Abstract
Background
On target 18F-Flortaucipir (FTP) binding of Alzheimer’s disease tau aggregates and off-target binding of melanocytes have been demonstrated with autoradiography.
Objective
We aimed to investigate the hypothesis that if binding in choroid plexus (CP) is due to melanocytes, the signal would be elevated in Black/African American (B/AA) compared to White (W) participants. In addition, we examined whether CP signal affects measurements in adjacent regions, and whether correcting for spill-in effects has an influence on associations between hippocampus (HC) FTP and amyloid or cognition.
Methods
FTP race differences in 147 Harvard Aging Brain Study participants (23 B/AA, 124W) were examined in CP, HC, HC covaried for CP, amygdala, inferior temporal gyrus, entorhinal cortex, and fusiform regions. Associations between CP FTP and other regions-of-interest (ROIs) were probed to assess spill-in effects. A statistical regression approach to attenuate CP spill-in was tested by relating adjusted HC SUVR residuals and unadjusted HC SUVR to race, cognition and amyloid. All analyses were covaried for age, sex, education and amyloid deposition, and Bonferroni-corrected for multiple comparisons.
Results
B/AA individuals had elevated CP and HC SUVR (p < 0.007), whereas other ROI SUVR and HC SUVR covaried for CP SUVR did not show race differences (p > 0.05). CP SUVR was associated with HC SUVR (p < 10−14), but with no other ROI SUVR (p > 0.05). When adjusting HC SUVR for CP SUVR, no race differences in residual HC SUVR were detected, and relationships with amyloid and memory became apparent.
Conclusion
Melanocyte FTP binding may account partially for high CP signal. This off-target binding affects mainly HC FTP measurements, which should be interpreted with caution.
Keywords: Alzheimer’s disease, choroid plexus, off-target binding, melanin, race, tau PET
INTRODUCTION
Alzheimer’s disease (AD) is defined by two proteinaceous brain lesions, amyloid plaques (Aβ) and tau neurofibrillary tangles, both accumulating before onset of clinical symptoms. Aβ and, more recently, tau pathologies are now readily detectable in vivo using positron emission tomography (PET) [1]. Tau PET acquired with 18F-Flortaucipir (FTP) reflects both the anatomic spread of AD-related tau pathology and its associations with clinical diagnosis and cognitive impairment [2–5]. Autoradiography studies using human brain tissue samples from multiple neurodegenerative disorders have largely confirmed the specific binding of FTP to paired helical filaments (PHF) tau-containing neurofibrillary tangles and dystrophic neurites in AD brains. In comparison, FTP binding is absent or very low in tau aggregates comprised by straight filaments as well as in alpha-synuclein or TDP-43 deposits [6–9].
In vivo and autoradiography studies suggest that FTP PET may be useful for detection of PHF-tau aggregates in AD; however, both have also identified other tracer binding substrates that appear not to be tau-related, so called “off-target” binding [7, 8, 10]. FTP PET signal is particularly prominent within the choroid plexus (CP) among a subset of individuals [5], but the substrate of this binding has not been established. This finding is of particular interest because of the close proximity of CP to the hippocam-pus (HC)—a region that is crucial in the study of AD tauopathy because its involvement is thought to occur at a critical stage of AD tauopathy progression [11]. CP consists of a dense collection of capillaries in an ependymal stroma surrounded by a layer of epithelium [12]. The CP of the lateral ventricles contain several materials that could possibly bind to FTP, including melanin [13], calcification/mineralization [14], Biondi rings [15, 16], and iron deposits [17]. Monoamine oxidase A has also been cited as a source of off-target binding [10, 18]. We hypothesized that if FTP were binding to melanocytes in CP, then CP FTP PET measures would be elevated in Black/African American (B/AA) participants compared to White (W) participants. We also compared FTP binding in CP to binding in nearby regions-of-interest (ROIs), to assess the relative impact of signal spill-in, and tested whether ROI group differences were related to proximity to CP. To further understand the influence of potential spill-in of CP FTP signal, we investigated whether associations between HC FTP measurement and Aβ or memory performance can be observed as expected based on the autopsy literature [19, 20], when using an unadjusted HC FTP measure or a HC FTP measure adjusted for CP binding.
MATERIALS AND METHODS
Participants
Participants included in this study were enrolled in the Harvard Aging Brain Study (HABS), a longitudinal study on aging and AD. All participants provided informed consent and all study procedures were approved by the Partners Human Research Committee. All participants underwent at least one comprehensive medical and neurological evaluation, and none had medical or neurological disorders that might contribute to cognitive dysfunction, including a history of alcoholism, drug abuse, or head trauma; or a family history of autosomal dominant AD. Presence of clinical depression (Geriatric Depression scale < 11) or other psychiatric illness, history of alcoholism, drug abuse, head trauma, or a family history of autosomal dominant AD was an exclusion criteria.
Participants underwent clinical and cognitive evaluations, including the Clinical Dementia Rating (CDR) Scale and the Mini-Mental State Examination (MMSE), and met the following criteria: global CDR of 0 at baseline, and MMSE >25; and a self-reported race of Black/African American (B/AA) or White (W). Other race and multi-race individuals (n = 5) were excluded due to insufficient sample size. Overall, 147 controls (23 B/AA, 124W) were included in analyses (Table 1).
Table 1.
