Abstract
Prevention of lower extremity fluid pooling (LEFP) is associated with improved sleep quality. Physical activity and compression stockings are non-invasive methods used to manage LEFP, but both are associated with low adherence. Calf muscle pump (CMP) stimulation is an alternative and more convenient approach. Convenience sampling was used to recruit 11 participants between ages 45 and 65 with poor sleep quality. A within-person single-group pre-test–post-test design was used to evaluate changes in sleep quality, daytime sleepiness, and functional outcomes sensitive to impaired sleep as measured by the Pittsburgh Sleep Quality Index (PSQI), Functional Outcomes of Sleep Questionnaire, and Epworth Sleepiness Scale after 4 weeks of CMP stimulation. Statistical analysis included effect size (ES) calculations. After daily use of CMP stimulation, participants demonstrated improvement in overall sleep quality (ES = = −.97) and a large reduction in daily disturbance from poor sleep (ES = −1.25). Moderate improvements were observed in daytime sleepiness (ES = −.53) and functional outcomes sensitive to sleepiness (ES = .49). Although causality could not be determined with this study design, these results support further research to determine whether CMP stimulation can improve sleep quality.
Keywords: sleep, muscle stimulation, fluid retention, quality of life
From 50 to 70 million Americans chronically suffer from a sleep or circadian disorder (Institute of Medicine Committee on Sleep Medicine and Research, 2006). Manifestations of sleep disturbances include fatigue, daytime sleepiness, and reduced daytime function (Bliwise & Scullin, 2017). Impaired sleep quality can adversely affect physiological, psychological, and well-being, and disturbances in sleep and poor sleep quality have been linked to heart disease, chronic obstructive pulmonary disease, depression, and obesity (Bliwise & Scullin). Risk factors for impaired sleep include physiological stressors, such as sleep-disordered breathing and pain and psychological stressors, such as anxiety and stress.
Lower extremity fluid pooling (LEFP) has been associated with impaired sleep quality (Chiu et al., 2006; Redolfi, Arnulf, Pottier, Bradley, & Similowski, 2011; Redolfi et al., 2009; Shiota et al., 2007; Yumino et al., 2010). Fluid that accumulates in the lower extremities during the day redistributes toward the head during sleep, which quickly increases volume in low-pressure areas, such as the veins and interstitial spaces in the neck (Bradley & Floras, 2003; Shepard, Pevernagie, Stanson, Daniels, & Sheedy, 1996). Fluid pressure in the neck may collapse the upper airway, which predisposes individuals to sleep-disordered breathing (Bradley & Floras; Shepard et al.).
Novel methods to reduce daytime LEFP may alleviate poor sleep quality. Calf muscle (soleus) pumps (CMPs) facilitate venous and interstitial fluid return to reduce LEFP and represent a target for improving sleep quality. In healthy individuals, CMPs prevent excessive venous and lymphatic pooling in the lower extremities during upright posture (Padberg, 2001). However, up to 40% of adult women have inadequate CMP activity (based on N = 54; Goddard, Pierce, & McLeod, 2008). Furthermore, the prevalence of venous disease, such as chronic venous insufficiency (e.g., leg edema, lymphedema, skin changes, ulcerations, and/or pain), increases with age (Lee et al., 2015; Robertson et al., 2013). In the Edinburgh Vein Study of over 1,400 men and women in Scotland, adults ages 55–64 years were almost eight times more likely to develop chronic vein insufficiency than those ages 18–34 years (Robertson et al., 2013), which leads to LEFP.
LEFP is typically treated with leg elevation, compression stockings (Christopoulous et al., 1989; Rooke & Felty, 2001; Rowell, 1993), and exercise. Although wearing compression stockings improves lower extremity blood flow and reduced lower leg edema, much of the potential benefit of compression stockings is not achieved in practice due to low patient adherence. Low adherence has been linked to discomfort and high cost of compression stockings. These stockings also increase risk of developing exudative lesions of the lower legs from incorrect fit and improper use (Ziaja, Kocełak, Chudek, & Ziaja, 2011).
Although exercise prevents LEFP by improving CMP activity (Kan & Delis, 2001; Padberg, Johnston, & Sisto, 2004), many adults susceptible to LEFP have sedentary lifestyles and fail to engage in adequate physical activity. In fact, only about one in five adults meets the 2008 Physical Activity Guidelines for Americans endorsed by the American Heart Association (American Heart Association, 2016). These guidelines recommend that adults and older adults without limiting chronic conditions engage in 150 minutes per week (e.g., 30 minutes, 5 days per week) of at least moderate-intensity aerobic physical activity (e.g., walking briskly or general gardening), which should include moderate-intensity muscle-strengthening activities (e.g., lifting weights or climbing stairs) 2 or more days a week (American Heart Association, 2016).
