Summary
Our bodies are mostly water, and this water is constantly being lost through evaporative and other means. Thus the evolution of robust mechanisms for finding and consuming water has been critical for the survival of most animals. In this Primer, we discuss how the brain monitors the water content of the body and then transforms that physical information into the motivation to drink.
A physiologic system maintains fluid balance
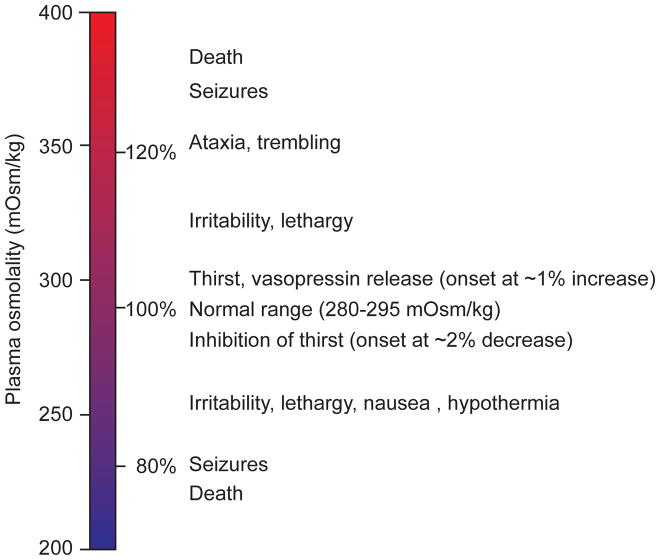
One of the most remarkable features of animals is their ability to sense their changing internal needs and then generate specific behavioral responses that restore homeostasis. A dramatic illustration of this phenomenon involves the regulation of fluid balance, where an increase in blood osmolality of as little as one percent can trigger the sensation of thirst (Figure 1). This sensation, in turn, is sufficient to orient and energize all of an animal’s actions toward the goal of finding and consuming water. Thus the study of thirst is the study of how the brain performs this remarkable transformation, such that small changes in the composition of the blood become a potent and specific motivational drive.
Figure 1.
Physiologic effects of deviations in plasma osmolality
Why are we so sensitive to small changes in fluid balance? One reason is that our cells are selectively permeable to certain ions, resulting in an electrochemical gradient across the plasma membrane that is exploited for numerous cellular functions. Changes in blood osmolality alter this gradient, changing intracellular water content and degrading normal cellular function. A second reason is that the blood is used as a vehicle to transport essential nutrients such as oxygen to distant sites in the body. As blood volume decreases, increased work is required to maintain the minimum blood pressure necessary for adequate circulation.
For these and other reasons, a physiologic system has evolved to maintain fluid balance. The key components of this system are specialized neurons that monitor the osmolality and volume of the blood and, when necessary, trigger a coordinated set of autonomic, neuroendocrine, and behavioral responses that defend these parameters against change. Many of these responses function at the level of the kidney, by modulating the rate at which water and salt are lost through excretion. However, there is a limit to the effectiveness of these renal mechanisms, because toxic substances must be periodically removed from the circulation through urination, and furthermore because sweating and other evaporative mechanisms cause continual loss of water and salt even in the absence of excretion. Thus at some point fluid balance must be restored by ingesting water.
Thirst is the motivation to find and consume water
Water is not always immediately available and finding water can be costly, since it may require performing work (e.g. foraging), taking risks (e.g. predation), or forgoing other opportunities (e.g. eating or mating). For this reason, water seeking and consumption have evolved to be motivated behaviors, meaning that animals are able to weigh their need for water against competing survival demands and then devise flexible behavioral strategies to meet these goals. The sensation of thirst plays a critical role in this process, by apprising animals on a moment by moment basis of their degree of water need.
Thirst motivates water seeking and consumption by both positive and negative valence mechanisms. Thirst positively reinforces drinking behavior by magnifying the rewarding sensory properties of water: a glass of water tastes wonderful when you are thirsty, but much less so when you are sated. At the same time, thirst negatively reinforces drinking behavior by virtue of the fact that thirst itself is an unpleasant state, and thus animals are motivated to consume water in order to eliminate this aversive feeling. These two motivational mechanisms act in unison to promote drinking behavior, but how they are instantiated in the brain remains unclear. Isolating the specific circuit elements responsible for these positive and negative valence signals is an active area of research.
