychopharmacol. 2002;22(6):584–91. doi: 10.1097/00004714-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Wang PW, Santosa C, Schumacher M, et al. Gabapentin augmentation therapy in bipolar depression. Bipolar Disorders. 2002;4:296–301. doi: 10.1034/j.1399-5618.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- 49.Biyik Z, Solak Y, Atalay H, et al. Gabapentin versus pregabalin in improving sleep quality and depression in hemodialysis patients with peripheral neuropathy: a randomized prospective crossover trial. Int Urol Nephrol. 2013;45:831–7. doi: 10.1007/s11255-012-0193-1. [DOI] [PubMed] [Google Scholar]
- 50.Roberto M, Gilpin NW, O’Dell LE, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28(22):5762–71. doi: 10.1523/JNEUROSCI.0575-08.2008. •• Seminal study of the mechanism of action of gabapentin in alcohol dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108–13. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Xiao W, Boroujerdi A, Bennet GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-s-delta-1 calcium channel subunit. Neuroscience. 2007;144(2):714–20. doi: 10.1016/j.neuroscience.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uchitel OD, Di Giulmi MN, Urbano FJ, Gonzalez-Inchauspe C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels (Austin) 2010;4(6):490–6. doi: 10.4161/chan.4.6.12864. [DOI] [PubMed] [Google Scholar]
- 54.Fink K, Meder W, Dooley DJ, Göthert M. Inhibition of neuronal Ca(2+) influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Br J Pharmacol. 2000;130(4):900–06. doi: 10.1038/sj.bjp.0703380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunningham MO, Woodhall GL, Thompson SE, et al. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci. 2004;20(6):1566–76. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- 56.Taylor CP, Gee NS, Su TZ, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29(3):233–49. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 57.Götz E, Feuerstein TJ, Lais A, Meyer DK. Effects of gabapentin on release of gamma-aminobutyric acid from slices of rat neostriatum. Arzneimittelforschung. 1993;43(6):636–8. [PubMed] [Google Scholar]
- 58.Bertrand S, Ng GY, Purisai MG, et al. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain gamma-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channels. J Pharmacol Exp Ther. 2001;298(1):15–24. [PubMed] [Google Scholar]
- 59.Jensen AA, Mosbacher J, Elg S, et al. The anticonvulsant gabapentin (neurontin) does not act through gamma-aminobutyric acid-B receptors. Mol Pharmacol. 2002;61(6):1377–84. doi: 10.1124/mol.61.6.1377. [DOI] [PubMed] [Google Scholar]
- 60.Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993;10:276–81. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- 61.Neurontin (gabapentin capsule; gabapentin tablet, film coated; gabapentin solution) human prescription drug label information. [Last accessed 6 December 2017];Parke-Davis Division of Pfizer Inc. 2016 Available at: https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388&type=pdf&name=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388.
- 62.Roberto M, Cruz MT, Gilpin NW, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67(9):831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–92. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katsura M, Shibasaki M, Hayashida S, et al. Increase in expression of alpha1 and alpha2/delta1 subunits of L-type high voltage-gated calcium channels after sustained ethanol exposure in cerebral cortical neurons. J Pharmacol Sci. 2006;102(2):221–30. doi: 10.1254/jphs.fp0060781. [DOI] [PubMed] [Google Scholar]
- 65.Farris SP, Arasappan D, Hunicke-Smith S, et al. Transcriptome organization for chronic alcohol abuse in human brain. Mol Psychiatry. 2015;20(11):1438–47. doi: 10.1038/mp.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend. 2006;83(1):25–32. doi: 10.1016/j.drugalcdep.2005.10.008. •• Seminal PK/PD alcohol-gabapentin interaction study. [DOI] [PubMed] [Google Scholar]
- 67.Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcoholism Clin Exp Res. 2007;31(2):221–7. doi: 10.1111/j.1530-0277.2006.00299.x. • Supports safety of alcohol administration in conjunction with gabapentin treatment. [DOI] [PubMed] [Google Scholar]