Demographic and cognitive characteristics of participants self-identified as Black/AA or White
| Total Mean (SD) or N,% |
Black/AA Mean (SD) or N,% |
White Mean (SD) or N,% |
t test χ2 |
p value | |
|---|---|---|---|---|---|
| N | 147 | 23 | 124 | — | — |
| Sex | 87F, 59.2% | 19F, 82.6% | 68F, 54.8% | 5.097 | 0.024 |
| Age (y) | 75.9 (6.9) | 73.1 (7.1) | 76.5 (6.8) | −2.076 | 0.047 |
| PiB DVR | 1.21 (0.22) | 1.14 (0.14) | 1.22 (0.24) | −2.316 | 0.025 |
| Aβ status | 46 Aβ+, 31.3% | 5 Aβ+, 21.7% | 41 Aβ+, 33.1% | 0.691 | 0.406 |
| Education (y) | 15.9 (2.9) | 14.4 (2.7) | 16.1 (2.9) | −2.699 | 0.011 |
| MMSE | 29.2 (0.97) | 29.0 (1.07) | 29.2 (0.96) | −1.165 | 0.254 |
| CDR Sum of Boxes | 0.19 (0.35) | 0.20 (0.29) | 0.19 (0.36) | 0.031 | 0.976 |
| (CDR = 0.5: N, %) | 12, 8.2% | 1, 4.3% | 11, 8.9% | −0.896 | 0.375 |
| Composite Memory | 0.31 (0.93); 3 missing | 0.33 (0.89) | (0.31 (0.95); 3 missing | 0.130 | 0.897 |
| Composite Executive | 0.16 (0.73); 3 missing | −0.33 (0.77) | 0.25 (0.69); 3 missing | −3.462 | 0.002 |
AA, African American; Aβ, amyloid-β; CDR, Clinical Dementia Rating; MMSE, Mini-Mental State Examination; PiB DVR, 11C-Pittsburg Compound B Distribution Volume Ratio; SD, standard deviation. NOTE: Chi-square (or Fisher) tests were used to test group differences on categorical variables; independent t tests were used to calculate group differences on continuous variables. All individuals received CDR 0 at study baseline. The proportion of CDR 0.5 reflects the number of individuals who received CDR 0.5 at the time of PET. Bold values indicate significant group differences (p < 0.05).
Image acquisition and processing
Magnetic resonance imaging (MRI) and 11C-Pittsburg Compound B (PiB) PET were collected within 8 months on average from FTP PET: mean (SD) days were 122 (112) and 103 (141), respectively.
MRI, including a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE), was performed on a 3T Tim Trio (Siemens) with a 12-channel phased-array head coil at the Massachusetts General Hospital, Athinoula A. Martinos Centre for Biomedical Imaging (repetition time = 2300 ms, echo time = 2.95 ms, inversion time = 900 ms, flip angle = 9°, resolution = 1.05 × 1.05 × 1.2 mm). The images were processed with FreeSurfer 5.1 using the default automated reconstruction as described previously [21–23]. ROIs from the Desikan atlas [24] and the subcortical atlas provided in FreeSurfer included: cerebellar gray matter, cerebral white matter, CP, HC, amygdala (AM), entorhinal (ER), inferior temporal (IT), and fusiform (FF), as depicted in Fig. 1.
Fig. 1.
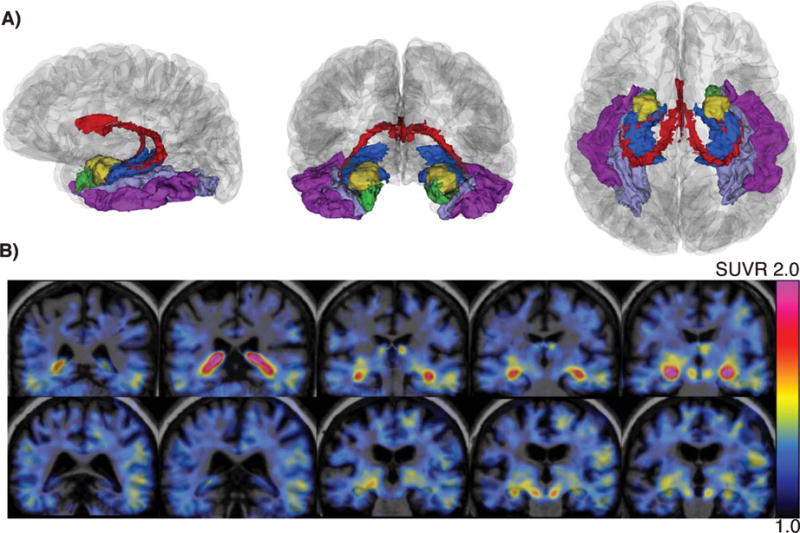
(A) Sagittal, coronal, and axial perspectives (left to right) of the FreeSurfer segmentation of a randomly chosen participant showing the anatomy of select ROI: CP (red), HC (blue), AM (gold), ER (green), FF (lavender), IT (violet). (B) Co-registered MR-PET SUVR image (scale range: 1.0–2.0; pvc data) of a Black/AA (Female, 71 years old; top row) and White participant (Female, 71 years old; bottom row).
FTP and PiB were prepared and PET data were acquired at Massachusetts General Hospital as previously reported [5]: FTP PET (80–100 min, 4 × 35-min frames) and PiB PET (60 min dynamic acquisition, 69 frames) were acquired on a Siemens/CTI ECAT HR1 scanner. PET data was reconstructed and attenuation-corrected, and each frame was evaluated to verify adequate count statistics and absence of head motion.