An alternative approach to prevent LEFP during inactivity is exogenous CMP activation, which can be accomplished via stimulation of mechanoreceptors (Meissner’s corpuscles) on the plantar surface of the feet (Madhavan, Stewart, & McLeod, 2006). Mechanoreceptor activation in the frontal plantar surface leads to soleus muscle pump (CMP) contraction through a postural reflex arc, while mechanoreceptor activation in the heel relaxes the soleus muscles (Goddard et al., 2008). The plantar stimulation is achieved by placing the feet on a wedge-shaped device that vibrates at a specific frequency (see Fig. 1). We examined changes in sleep quality, daytime sleepiness, and functional outcomes related to impaired sleep after a 4-week intervention that featured CMP stimulation.
FIGURE 1.
Calf muscle pump stimulation device featured in this study (MM-100 Calf Muscle Pump Stimulator, Sonostics, Inc., Vestal, NY), which provides plantar (i.e., sole of the foot) micro-mechanical stimulation through vibration—specifically, a 45 Hz, 50 μm displacement of the frontal portion of the plantar surface.
Methods
Design and Sample
This study used a within-person, pre-test–post-test design. A convenience sample of community-dwelling participants with subjective poor sleep quality was recruited at Binghamton University via Dateline (a daily listserv of campus news and events directed to faculty and staff) and word of mouth. Participants eligible for inclusion were between the ages of 40 and 65 years and had poor sleep quality, defined as either frequent awakening during the night, difficulty initiating sleep, not feeling refreshed in the morning, or snoring. Because age is a risk factor for venous insufficiency (Robertson et al., 2013), and the rate of venous thromboembolism is substantially higher among adults over 65 (Johansson, Johansson, & Lind, 2014), participants older than 65 years were excluded. Additional exclusion criteria were nocturnal oxygen use, hypertension uncontrolled with medications, a history of deep vein thrombosis, current use of continuous positive airway pressure (CPAP), nighttime use of sleep aids, and pregnancy. A total of 21 participants responded to the advertisement and were screened over the telephone for eligibility using a screening script. Ten of these were excluded because of current CPAP use or inability to use the CMP stimulation device daily.
Eleven participants were enrolled; demographic characteristics are presented in Table 1. Based on the Berlin Questionnaire, more than half were at high risk for sleep apnea. Two of these had been diagnosed with obstructive sleep apnea (OSA) but were not using CPAP because of mask discomfort. The majority of participants were white, female, middle-aged, and obese. Self-reported medical diagnoses included hypertension (36%; n = 4), hypercholesterolemia (45%; n = 5), and hypothyroidism (45%; n = 5).
Table 1.
Participant Characteristics at Baseline (N=11)
| Characteristic | Mean ± SD | n (%) |
|---|---|---|
| Female | 6 (55) | |
| Married or partnered | 8 (73) | |
| Educational level high school or greater | 3 (27) | |
| Berlin questionnaire—high risk for OSAa | 7 (64) | |
| Age in years | 60 ± 2.0 | |
| Body mass index (kg/m2) | 30.3 ± 1.3 | |
| Neck circumference (cm) | 38.3 ± 1.3 |
Note. OSA, obstructive sleep apnea.
High risk equals two out of three positive subscores on the questionnaire.
Measures
Pittsburgh Sleep Quality Index (PSQI)
The PSQI measures subjective global sleep quality over the previous month. A PSQI score greater than 5 on a range of 0–21 indicates severe difficulties in at least two areas or moderate difficulties in more than three areas. The PSQI has had a diagnostic sensitivity of 89.6% and specificity of 86.5 and a reliability coefficient of 0.83. Test–retest reliability revealed no differences between time 1 and time 2 total scores. Seven component scores are used to quantify (1) the time it takes after deciding to fall asleep to actually initiate sleep (sleep latency); (2) time actually asleep (sleep duration); (3) the percentage of time in bed that is associated with being asleep (habitual sleep efficiency); (4) sleep disturbances (hot flashes, nocturia, and feeling cold); (5) the use of sleep medication; (6) sleep quality; and (7) daytime dysfunction (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The seven component scores are correlated at an average of 0.85 (p <.001; Buysse et al., 1989).
