Abstract
The human default network (DN) plays a critical role in internally-directed cognition, behavior, and neuropsychiatric disease. Despite much progress with functional neuroimaging, persistent questions still linger concerning the electrophysiological underpinnings, fast temporal dynamics, and causal importance of the DN. Here, we review how direct intracranial recording and stimulation of the DN provides a unique combination of high spatiotemporal resolution and causal information that speaks directly to many of these outstanding questions. Our synthesis highlights the electrophysiological basis of activation, suppression, and connectivity of the DN, each key areas of debate in the literature. Integrating these unique electrophysiological data with extant neuroimaging findings will help lay the foundation for a mechanistic account of DN function in human behavior and cognition.
Keywords: default network, intracranial electroencephalography, iEEG, electrocorticography, ECoG
Toward a deeper understanding of the default network
Serendipity has played an eminent role in the history of scientific discovery: chance observations and insights are alleged to lie at the root of many major and minor breakthroughs [1]. Every innovation must subsequently be refined and scrutinized, however; every paradigm shift in scientific thinking must be followed by years or decades of rigorous and painstaking ‘normal science’ [2]. The fortuitous discovery of the default mode network (or default network, DN) some 20 years ago provides a prototypical example [3–5]: the unexpected observation that a consistent set of brain regions showed reduced cerebral blood flow (‘deactivated’) during a variety of cognitive tasks (compared to rest [4, 5]) seeded a paradigm shift in our view of the functional architecture of the brain [6–8].
The human DN is now well known as a collection of associative brain regions distributed over the temporal, parietal and frontal lobes (Fig. 1), readily identifiable by correlated spontaneous fluctuations in the functional magnetic resonance imaging (fMRI) blood oxygen level-dependent (BOLD) signal. The DN is just one of numerous distributed intrinsic brain networks [9–12] identified during the resting state and persistently correlated during a variety of cognitive tasks [13, 14] and states of consciousness [15–17]. Despite much progress with neuroimaging [18], persistent questions still linger concerning the electrophysiological underpinnings, fast temporal dynamics, and causal importance of the human DN. We require a fuller understanding of how this canonical brain network contributes to human cognition and behavior, and how its pathological modulation relates to neuropsychiatric disorders. Such an understanding calls for a detailed neuromechanistic account that explains the DN’s internal dynamics and interactions with other intrinsic networks with high anatomical precision and temporal resolution. Moreover, the relationships between DN activity and human subjective experience and behavior need to be explored with causal methods that go beyond mere correlative observations.
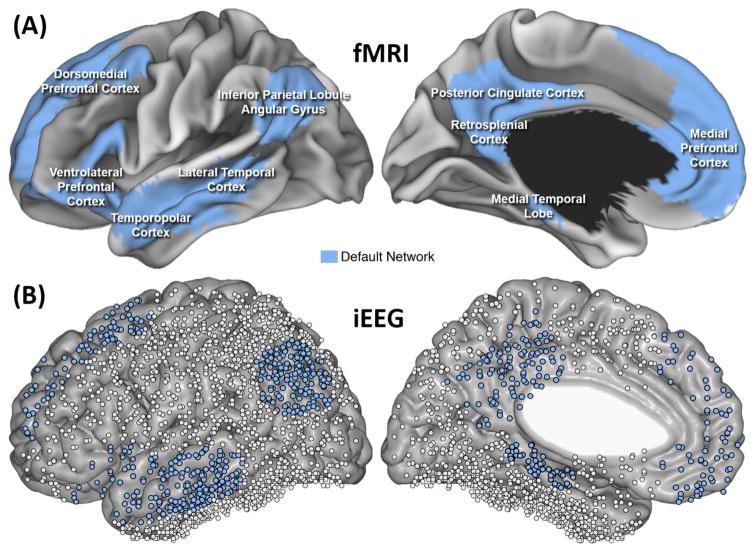
Figure 1. Intracranial electrophysiology of the human default network.
(A) The human DN as assessed using intrinsic connectivity fMRI during the resting state in 1000 participants [9]. The network is distributed throughout the brain: in the frontal lobe, medial prefrontal cortex (PFC), dorsomedial PFC, and ventrolateral PFC; in the temporal lobe, temporopolar cortex, lateral temporal cortex, and aspects of the medial temporal lobe (MTL); and in the parietal lobe, the posterior cingulate cortex and retrosplenial cortex medially, and angular gyrus laterally. Although there is general consensus on this collection of regions, specific network borders vary depending on methods and modality used, and the notion of what constitutes the DN continues to evolve [55]. Because much of the MTL falls outside DN boundaries using standard methods, as a rule we do not discuss iEEG investigations of the MTL throughout this review unless they bear directly on questions relating to the function of other DN regions (for a reviewing covering many iEEG studies involving the MTL, see ref. [103]). (B) Combining iEEG data from cohorts of patients can provide comprehensive coverage of the brain, including all cortical DN regions (here we show 1,955 electrode sites from 16 patients). There are two basic types of iEEG recording [19]. (i) Electrocorticography (ECoG), using subdural grids or strips of circular (plate-shaped) electrodes placed on the brain’s surface, can record from lateral or medial cortical DN areas. Grids often contain dozens of contacts, allowing coverage of, and simultaneous recording from, a substantial proportion of cortex. (ii) Stereotactic EEG (sEEG) uses cylindrical depth electrodes which penetrate through the skull and brain tissue and can reach deep medial cortical and subcortical structures, including midline DN regions. Each depth electrode typically contains 6–10 contacts. For more details, see [19] and Box 1.
Intracranial electroencephalography (iEEG; Box 1) has much to contribute to this endeavor, by providing direct recording and stimulation data from the human brain. Typically, iEEG is undertaken in patients undergoing monitoring for epilepsy surgery, and involves the placement of electrode grids directly on the cortical surface (Box 1, Fig. I), and/or the insertion of depth electrodes which can contact deep cortical and subcortical structures. With high anatomical precision (at the level of neuronal populations), high temporal resolution (at the millisecond scale), and high signal-to-noise ratio (at the level of several-fold signal increase) [19], iEEG provides the necessary fine-scale view of the human brain to both advance and refine our knowledge of the DN. Although the tools of iEEG and electrical brain stimulation have been available for decades, recent advances in computational methods have given rise to entirely novel means of charting and perturbing the engagement of local neuronal populations and larger functional networks. For example, gauging the power of the electrophysiological signal in the high frequency broadband (HFB) (>50 Hz, also known as ‘high gamma’; see Box 4) range has provided a new metric of cortical activation that correlates well with both fMRI BOLD signals [20, 21] and averaged neuronal spiking activity [22], providing a potent means of replicating and extending findings from human neuroimaging and animal neurophysiology, respectively (Box 2).
Box 1. An overview of the intracranial EEG method.
Functional and morphometric MRI have undoubtedly played the starring role in the discovery and subsequent investigation of the DN [4, 8, 42, 105]. Yet even as the DN continues to occupy the spotlight in much of contemporary cognitive and clinical neuroscience research, non-invasive and correlational neuroimaging methods continue to confront important technological barriers. Limited temporal resolution, poor signal-to-noise ratio, and lack of causal data from neuroimaging have left in their wake many persistent and critical questions that iEEG can make a unique contribution to answering.
The spatial precision of the iEEG signal lies between that of classic local field potentials (LFPs) and scalp EEG [106]. Similar to scalp EEG, iEEG records the brain’s endogenously produced electrical potentials, but by bypassing the filtering properties of the meninges, skull, and scalp, iEEG gains a huge increase in signal-to-noise ratio and a widening of the detectable spectrum of neural activity. Moreover, the recorded signal has great anatomical precision since the inverse problem (i.e., inferring the precise source of recorded signals) is greatly mitigated. The fundamental recorded activity is the cortical field potential, a rich signal thought to represent a summation primarily of synaptic currents and neuron spiking near the electrode [19, 106, 107]. Although the recorded signal does not reveal the precise spiking of specific neurons (the ‘trees’), it does reliably measure the engagement of the ‘forest’ (population) of neurons (an estimated 200,000–500,000 neurons [19]). IEEG therefore provides mesoscale-level data uniquely complementary to the cellular level of mapping with single-neuron recordings in animals and the regional/whole-brain level of mapping in humans with neuroimaging.
IEEG’s powerful combination of high anatomical and temporal resolution can contribute unique and hitherto unknown information to the field of human neuroscience. With electrode contacts placed directly on the surface of the cortex (in grids; Fig. I) or deep within brain structures (with cylindrical depth electrodes), unlike single-unit recordings, iEEG can often capture simultaneous recordings across a large mantle of the human brain (up to ~200 electrodes in a single patient). By recording simultaneously from several nodes of a network, iEEG signals can reveal information about functional interactions within and across networks during different stages of neural computation even during complex cognitive, affective, and perceptual tasks requiring human participants. Furthermore, the method allows delivery of a volley of electrical discharges directly to a brain region in awake human participants [93, 94], providing the ability to causally perturb subjective experience and behavioral performance, as well as modulate network activity (Box 2) and potentially treat neuropsychiatric disease (Box 3).