Each individual PET data set was rigidly coregistered to the participant’s MPRAGE data, and transformed into the PET native space. Data using cerebellar gray or cerebral white matter as reference region were both included in analyses. FTP PET data were represented as standardized uptake value ratio (SUVR) values of the average left and right ROIs, while PiB PET data were represented as distribution volume ratio (DVR) values. PiB retention was assessed using a large cortical ROI aggregate that included frontal, lateral temporal, and retros-plenial cortices as described previously. PiB DVR was used as a continuous measure of Aβ, and a Gaussian mixture modeling approach following previously described methods [25] was used to classify the participants as Aβ+ or Aβ− (cutoff value, 1.186). Data with and without partial volume correction (pvc) using the geometric transfer matrix method were used for analyses [26].
Cognitive performance
Cognitive assessments (MMSE, CDR and composite scores) were collected within a mean (SD) of 90.2 (48.7) days from FTP PET. Composite scores for memory and executive function were created using factor analyses from the entire HABS cohort (N = 284). The memory composite (with factor loading weights between parentheses) included the z-score transformations of the delayed recall scores of the 6-Trial Selective Reminding test [27] (0.739), the free recall of the Free and Cued Selective Reminding Test (0.605) and the delayed recall of the Logical Memory Test [28] (0.534). The executive function composite included the z-score transformations of the Trail Making Test form B minus A [29] (0.666), the Letter Number Sequencing test [30] (0.533) and the phonemic fluency FAS test [31] (0.622).
Statistical analyses
Analyses were performed using R v3.3 (https://www.r-project.org), and statistical significance was set at p < 0.05 (two-sided). Group differences were assessed with Welch’s Two Sample t-test for continuous variables, and χ2 tests (or Fisher) for dichotomous variables.
To investigate the effect of race on FTP SUVR, linear regression models were used (separate model for each ROI). All regression models performed as part of this study were controlled for the covariates of race, age, sex, education, and Aβ burden (Aβ status, or continuous PiB DVR).
To examine the associations between CP SUVR and nearby ROI SUVR, linear regression models were used (separate model for each ROI). The interaction of race by CP SUVR was investigated. If the interaction was not significant, we removed it from the model and examined the main effect of CP SUVR keeping the main effect of race as a covariate in the model. Regression models assessing regional associations with non-pvc data were repeated with the addition of cerebral white matter SUVR as covariate.
Next, we created a partial residual for HC FTP signal as previously reported [21] in order to reduce the confounding of CP signal on HC PET measurement [32]. This involved extracting the residuals from the univariate regression between CP SUVR and HC SUVR (n = 147). This adjusted HC measure (residual HC SUVR) can be interpreted as the difference in tau binding in the HC from the expected level for a person given his or her level of CP FTP binding.
Subsequently, we investigated whether CP signal influenced the association between HC measurements and Aβ, through linear regression models that used either unadjusted HC SUVR or residual HC SUVR. In the same way, we investigated whether CP signal influenced race differences in the slope between HC measurements and Aβ.
Linear regression models were run to test the associations of HC SUVR (unadjusted and residuals) with memory and executive performance. Analyses were run within the entire sample, as well as within the Aβ+ and Aβ− cohorts.
All analyses were performed with either pvc or non-pvc data to assess the impact of volume correction on possible spill-in effects. Manuscript tables and figures show both pvc and non-pvc data, under the following main conditions: cerebellar gray reference region, Aβ treated as continuous covariate (PiB DVR), and inclusion of CDR > 0 participants.
In addition, analyses were repeated with cerebral white matter as reference, since cerebral white matter has shown to be more sensitive to detect treatment differences than cerebellar gray matter [33]. Analyses were repeated with the exclusion of participants who progressed from baseline CDR score of 0 to 0.5 (n = 12), to ensure that our findings were not driven by individuals who progressed in CDR scoring. Analyses were also repeated using dichotomous Aβ status as measures of Aβ burden. Results of analyses using alternate conditions are referred to in the Results, Discussion, or Supplementary Materials, when they differ from the main conditions. Histograms, residual-versus-predicted values and Q-Q-plots were inspected to test principal assumptions and the variance inflation factor (<5) was inspected for multi-collinearity. Probability values were corrected for multiple comparisons using the Bonferroni method (max 15 models, p < 0.003).
RESULTS
Demographics
Participant demographics across the full sample and within race groups are shown in Table 1. B/AA participants in comparison to W were younger (p = 0.05), had proportionally more women (p = 0.02), lower PiB DVR (p = 0.03), fewer years of education (p = 0.01), and lower executive function scores (p < 0.01). There were no race differences (p > 0.05) in MMSE scores, memory scores, the number of participants with CDR > 0 (B/AA, 1; W, 11), or the proportion of Aβ+ participants (B/AA, 21.7%; W, 33.1%).
B/AA participants have elevated FTP SUVR in CP and HC
In comparison to W, B/AA participants had elevated SUVR in the CP (β = −0.61, T-value = −3.62, p-value = 0.006; unadjusted mean difference = 0.57 SUVR). The ranges in CP SUVR for the two races were overlapping: B/AA, 0.87–4.18; W, 0.65–4.04. Individuals with high CP SUVR (SUVR>full sample mean) were found in both the B/AA (73.9%) and W (37.1%) groups (see Fig. 2).
Fig. 2.