Two kinds of dehydration trigger thirst
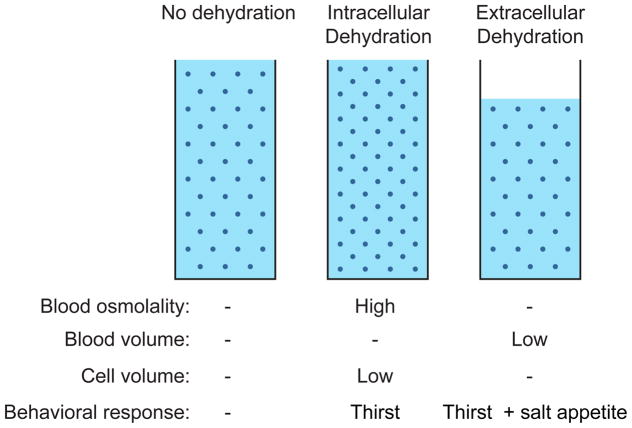
All water in the body is present either inside or outside of cells, and the loss of water from these two compartments triggers different behavioral responses (Figure 2). Intracellular dehydration refers to the loss of water from inside cells and is typically caused by an increase in blood osmolality, which draws water out of cells by osmosis and causes them to shrink. By contrast, extracellular dehydration refers to a decrease in the total blood volume, such as occurs during bleeding. Whereas intracellular dehydration can be corrected by drinking water alone, extracellular dehydration requires consumption of both water and salt in order to regenerate the blood at its correct osmolality. For this reason extracellular dehydration triggers not only thirst but also salt appetite.
Figure 2.
Intracellular versus extracellular dehydration.
Many stimuli that trigger thirst cause both intracellular and extracellular dehydration simultaneously. For example, sweating causes extracellular dehydration, because it reduces the volume of water available to the blood and interstitial fluids. Sweating also causes intracellular dehydration, because sweat is less salty than the blood and therefore leaves behind excess salt that increases blood osmolality. Thus these two mechanisms often go hand in hand in normal circumstances. Nevertheless, it is useful to think about these two types of dehydration separately, because the brain utilizes distinct mechanisms for detecting them.
Osmolality is sensed directly by the brain
Changes in blood osmolality correlate well with the subjective feeling of thirst in humans, and increased blood osmolality is probably the most important homeostatic signal for drinking in everyday life. When defense of blood osmolality and volume are placed in conflict – such as when the blood volume and osmolality increase simultaneously – the body prioritizes defense of osmolality, suggesting that blood hypertonicity is the more immediate threat to survival under most circumstances.
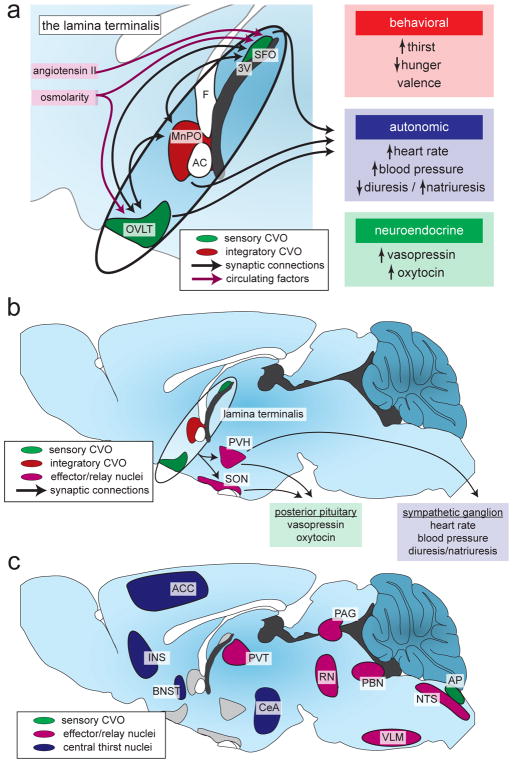
Changes in blood osmolality are detected by two small structures in the forebrain known as the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT) (Figure 3). These two structures are located outside the blood-brain-barrier and therefore have enhanced access to the circulation. Specialized neurons in these two brain regions are activated by increases in blood osmolality, and their activation is necessary and sufficient for generation of thirst. It is thought that these SFO and OVLT neurons monitor the blood osmolality directly, possibly via stretch-sensitive ion channels embedded in their plasma membranes that detect changes in cell volume following intracellular dehydration. However the identity of the specific ion channel or other protein responsible for osmosensing by these neurons is unknown. Furthermore, the possibility cannot be excluded that other cell types, such as glia, play an important role in osmosensation. Identification of the molecular mechanism by which the brain detects changes in blood osmolality would be an important advance in this field.