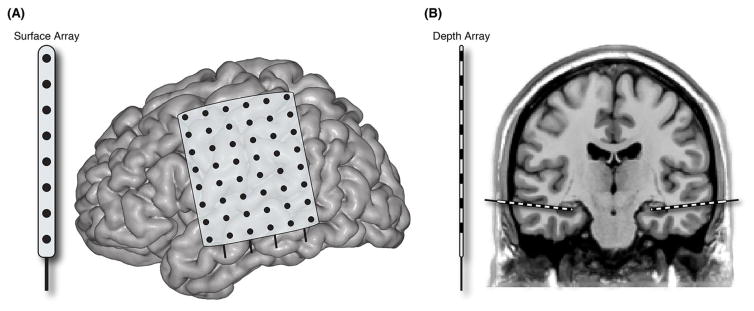
Figure I. Intracranial EEG refers to the invasive measure of electrical brain potentials via two common methods.
A) Electrocorticography (ECoG) utilizes strips or grids of electrode arrays placed subdurally on the cortical surface. B) Stereo-EEG (sEEG) utilizes penetrating depth electrode arrays based on stereotactic coordinates for targeting deeper brain structures.
Box 4. Understanding high frequency activity in the brain.
The high frequency broadband (HFB) signal is currently interpreted as a reflection of a non-oscillatory broadband signal [146, 147]. Importantly, HFB differs from pathological high frequency oscillations (HFOs) that are seen in epileptic recording sites. Unlike HFB signals, HFOs are associated or coupled with interictal epileptiform discharges and/or pathological background activity at the recording site and can be present across several adjacent electrodes [148, 149] (see Box 1).
A growing body of evidence suggests that high frequency activity is a reliable electrophysiological correlate of underlying population spiking activity generated by thousands of neurons [21, 150–153]. HFB signals also correlate with hemodynamic signals detected with fMRI [20–22, 154, 155]. Thus, an increase in the HFB power in a recording site represents the local engagement of the cortical tissue underneath or around the electrode. There is strong evidence that, unlike slow oscillatory activity, the HFB signal has a remarkably localized anatomical precision and originates from the cortical tissue immediately around or underneath the recording electrode (for human iEEG evidence, see refs. [28, 30, 47, 156–159], and for direct measures from non-human primates, see refs. [151–154]).
There is evidence from non-human brains that the number of cells contributing to the high frequency signal may be as little as ~16% of neurons sampled by a given electrode [160] and that the source of the high frequency signal is often within several hundreds of micrometers from the electrode tip [152, 161, 162]. Given the specifics of iEEG electrodes and recording parameters, our assumption is that the iEEG signal is rooted in the activity of a diverse population of cells in the millimeter space (for a recent, in-depth review, see [19]).
Box 2. Advantages of combining iEEG with other modalities.
There are many applications of combining iEEG with other methods, particularly fMRI. Here we highlight several examples, but the possibilities of such multimodal investigations are still just beginning to be explored.
Providing functional context for electrode localization: Although the anatomical location of intracranial electrodes can be reconstructed with relatively high precision, functional brain networks vary considerably across individual neuroanatomy [108, 109]. Anatomical landmarks alone are therefore at best a coarse indicator of regional function, but resting-state fMRI scans can provide relatively reliable estimates of subject-specific brain networks [110, 111], allowing particular electrodes to be assigned with some confidence to individually-specified functional networks [87, 111, 112].
Putting novel iEEG-elicited effects into broader context: While iEEG can discover novel effects, its sparse coverage of the brain often renders it uninformative about the whole-brain relevance of these unique findings. FMRI can supplement the paucity of recording sites in iEEG and provide complementary whole-brain data that clarifies and corroborates iEEG findings (e.g., [112]).
Understanding effects of focal electrical stimulation in brain disease: With the increasing use of intracranial methods to treat neurological and psychiatric disease, combined pre- and post-intervention PET and fMRI scanning has already been employed to elucidate how focal electroceutical interventions affect functioning of the DN [113–115]. Neuroimaging will continue playing a key role in corroborating and understanding the brain-wide and long-term effects of targeted intracranial stimulation (see Box 3).
Simultaneous multimodal investigations: Progress is being made in developing ways of safely combining iEEG with both fMRI [116, 117] and MEG [118, 119] in humans, which could open up many new possibilities, for instance directly observing the whole-brain effects of focal electrical stimulation [120].
This article provides an accessible synthesis of the most recent iEEG findings related to the DN for researchers both in and outside the field of human intracranial electrophysiology. We aim to promote cross-talk among disciplines and explore how iEEG findings can help transform our understanding of the DN. In the text that follows, we synthesize the DN iEEG literature pertinent to i) task-induced deactivations, ii) task-induced activations, (iii) intra- and internetwork connectivity, and iv) the causal effects of electrical stimulation.
Electrophysiological correlates of task-induced default network deactivations
The first finding from neuroimaging suggesting the DN was a coordinated functional system was the observation of reduced cerebral blood flow in DN regions during engagement in externally-directed tasks, such as visual search [4, 5], compared to a resting or baseline state. Some of the earliest intracranial investigations therefore explored whether parallel electrophysiological markers of deactivation could be observed with iEEG (Fig. 2A–C). For iEEG data, ‘deactivation’ would be reflected in reductions in power of high frequency activity (such as HFB) relative to the pre-stimulus period (typically, 100–200 ms prior to the onset of a given stimulus). Beginning in 2008, researchers began to note suppression of HFB power in response to externally-directed tasks in isolated DN regions, such as ventrolateral [23] (20–150 Hz) and rostromedial [24] prefrontal cortex (PFC) (50–150 Hz). Subsequent studies reported similar deactivations in HFB activity while recording from multiple DN regions simultaneously [25–27]. Critically, some studies have explored the various cognitive and behavioral correlates of electrophysiological DN deactivations [27, 28], and parallel work has confirmed that anatomical sites of iEEG deactivation are indeed localized within subject-specific DN boundaries as identified with resting-state fMRI [29, 30].
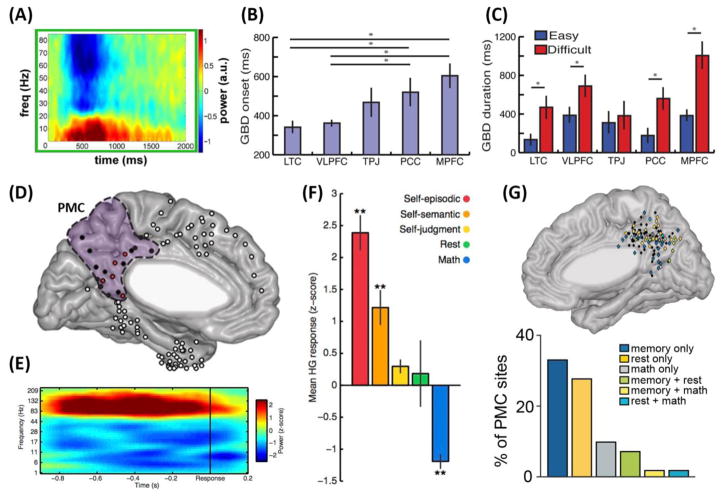
Figure 2. Unique contributions to understanding default network deactivations and activations from intracranial EEG.
(A) Default network deactivations in response to a visuomotor task consists largely of rapid suppression of power in higher frequency ranges (>30 Hz). Hot colors indicate increased power, and cool colors decreased power, at given frequencies. These and similar findings from other studies constitute a major advance in understanding the electrophysiological basis of DN deactivations observed with neuroimaging. (B) Timing of deactivation matters: whereas neuroimaging findings might suggest that the entire DN deactivates simultaneously, iEEG shows that there are subtle (yet probably important) timing differences in the onset of higher frequency ‘gamma band deactivations’ (GBD) in response to the presentation of complex visual task stimuli. (C) The duration of deactivations is also of functional significance: a more difficult visual search task elicited significantly longer suppressions of high frequency power in default regions. (D) Certain neuronal populations in posteromedial cortex (PMC) are significantly activated (electrodes with red fill color) by a variety of internally-directed cognitive tasks, (E) eliciting power increases specifically at higher frequencies (hot colors indicate increased power, cool colors decreased power). (F) The mean magnitude of PMC activations in the high-gamma band (70–180 Hz) can differentiate between conditions, with the largest activations for the most episodic/memory-like judgments. In contrast, significant suppression of high-gamma power is evident during an externally-directed math task (i.e., judging the accuracy of simple arithmetic equations). (G) Intraregional functional heterogeneity within the PMC. Certain populations show preferential activation in response to memory retrieval, others are selectively active during rest periods, and yet others show mixed patterns. Default regions often treated as functionally homogeneous in neuroimaging investigations in fact contain populations with very diverse response profiles. Panel A reproduced, with permission, from [34]. Panels B and C reproduced, with permission, from [27]. Panels D–F reproduced, with permission, from [28]. Panel I modified, with permission, from [35].