The PSQI three-factor scoring model was used to further assess sleep disturbance (Cole et al., 2006). Sleep Efficiency is the average of sleep duration and sleep efficiency, Perceived Sleep Quality is the average of subjective sleep quality, sleep latency, and use of sleep medication, and Daily Disturbance is the average of sleep disturbance and daytime dysfunction.
Epworth Sleepiness Scale (ESS)
The ESS is a subjective measure of daytime sleepiness. Participants rate their tendency to become sleepy in eight situations on a 0–3 scale, where 0 = no chance of dozing and 3 = high chance of dozing. Excessive sleepiness is indicated by total score of 10 or greater. Total ESS scores range from 0 to 24 (Johns, 1991). Test–retest reliability in 87 healthy medical students was r = .82, while internal consistency as measured by Cronbach alpha was .88 (Johns, 1991).
Functional Outcomes of Sleep Questionnaire (FOSQ)
The FOSQ provides a rating of impairment in daily behaviors that are sensitive to sleepiness (Weaver et al., 1997). The FOSQ is a 30-item scale with five subscales (activity level, vigilance, intimacy and sexual relationships, general productivity, and social outcomes). The total score ranges from 5 to 20. Higher scores indicate better functional status. A normal total score on the FOSQ is 17.9 or greater (Weaver et al., 2007). Internal consistency was α = .95 for the total score; test–retest reliability of the FOSQ was r = .81–.91 for the five subscales and r = .90 for the total score (Weaver et al., 2007).
Berlin Questionnaire
The 10-item Berlin Questionnaire predicts the presence of sleep apnea in patients in primary care settings (Netzer, Stoohs, Netzer, Clark, & Strohl, 1999). The items address the presence and frequency of snoring, wake-time sleepiness and fatigue, and history of obesity or hypertension. Patients with a high severity and/or frequency in any two of the three domains are considered to be at high risk for sleep apnea. The Berlin Questionnaire has a sensitivity of 86% in predicting sleep apnea, a specificity of 77%, positive predictive value of 0.89, and a likelihood ratio of 3.79. Cronbach alpha coefficients of .86–.92 have been reported (Netzer et al., 1999).
Body mass index (BMI kg/m2)
The BMI of each participant was calculated from his or her height and weight, which were measured by a single member of the research team. The participants’ weight in light street clothes and without shoes was measured using a calibrated eye-level scale (Detecto 338). Calibration was performed before each weight measurement by placing both the small and large weight indicators to zero. Measurements were recorded to the nearest half pound. To measure height, a stadiometer was used. Participants stood on the scale backwards, so that their heels, buttocks, back, and head were touching the backboard of the stadiometer. The headpiece was lowered to the crown of the head until it lay flat, and the measurement was rounded to the nearest quarter inch.
Procedures
All assessments were performed in the Clinical Science and Engineering Research Center at Binghamton University. Data collection occurred from May 2014 to January 2015. Approval for the study was granted by the institutional review board at Binghamton University. After obtaining consent, baseline demographic and clinical measures were collected (age, sex, medical history, and height and weight), and participants completed the self-report baseline measures.
Calf muscle pump (CMP) stimulation
Once the baseline assessment was complete, the participants were seated in a standard office chair and placed their feet on the CMP stimulation device (MM-100, Sonostics, Inc., Vestal, NY; see Fig. 1). The CMP stimulation device was activated for 30 minutes, and the participants’ brachial blood pressure was monitored every 5 minutes by a registered nurse to confirm the subject’s safety and comfort. Participants who had an increase in systolic blood pressure to over 140 mm/Hg or an increase in diastolic blood pressure to over 90 mm/Hg during the trial CMP session would have been excluded from further participation.
The CMP stimulation device, the MM-100 Calf Muscle Pump Stimulator (Sonostics, Inc., Vestal, NY), is a wedge-shaped rectangular box with a top plate (see Fig. 1). The individual sits in a relaxed position with his or her spine against the back of the chair and knees bent at approximately a 90° angle. The plantar surface of the forefoot is placed on the CMP device while the heel rests on the floor. The device delivers vibration to the plantar surface of the foot, which results in contraction of the soleus muscles in the calves. The effectiveness of this non-invasive device to reduce lower extremity venous pooling has been demonstrated in a number of studies in samples with fibromyalgia, postural hypotension, delayed hypotension, dialysis-related hypotension, lower limb edema, and congestive heart failure (Baniak, Pierce, Hiester, & McLeod, 2014; Madhavan, Goddard, & McLeod, 2008; Madhavan, Nemcek, Martinez, & McLeod, 2009; Madhavan, Stewart, & McLeod, 2005; Pierce & McLeod, 2009; Stewart, Karman, Montgomery, & McLeod, 2005).