- 68.Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Bio. 2009;14(1):73–83. doi: 10.1111/j.1369-1600.2008.00133.x. • Effects of gabapentin in human laboratory model of protracted alcohol abstinence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karem-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry. 2000;157(1):151. doi: 10.1176/ajp.157.1.151. • Case reports of gabapentin effects on alcohol-related insomnia. [DOI] [PubMed] [Google Scholar]
- 70.Karem-Hage M, Brower KJ. An Open Pilot Study of Gabapentin vs. Trazodone to Treat Insomnia in Alcoholic Outpatients. Psychiatry Clin Neurosci. 2003;57(5):542–4. doi: 10.1046/j.1440-1819.2003.01161.x. • Comparison of gabapentin and trazodone for alcohol-related insomnia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429–38. doi: 10.1111/j.1530-0277.2008.00706.x. • Effects of gabapentin on alcohol use and sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691–1700. doi: 10.4088/jcp.v68n1108. •• Seminal study of gabapentin treatment of alcohol dependence. [DOI] [PubMed] [Google Scholar]
- 73.Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence. A randomized clinical trial. JAMA Intern Med. 2014;174(1):70–7. doi: 10.1001/jamainternmed.2013.11950. •• Seminal study of gabapentin treatment of alcohol dependence and symptoms of protracted abstinence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cundy KC, Annamalai T, Bu L, et al. XP13512 [(+/−)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311(1):324–33. doi: 10.1124/jpet.104.067959. [DOI] [PubMed] [Google Scholar]
- 75.Anton RF, Myrick H, Baros AM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29(4):334–42. doi: 10.1097/JCP.0b013e3181aba6a4. [DOI] [PubMed] [Google Scholar]
- 76.Anton RF, Myrick H, Wright TM, et al. Gabapentin Combined with Naltrexone for the Treatment of Alcohol Dependence. Am J Psychiatry. 2011;168(7):709–17. doi: 10.1176/appi.ajp.2011.10101436. • Comparison of gabapentin combined with naltrexone vs. naltrexone alone vs. placebo for alcohol dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addiction. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. • A scale for the systematic assessment of alcohol withdrawal severity. [DOI] [PubMed] [Google Scholar]
- 78.Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focu on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1106–17. doi: 10.1016/j.pnpbp.2007.09.021. • Review includes gabapentin as a treatment for acute alcohol withdrawal. [DOI] [PubMed] [Google Scholar]
- 79.Hammond CJ, Niciu MJ, Drew S, et al. Anticonvulsants for the Treatment of Alcohol Withdrawal Syndrome and Alcohol Use Disorders. CNS Drugs. 2015;29:293–311. doi: 10.1007/s40263-015-0240-4. •Review includes gabapentin as a treatment for acute alcohol withdrawal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leung JG, Hall-Flavin D, Nelson S, et al. Role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897–906. doi: 10.1177/1060028015585849. •Review of gabapentin as a treatment for acute alcohol withdrawal. [DOI] [PubMed] [Google Scholar]
- 81.Myrick H, Malcolm R, Randall PK, et al. A double blind trial of gabapentin vs. lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582–8. doi: 10.1111/j.1530-0277.2009.00986.x. •Comparison of gabapentin and lorazepam for acute alcohol withdrawal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trevisan LA, Ralevski E, Keegan K, et al. Alcohol detoxification and relapse prevention using valproic acid versus gabapentin in alcohol-dependent patients. Addiction Disorders and Their Treatment. 2008;7(3):119–28. [Google Scholar]
- 83.How gabapentin differs from pregabalin. Pharmacy Times. Cranbury, NJ: Pharmacy & Healthcare Communications, LLC; 2015. [Last accessed 6 December 2017]. Available at: http://www.pharmacytimes.com/contributor/jeffrey-fudin/2015/09/how-gabapentin-differs-from-pregabalin. [Google Scholar]
- 84.Di Nicola M, Martinotti G, Tedeschi D, et al. Pregabalin in outpatient detoxification of subjects with mild-to-moderate alcohol withdrawal syndrome. Hum Psychopharmacol Clin Exp. 2010;25:268–75. doi: 10.1002/hup.1098. [DOI] [PubMed] [Google Scholar]
- 85.Martinotti G, Di Nicola M, Frustaci A, et al. Pregabalin, tiapride and lorazepam in alcohol withdrawal syndrome: a multi-centre, randomized, single-blind comparison trial. Addiction. 2010;105:288–99. doi: 10.1111/j.1360-0443.2009.02792.x. [DOI] [PubMed] [Google Scholar]
- 86.Becker HC, Myrick H, Veatch LM. Pregabalin is effective against behavioral and electrographic seizures during alcohol withdrawal. Alcohol Alcohol. 2006;41(4):399–406. doi: 10.1093/alcalc/agl029. [DOI] [PubMed] [Google Scholar]