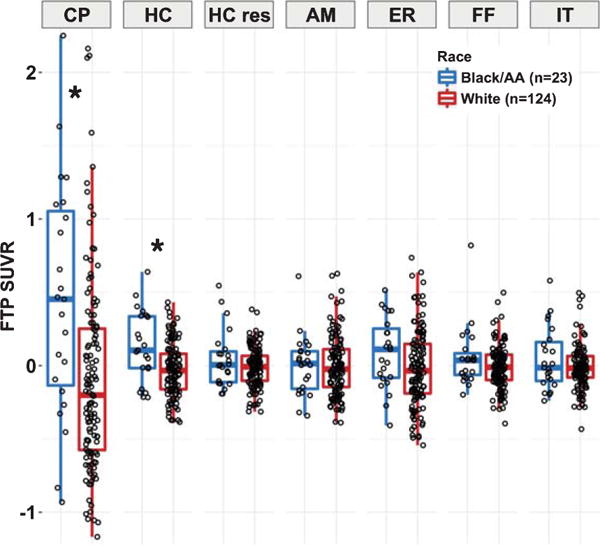
Regional FTP SUVR comparisons between race groups. FTP SUVR (pvc; with PiB DVR, age, sex, and education regressed out) is presented for the Black/AA (n = 23, blue) and White (n = 124, red) race groups. Significant differences (p < 0.05) between races are noted by the asterisks. Elevated FTP measurements are observed in the CP and HC, but not the HC residuals or other ROIs. CP, choroid plexus; HC, hippocampus; HC res, residualized hippocampus; AM, amygdala; ER, entorhinal; FF, fusiform; IT, inferior temporal.
B/AA participants had elevated unadjusted SUVR within the adjacent HC compared to W (β = −0.18, T-value = −3.90, p-value = 0.002; unadjusted mean difference = 0.12 SUVR). The ranges in unadjusted HC SUVR for the two races were overlapping: B/AA, 1.10–2.12; W, 1.01–1.94. Individuals with high unadjusted HC SUVR (SUVR>full sample mean) were found in both the B/AA (60.9%) and W (41.1%) groups as was seen for CP SUVR.
In contrast, no race effect was seen in the nonadjacent IT, ER, AM, or FF regions (p > 0.05, see Table 2). Also, no race difference was seen in the residual HC SUVR (p > 0.05; Fig. 2), which were used as outcome measure to better understand the extent to which the CP-HC association contributes to race differences in these regions.
Table 2.
Race differences in regional FTP measurements
| Estimate | SE | t value | p value | Adj. p (14 tests) |
|
|---|---|---|---|---|---|
|
SUVR (pvc)
|
|||||
| CP | −0.606 | 0.168 | −3.619 | <10−3 | 0.006 |
| HC | −0.182 | 0.047 | −3.902 | <10−3 | 0.002 |
| HC residuals | −0.060 | 0.036 | −1.648 | 0.102 | >1.000 |
| AM | −0.001 | 0.050 | −0.011 | 0.991 | >1.000 |
| ER | −0.119 | 0.064 | −1.876 | 0.063 | 0.878 |
| FF | −0.077 | 0.039 | −1.986 | 0.049 | 0.686 |
| IT | −0.062 | 0.039 | −1.578 | 0.117 | >1.000 |
|
SUVR (non-pvc)
|
|||||
| CP | −0.194 | 0.056 | −3.442 | 0.001 | 0.011 |
| HC | −0.106 | 0.033 | −3.215 | 0.002 | 0.023 |
| HC residuals | −0.020 | 0.022 | −0.890 | 0.375 | >1.000 |
| AM | −0.026 | 0.027 | −0.945 | 0.346 | >1.000 |
| ER | −0.044 | 0.026 | −1.719 | 0.088 | >1.000 |
| FF | −0.025 | 0.019 | −1.330 | 0.186 | >1.000 |
| IT | −0.020 | 0.020 | −0.986 | 0.326 | >1.000 |
CP, choroid plexus; HC, hippocampus; HC residuals, hippocampus residualized for CP signal; AM, amygdala; ER, entorhinal; FF, fusiform; IT, inferior temporal; SE, standard error; Adj. p, p values adjusted for multiple comparison using the Bonferroni method; SUVR, standardized uptake value ratio; pvc, partial volume correction using geometric transfer matrix method. NOTE: Cerebellar cortex was used as reference for SUVR shown. Separate multiple linear regression models were run for each region, all models covarying for age, sex, PiB DVR, and education. Bold values indicate significant race differences in FTP measurements (p < 0.05).
Race differences in CP and unadjusted HC SUVR were consistent using pvc or non-pvc data. Results did not differ under alternate conditions of reference region, Aβ measurement scale, or exclusion of CDR > 0 individuals (see Table 2, non-pvc data; Supplementary Table 1, cerebral white reference data).
CP FTP SUVR is associated with FTP SUVR in HC but no other regions
CP SUVR was positively associated with SUVR in the HC (p < 10−14) but not with SUVR in other ROIs (p > 0.05) (Table 3 and Fig. 3). There was no interaction effect of race by CP SUVR on SUVR in any of the selected regions (all interaction term ps > 0.39, >0.22 for pvc, non-pvc data respectively; data not shown) indicating that the association between CP SUVR and SUVR in the other ROIs (reflecting spill-in effect) was similar for B/AA and W participants.
Table 3.