Collectively, these investigations have revealed essential information about the DN specifically and the fMRI BOLD signal more generally. First, these data have helped clarify the neurophysiological contributions to fMRI BOLD signal decreases. Early concerns had been raised that apparent deactivations in neuroimaging data were of non-neural origin, for instance caused by cardiac or respiratory artifacts [31] or vascular ‘stealing’ [32]. Task-induced suppression of iEEG HFB activity, a correlate of averaged neuronal firing, provides compelling evidence that DN deactivations seen in neuroimaging studies have a firm grounding in the electrophysiological activity of neuronal populations within DN regions (Fig. 2A) – a conclusion further supported by single-neuron data from non-human primates [33].
Second, HFB suppression in various DN areas is observed in response to a wide variety of externally-directed tasks (as with neuroimaging [5]), including visual search [27], reading [23, 26], a backward-masking visual categorization task [34], the Navon ‘global vs. local’ attention task [26], and arithmetic calculation [28–30, 35]. These findings support the conclusion from neuroimaging studies that DN deactivations are a common, domain-general response to the demands of externally-directed attention.
Third, not all DN areas deactivate at the same time [26, 27] (Fig. 2B; but for an exception, see [34]). The finding of subtle differences in mean timing of iEEG deactivations across DN nodes highlights an important contribution made possible by the high temporal resolution of iEEG. Indeed, the ability to temporally dissociate responses within the DN is a critical domain of progress uniquely served by iEEG (see ‘Electrophysiological correlates of intra- and internetwork interactions,’ below).
Finally, the timing and magnitude of HFB suppression have functional significance. The duration of HFB suppression correlates with reaction time [27, 28] (consistent with magnitude-reaction time correlations with fMRI [36]). Additionally, both the duration and magnitude of HFB deactivations are associated with task complexity, with more complex tasks inducing longer and larger iEEG deactivations [27] (Fig. 2C) (a finding observed early on with fMRI [5, 37]). Both findings suggest that deactivation represents not merely the relative quiescence of a network not participating in a given task, but instead might be an active process of functional suppression important for successful task performance. Increasing task complexity/difficulty also reliably leads to reductions in self-reported mind-wandering and other internally-directed thought processes (e.g., [38, 39]); whether or not these psychological effects are directly related to differential duration and magnitude of deactivation in default areas is an important question that should be explored in future research (cf. [37]).
Electrophysiological correlates of task-induced default network activations
Whereas externally-directed cognitive tasks often evoke DN deactivation, DN activation (i.e., relative increases of cerebral blood flow or BOLD signal strength compared to the control condition) is linked to many forms of internally-directed cognition [40, 41] such as self-referential thinking [42], mind-wandering [43, 44], simulation of the future [45], and remembrance of the past [46]. The unique strengths of iEEG are helping to disentangle the complex functionality and electrophysiology of DN activation by providing detailed information about the time course, magnitude, and selectivity of signals from recording sites throughout the network. Collectively, investigations of DN activation have allowed us to reach several broad conclusions about the temporal dynamics of, and role(s) played by, different regions and neuronal populations.
First, the electrophysiology of DN activations comprises increases in HFB power (compared to pre-stimulus baseline) concomitant with decreases in lower frequency ranges, such as alpha and theta [29, 30] (Fig. 2E) – mirroring results from intracranial investigations of motor and sensory cortices [47]. This finding complements the spectral changes that characterize DN deactivations, which typically show the opposite pattern of power change [27, 34] (Fig. 2A), and contrasts with intrinsic resting state DN activity, where theta oscillations predominate [48].
Second, similar to the breadth of internally-directed processes that activate the DN in neuroimaging [49, 50], a variety of internally-directed tasks increase HFB power in DN neuronal populations [28–30, 51, 52] (Fig. 2F). Increased high frequency (30–180 Hz) power was first reported [29] in the posterior cingulate cortex (PCC) and retrosplenial cortex (RSC) during self-referential judgments (consistent with fMRI [53]), with numerous iEEG studies having since replicated these observations in these and other default areas [28, 30, 51, 52].
Third, the magnitude of DN activations has functional significance (as it does for deactivations). Specific neuronal populations (i.e., electrode sites) showing larger HFB power increases for a given type of internally-directed cognition (such as episodic memory recall) tend to be more active for similar kinds of mentation, such as self-referential judgments [28]. Activation magnitude can also distinguish between different types of internally-directed cognition: for instance, a recent study [28] found a clear tuning of HFB activations for self-referential judgments in PCC and RSC: larger responses were elicited by more episodic/autobiographical judgments (e.g., “I ate fruit yesterday”), compared to more general semantic (e.g., “I eat fruit often”) or personal judgments (e.g., “I am kind”) (Fig. 2F). Moreover, PCC/RSC neuronal populations more active for autobiographical judgments also showed larger deactivations during an externally-directed attentional task (arithmetic calculation) [28].
Fourth, not all populations within DN areas respond in the same way (Fig. 2G), suggesting that large-scale parallels between DN activation across fMRI and iEEG represent only a coarse picture of averaged signals in a given region. Although DN regions, and even the entire network, are often treated as functionally homogenous in the neuroimaging literature, some pioneering efforts at finer functional-anatomic fractionations have been undertaken in recent years [54–57]. These fMRI studies hint at the possibility that particular DN regions, too, might contain functionally heterogeneous neuronal populations. In keeping with this, a study [29] using direct recordings from the DN hub in the posteromedial cortex (PMC) found only a small subset of recording sites displaying high frequency (30–180 Hz) power increases to self-referential judgments. A more recent study [52] involved a larger cohort of 13 subjects who alternated between evaluating autobiographical memory and arithmetic statements, and staring at a fixation cross at the center of a dark blank screen (‘fixation rest’). Recording from multiple sites within each individual patient’s PMC revealed clear functional heterogeneity across three distinct populations of neurons within this DN hub. Anatomically distinct clusters of neuronal populations in the PMC showed activations (in the HFB range) during either memory processes or fixation rest, and within-subject analysis showed that rest-active sites were (on average) located more dorsally than memory-active sites. Such spatially overlapping but functionally heterogeneous populations (Fig. 2G) can only be appreciated with a method that offers a unique combination of subject-specific anatomical precision, high signal-to-noise ratio, and simultaneous recordings with tens of electrodes across a cortical region (Box 1). Moreover, there were subtle differences in the temporal domain as well. Some populations showed fast, time-locked activations at the beginning of fixation rest trials (i.e., ‘switch sites’). These neuronal populations might help shift the brain from an externally-directed attentional state to a more internally-focused one, as subjects become free to explore their own thoughts and surroundings (cf. [58, 59]). Conversely, this fast, time-locked, and seemingly automatic activity following the switch from externally-focused attention to fixation rest is unlikely to reflect the re-emergence of spontaneous thoughts (as the time of onset of HFB activity is too quick for any spontaneous thought to occur). Other clusters of PMC neurons showed a slow, temporally-jittered rise of activity during fixation rest. Such variable and delayed temporal dynamics suggest that these populations of PMC neurons could be engaged in the arising of stimulus-independent spontaneous cognition, which is unlikely to occur with consistent timing across trials [60]. Finally, a third type of neuronal population in the PMC showed time-locked activations during the memory condition, and often deactivated during the math condition; the relatively late (>300 ms) recruitment of these populations suggests that the PMC is likely engaged after the MTL.
Although high-resolution/long-duration single-subject recordings and multi-voxel pattern analysis with fMRI have begun to provide considerably greater spatial precision, the distinct temporal profiles described above would be impossible to isolate with neuroimaging methods.
Electrophysiological correlates of intra- and internetwork interactions
Network neuroscience views the brain as functioning via widely-dispersed but interconnected regions coordinating their activity across multiple timescales [61, 62]. Fine temporal resolution methods are therefore needed to track how neocortical dynamics are modulated at both fast (>40 Hz) and slow (<1 Hz) frequencies. IEEG is now beginning to furnish detailed data about fast temporal dynamics to complement slower-timescale data from neuroimaging. These new data can advance our understanding of intra- and internetwork interactions in several ways. First, iEEG can explore the electrophysiological correlates of intrinsic activity and connectivity and how these relate to network models from neuroimaging. Second, iEEG can explore the temporal priority of network recruitment during DN activation by examining the time course of activity throughout the brain. Third, direct electrical stimulation can be utilized to probe the directionality and timecourse of the DN’s intra- and internetwork interactions using cortico-cortical evoked potentials (CCEPs). We discuss each approach sequentially in the following sections.