We provided a CMP stimulation device with printed instructions for each participant to use at home. Subjects were instructed to use the device for at least 1 hour each day (either 60 minutes continuously or two 30-minute sessions) for at least 5 days each week and record their daily device usage in a spiral notebook. Weekly telephone calls to the participants were used to monitor their self-reported compliance with using the CMP device. During these calls, the PI asked general questions about overall changes in sleep and responses to the CMP stimulation. On average, the participants self-reported using the CMP stimulation device 1 hour per day, typically in two 30-minute sessions. No adverse effects were reported or observed during the intervention. No data were collected on what participants may have done to improve sleep quality in addition to using the CMP stimulation device. Following the intervention, participants returned to the Clinical Science and Engineering Research Center, where the clinical assessments were made and the questionnaires were completed a second time.
Data Analysis
Descriptive statistics were computed. Distribution of continuous variables was described using mean and standard deviation; distribution of categorical variables was reported as frequencies and percentages. Measures were not evaluated for reliability in this sample due to the small sample size.
Differences in pre- and post-intervention scores on the PSQI (global and 3-factor), FOSQ, and ESS were evaluated using effect size. Standardized effect sizes were calculated by averaging the pre-treatment (baseline) scores, subtracting this average from the average post-intervention scores, and then dividing the difference by the standard deviation of the pre-treatment scores of the sample (Becker, 1988). The relative magnitude of change was based on Cohen’s definitions of a small (0.2), moderate (0.5), and large (>0.8) effect size (Cohen, 1992). Analyses were conducted using IBM SPSS Statistics Version 19, and effect sizes were computed using Microsoft Excel.
Results
During the weekly phone calls and at the final assessment, all 11 participants reported an improvement in sleep quality. Two participants who initially complained of leg cramps attributed their improved sleep quality to a decrease in the severity of their leg cramps. Of the five participants who reported nocturia at baseline, four reported a reduction in the frequency of nocturia during the intervention.
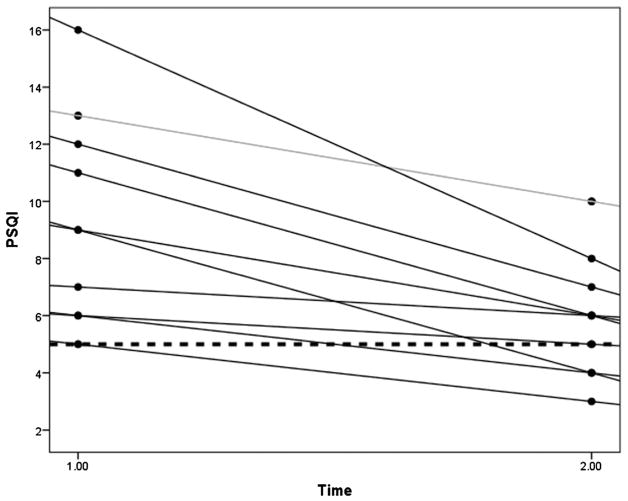
At baseline, 10 of the 11 participants at baseline were poor sleepers, as indicated by a total PSQI score >5. Every participant had an improved PSQI total score after CMP stimulation use (Fig. 2).
FIGURE 2.
Plot of differences in PSQI scores of each participant (N = 11) before and after using calf muscle pump (CMP) stimulation for 4 weeks. Changes in PSQI scores from baseline to follow-up reflect changes in sleep quality for each participant after 4 weeks of CMP stimulation. Each line represents a participant. Lower PSQI scores indicate better sleep quality. At baseline (time 1.00), all participants were categorized as poor sleepers (PSQI >5—threshold indicated by the dotted horizontal line). At follow-up (time 2.00), four participants became good sleepers (PSQI ≤ 5).
While all participants reported subjective daytime sleepiness during the telephone screening, only 18% (n = 2) were excessively sleepy (ESS ≥10) at baseline. Eight participants reported decreases in daytime sleepiness at follow-up, two participants had the same level of sleepiness, and one had increased daytime sleepiness at follow-up (Fig. 3).