Figure 3. The neural circuit that controls thirst.
(A) A set of integrated brain structures called the lamina terminalis monitors the state of the blood and generates appropriate motivational, autonomic, and hormonal responses. The subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT) directly sense circulating factors and, along with the median preoptic nucleus (MnPO), integrate this information and communicate with downstream brain regions. 3V, third ventricle; AC, anterior commissure; F, fornix.
(B) The downstream circuit that controls the autonomic and hormonal responses to fluid imbalance relies on projections from the lamina terminalis to the paraventricular nucleus of the hypothalamus (PVH) and the supraoptic nucleus (SON).
(C) The downstream circuit that generates thirst is less clear, although many brain regions have been implicated in the central representation of thirst and as relay and effector/output nuclei. These brain regions were identified using Fos mapping studies in rodents and fMRI/PET mapping studies in humans. ACC, anterior cingulate cortex; AP, area postrema; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; INS, insular cortex; NTS, nucleus tractus solitarii; PAG, periaqueductal gray; PBN, parabrachial nucleus; PVT, paraventricular nucleus of the thalamus; RN, raphe nuclei; VLM, ventrolateral motor nucleus.
Extracellular dehydration is communicated in part by angiotensin
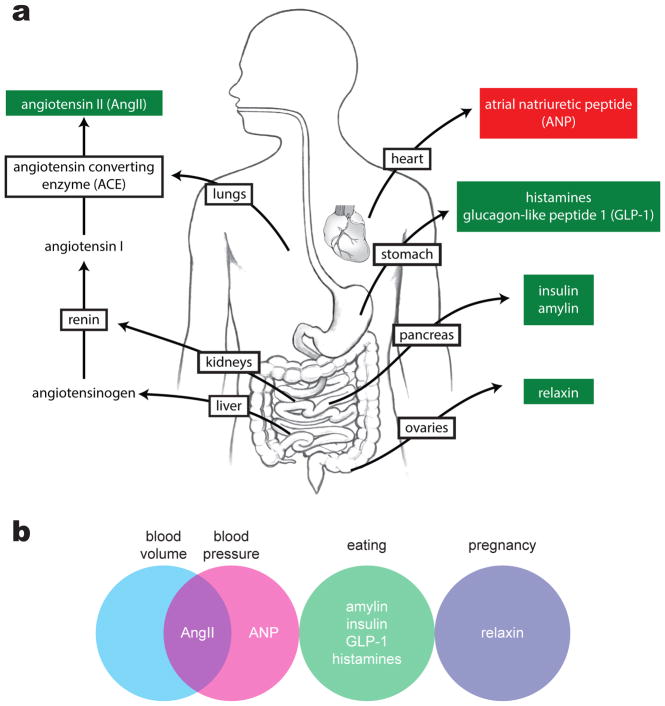
Extracellular dehydration is manifest by a decrease in blood volume (hypovolemia), which is typically accompanied by a decrease in blood pressure (hypotension). One of the most important responses to hypovolemia and hypotension is generation of the peptide hormone angiotensin II (ANGII) (Figure 4). ANGII is produced through a multistep mechanism in which a precursor, angiotensinogen, is secreted into the bloodstream by the liver. When specialized cells in the kidneys detect low blood pressure, they release an enzyme called renin that cleaves circulating angiotensinogen to create angiotensin I (ANGI). ANGI is cleaved again by angiotensin converting enzyme to form ANGII. ANGII has multiple physiologic effects, including acting in the brain to promote drinking and salt consumption and acting in the periphery to constrict blood vessels and promote water reuptake by the kidneys.
Figure 4. Hormonal stimuli for thirst.
(A) The most potent hormonal stimulus for thirst is angiotensin II (AngII), which is generated when the rate-limiting enzyme renin is secreted by the kidneys in response to hypovolemia or hypotension. Other hormonal stimuli for thirst are secreted by the stomach and pancreas during eating, as well as by the ovaries during pregnancy. Atrial natriuretic peptide, a potent inhibitor of thirst, is secreted by the heart in response to hypertension.
(B) The physiological stimuli that induce secretion of thirst-related hormones include changes in plasma volume and pressure, as well as eating and pregnancy. Decreases in blood volume and pressure increase levels of the dipsogenic hormone AngII, whereas increases in blood volume increase levels of the thirst-inhibiting hormone ANP.