CP FTP SUVR associated with SUVR in nearby ROI
| Estimate | SE | t value | p value | Adj. p (15 tests) |
|
|---|---|---|---|---|---|
|
SUVR (pvc)
|
|||||
| HC | 0.177 | 0.018 | 9.760 | <10−15 | <10−14 |
| AM | −0.012 | 0.025 | −0.465 | 0.643 | >1.000 |
| ER | 0.021 | 0.032 | 0.667 | 0.506 | >1.000 |
| FF | 0.019 | 0.019 | 1.001 | 0.319 | >1.000 |
| IT | 0.020 | 0.020 | 1.032 | 0.304 | >1.000 |
|
SUVR (non-pvc)
|
|||||
| HC | 0.439 | 0.033 | 13.346 | <10−15 | <10−14 |
| AM | 0.163 | 0.039 | 4.193 | <10−4 | 0.001 |
| ER | 0.111 | 0.038 | 2.950 | 0.004 | 0.056 |
| FF | 0.084 | 0.027 | 3.111 | 0.002 | 0.034 |
| IT | 0.078 | 0.029 | 2.685 | 0.008 | 0.122 |
|
SUVR (non-pvc, covarying cerebral WM SUVR)
|
|||||
| HC | 0.350 | 0.032 | 10.937 | <10−15 | <10−14 |
| AM | 0.041 | 0.036 | 1.155 | 0.250 | >1.000 |
| ER | 0.021 | 0.038 | 0.552 | 0.582 | >1.000 |
| FF | −0.013 | 0.023 | −0.558 | 0.578 | >1.000 |
| IT | −0.020 | 0.026 | 0.792 | 0.430 | >1.000 |
CP, choroid plexus; HC, hippocampus; HC residuals, residualized hippocampus; AM, amygdala; ER, entorhinal; FF, fusiform; IT, inferior temporal; SE, standard error; Adj. p, p values adjusted for multiple comparison using the Bonferroni method; SUVR, standardized uptake value ratio; pvc, partial volume correction using geometric transfer matrix method; WM, white matter. NOTE: Cerebellar cortex was used as reference for all SUVR shown. Separate multiple linear regression models were run for each region, all models covarying for race, age, sex, PiB DVR, and education. Bold values indicate significant association between FTP measurements in the CP and nearby ROI (p < 0.05).
Fig. 3.
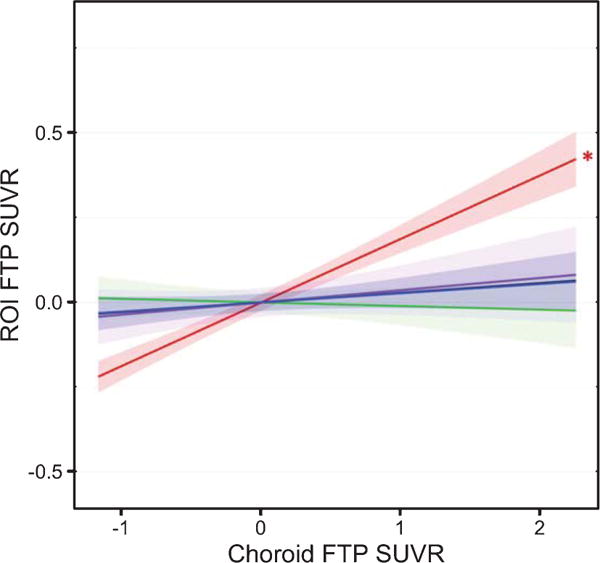
Regional FTP associations. Linear regressions models (covarying PiB DVR, age, sex, and education) across the combined race sample (N = 147) show the associations between CP FTP SUVR and FTP SUVR in the HC (red), AM (green), ER (violet), FF (black) and IT (blue), using GTM-corrected data. Shading represents 95% confidence intervals. The asterisk denotes the only significant association (p < 0.05), between the CP and HC FTP SUVR. Similar associations were observed between B/AA and W individuals (no significant interaction).
We repeated these analyses using non-pvc data (Table 3). Results showed significant associations between CP SUVR and SUVR in the HC, AM, ER, FF, and IT (p < 0.05). On closer inspection of the data, the differences between pvc and non-pvc analyses were likely driven by cerebral white matter spill-in effects. First, adding cerebral white matter SUVR as covariate to the models showed that the association between the CP and ROIs was no longer significant, except for the HC (p < 10−14). Second, performing analyses using cerebral white matter reference data led to the findings consistent with pvc data (see Supplementary Table 2).
Results of regional associations remained consistent regardless of Aβ measurement scale, or exclusion of CDR > 0 individuals.
CP FTP SUVR influences HC FTP SUVR association with memory
Shown in Table 4, residual HC SUVR was negatively associated with memory scores across Aβ+ participants (p = 0.025,0.027; pvc, non-pvc) but not across Aβ− participants (p > 1.0; both pvc and non-pvc). In contrast, unadjusted HC SUVR was not associated with memory scores in either Aβ+ or Aβ− participants (p > 0.13; both pvc and non-pvc). Across the entire sample, residual HC SUVR (pvc) was associated with memory (p = 0.028), while residual HC SUVR (non-pvc) (p = 0.027) did not survive multiple comparison correction (p = 0.11). Unadjusted HC SUVR was not associated with memory (p > 0.24; both pvc and non-pvc) across the entire sample. Both residual HC SUVR and unadjusted HC SUVR showed no association to executive function scores, in the Aβ+, Aβ− or entire sample (p > 1.0; both pvc and non-pvc). Associations with memory were consistent regardless of Aβ measurement scale, reference region (see Supplementary Table 3), or exclusion of CDR > 0 individuals.
Table 4.