Intrinsic activity of the default network
Scalp-based EEG has a long history of investigating spontaneous fluctuations in the brain’s electrical activity and identifying canonical oscillations reliably associated with various parts of the brain [63, 64]. Can similar observations be made for the DN? A study in 2012 [48] was the first to report that the PMC displayed strong intrinsic theta-band oscillations (~4–5 Hz peak) at rest, in contrast to nearby visual areas, which instead showed canonical alpha band oscillations (~8–10 Hz peak). Analysis of cross-frequency coupling revealed that HFB (70–180 Hz) amplitude at rest was coupled with the phase of ongoing theta oscillations in the PMC (i.e., phase-amplitude coupling, or PAC), suggesting that patterns of intrinsic activity in DN areas (at least PMC) are readily distinguishable from other brain regions [48]. This study demonstrated that the PMC shows patterns of intrinsic oscillatory activity that are distinct from nearby sensorimotor brain regions, yet similar to distal areas like the MTL [65], with which PMC likely cooperates to instantiate memory recall and other forms of internally-directed cognition [48]. More speculatively, the authors noted that theta/HFB coupling displayed slow modulation (<1 Hz), providing a potential link between local population coordination and slower intrinsic connectivity dynamics [48]. These findings were an important step toward identifying a potential oscillatory ‘signature’ for the DN, but did not test if these motifs are correlated across multiple DN regions.
Intrinsic connectivity of the default network
The defining feature of intrinsic brain networks identified with BOLD fMRI is the spontaneous correlation of slow (<0.1 Hz) signal fluctuations [66, 67]. Given that iEEG signals can vary on a much faster timescale than fMRI BOLD signals, how can these different timescales be linked? One common solution is to examine the low frequency components of fast activity by extracting the slow fluctuations (i.e., low-pass filtering) of the amplitude envelope of a frequency band of interest (e.g., the HFB range; [68, 69]).
To date, this approach has proven to be a fruitful assay of fMRI-like connectivity patterns [30]. For instance, taking such an approach to spontaneous iEEG connectivity, it has been shown [68] that slow spontaneous fluctuations (<0.1 Hz) in high-gamma (40–100 Hz) envelope signals were highly correlated between the left and right auditory cortex. Early on, researchers working with non-human primates had proposed that the anatomical selectivity of such correlations might mimic functional connectivity patterns seen in fMRI [69], a hypothesis echoed by others [68]. A number of follow-up studies have confirmed this prediction, showing consistent spatial patterns of BOLD fMRI and iEEG-envelope functional connectivity within subjects, including throughout many nodes of the DN [30, 70–72].
Employing a related yet analytically distinctive approach, intracranial recordings from medial PFC and PCC showed that these spatially distributed DN regions exhibited coherent activity peaking at ~0.017 Hz at higher frequencies (65–110 Hz) (i.e., envelope spectral coherence) [73]. This frequency of inter-regional coherence was almost identical to the peak power observed in intrinsic BOLD signal fluctuations recorded from the same patients [73]. Using methods more akin to earlier work [68], more recent research [30] showed that correlation patterns over medial and lateral parietal cortex using iEEG consistently identified DN subregions, specifically the PCC/RSC and angular gyrus (AG). These patterns of iEEG connectivity, using slow (<1 Hz) fluctuations in HFB amplitude, showed high spatial correlation with fMRI connectivity patterns within subjects [30]. Importantly, when the slow fluctuations of other frequency bands were explored (e.g., theta, alpha, beta), iEEG connectivity patterns, although observed, were anatomically coarse (extending into non-DN regions). This observation of significant, but anatomically non-specific, iEEG correlations for slow fluctuations of lower frequency bands is consistent with earlier observations [68]. However, more recent reports, focused on different DN regions, suggest that while slow fluctuations of high frequency activity is the most reliable correlate of resting state fMRI connectivity, other frequency bands might also play a role, and low frequency slow fluctuations may differ between intrinsic networks [71] (Fig. 3C). This work requires further exploration, and extension to other brain states where low frequency activity is more varied.
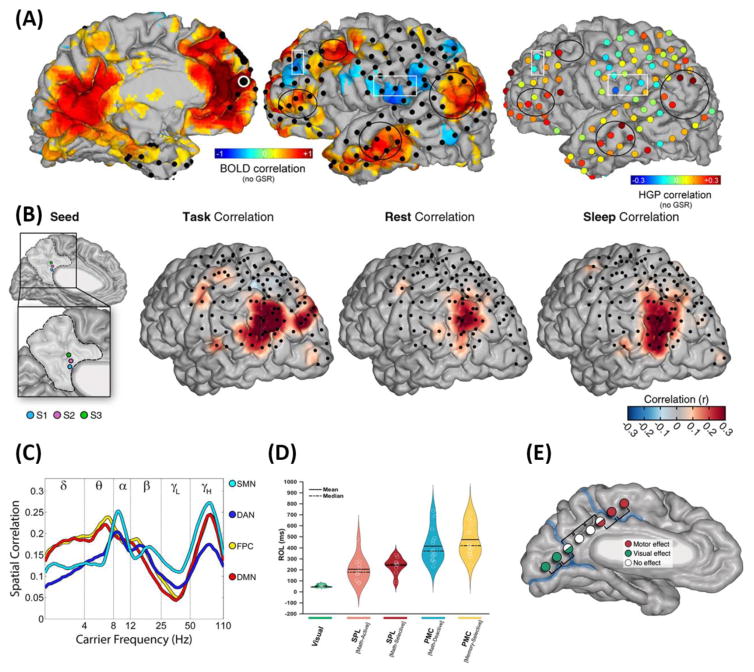
Figure 3. Intrinsic connectivity, internetwork interactions, and electrical stimulation of the default network.
(A) Considerable correspondence between maps of intrinsic functional connectivity as measured with fMRI BOLD (two leftmost panels) and iEEG power restricted to high gamma power (50–150 Hz) (rightmost panel) across lateral DN regions as well as other brain networks. Color indicates strength of correlated spontaneous activity at each point with a seed region in the medial PFC DN hub (white-rimmed circle in leftmost panel). Black circles highlight positive correlations with other default regions for both methods, and conversely, white boxes underscore anticorrelations with various non-default regions. (B) Using electrodes in the posterior cingulate cortex as seed regions, significant intrinsic connectivity is observed with the angular gyrus while performing an episodic memory task, at rest, and during sleep. (C) Spatial correlation of intrinsic connectivity maps across iEEG and fMRI BOLD in the default mode network (DMN) is driven most strongly by power in the theta (4–8 Hz) and higher frequency (50–100 Hz) ranges. Other networks (somatomotor network, SMN; dorsal attention network, DAN; and frontoparietal control network, FPC) show similarities but also differences in their correspondence spectra. (D) Precise timing of HFB activations in a DN hub (here, PMC) during an episodic memory task compared to its deactivation during math and activation of superior parietal lobule (SPL) regions during the same conditions. The delayed activation and deactivation of the PMC compared to SPL suggests that the DN lies higher in a hierarchy of information flow through the brain. (E) HF-EBS along the medial occipital and dorsomedial parietal cortical surface often elicits visual and motor effects, respectively, but has no effect within posterior parietal DN regions (PCC/RSC). Panel A reproduced, with permission, from [70]. Panel B reproduced, with permission, from [30]. Panel C reproduced, with permission, from [71]. Panel D reproduced, with permission, from [104]. Panel E reproduced, with permission, from [95].
If functional connectivity patterns truly reflect intrinsic network organization, they should exhibit some stability across brain states. Partially addressing this question, a recent study [30] investigated iEEG intrinsic connectivity between posterior nodes of the DN (PCC/RSC and AG) and found that, as anticipated, intrinsic connectivity was intact across rest, an internally-directed memory task, and sleep (Fig. 3B). This suggests that these slow fluctuations reflect intrinsic network properties that are subsequently modulated by behavioral state and cognition [74]. Nonetheless, although these regions were correlated across all conditions, they showed the highest correlation during the self-referential episodic memory condition, during which these regions are most actively engaged (Fig. 2F).
Collectively, iEEG has provided several important insights about the electrophysiology of DN intrinsic connectivity. First, a variety of methods have confirmed that the iEEG signal shows correlated slow intrinsic fluctuations (<1 Hz) between DN areas commensurate with slow spontaneous fluctuations in the fMRI BOLD signal [30, 48, 73, 75]. Second, these patterns of iEEG intrinsic connectivity correlate well with connectivity patterns in the fMRI BOLD signal at the same sites in the same subjects [30, 70, 71] (Fig. 3A). Third, power in specific frequency bands contributes most to slow iEEG fluctuations in intrinsic connectivity, with HFB the most consistent and anatomically specific correlate [30, 70, 71] – although other frequency bands (<20 Hz) might also play a role [71, 76] (Fig. 3C). These findings are important because different frequency bands reflect distinctive neurophysiological processes: whereas changes in HFB amplitude are likely to reflect population spiking, oscillatory activity in lower frequencies might reflect neurophysiological events such as excitability fluctuations that coordinate long-distance communication [77]. Finally, intrinsic connectivity between DN areas is preserved across multiple cognitive states (Fig. 3B) – a major corroboration of findings from neuroimaging [13–16]. Going beyond mere replication, however, iEEG has shown that cross-state patterns of connectivity similarity are largely limited to high beta and HFB power [30].