FIGURE 3.
Plot of differences in Epworth Sleepiness Scale (ESS) scores of each participant (N = 11) before and after using calf muscle pump (CMP) stimulation for 4 weeks. Changes in ESS scores from baseline to follow-up reflect changes in daytime sleepiness for each participant after 4 weeks of CMP stimulation. Each line represents one participant. Higher ESS scores indicate increased daytime sleepiness. Excessive sleepiness is indicated by an ESS score of 10 or greater (dotted horizontal line). Eight participants experienced decreases in daytime sleepiness at follow-up (time 2.00), two experienced no change, and one experienced an increase.
The majority of participants (n = 7) reported impairment in sleep-related functional outcomes, defined as FOSQ scores less than 17.9 (Fig. 4). Five of those seven demonstrated an improvement in FOSQ scores after CMP stimulation use.
FIGURE 4.
Plot of differences between FOSQ scores for each participant (N = 11) before and after using calf muscle pump (CMP) stimulation for 4 weeks. Each line represents one participant. The increase in FOSQ scores from baseline (time 1.00) to follow-up (time 2.00) demonstrates improved functional status in all but two participants after 4 weeks of CMP stimulation. The group mean score increased from an abnormal level of function (FOSQ = 16.8 ± 2.2) to a normal level of function (FOSQ = 17.9 ± 1.7).
Mean change scores and standard deviations from pre-intervention to post-intervention for PSQI total score, the three PSQI factor scores, ESS, and FOSQ are presented in Table 2. The group mean PSQI improved from pre-intervention to post-intervention, and this score was associated with a large improvement in overall sleep quality (effect size [ES] = −0.97). In the components of sleep quality, a large reduction from before to after the intervention in Daily Disturbance (ES = −1.25) and moderate improvements in both Sleep Efficiency (ES = −0.51) and Perceived Sleep Quality (ES = −0.59) were seen. The group demonstrated moderate reductions in subjective sleepiness (ES = −0.53), and improved functional outcomes (ES = 0.49) after using the CMP stimulation device for 4 weeks. The mean FOSQ score increased by almost a full point, which moved the average from an abnormal functional level to a normal functional level (Weaver et al., 2007).
Table 2.
Changes in Each Outcome Variable After 4 Weeks of CMP Stimulation (N = 11)
| Outcome Variable | Baseline
|
4 Weeks
|
Change
|
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean Difference | Effect Size | |
| PSQI Global Score | 9.70 | 3.6 | 6.30 | 2.3 | −3.45 | −0.97 |
| PSQI factors | ||||||
| Sleep Efficiency | 1.59 | 0.8 | 1.18 | 0.6 | −0.41 | −0.51 |
| Perceived Sleep Quality | 1.24 | 0.9 | 0.73 | 0.6 | −0.51 | −0.59 |
| Daily Disturbance | 1.41 | 0.4 | 0.86 | 0.5 | −0.55 | −1.25 |
| Epworth Sleepiness Scale | 7.70 | 4.6 | 5.30 | 4.3 | −2.45 | −0.53 |
| Functional Outcomes of Sleep Questionnaire | 16.80 | 2.2 | 17.90 | 1.7 | 1.10 | 0.49 |
Note. Standardized effect size for each outcome measure was calculated by dividing the mean difference by the standard deviation at pre-intervention (Becker, 1988). PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation.
Discussion
This study represents the first known study of CMP stimulation as an intervention to improve sleep. Improvements in daytime sleepiness and daytime functional outcomes were observed following just 4 weeks of using the CMP stimulation device. The results suggest that CMP stimulation via plantar micro-mechanical stimulation may result in improved overall sleep quality.
Because the sleep measures for most participants were in the normal or near-normal range at baseline, it is unclear whether or not these improvements would be clinically significant in other healthcare contexts. Furthermore, in this single-group design, unmeasured factors may have led to improved sleep, and no causality can be presumed. Nonetheless, the large effect size for daily disturbance due to inadequate sleep is noteworthy. This daily disturbance component of sleep quality includes questions on sleep fragmentation and daytime dysfunction related to poor sleep. Reduction in daily disturbance coincides with the known therapeutic effects of exercise, which include improvement in sleep fragmentation and daytime fatigue.