- 87.Martinotti G, Di Nicola M, Tedeschi D, et al. Efficacy and safety of pregabalin in alcohol dependence. Adv Ther. 2008;25(6):608–18. doi: 10.1007/s12325-008-0066-2. [DOI] [PubMed] [Google Scholar]
- 88.Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharm. 2010;24(9):1367–74. doi: 10.1177/0269881109102623. [DOI] [PubMed] [Google Scholar]
- 89.Häkkinen M, Vuori E, Kalso E, et al. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Sci Int. 2014;241:1–6. doi: 10.1016/j.forsciint.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 90.Schjerning O, Pottegård A, Damkier P, et al. Use of pregabalin – a nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry. 2016;49(4):155–61. doi: 10.1055/s-0042-101868. [DOI] [PubMed] [Google Scholar]
- 91.Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J Clin Psychiatry. 2001;62:19–23. doi: 10.4088/jcp.v62n0105. [DOI] [PubMed] [Google Scholar]
- 92.Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84–6. doi: 10.4088/jcp.v65n0114. [DOI] [PubMed] [Google Scholar]
- 93.Hart CL, Haney M, Voxburg SK, et al. Gabapentin does not reduce smoked cocaine self-administration: employment of a novel self-administration procedure. Behav Pharmacol. 2007;18(1):71–5. doi: 10.1097/FBP.0b013e328014139d. [DOI] [PubMed] [Google Scholar]
- 94.González G, Desai R, Sofuoglu M, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87(1):1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2005;81(3):267–74. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 96.Urschel HC, 3rd, Hanselka LL, Gromov I, et al. Open-label study of a proprietary treatment program targeting type A gamma-aminobutyric acid receptor dysregulation in methamphetamine dependence. Mayo Clin Proc. 2007;82(10):1170–8. doi: 10.4065/82.10.1170. [DOI] [PubMed] [Google Scholar]
- 97.Urschel HC, 3rd, Hanselka LL, Baron M. A controlled trial of flumazenil and gabapentin for initial treatment of methylamphetamine dependence. J Psychopharmacol. 2011;25(2):254–62. doi: 10.1177/0269881109349837. [DOI] [PubMed] [Google Scholar]
- 98.Ling W, Shoptaw S, Hillhouse M, et al. Double-blind placebo-controlled evaluation of the PROMETA™ protocol for methamphetamine dependence. Addiction. 2012;107(2):361–9. doi: 10.1111/j.1360-0443.2011.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martínez-Raga J, Sabater A, Perez-Galvez B, et al. Add-on gabapentin in the treatment of opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):599–601. doi: 10.1016/j.pnpbp.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 100.Kumar P, Jain MK. Gabapentin in the management of pentazocine dependence: a potent analgesic-anticraving agent. J Assoc Physicians India. 2003;51:673–6. [PubMed] [Google Scholar]
- 101.Ziaaddini H, Ziaaddini A, Asghari N, et al. Trial of tramadol plus gabapentin for opioid detoxification. Iran Red Crescent Med J. 2015;17(1):e18202. doi: 10.5812/ircmj.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodríguez de Fonseca F, Carrera MR, Navarro M, et al. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276(5321):2050–4. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 103.Salinsky MC, Storzbach D, Spencer DC, et al. Effects of topirimate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64(5) doi: 10.1212/01.WNL.0000152877.08088.87. pp-pp, supplement tables E1 and E2. [DOI] [PubMed] [Google Scholar]
- 104.Freynhagen R, Backonja M, Schug S, et al. Pregabalin for the treatment of drug and alcohol withdrawal syndrome: a comprehensive review. CNS Drugs. 2016;30(12):1191–1200. doi: 10.1007/s40263-016-0390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bastiaens L, Galus J, Mazur C. Abuse of gabapentin is associated with opioid addiction. Psychiatr Q. 2016;87(4):763–7. doi: 10.1007/s11126-016-9421-7. [DOI] [PubMed] [Google Scholar]
- 106.Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647–54. doi: 10.1007/s40263-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 107.Wilens T, Zulauf C, Ryland D, et al. Prescription medication misuse among opioid dependent patients seeking inpatient detoxification. Am J Addict. 2015;24(2):173–7. doi: 10.1111/ajad.12159. [DOI] [PubMed] [Google Scholar]
- 108.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–74. doi: 10.1111/add.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]