Temporal priority and internetwork coordination
The millisecond temporal resolution of iEEG allows for detailed examination of the timing of DN region recruitment, as well as DN communication with other networks, providing important clues about the mechanisms of intra- and internetwork communication. For instance, there are differences in mean deactivation latencies across DN areas (Fig. 2B) on the order of tens to hundreds of milliseconds [26–28] – far too fast to detect with present fMRI methods. With respect to activations, although the AG and PCC/RSC show no significant differences in their mean response times [30], this might not be true for more widely-dispersed regions within a network – a question that needs to 9be examined in future work. IEEG can also answer questions about the timing of internetwork interactions and information flow and their relation to DN activations. For instance, visual and attention networks are recruited faster than PCC and RSC during various forms of cognition (Fig. 3D). Taken together, subtle timing differences suggest that DN regions might not simply activate and deactivate en masse, despite appearances from functional neuroimaging; and internetwork timing differences hint at hierarchies of information flow, and possibly also control, within and between intrinsic networks – each of which may have a specific ‘temporal receptive window’ related to its optimal functioning [78].
Additionally, significant phase synchronization (specifically in the 3–5 Hz theta range) occurs between RSC and more anterior regions of the MTL (outside the DN) for episodic memory judgments, but not control conditions [51]. Critically, the theta-band synchronization between RSC and MTL consistently peaks prior to the onset of the relatively late (~400 ms) HFB responses in RSC, suggesting that internetwork interactions precede task-specific DN activations. These results are consistent with the finding (discussed above) that populations in the more ventral regions of the PMC, which have stronger anatomical connections to MTL regions [79, 80], display earlier activations in response to episodic memory recall than populations in more dorsal aspects of the PMC [28]. Together, the iEEG evidence strongly suggests that the DN activation consistently observed during memory recall is in fact preceded by activity in, and communication with, areas outside the DN, such as anterior aspects of the MTL [51, 81, 82]. Collectively, these fine-scale differences in temporal priority (Fig. 3D) have implications not only for our understanding of which regions initiate internally-directed forms of cognition and how [83], but also for models seeking to elucidate which regions preferentially ‘drive’ network activity and how these can be perturbed in the service of clinical interventions ([62]; Box 3).
Box 3. Electroceutical interventions for clinical dysfunctions of the default network.
Numerous psychiatric, neurological, and neurodegenerative diseases implicate the DN [121–124]. Developing a deeper understanding of the DN’s functioning in healthy people therefore lays the groundwork for better models of, and interventions for, neuropsychiatric disease. Non-invasive electroceutical interventions like transcranial magnetic stimulation are leading the way [125], but currently have limited ability to reach and specifically target deep brain structures (including many DN regions) in large brains, such as those of humans. As the limitations of lesioning, pharmaceuticals, and non-invasive electrical interventions for mental health disorders are increasingly appreciated [126–128], the use of iEEG methods for focal neuromodulation is also becoming increasingly common and sophisticated [114, 129–133] – and might also result in fewer long-term adverse effects [127].
Stimulation of DN regions per se is neither necessary for modulating DN function, nor necessarily the best means of doing so, because focal electrical stimulation has widespread effects on even distal brain regions [134, 135]. Pilot studies have already investigated how focal stimulation of particular regions influences DN activity and connectivity and how these changes might relate to symptom improvements in neuropsychiatric disease such as depression and obsessive-compulsive disorder [114, 115, 136–138], but these studies should be viewed as valuable proofs-of-concept rather than proven therapies. Nonetheless, DBS interventions are reversible, adaptable, and can be continually ameliorated as both technological expertise and understanding of brain disease improve [132, 133, 139]. Challenges on the immediate horizon include minimizing adverse side-effects of surgical implantation and subsequent stimulation [140, 141]; developing an understanding of neural mechanisms of action [134, 142]; exploring whether genetics predisposes particular individuals to being more or less responsive to intracranial stimulation, as is suggested for transcranial stimulation methods [143, 144]; and building ‘smart’ stimulation devices that act preemptively and/or adaptively [130, 145].
Directionality and timecourse of intra- and internetwork communication
Interactions between nodes within the DN, and between the default and other networks, can be directly investigated with iEEG using single pulse electrical stimulation (also known as cortico-cortical evoked potentials, or CCEPs; [84]). In this approach, repeated single-pulses of electrical charge are delivered at a target electrode site while recording the responses elicited at other electrodes throughout the brain [84–87]. Research has shown that the pattern and magnitude of evoked responses predicts subject-specific intrinsic network connectivity recorded independently with resting-state fMRI [85]. Many questions about DN function and connectivity could be explored using similar paradigms to investigate both the magnitude and timing of CCEPs. For instance, several detailed explorations of the magnitude of CCEPs suggest that many bidirectional connections are asymmetrical (i.e., show differential strength) [86, 88, 89]. A straightforward interpretation of this asymmetry is that for any given pair of brain regions, certain regions (‘projectors’) are better at driving activity in the reciprocally connected brain area, whereas others (‘integrators’) play a more integrative or computational role [86, 88, 89].
A recent study went beyond investigation of the magnitude of CCEPs to examine their detailed directionality and temporal dynamics [87]. The researchers functionally identified iEEG electrode locations within subject-specific networks of interest (default, frontoparietal, and salience networks) using pre-surgical resting-state fMRI intrinsic connectivity in the same subjects. The CCEPs that resulted from stimulating sites within each network revealed two important findings. First, interactions among the three intrinsic networks were not mutually bidirectional: evoked responses elicited across networks were clearly asymmetrical. Second, temporal patterns of signal propagation within and between networks were not equivalent: stimulation of the frontoparietal and salience networks evoked strong responses at an earlier processing stage in all networks, whereas stimulating the DN influenced the frontoparietal and salience networks at a significantly later stage (>100 ms difference) [87]. These findings suggest differences in ‘temporal receptive window’ across networks [78]: the DN may be influencing other networks after they have finished their own local processing, whereas the engagement of salience and frontoparietal networks might make a more instantaneous impact on other networks – a hypothesis needing further exploration.
Probing the causal importance of the default network
Injecting high frequency electrical current (typically ≥50 Hz, ~1–10 mA) into the brain has long been known to both elicit subjective experiences [90, 91] and sometimes disrupt ongoing cognitive or motor processes [92]. High frequency electrical brain stimulation (hf-EBS) has been applied widely throughout cortical and subcortical structures in clinical ‘functional mapping’ sessions [93, 94], but despite providing uniquely causal information about a region’s functioning, reports of hf-EBS to DN areas, especially the major hubs along the medial surface of the brain, have been scarce.
Administering more than 800 electrical stimulations throughout medial posterior brain regions in 25 passively resting epilepsy patients, our group [95] found that stimulation to PCC and RSC never yielded subjective experiences or disturbances of any kind (Fig. 3E). Given the role of these areas in memory recall [96], and their selective iEEG activity both at rest and during self-referential thinking and memory recall [28–30, 52], it seems likely that hf-EBS administered during internally-directed tasks could cause an interruption in these cognitive functions, albeit without eliciting effects in the subjective or perceptual domain.
HF-EBS has been applied to various other putative default regions in scattered case reports [93], but virtually none of these studies has confirmed that stimulation sites were specifically within DN boundaries or undertaken a systematic investigation including reporting of null effects. A thorough understanding of the effects of hf-EBS to DN regions is an important project for future research, and will require targeted studies employing large cohorts, precise electrode localization, and detailed subjective reports.
Intracranial electrophysiology of the non-human default network
Although the explicit focus of this review has been on the human default network, recent years have seen the discovery of parallel (and possibly homologous) ‘default’ networks in the macaque [17], chimpanzee [97], cat [98], rat [99], and mouse [100]. A deep understanding of the human DN will need to include an account of the ways in which it is similar to potentially homologous networks in other mammals, but also what makes it unique. Here we provide a brief sketch of what is known so far about such similarities and differences, as well as highlighting how human iEEG provides unique information that goes beyond findings from animal studies.
First, functional neuroimaging methods have delineated intrinsically connected networks in animals that roughly parallel the human DN, including nodes in medial PFC, PMC, lateral parietal cortex, and lateral temporal cortex [17, 97–100]. Despite the importance of these findings, cross-state comparisons have so far been sparse in animal studies because fMRI without anesthesia is challenging in most species (for an exception, see [101]). In humans, conversely, it has been straightforward to demonstrate the persistence of DN intrinsic connectivity across multiple conscious states with both fMRI and iEEG (Fig. 3B; [30]).
Second, task-induced deactivation of a DN region has been reported in monkeys: having macaques engage in attention or working memory tasks reliably suppressed neuron spiking in CGp [33] (a homolog of human PCC). Moreover, and reminiscent of findings in humans discussed above, less suppression of activity in CGp predicted performance errors as well as slower reaction times, again corroborating the findings from human research that DN deactivation has functional significance.
While animal studies have therefore been able, in a limited way, to study DN intrinsic connectivity (usually under anesthesia) and task-induced deactivations (in a single DN region), we are aware of no reports of task-induced activations specifically investigating DN function in the animal literature. The growing body of iEEG research investigating the many complex forms of cognition that activate DN regions in humans (such as episodic memory recall and self-referential judgments; [28–30, 51, 52]) will therefore provide a unique contribution to our understanding of the DN.