Although many factors are involved in poor sleep quality, and we do not know the specific mechanism by which CMP stimulation may improve sleep quality, several possibilities exist. The first is exercise response. Reflex-mediated stimulation of the CMP can be viewed as a type of passive exercise, which promotes not only increased soleus muscle tone, improved function, and increased systemic perfusion, but also increased cardiac output and tissue perfusion associated with voluntary exercise (Padberg, 2001). Traditional exercise regimens have known benefits for improving circulation, muscle tone, sleep quality, daytime sleepiness, fatigue, and mood. Exercise regimens also protect against sleep-disordered breathing such as OSA (Chen, Liu, Huang, & Chiou, 2012; King, Oman, Brassington, Bliwise, & Haskell, 1997; Kline et al., 2012, 2013). Exercise has been independently associated with a reduction in OSA severity among sedentary overweight adults, even when no marked decrease in body weight was recorded (Kline et al., 2011). Conversely, decreased exercise resulted in worse sleep-disordered breathing (Awad, Malhotra, Barnet, Quan, & Peppard, 2012). Sedentary activity leads to inadequate CMP activity, which leads to LEFP and decreased systemic perfusion (Padberg, 2001; Redolfi et al., 2009).
Another explanation for the improvement in sleep quality observed in this study is mobilization of LEFP and resulting rostral fluid shifts (White et al., 2014). When a human lies down to sleep, the veins and interstitial spaces above the heart, including the neck veins, will re-fill, increasing vessel pressure from 0 mm/Hg to 20 mm/Hg within seconds (Rowell, 1993). This increased pressure can lead to extravasation into the interstitial spaces surrounding the pharynx, which compromises the upper airway in those susceptible to sleep disordered breathing, particularly OSA (Rowell, 1993; Shiota et al., 2007; Su et al., 2008). The results of the current study are consistent with a small (N = 6) clinical trial (Redolfi et al., 2011) in which compression stockings improved sleep (37% reduction in the apnea/hypopnea index) among persons with obstructive sleep apnea. Similar results have been observed in recent research (e.g., White, Lyons, Yadollahi, Ryan, & Bradley, 2015). Although a diagnosis of OSA was not required for inclusion in this study, the high prevalence of participants who were at high risk for OSA according to the Berlin Questionnaire suggests that mobilization of LEFP may be a plausible mechanism for the improved sleep quality seen after our intervention.
Limitations
First, this study was of a small convenience sample with the participants acting as their own controls, which limited statistical power and restricted the generalizability of and the inferences that can be made from the data we collected. The specific mechanism of action for this improvement remains undetermined, and randomized controlled designs are required to affirm that changes in sleep quality were caused by the intervention. Also, participants with OSA or LEFP were not recruited specifically, and objective measures of changes in OSA severity or leg volume were not included in our protocol. Furthermore, the CMP stimulation device featured in this study did not contain an internal monitor to record actual usage, which certainly limits the generalizability of our results.
In addition to randomized controlled designs, dose-response studies are recommended to confirm the most effective length of time needed to receive maximum benefit from CMP stimulation. Moreover, to identify the mechanism for improved sleep quality and decreased OSA severity, additional research is needed that incorporates objective measures of sleep apnea with overnight polysomnography, LEFP quantification with air plethysmography, and physical activity with personal activity monitors.
Conclusion
This study is the first known examination of CMP stimulation as an intervention to improve sleep quality and outcomes related to sleep in persons who report poor subjective sleep quality. CMP stimulation was followed by a modest improvement in sleep quality, functional outcomes sensitive to sleepiness, and daytime sleepiness. Adequate sleep quality increasingly has been recognized as a vital component of health promotion, chronic disease prevention, and overall well-being. Although further study is needed, the results of this study suggest that CMP stimulation, a non-invasive, non-pharmacologic intervention that can be implemented in the home or workplace, could be helpful to persons struggling to manage sleep disturbance. Moreover, CMP stimulation could be employed as an adjunctive therapy in concert with established treatment regimens to improve sleep quality for persons suffering from impaired sleep.
Acknowledgments
This research was supported by the Clinical Science and Engineering Research Center at Binghamton University, the Decker School of Nursing at Binghamton University, and the T32, Technology: Research in Chronic and Critical Illness (2T32 NR008857) at the University of Pittsburgh, School of Nursing.
Footnotes
Baniak, Pierce, and Chasens report no conflicts of interest. McLeod is a stakeholder in Sonostics, Inc., which produces the device featured in this study.