The SFO is the principal site in the brain where ANGII acts to promote drinking behavior (Figure 3a). In rodents, centrally delivered ANGII stimulates profound water consumption, and this dramatic drinking response is commonly used as a positive control to verify the correct placement of intracerebroventricular cannulae. However in humans ANGII levels do not correlate well with the perception of thirst, and infusions of physiologic levels of ANGII do not stimulate drinking. While this suggests that ANGII might be less important for regulation of drinking in humans, interpretation of these negative results is complicated by the fact that peripheral infusion of ANGII rapidly increases blood pressure, which can then feed back to counteract any effects of ANGII on thirst.
Extracellular dehydration is also detected by baroreceptors
Blood pressure is also monitored by stretch-sensitive mechanoreceptor neurons called baroreceptors that innervate blood vessels and the heart. Baroreceptors respond to decreases or increases in blood pressure by inducing or inhibiting thirst, respectively, through their projections via cranial nerves IX and X to a brainstem region called the nucleus of the solitary tract. In addition, baroreceptors initiate a brainstem-mediated response called the baroreflex, in which they counteract acute changes in blood pressure by controlling autonomic nervous system activity in the heart to alter cardiac output. However, much remains unclear about baroreceptor signaling and thirst, including the molecular mechanism of baroreceptors’ stretch sensitivity and their importance relative to the renin-angiotensin system during hypovolemia. In addition, sensory neurons in the kidneys may also monitor blood pressure and affect thirst under some circumstances, though the mechanisms involved are poorly defined.
Dehydration triggers a coordinated homeostatic response
The brain processes these signals and then activates a coordinated set of behavioral, autonomic, and neuroendocrine responses that restore fluid balance. As described above, the SFO and OVLT are the primary sites that detect increases in osmolality and ANGII. The SFO and OVLT are highly interconnected with each other and a third brain region that is located between them, the median preoptic nucleus (MnPO). These three regions together comprise the lamina terminalis, which represents a forebrain hub that processes information about fluid balance (Figure 2b). Activation of these regions is sufficient to trigger multiple homeostatic responses, including the generation of thirst, activation of the sympathetic nervous system to increase blood pressure, and release of the hormones vasopressin (AVP) and oxytocin (OXT).
The neural pathways that lead from activation of the lamina terminals to sympathetic activation and hormone release are relatively well understood. The lamina terminalis and autonomic nervous system are separated by just two synapses: excitatory neurons in the lamina terminalis project to neurons in the paraventricular hypothalamus (PVH) which in turn sends descending projections to autonomic regions of the hindbrain and spinal cord (Figure 3b). Sympathetic nervous system activation in response to dehydration results in vasoconstriction, increased water reuptake in the kidneys, and renin release leading to ANGII production. Excitatory lamina terminalis neurons also synapse directly on neurons in the PVH and supraoptic nucleus (SON) that release AVP and OXT into the bloodstream via their axon terminals in the posterior pituitary gland (Figure 3b). AVP and OXT affect fluid balance in large part through their effects on the kidney, where they promote water retention.
In contrast to these well-established autonomic and neuroendocrine pathways, the neural circuits responsible for the generation of thirst remain poorly defined. While thirst is thought to originate within the lamina terminalis, it remains unknown how the lamina terminalis connects to downstream brain regions important for motivation, learning, and other aspects of behavior. In this regard, many brain regions spanning the cortex, hypothalamus, thalamus, amygdala, midbrain and hindbrain have been shown to either receive axonal projections from the lamina terminalis or have been functionally connected to thirst in other ways (Figure 3c). Among these, activity in cingulate cortex has been shown to correlate especially well with the feeling of thirst in functional magnetic resonance imaging (fMRI) studies in humans. An important task for the future will be to elucidate the neural pathway that connects the lamina terminalis with these downstream structures and then define their specific functional roles (Figure 3c).
Thirst can also be regulated by anticipatory signals
Thus far, we have characterized thirst as a purely homeostatic response to deviations in the blood osmolality or volume. However, a substantial fraction of normal drinking behavior is not regulated by changes in the blood directly, and instead appears to anticipate those changes before they occur. This anticipatory, or pre-systemic, thirst enables the regulation of drinking behavior in a way that prevents the development of potentially dangerous physiologic imbalances. In the following sections, we discuss two examples of anticipatory signals that can regulate thirst.