Concluding remarks and future perspectives
Investigation of the DN has progressed rapidly since its initial discovery and has now become a major research program. The success of this project depends critically on a clear recognition of the strengths and weaknesses of the various tools at our disposal – and on their resourceful combination in research contexts. While iEEG offers spatially and temporally precise neural information at the implanted electrode sites, electrode coverage can be sparse in any given patient, and standard iEEG electrodes simultaneously record the activity of hundreds of thousands of neurons [19]. Specialized microelectrodes can resolve the differential responses of individual neurons in the human brain [102], but such methods are still employed only rarely in iEEG research.
Combining iEEG with other methods, such as fMRI, can often mitigate these concerns by providing broader context and mutually corroborative data (Box 2). And despite its limitations, human iEEG goes beyond simply replicating what is already known, or can be known, from non-invasive lines of research in humans or from invasive recordings in non-human mammalian brains. By implementing intracranial methods in human participants who can provide self-reports and execute complex cognitive-affective tasks, iEEG is making a unique contribution by illuminating what DN activation, suppression, and connectivity are (in terms of underlying electrophysiology) and what they mean (in terms of behavioral and cognitive consequences for ‘higher’ mental processes).
The major task ahead for the field is to continue moving beyond a purely correlational understanding of the DN toward high spatiotemporal resolution models of (i) electrophysiological foundations, (ii) intraregional functional heterogeneity, (iii) intra- and internetwork communication, (iv) neural and subjective effects of direct electrical perturbation, and (iv) relationships with cognition and behavior (see Outstanding Questions). Clinicians can then leverage the knowledge gleaned from this developing neuromechanistic account to (v) better understand, and hopefully treat, neurological and psychiatric conditions involving DN dysfunction (Box 3). Advancing our understanding of the DN will therefore have many ramifications both for fathoming the marvelous complexity of the healthy brain at work, as well as treating the many ways this functioning can go awry in brain disease.
Outstanding Questions Box.
Beyond suppression of HFB power, how is ‘deactivation’ instantiated at the single-cell level in the DN and elsewhere? Can single-neuron studies in humans [163] clarify whether reduced neuronal activity (i.e., spiking) plays a role and whether particular neuronal types (e.g., interneurons) are involved?
DN regions show fast (~300 ms) responses during disengagement from externally-directed tasks and the return to baseline [29]. What is the functional significance of these positive responses and what neural mechanism drives them? How are they related to later (~500 ms) DN responses evoked by internally-directed cognition [28, 30]?
Distinct neuronal populations with unique response profiles are interlaced throughout the medial parietal DN hub. What are the extent and significance of this intraregional functional heterogeneity in other DN regions?
Functional neuroimaging has recently linked DN activation to a variety of behavioral correlates, including attentional stability [164], tracking changing narratives [165], and functioning on ‘autopilot’ [166]. What is the electrophysiological basis of these behavioral correlates and how does it relate to DN activations in relation to internally-directed forms of cognition?
What is the electrophysiological basis of anticorrelated network activity, and are specific frequency ranges of particular importance? How are both competitive and cooperative internetwork interactions mediated and instantiated at the electrophysiological level at rest and during tasks?
What mechanism drives the slow dynamics of DN intrinsic connectivity? Is this slow variation causally linked to other important psychophysiological processes? Are glial cells important?
Reports of direct electrical stimulation of default areas have mostly been haphazard. What are the subjective effects of directly stimulating default areas, and does stimulation affect or impair performance on externally- and/or internally-directed tasks?
There is mounting evidence for homologous ‘default’ networks in non-human primates and rodents. How do these networks differ functionally and electrophysiologically from the human DN? What other species exhibit such a network?
How can iEEG methods best be harnessed to understand and treat the many neuropsychiatric disorders that implicate the DN? What effect does focal neuromodulation have on the wider dynamics of the default and other brain networks?
The DN includes aspects of several subcortical structures, particularly the cerebellum [167]. What are the electrophysiological dynamics of these subcortical DN areas? Can iEEG methods clarify how, and on what timescale, they participate in DN functioning?
Outstanding Questions Box.
How is ‘deactivation’ instantiated at the single-cell level in the DN and elsewhere? Can single-neuron studies in humans clarify whether reduced single neuronal activity (i.e., firing rate) plays any functional role and whether particular neuronal types (e.g., interneurons) are involved?
What is the functional significance of the rapid (<300ms) responses in DN regions during the switch from engagement in externally-directed tasks to rest? What neural mechanisms are involved? Is this “switch signal” the cause or the result of disengagement from externally oriented tasks?
What is the significance of functionally heterogeneous neuronal populations within other DN regions beyond the PMC?
What is the electrophysiological basis of DN involvement in behavioral correlates such as attentional stability, tracking changing narratives, and functioning on ‘autopilot’?
How are both competitive and cooperative internetwork interactions mediated and instantiated at the electrophysiological level, at rest and during tasks? Are specific frequency ranges of electrophysiological activity of particular importance?
What mechanisms drive the slow dynamics of DN intrinsic connectivity? Is this slow variation causally linked to other important psychophysiological processes? Are glial cells important?
What are the subjective effects of direct electrical stimulation to DN areas beyond the PMC? Does stimulation affect or impair performance on externally- and/or internally-directed tasks?
How do putatively homologous ‘default’ networks in non-human primates and rodents differ functionally and electrophysiologically from the human DN?
How can iEEG methods best be harnessed to understand and treat the many neuropsychiatric disorders that implicate the DN? What effect does focal neuromodulation have on the wider dynamics of the default and other brain networks?
What are the electrophysiological dynamics and functional role of subcortical components of the DN, such as the thalamus and cerebellum?
Trends Box.
Understanding of the default network (DN) is rapidly progressing from a relatively coarse picture toward more detailed models in which network nodes contain functionally heterogeneous neuronal populations with distinctive intra- and internetwork connectivity
Intracranial electroencephalography (iEEG) is providing unique data about the precise temporal profiles of neuronal populations throughout the DN, and their functional role(s) in the network’s deactivations, activations, intrinsic activity, and interactions with other brain networks
IEEG is also providing critical corroborative data elucidating the electrophysiological foundations of signals observed with neuroimaging
IEEG methods are being used not only to passively record but also to causally probe and perturb the DN’s functioning and interactions with other networks
Intracranial methods are being developed as electroceutical interventions for neuropsychiatric disorders implicating the DN
Acknowledgments
The authors are grateful to the many patients who have volunteered to participate in the studies described here, without whom this research would be impossible, as well as numerous funding agencies for their generous support. K.C.R.F. is supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council (NSERC) of Canada. B.L.F. is supported by a research grant from the U.S. National Institute of Mental Health (R00MH103479). A.K. is supported by a Banting Postdoctoral Fellowship from the Canadian Institutes of Health Research (CIHR). A.L.D. is supported by a postdoctoral fellowship from the National Institute of Child Health and Human Development (1F32HD087028-01). J.P. is supported by research grants from the U.S. National Institute of Neurological Disorders and Stroke (R01NS078396), U.S. National Institute of Mental Health (1R01MH109954-01) and the U.S. National Science Foundation (BCS1358907).
Glossary
- Default network (DN)
a collection of associative brain regions that consistently deactivate during externally-directed tasks (e.g., visual search), while conversely showing activation during internally-directed tasks (e.g., memory retrieval). Like other intrinsic networks, DN regions display robustly correlated hemodynamic activity during resting states. Anatomically, key nodes of the DN include the medial prefrontal cortex, medial parietal cortex (retrosplenial cortex, posterior cingulate cortex), aspects of the medial temporal lobe, inferior lateral parietal cortex (angular gyrus), middle temporal gyrus, and parts of ventrolateral and dorsolateral frontal cortex (Fig. 1).
- Electroceutical interventions
clinical interventions utilizing intracranial electrical stimulation and modulation to ameliorate neurodegenerative (e.g., Parkinson’s disease) and psychiatric (e.g., major depression) illness; focal neuromodulation nonetheless has indirect effects on the metabolism and functioning of widespread brain networks.
- Envelope
an alternative term for the time varying ‘analytic amplitude’ of a frequency band limited signal, typically squared to obtain ‘power’. A common metric used for correlating task or resting state activity between iEEG recording sites.
- High frequency broadband (HFB)
non-oscillatory, high frequency (>50 Hz) activity in the cortical field potential; a primary signal of interest in iEEG studies, known to be correlated with the fMRI BOLD signal as well as averaged neuronal population spiking activity.
- High frequency electrical brain stimulation (hf-EBS)
injection of electrical current into brain tissue via intracranial electrodes, typically using square-wave pulses at ≥50 Hz and 1–10 mA. Routinely used in clinical mapping sessions to determine localization of function, hf-EBS often elicits subjective experiences or perturbs ongoing cognition and perception, providing a potent tool for causally exploring regional functionality.