Contributor Information
Lynn M. Baniak, Postdoctoral Fellow, University of Pittsburgh, School of Nursing, 3500 Victoria St., Victoria Bldg. 363A, Pittsburgh, PA 15261
Carolyn S. Pierce, Associate Professor, Binghamton University, School of Nursing, Binghamton, NY
Kenneth J. McLeod, Professor, Director, Clinical Science and Engineering, Research Center, Binghamton University, Binghamton, NY
Eileen R. Chasens, Associate Professor, University of Pittsburgh, School of Nursing, Pittsburgh, PA
References
- American Heart Association. American Heart Association recommendations for physical activity in adults. 2016 Retrieved from http://www.heart.org/HEARTORG/HealthyLiving/PhysicalActivity/FitnessBasics/American-Heart-Association-Recommendations-for-Physical-Activity-in-Adults_UCM_307976_Article.jsp#.VrOVDrIrJ9M.
- Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE. Exercise is associated with a reduced incidence of sleep-disordered breathing. The American Journal of Medicine. 2012;125:485–490. doi: 10.1016/j.amjmed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniak LM, Pierce CS, Hiester E, McLeod KJ. Calf muscle pump stimulation as a means to reduce symptoms of fibromyalgia syndrome. Biological Research for Nursing. 2014;17:334–339. doi: 10.1177/1099800414546893. [DOI] [PubMed] [Google Scholar]
- Becker BJ. Synthesizing standardized mean change measures. British Journal of Mathematical and Statistical Psychology. 1988;41:257–278. [Google Scholar]
- Bliwise DL, Scullin MK. Normal aging. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 6. Philadelphia: Elsevier, Inc; 2017. pp. 25–38. [Google Scholar]
- Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: Obstructive sleep apnea. Circulation. 2003;107:1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chen MC, Liu HE, Huang HY, Chiou AF. The effect of a simple traditional exercise programme (Baduanjin exercise) on sleep quality of older adults: A randomized controlled trial. International Journal of Nursing Studies. 2012;49:265–273. doi: 10.1016/j.ijnurstu.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, … Bradley TD. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. American Journal of Respiratory and Critical Care Medicine. 2006;174:1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- Christopoulous D, Nicolaides AN, Cook A, Irvine A, Galloway JM, Wilkinson A. Pathogenesis of venous ulceration in relation to the calf muscle pump function. Surgery. 1989;106:829–835. [PubMed] [Google Scholar]
- Cohen J. A power primer. Quantitative Methods in Psychology. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep. 2006;29:112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- Goddard AA, Pierce CS, McLeod KJ. Reversal of lower limb edema by calf muscle pump stimulation. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28:174–179. doi: 10.1097/01.HCR.0000320067.58599.ac. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Sleep Medicine and Research. Sleep disorder and sleep deprivation: An unmet public health problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- Johansson M, Johansson L, Lind M. Incidence of venous thromboembolism in northern Sweden (VEINS): A population-based study. Thrombosis Journal. 2014;12:6. doi: 10.1186/1477-9560-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1016/j.sleep.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kan YM, Delis KT. Hemodynamic effects of supervised calf muscle exercise in patients with venous leg ulceration. Archives of Surgery. 2001;136:1364. doi: 10.1001/archsurg.136.12.1364. [DOI] [PubMed] [Google Scholar]
- King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults: A randomized controlled trial. Journal of the American Medical Association. 1997;277:32–37. doi: 10.1001/jama.1997.03540250040029. [DOI] [PubMed] [Google Scholar]
- Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, … Youngstedt SD. The effect of exercise training on obstructive sleep apnea and sleep quality: A randomized controlled trial. Sleep. 2011;34:1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Ewing GB, Burch JB, Blair SN, Durstine JL, Davis JM, Youngstedt SD. Exercise training improves selected aspects of daytime functioning in adults with obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2012;8:357–365. doi: 10.5664/jcsm.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline CE, Irish LA, Krafty RT, Sternfeld BS, Kravitz HM, Buysse DJ, … Hall MH. Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: The SWAN sleep study. Sleep. 2013;36:1279–1288. doi: 10.5665/sleep.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJ, Robertson L, Boghossian S, Allan P, Ruckley V, Fowkes G, Evans C. Progression of varicose veins and chronic venous insufficiency in the general population in the Edinburgh Vein Study. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2015;3:18–26. doi: 10.1016/j.jvsv.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Madhavan G, Goddard AA, McLeod KJ. Prevalence and etiology of delayed orthostatic hypotension in adult women. Archives of Physical Medicine and Rehabilitation. 2008;89:1788–1794. doi: 10.1016/j.apmr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Madhavan G, Nemcek MA, Martinez DG, McLeod KJ. Enhancing hemodialysis efficacy through neuromuscular stimulation. Blood Purification. 2009;27:58–63. doi: 10.1159/000167010. [DOI] [PubMed] [Google Scholar]