Drinking quenches thirst in anticipation of water absorption
There is a delay of tens of minutes between the ingestion of water and its full absorption into the bloodstream. However drinking can quench thirst within seconds, long before the ingested water has had time to alter the blood volume or osmolality. Thus thirst is not quenched by the reverse of the process that generates it. Instead, the brain terminates thirst by using sensory cues from the oropharynx to track ongoing water consumption and then estimate how this water intake will alter fluid balance in the future, after the water has been absorbed. These anticipatory signals are transmitted from the oral cavity to the SFO, where they inhibit the same thirst neurons that respond to change in the blood volume and osmolality. This circuit organization allows SFO thirst neurons make a comparison between the physiologic need for water, which they measure directly by monitoring the blood, and the amount of water that has recently been consumed, which they measure by tracking oropharyngeal signals of fluid intake. SFO thirst neurons then compare these two values to decide when drinking should be terminated. It is likely that a similar integration occurs within other structures of the lamina terminalis that control drinking behavior and hormone release.
The specific oropharyngeal mechanisms that are used to track water consumption are not well defined. One signal appears to be temperature, since cold liquids inhibit SFO thirst neurons more efficiently than warm liquids, and oral cooling alone can reduce both thirst and the activity of these SFO cells. One explanation for this temperature-dependence is that water ingestion tends to cool the oropharynx, and as a result animals may learn to associate changes in oral temperature with the post-ingestive effects of water consumption. In addition to temperature, other somatosensory signals that report on the sensation of water in the oral cavity are likely to be important. There is also evidence that signals from further down the gastrointestinal tract, such as stretch receptors and osmosensors in the stomach, may play a role in thirst satiation. However in all cases the identity of the relevant sensory neurons and the neural pathway by which they transmit information to the lamina terminalis remains nebulous.
Eating generates thirst in anticipation of food absorption
Eating is an important stimulus for thirst in many animals. In rodents, water consumption is distributed across numerous small meals throughout the dark cycle (subjective daytime), whereas in humans drinking is typically concentrated around a few large meals. Eating increases the need for water for two reasons: (1) in order to replace the fluid utilized for swallowing (e.g. saliva) and digestion (e.g. water diverted from the circulation into the gastrointestinal tract), and (2) in order to counteract the increase in blood osmolality caused by the absorption of salts and other osmolytes from food. In principle, meal-associated (prandial) thirst could be secondary to changes in the blood that result from these water demands imposed by food digestion and absorption. However this does not seem to be the case, since prandial thirst develops before any change in blood osmolality or volume can occur. Instead, the act of eating itself triggers thirst, in a way that anticipates the impending homeostatic burden imposed by ongoing food consumption. As a result, animals drink at the same time that they eat, and changes in blood osmolality and volume are mitigated.
Eating activates the same thirst neurons in the lamina terminalis that monitor blood osmolality and volume, and activation of these neurons is necessary and sufficient for prandial thirst. However, as with thirst quenching, the mechanism by which anticipatory signals about ongoing food ingestion are communicated to the lamina terminalis remains unclear. Possible mechanisms include somatosensory signals from the oral cavity that report on food swallowing or its effects on the saliva; osmosensory signals from the gastrointestinal tract, portal vein, or other organs that report on the early effects of food ingestion on the osmolality of internal fluids; and learned associations between food consumption and its eventual effects on the blood that enable animals use arbitrary sensory cues to anticipate their water needs. In addition to these neural mechanisms, several hormones associated with eating and satiety have been proposed to modulate thirst neurons, including amylin, cholecystokinin (CCK), ghrelin, glucagon-like peptide-1 (GLP-1), histamines, insulin, and leptin. It is possible that one or more of these circulating nutritional signals is also critical for the regulation of prandial thirst.
If prandial thirst is not quenched by drinking, then further food consumption is reduced, a phenomenon known as dehydration-induced anorexia. This is a general phenomenon, since any kind of experimentally induced dehydration can inhibit food intake. Dehydration anorexia requires activation of thirst neurons in the SFO, since silencing of these cells can rescue food intake in the absence of water, and it involves a decrease in meal size but not meal number, indicating that it affects primarily meal termination. An important area for future research will be to identify the points of convergence between the neural circuits that control hunger and thirst, so that these and other mechanisms by which eating and drinking are coordinated can be more clearly defined. It will also be important to investigate how these behaviors are influenced by other signals that can modulate thirst, such as changes in circadian rhythms, thermoregulation, and pregnancy.