Cognitive measures associated with unadjusted or residual HC SUVR
| Sample | Data type | Predictor | Estimate | SE | t value | p value | Adj. p value (4 tests) |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Memory factor score as outcome variable | |||||||
|
| |||||||
| All(N = 147) | pvc | HC SUVR | −0.677 | 0.359 | −1.887 | 0.061 | 0.245 |
| HC SUVR residuals | −1.237 | 0.451 | −2.741 | 0.007 | 0.028 | ||
| non-pvc | HC SUVR | −0.496 | 0.517 | −0.960 | 0.339 | >1.000 | |
| HC SUVR residuals | −1.696 | 0.759 | −2.234 | 0.027 | 0.108 | ||
| Aβ+ (n = 46) | pvc | HC SUVR | −1.324 | 0.600 | −2.208 | 0.033 | 0.133 |
| HC SUVR residuals | −2.054 | 0.710 | −2.894 | 0.006 | 0.025 | ||
| non-pvc | HC SUVR | −1.450 | 0.874 | −1.659 | 0.105 | 0.421 | |
| HC SUVR residuals | −3.218 | 1.121 | −2.870 | 0.007 | 0.027 | ||
| Aβ− (n = 101) | pvc | HC SUVR | 0.018 | 0.440 | 0.042 | 0.967 | >1.000 |
| HC SUVR residuals | −0.051 | 0.636 | −0.080 | 0.937 | >1.000 | ||
| non-pvc | HC SUVR | 0.365 | 0.610 | 0.599 | 0.551 | >1.000 | |
| HC SUVR residuals | 0.917 | 1.049 | 0.874 | 0.384 | >1.000 | ||
|
Executive factor score as outcome variable
|
|||||||
| All(N = 147) | pvc | HC SUVR | −0.019 | 0.269 | −0.069 | 0.945 | >1.000 |
| HC SUVR residuals | −0.219 | 0.342 | −0.641 | 0.522 | >1.001 | ||
| non-pvc | HC SUVR | −0.011 | 0.383 | −0.029 | 0.977 | >1.002 | |
| HC SUVR residuals | −0.603 | 0.569 | −1.060 | 0.291 | >1.003 | ||
| Aβ+ (n = 46) | pvc | HC SUVR | −0.176 | 0.483 | −0.365 | 0.717 | >1.000 |
| HC SUVR residuals | −0.327 | 0.593 | −0.552 | 0.584 | >1.000 | ||
| non-pvc | HC SUVR | −0.326 | 0.685 | −0.475 | 0.637 | >1.000 | |
| HC SUVR residuals | −0.937 | 0.927 | −1.011 | 0.319 | >1.000 | ||
| Aβ− (n = 101) | pvc | HC SUVR | 0.018 | 0.358 | 0.051 | 0.959 | >1.000 |
| HC SUVR residuals | −0.385 | 0.516 | −0.747 | 0.457 | >1.000 | ||
| non-pvc | HC SUVR | 0.133 | 0.497 | 0.268 | 0.789 | >1.000 | |
| HC SUVR residuals | −0.622 | 0.854 | −0.729 | 0.468 | >1.000 | ||
Aβ+/−, elevated/low amyloid-β groups; Adj. p, p values adjusted for multiple comparison using the Bonferroni method; HC, hippocampus; pvc, partial volume correction using geometric transfer matrix method; SE, standard error; SUVR, standardized uptake value ratio. NOTE: Cerebellar cortex was used as reference for all SUVR shown. Separate multiple linear regression models were run. All models covarying for race, age, sex, and education. Bold values indicate significant association between FTP measurements in the CP and nearby ROI (p < 0.05).
CP FTP SUVR influences HC FTP SUVR association with Aβ
Table 5 shows residual HC SUVR, unadjusted HC SUVR and CP SUVR tested for association with Aβ. Residual HC SUVR was associated with Aβ (p = 0.011,0.007; pvc,non-pvc), whereas unadjusted HC SUVR and CP SUVR were not related to Aβ (p > 0.05 after multiple comparison correction; pvc and non-pvc). The interaction of Aβ by race on residual HC SUVR (pvc) (p = 0.022) did not survive multiple comparison correction (p > 0.05). The interaction was not significant for residual HC SUVR (non-pvc) or unadjusted HC SUVR (pvc or non-pvc).
Table 5.
Influence of Race on association between FTP SUVR and amyloid
| Data type | Predictor | Estimate | SE | t value | p value | Adj. p value (10 tests) |
|---|---|---|---|---|---|---|
| CP SUVR
|
||||||
| pvc | PiB DVR | 0.030 | 0.261 | 0.114 | 0.910 | >1.000 |
| non-pvc | PiB DVR | 0.004 | 0.088 | 0.046 | 0.963 | >1.000 |
| HC SUVR
|
||||||
| pvc | PiB DVR | 0.194 | 0.073 | 2.671 | 0.008 | 0.085 |
| non-pvc | PiB DVR | 0.120 | 0.051 | 2.331 | 0.021 | 0.212 |
| residual HC SUVR
|
||||||
| pvc | PiB DVR | 0.188 | 0.056 | 3.335 | 0.001 | 0.011 |
| non-pvc | PiB DVR | 0.118 | 0.034 | 3.459 | 0.001 | 0.007 |
| HC SUVR
|
||||||
| pvc | PiB DVR * Race | −0.204 | 0.301 | −0.677 | 0.499 | >1.000 |
| non-pvc | PiB DVR * Race | 0.114 | 0.213 | 0.536 | 0.593 | >1.000 |
| residual HC SUVR
|
||||||
| pvc | PiB DVR * Race | −0.532 | 0.230 | −2.317 | 0.022 | 0.219 |
| non-pvc | PiB DVR * Race | −0.176 | 0.141 | −1.248 | 0.214 | >1.000 |
Adj. p, p values adjusted for multiple comparison using the Bonferroni method; CP, choroid plexus; HC, hip-pocampus; PiB DVR, 11C-Pittsburg Compound B Distribution Volume Ratio; pvc, partial volume correction using geometric transfer matrix method; SE, standard error; SUVR, standardized uptake value ratio. NOTE: CDR > 0 participants were included in the sample used here (N = 147). Cerebellar cortex was used as reference for all SUVR shown. Separate multiple linear regression models were run. All models covarying for race, age, sex, and education. Bold values indicate significant association between FTP measurements in the CP and nearby ROI (p < 0.05).