- Madhavan G, Stewart JM, McLeod KJ. Effect of plantar micromechanical stimulation on cardiovascular responses to immobility. American Journal of Physical Medicine & Rehabilitation. 2005;84:338–345. doi: 10.1097/01.PHM.0000159970.81072.8B. [DOI] [PubMed] [Google Scholar]
- Madhavan G, Stewart JM, McLeod KJ. Cardiovascular systemic regulation by plantar surface stimulation. Biomedical Instrumentation and Technology. 2006;40:78–84. doi: 10.2345/0899-8205(2006)40[78:CSRBPS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine. 1999;131:485–491. doi: 10.1001/jama.1966.03110240153072. [DOI] [PubMed] [Google Scholar]
- Padberg F. The physiology and hemodynamics of the normal venous circulation. In: Gloviczki P, Yao J, editors. Handbook of venous disorders. 2. New York: Arnold Publishers; 2001. pp. 25–35. [Google Scholar]
- Padberg FT, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: A randomized trial. Journal of Vascular Surgery. 2004;39:79–87. doi: 10.1016/j.jvs.2003.09.036. [DOI] [PubMed] [Google Scholar]
- Pierce C, McLeod KJ. Feasibility of treatment of lower limb edema with calf muscle pump stimulation in chronic heart failure. European Journal of Cardiovascular Nursing. 2009;8:345–348. doi: 10.1016/j.ejcnurse.2009.07.001. doi: http://doi.org/doi:10.1016/j.ejcnurse.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respiratory Physiology & Neurobiology. 2011;175:390–393. doi: 10.1016/j.resp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, Bradley TD. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. American Journal of Respiratory and Critical Care Medicine. 2009;179:241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- Robertson L, Lee AJ, Evans CJ, Boghossian S, Allan PL, Ruckley CV, Fowkes F. Incidence of chronic venous disease in the Edinburgh Vein Study. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2013;1:59–67. doi: 10.1016/j.jvsv.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Rooke T, Felty C. Lymphedema: Medical and physical therapy. In: Gloviczki P, Yao J, editors. Handbook of venous disorders. 2. New York: Arnold Publishers; 2001. pp. 473–482. [Google Scholar]
- Rowell LB. Human cardiovascular control. New York: Oxford University Press, Inc; 1993. [Google Scholar]
- Shepard JW, Pevernagie DA, Stanson AW, Daniels BK, Sheedy PF. Effects of changes in central venous pressure on upper airway size in patients with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 1996;153:250–254. doi: 10.1164/ajrccm.153.1.8542124. [DOI] [PubMed] [Google Scholar]
- Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight JS, Arzt M, … Bradley TD. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–872. doi: 10.1136/thx.2006.071183PMCID:PMC2094267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Karman C, Montgomery LD, McLeod KJ. Plantar vibration improves leg fluid flow in perimenopausal women. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2005;288:R623–R629. doi: 10.1152/ajpregu.00513.2004. [DOI] [PubMed] [Google Scholar]
- Su MC, Chiu KL, Ruttanaumpawan P, Shiota S, Yumino D, Redolfi S, … Bradley TD. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respiratory Physiology & Neurobiology. 2008;161:306–312. doi: 10.1016/j.resp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh, Deepak K, Lyon K, … Dinges DF. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CFP, Greenberg H, … Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1016/S8756-3452(08)70707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LH, Lyons OD, Yadollahi A, Ryan CM, Bradley TD. Effect of below-the-knee compression stockings on severity of obstructive sleep apnea. Sleep Medicine. 2015;16:258–264. doi: 10.1016/j.sleep.2014.12.005. [DOI] [PubMed] [Google Scholar]
- White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respiratory Physiology and Neurobiology. 2014;192:17–22. doi: 10.1016/j.resp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, … Bradley TD. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–1605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- Ziaja D, Kocelak P, Chudek J, Ziaja K. Compliance with compression stockings in patients with chronic venous disorders. Phlebology: Venous Forum of the Royal Society of Medicine. 2011;26:353–360. doi: 10.1258/phleb.2010.010086. [DOI] [PubMed] [Google Scholar]