- Intracranial electroencephalography (iEEG)
a suite of methods for invasively monitoring the brain’s electrical activity and directly stimulating brain tissue. Includes grids and strips of electrodes placed subdurally on the cortical surface (also known as electrocorticography, or ECoG), as well as depth electrodes that penetrate brain tissue and reach deep cortical and subcortical structures (also known as stereotactic EEG, or SEEG).
- Intrinsic brain network
a set of spatially segregated brain regions displaying inter-regional correlated spontaneous activity (on the scale of minutes and longer) that is persistent across cognitive and conscious states (e.g., tasks and rest, waking and sleep).
- Neuronal population
the group of nearby neurons (~200,000–500,000) thought to make the main contribution to the local signal recorded from a given intracranial electrode contact.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts RM. Serendipity: Accidental discoveries in science. Wiley; 1989. [Google Scholar]
- 2.Kuhn TS. The Structure of Scientific Revolutions. 2. University of Chicago Press; 1975. [Google Scholar]
- 3.Buckner RL. The serendipitous discovery of the brain’s default network. Neuroimage. 2012;62(2):1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98(2):678–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 6.Raichle ME. A paradigm shift in functional brain imaging. J Neurosci. 2009;29(41):12729–34. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14(4):180–90. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature reviews Neuroscience. 2001;2(10):685. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 9.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckmann CF, et al. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damoiseaux J, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greicius MD, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole MW, et al. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krienen FM, et al. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Phil Trans R Soc B. 2014;369(1653):20130526. doi: 10.1098/rstb.2013.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson-Prior LJ, et al. Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences. 2009;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhaudenhuyse A, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–71. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 18.Raichle ME. The brain’s default mode network. Annual Review of Neuroscience. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Kastner S. Mapping the functional architecture of the human brain with intracranial EEG: The past, the present, and the promises ahead. Nature Neuroscience 2018 [Google Scholar]
- 20.Logothetis NK, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 21.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309(5736):951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 22.Nir Y, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Current Biology. 2007;17(15):1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 23.Lachaux JP, et al. Silence is golden: transient neural deactivation in the prefrontal cortex during attentive reading. Cerebral Cortex. 2008;18(2):443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- 24.Jung J, et al. Brain responses to success and failure: direct recordings from human cerebral cortex. Human Brain Mapping. 2010;31(8):1217–1232. doi: 10.1002/hbm.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller KJ, et al. Direct electrophysiological measurement of human default network areas. Proceedings of the National Academy of Sciences. 2009;106(29):12174–12177. doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerbi K, et al. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Frontiers in Systems Neuroscience. 2010:4. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossandón T, et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. The Journal of Neuroscience. 2011;31(41):14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster BL, et al. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. Proceedings of the National Academy of Sciences. 2012;109(38):15514–15519. doi: 10.1073/pnas.1206580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dastjerdi M, et al. Differential electrophysiological response during rest, self-referential, and non–self-referential tasks in human posteromedial cortex. Proceedings of the National Academy of Sciences. 2011;108(7):3023–3028. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster BL, et al. Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron. 2015;86(2):578–590. doi: 10.1016/j.neuron.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birn RM, et al. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Human Brain Mapping. 2008;29(7):740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shmuel A, et al. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36(6):1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- 33.Hayden BY, et al. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proceedings of the National Academy of Sciences. 2009;106(14):5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramot M, et al. A widely distributed spectral signature of task-negative electrocorticography responses revealed during a visuomotor task in the human cortex. Journal of Neuroscience. 2012;32(31):10458–10469. doi: 10.1523/JNEUROSCI.0877-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daitch AL, Parvizi J. Heterogeneous memory- and rest-related activity within the human posteromedial cortex. Annual Meeting of the Organization for Human Brain Mapping; 2017. [Google Scholar]
- 36.Weissman D, et al. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 37.McKiernan KA, et al. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 38.Mason MF, et al. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teasdale JD, et al. Stimulus-independent thought depends on central executive resources. Memory & Cognition. 1995;23(5):551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 40.Dixon ML, et al. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia. 2014;62:321–330. doi: 10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Buckner RL, et al. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 42.Gusnard DA, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox KCR, et al. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 44.Christoff K, et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106(21):8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Addis DR, et al. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svoboda E, et al. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller KJ, et al. Spectral changes in cortical surface potentials during motor movement. Journal of Neuroscience. 2007;27(9):2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foster BL, Parvizi J. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. Neuroimage. 2012;60(1):384–391. doi: 10.1016/j.neuroimage.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spreng RN, et al. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 50.Andrews-Hanna JR, et al. The default network and self-generated thought: Component processes and dynamic control. Annals of the New York Academy of Sciences. 2014;1316(1):29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster BL, et al. Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. The Journal of Neuroscience. 2013;33(25):10439–10446. doi: 10.1523/JNEUROSCI.0513-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daitch AL, Parvizi J. Functional heterogeneity within the human posteromedial cortex; Proceedings of the National Academy of Sciences; under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Northoff G, et al. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Andrews-Hanna JR, et al. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braga RM, Buckner RL. Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity. Neuron. 2017;95(2):457–471. e5. doi: 10.1016/j.neuron.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leech R, et al. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. Journal of Neuroscience. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margulies DS, et al. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Crittenden BM, et al. Recruitment of the default mode network during a demanding act of executive control. Elife. 2015;4:e06481. doi: 10.7554/eLife.06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayden BY, et al. Cognitive control signals in posterior cingulate cortex. Frontiers in Human Neuroscience. 2010:4. doi: 10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellamil M, et al. Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. NeuroImage. 2016;136:186–196. doi: 10.1016/j.neuroimage.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 61.Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nature Neuroscience. 2014;17(5):652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- 62.Bassett DS, Sporns O. Network neuroscience. Nature Neuroscience. 2017;20(3):353. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adrian ED, Matthews BH. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57(4):355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- 64.Berger H. Über das elektrenkephalogramm des menschen. European Archives of Psychiatry and Clinical Neuroscience. 1929;87(1):527–570. [Google Scholar]
- 65.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 66.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo planar mri. Magnetic resonance in medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 67.Buckner RL, et al. Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 68.Nir Y, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nature Neuroscience. 2008;11(9):1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leopold DA, et al. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cerebral Cortex. 2003;13(4):422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 70.Keller CJ, et al. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. Journal of Neuroscience. 2013;33(15):6333–6342. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hacker CD, et al. Frequency-specific electrophysiologic correlates of resting state fMRI networks. Neuroimage. 2017;149:446–457. doi: 10.1016/j.neuroimage.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kucyi A, et al. Cosistent ECoG-fMRI correspondence of intrinsic networks across fMRI denoising strategies. 47th Annual Meeting of the Society for Neuroscience; Washington, D.C. 2017. [Google Scholar]
- 73.Ko AL, et al. Quasi-periodic fluctuations in default mode network electrophysiology. The Journal of Neuroscience. 2011;31(32):11728–11732. doi: 10.1523/JNEUROSCI.5730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. [Google Scholar]
- 75.Ko AL, et al. Identifying functional networks using endogenous connectivity in gamma band electrocorticography. Brain Connectivity. 2013;3(5):491–502. doi: 10.1089/brain.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, et al. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76(5):1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Honey CJ, et al. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. 2012;76(2):423–434. doi: 10.1016/j.neuron.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. Journal of Comparative Neurology. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 80.Parvizi J, et al. Neural connections of the posteromedial cortex in the macaque. Proceedings of the National Academy of Sciences. 2006;103(5):1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke JF, et al. Theta and high-frequency activity mark spontaneous recall of episodic memories. The Journal of Neuroscience. 2014;34(34):11355–11365. doi: 10.1523/JNEUROSCI.2654-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gelbard-Sagiv H, et al. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322(5898):96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox KCR, et al. The neurobiology of self-generated thought from cells to systems: Integrating evidence from lesion studies, human intracranial electrophysiology, neurochemistry, and neuroendocrinology. Neuroscience. 2016;335:134–150. doi: 10.1016/j.neuroscience.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 84.Matsumoto R, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127(10):2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- 85.Keller CJ, et al. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proceedings of the National Academy of Sciences. 2011;108(25):10308–10313. doi: 10.1073/pnas.1019750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keller CJ, et al. Corticocortical evoked potentials reveal projectors and integrators in human brain networks. Journal of Neuroscience. 2014;34(27):9152–9163. doi: 10.1523/JNEUROSCI.4289-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shine JM, et al. Distinct patterns of temporal and directional connectivity among intrinsic networks in the human brain. The Journal of Neuroscience. 2017;37(40):9667–9674. doi: 10.1523/JNEUROSCI.1574-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Entz L, et al. Evoked effective connectivity of the human neocortex. Human Brain Mapping. 2014;35(12):5736–5753. doi: 10.1002/hbm.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keller CJ, et al. Mapping human brain networks with cortico-cortical evoked potentials. Phil Trans R Soc B. 2014;369(1653):20130528. doi: 10.1098/rstb.2013.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bartholow R. Art. I.—experimental Investigations into the Functions of the Human Brain. The American Journal of the Medical Sciences. 1874;66(134):305–313. [Google Scholar]