Shown in Supplementary Table 5, these findings differed when participants with CDR > 0 were excluded. Both unadjusted and residual HC SUVR (pvc or non-pvc) were associated with Aβ, but these significances did not survive multiple comparisons correction (p > 0.05). The interaction of Aβ by race on residual HC SUVR (pvc or non-pvc) was significant even after correcting for multiple comparisons (p = 0.002,0.034; pvc, non-pvc). Specifically, the interaction showed a positive association between residual HC SUVR and Aβ that was stronger in B/AA than W.
Figure 4 compares the interaction of Aβ by race on unadjusted or residual HC SUVR, including or excluding CDR > 0 participants. Findings were consistent regardless of reference region (see Supplementary Tables 4 and 6), or Aβ measurement scale.
Fig. 4.
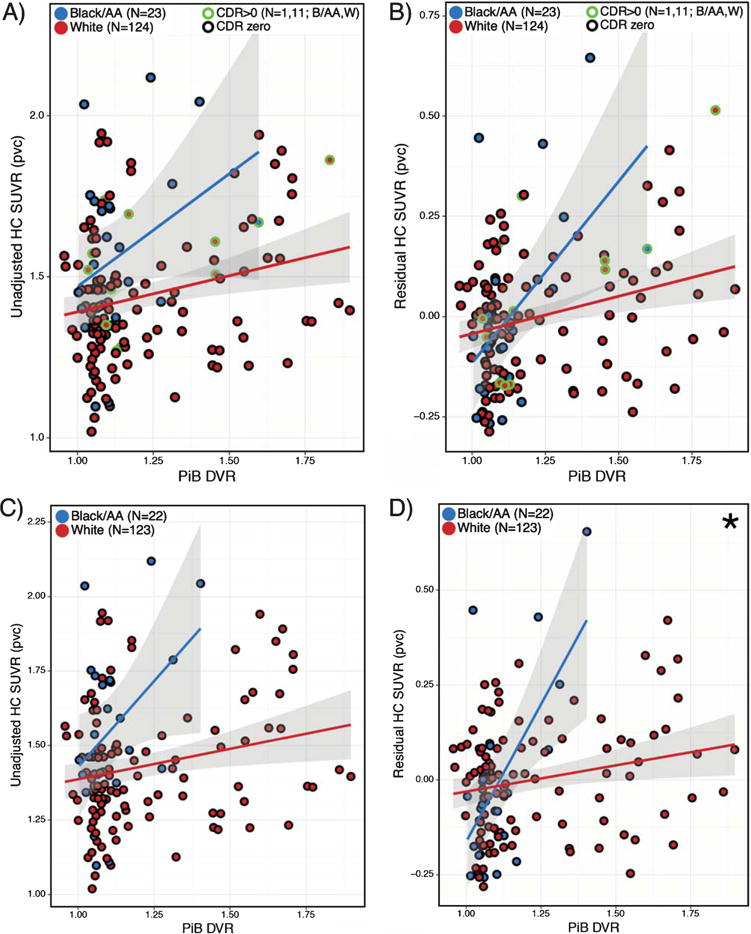
Association between residual HC SUVR and Aβ status differs by race. Plots A-D show unadjusted HC SUVR (A, C) or residual HC SUVR (B, D), either with (A-B) or without (C-D) the inclusion of CDR > 0 participants. Linear regressions models (covarying age, sex, and education) within B/AA (blue line) and W (red line) race groups show interaction of Aβ by race on HC signal. Interaction reached significance using residual HC SUVR (∗p = 0.002). PVC data is shown, values represent estimated marginal means corrected for covariates. Shading represents 95% confidence intervals.
DISCUSSION
The aim of this study was to further understand the nature and impact of FTP binding in the CP. We investigated race differences in CP SUVR as an indication of possible off-target binding to melanin. Additionally, we examined the influence of CP SUVR on SUVR in surrounding regions to assess spill-in effects of the off-target binding. To understand the extent to which CP spill-in effects influence the HC, we evaluated whether the expected associations with memory and Aβ were observed using either unadjusted HC SUVR or residual HC SUVR corrected for CP signal.
Our results add three important findings to the current understanding of FTP binding behavior in older adults. First, we observed elevated CP SUVR in B/AA participants compared to W, which could potentially indicate off-target FTP binding to melanin. This interpretation is likely, considering that recent neuropathology studies [34, 35] have found no race differences in tau pathology, while FTP autoradiography studies have shown tracer binding to melanin present in the leptomeningeal, and skin melanocytes of healthy adults [6, 8]. Second, we found that CP SUVR was associated with SUVR in the adjacent HC but not in non-adjacent regions, suggesting spill-in effects from the CP to the HC specifically. Third, by using a statistical method to reduce CP spill-in effects, we found that adjusted HC SUVR residuals followed the behavior expected of HC tau in three ways: 1) we found no group race differences in adjusted HC SUVR residuals; 2) we observed a stronger positive association between adjusted HC FTP and Aβ for B/AA than for W, something that was not observed when using the unadjusted HC FTP; and 3) adjusted HC SUVR residuals—and not unadjusted HC SUVR—were associated with memory performance.