Animals use diverse strategies for achieving fluid balance
In this Primer we have focused on thirst and fluid balance from the perspective of human physiology and the rodent model systems that are used to study it. However it is important to emphasize that these mechanisms that control drinking behavior are not universally conserved, and a wide variety of strategies have evolved that enable animals to maintain fluid balance. For example, while prandial drinking is common in humans and rodents, many grazing mammals eat nearly continuously but drink only a few times each day. Other species, such as many amphibians, do not need to drink at all because they are able to absorb water percutaneously, whereas most desert animals get water exclusively by consuming water-rich foods. Thus while blood osmolality and volume are tightly regulated across much of the animal kingdom, different species have evolved unique strategies to achieve this goal.
Future directions for thirst research
We have tried to emphasize in this Primer not only what is known about thirst, but also where there remain important gaps in our understanding. Many of these gaps surround the identity of the specific molecules, cell types, and circuits that act as sensors or effectors in the control of drinking behavior. The availability of genetically targeted methods for monitoring and manipulating neural circuits is likely to enable rapid progress toward addressing these questions in the near future.
Acknowledgments
D.E.L. acknowledges support from an NIH NRSA predoctoral fellowship (F31-HL131463) and a UCSF Discovery Fellowship. C.A.Z. acknowledges support from a UCSF Discovery Fellowship, a Genentech Foundation Predoctoral Fellowship, and an NSF Graduate Research Fellowship. Z.A.K. is a New York Stem Cell Foundation-Robertson Investigator. Z.A.K. acknowledges support from the New York Stem Cell Foundation, the American Diabetes Association Pathway Program, the Rita Allen Foundation, the McKnight Foundation, the Alfred P. Sloan Foundation, the Brain and Behavior Research Foundation, the Esther A. & Joseph Klingenstein Foundation, the Program for Breakthrough Biological Research, and the UCSF DERC (P30 DK06372) and NORC (P30DK098722). This work was supported by an NIH New Innovator Award (DP2-DK109533), R01-NS094781, and R01-DK106399.
FURTHER READING
- Antunes-Rodrigues J, De Castro M, Elias LLK, Valença MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. http://dx.doi.org/10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. http://dx.doi.org/10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. http://dx.doi.org/10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The physiological basis of thirst. Proc R Soc Lond B: Biol Sci. 1918;90:283–301. http://rspb.royalsocietypublishing.org/cgi/doi/10.1098/rspb.1918.0017. [Google Scholar]
- Fitzsimons JT. Thirst Physiol Rev. 1972;52:468–561. doi: 10.1152/physrev.1972.52.2.468. http://physrev.physiology.org/content/52/2/468. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. http://physrev.physiology.org/content/78/3/583. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. http://dx.doi.org/10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Kraly FS. Physiology of drinking elicited by eating. Psychol Rev. 1984;91:478–490. http://dx.doi.org/10.1037/0033-295X.91.4.478. [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Oldfield BJ, De Oliveira LB, Mathai ML. Water intake and the neural correlates of the consciousness of thirst. Semin Nephrol. 2006;26:249–257. doi: 10.1016/j.semnephrol.2006.02.001. http://dx.doi.org/10.1016/j.semnephrol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. http://dx.doi.org/10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Rolls BJ, Ledingham JGG, Morton JJ. Body fluid changes, thirst and drinking during free access to water. Physiol Behav. 1983;33:357–363. doi: 10.1016/0031-9384(84)90154-9. http://dx.doi.org/10.1016/0031-9384(84)90154-9. [DOI] [PubMed] [Google Scholar]
- Ramsay DJ, Booth DA. Thirst: physiological and Psychological Aspects. London: Springer-Verlag; 1991. http://www.springer.com/gp/book/9781447118190. [Google Scholar]
- Sterns RH. Disorders of plasma sodium: causes, consequences, and correction. N Engl J Med. 2015;372:55–65. doi: 10.1056/NEJMra1404489. http://dx.doi.org/10.1056/nejmra1404489. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Hoffmann ML. Presystemic signals in the control of thirst, salt appetite, and vasopressin secretion. Physiol Behav. 2007;91:404–412. doi: 10.1016/j.physbeh.2007.04.007. http://dx.doi.org/10.1016/j.physbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, Knight ZA. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016;537:860–864. doi: 10.1038/nature18950. http://dx.doi.org/10.1038/nature18950. [DOI] [PMC free article] [PubMed] [Google Scholar]