- 91.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain: A journal of neurology. 1937;60:389–443. [Google Scholar]
- 92.Ojemann G, et al. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. Journal of neurosurgery. 1989;71(3):316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 93.Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience. 2010:4. doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed]
- 94.Borchers S, et al. Direct electrical stimulation of human cortex--the gold standard for mapping brain functions? Nature reviews. Neuroscience. 2012;13(1):63. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- 95.Foster BL, Parvizi J. Direct cortical stimulation of human posteromedial cortex. Neurology. 2017;88(7):685–691. doi: 10.1212/WNL.0000000000003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience. 2004;24(45):10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rilling JK, et al. A comparison of resting-state brain activity in humans and chimpanzees. Proceedings of the National Academy of Sciences. 2007;104(43):17146–17151. doi: 10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Popa D, et al. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. Journal of Neuroscience. 2009;29(4):1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu H, et al. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences. 2012;109(10):3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stafford JM, et al. Large-scale topology and the default mode network in the mouse connectome. Proceedings of the National Academy of Sciences. 2014;111(52):18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barttfeld P, et al. Signature of consciousness in the dynamics of resting-state brain activity. Proceedings of the National Academy of Sciences. 2015;112(3):887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rutishauser U, et al. Single-neuron representation of memory strength and recognition confidence in left human posterior parietal cortex. Neuron. 2018;97(1):209–220. e3. doi: 10.1016/j.neuron.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends in cognitive sciences. 2010;14(4):162–171. doi: 10.1016/j.tics.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raccah O, et al. Relative timing of opposing responses in default mode and dorsal attention networks. Annual Meeting of the Organization for Human Brain Mapping; Vancouver, B.C., Canada. 2017. [Google Scholar]
- 105.Greicius MD, et al. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2008;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buzsáki G, et al. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nature Reviews Neuroscience. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuste R. From the neuron doctrine to neural networks. Nature Reviews Neuroscience. 2015;16(8):487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 108.Mueller S, et al. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77(3):586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gordon EM, et al. Individual variability of the system-level organization of the human brain. Cerebral Cortex. 2017;27(1):386–399. doi: 10.1093/cercor/bhv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laumann TO, et al. Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87(3):657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang D, et al. Parcellating cortical functional networks in individuals. Nature Neuroscience. 2015;18(12):1853. doi: 10.1038/nn.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parvizi J, et al. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80(6):1359–1367. doi: 10.1016/j.neuron.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Figee M, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nature Neuroscience. 2013;16(4):386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- 114.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Lozano AM, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 116.Carmichael DW, et al. Functional MRI with active, fully implanted, deep brain stimulation systems: safety and experimental confounds. Neuroimage. 2007;37(2):508–517. doi: 10.1016/j.neuroimage.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 117.Carmichael DW, et al. Feasibility of simultaneous intracranial EEG-fMRI in humans: a safety study. Neuroimage. 2010;49(1):379–390. doi: 10.1016/j.neuroimage.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 118.Dalal SS, et al. Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage. 2009;45(4):1289–1304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 119.Dubarry AS, et al. Simultaneous recording of MEG, EEG and intracerebral EEG during visual stimulation: from feasibility to single-trial analysis. Neuroimage. 2014;99:548–558. doi: 10.1016/j.neuroimage.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 120.Logothetis NK, et al. The effects of electrical microstimulation on cortical signal propagation. Nature neuroscience. 2010;13(10):1283–1291. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- 121.Christoff K, et al. Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 122.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 123.Kaiser RH, et al. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anticevic A, et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuo MF, et al. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2014;85:948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 126.Underwood E. Cadaver study casts doubts on how zapping brain may boost mood, relieve pain. Sciencemag.org 2016 [Google Scholar]
- 127.Kern DS, Kumar R. Deep brain stimulation. The Neurologist. 2007;13(5):237–252. doi: 10.1097/NRL.0b013e3181492c48. [DOI] [PubMed] [Google Scholar]
- 128.Cipriani A, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. The Lancet. 2009;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 129.Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. New England Journal of Medicine. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 130.Fischell RE, et al. System for treatment of neurological disorders. Google Patents. 2000 [Google Scholar]
- 131.Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annual Review of Neuroscience. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Ridder D, et al. Neuromodulation: Technology at the Neural Interface. 2017. State of the Art: Novel Applications for Cortical Stimulation. [DOI] [PubMed] [Google Scholar]
- 133.Roy HA, et al. State of the Art: Novel Applications for Deep Brain Stimulation. Neuromodulation: Technology at the Neural Interface. 2017 doi: 10.1111/ner.12604. [DOI] [PubMed] [Google Scholar]
- 134.McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiology of disease. 2010;38(3):329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barabási AL, et al. Network medicine: a network-based approach to human disease. Nature reviews Genetics. 2011;12(1):56. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Figee M, et al. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biological psychiatry. 2011;69(9):867–874. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 137.Menzies L, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harrison BJ, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66(11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 139.Morishita T, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475–484. doi: 10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kisely S, et al. Deep brain stimulation for obsessive–compulsive disorder: a systematic review and meta-analysis. Psychological medicine. 2014;44(16):3533–3542. doi: 10.1017/S0033291714000981. [DOI] [PubMed] [Google Scholar]
- 141.Saleh C, Fontaine D. Deep brain stimulation for psychiatric diseases: what are the risks? Current psychiatry reports. 2015;17(5):33. doi: 10.1007/s11920-015-0565-1. [DOI] [PubMed] [Google Scholar]
- 142.Fox MD, et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proceedings of the National Academy of Sciences. 2014;111(41):E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chhabra H, et al. Transcranial direct current stimulation and neuroplasticity genes: implications for psychiatric disorders. Acta neuropsychiatrica. 2016;28(1):1–10. doi: 10.1017/neu.2015.20. [DOI] [PubMed] [Google Scholar]
- 144.Cheeran B, et al. A common polymorphism in the brain derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of physiology. 2008;586(23):5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Ridder D, Vanneste S. Visions on the future of medical devices in spinal cord stimulation: what medical device is needed? Expert review of medical devices. 2016;13(3):233–242. doi: 10.1586/17434440.2016.1136560. [DOI] [PubMed] [Google Scholar]
- 146.Miller KJ. Broadband spectral change: evidence for a macroscale correlate of population firing rate? Journal of Neuroscience. 2010;30(19):6477–6479. doi: 10.1523/JNEUROSCI.6401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Winawer J, et al. Asynchronous broadband signals are the principal source of the BOLD response in human visual cortex. Current Biology. 2013;23(13):1145–1153. doi: 10.1016/j.cub.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiruska P, et al. Update on the mechanisms and roles of high frequency oscillations in seizures and epileptic disorders. Epilepsia. 2017;58(8):1330–1339. doi: 10.1111/epi.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schevon CA, et al. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132(11):3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ray S, et al. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. Journal of Neuroscience. 2008;28(45):11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS biology. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu J, Newsome WT. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. Journal of Neuroscience. 2006;26(30):7779–7790. doi: 10.1523/JNEUROSCI.5052-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kreiman G, et al. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron. 2006;49(3):433–445. doi: 10.1016/j.neuron.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 154.Manning JR, et al. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Niessing J, et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309(5736):948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 156.Flinker A, et al. Sub-centimeter language organization in the human temporal lobe. Brain and language. 2011;117(3):103–109. doi: 10.1016/j.bandl.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mesgarani N, et al. Phonetic feature encoding in human superior temporal gyrus. Science. 2014 doi: 10.1126/science.1245994. 1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hermes D, et al. Neurophysiologic correlates of fMRI in human motor cortex. Human Brain Mapping. 2012;33(7):1689–1699. doi: 10.1002/hbm.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. science. 2006;313(5793):1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park SH, et al. Functional subpopulations of neurons in a macaque face patch revealed by single-unit fMRI mapping. Neuron. 2017;95(4):971–981. e5. doi: 10.1016/j.neuron.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Katzner S, et al. Local origin of field potentials in visual cortex. Neuron. 2009;61(1):35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Engel AK, et al. Stimulus Dependent Neuronal Oscillations in Cat Visual Cortex: Inter Columnar Interaction as Determined by Cross Correlation Analysis. European Journal of Neuroscience. 1990;2(7):588–606. doi: 10.1111/j.1460-9568.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 163.Fried I, et al. Single Neuron Studies of the Human Brain: Probing Cognition. MIT Press; 2014. [Google Scholar]
- 164.Kucyi A, et al. Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proceedings of the National Academy of Sciences. 2016;113(48):13899–13904. doi: 10.1073/pnas.1611743113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Simony E, et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nature communications. 2016:7. doi: 10.1038/ncomms12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vatansever D, et al. Default mode contributions to automated information processing. Proceedings of the National Academy of Sciences. 2017 doi: 10.1073/pnas.1710521114. 201710521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of neuroscience. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]