The observation of increased FTP SUVR in the CP of B/AA participants compared to W was a robust finding, controlling for age, Aβ, and demographic differences. Findings remained consistent between pvc and non-pvc data, choice of reference region (cerebellar gray or cerebral white), and using Aβ as continuous or dichotomous covariate. This finding suggests melanin as a possible FTP substrate in the CP, and agrees with our recent preliminary data from autoradiography studies [36].
It is important to note that high SUVR (SUVR>mean SUVR) in the CP was present in individuals from both race groups (see Fig. 2). This observation agrees with human physiology, since melanin is produced by melanocytes in the skin, meninges, and CP [13] in both B/AA and W individuals, but to a greater extent in B/AA individuals [37]. However, high CP SUVR in light-skinned individuals could also point to additional factors contributing to CP FTP signal, which may include both off-target binding (e.g., iron deposits, Biondi rings, calcifications/mineralizations, MAO-A) and on-target binding (as observed within CP epithelial cells by Ikonomovic and colleagues) [38].
Ultimately, autopsy studies are necessary to validate melanin as substrate for CP FTP binding. Furthermore, the findings of this in vivo study might be supported by future in vitro studies, such as examination of FTP binding in the meninges of albino and darkly pigmented rodents or in specific cell lines.
Interestingly, B/AA participants also had elevated SUVR in the HC (not in the AM, ER, FF, IT) compared to W participants. This HC race difference contrasts with the neuropathology studies that observed no race difference in the prevalence of neurofibrillary tangles in any brain regions including HC [34, 35, 39]. This inconsistency with neuropathology studies may suggest CP spill-in effects on the HC. Similarly, the lack of race difference seen in adjusted HC SUVR residuals supports the explanation of CP spill-in. Although, it is important to note that the method for residualizing HC SUVR is a statistical correction, therefore the residual HC SUVR may not fully reflect the interindividual biological variability in HC signal, and a lack of finding requires further examination.
In order to look more closely at the possibility of CP-HC spill-in effects, we examined the association between CP SUVR and SUVR in nearby regions. We found that CP SUVR had a strong relationship with HC SUVR. The presence of a CP-HC association was found across the entire sample and within both race groups, and there was no significant interaction of race by CP SUVR on HC SUVR. Therefore, CP influences HC signal in similar ways for both races but at different offsets. Other regions (IT, ER, FF, AM) were not related to CP SUVR, showing that CP off-target binding is specific to the nearby HC.
The extent to which CP spill-in can be corrected for by statistical approaches is highly important for the Alzheimer’s disease research field given the connection HC tau has with memory decline and Aβ. Partial volume corrected HC SUVR differed by race (Black/AA participants had elevated HC SUVR), and was highly associated with CP signal. These findings indicate that partial volume correction (GTM method) is not sufficient for reducing CP spill-in.
We now show that using residual HC SUVR seems to be at least to some extent successful in removing the impact of CP spill-in. To begin, the residual HC SUVR did not differ by race, and was no longer associated with CP SUVR. The lack of race difference or association with CP SUVR is suggestive of the residual method’s ability to attenuate CP spill-in. In addition, residual HC SUVR (but not unadjusted HC SUVR) was positively associated with Aβ.
Furthermore, there was an apparent race difference in the association between residual HC SUVR and Aβ, when excluding CDR > 0 participants. Of note, though the inclusion/exclusion of CDR > 0 participants did not impact other analyses, CDR likely impacted this analysis as individuals with higher CDR-scores are more likely to have a higher Aβ burden (see Fig. 4). The significant interaction provides strong evidence that this approach to correcting HC signal improves spill-in from the CP. A recent population study of non-demented individuals showed a two-fold increase in Aβ burden rates of B/AA compared to W [40]. Also, increasing levels of HC tau burden are in accordance with at least Braak stage III tau pathology [41], when Aβ burden is also expected to rise. Thus, the positive association between residual HC SUVR and Aβ becomes more apparent when using this method validates this approach.
In addition, we observed that residual HC SUVR were inversely related to memory scores in high Aβ participants, whereas this relationship did not exist for low Aβ participants and at trend-level across the entire sample. The fact that we did not observe these relationships for the unadjusted HC SUVR and the non-pvc data, further stresses that removing spill-in effects through these methods should be further explored. Notably, residual HC SUVR showed no relationship to executive functioning in the Aβ−, Aβ+ or entire sample. The fact that the residual HC SUVR-memory relationship was specific to Aβ+ individuals, and specific to tests of memory over executive functioning, indicates that the method reduces the noise from spill-in effects to observe expected relationships in our population.
A limitation to this study may be the moderate sample size of the B/AA group. However, the percentage B/AA individuals compared to W are proportional to the national race demographics (2010 U.S. Census Data). The proportion of females was larger in B/AA compared to W individuals. Therefore, we could not assess differences between males and females, but in all our regression models, sex was not a significant covariate. In addition, while group differences were observed using an indirect measure of melanin, more sensitive and objective measures of melanin levels than the proxy of self-reported race will empower future studies to detect subtle relationships in small cohorts [42]. Furthermore, it is important to note that the presence of melanin appears to be a partial source of off-target binding in the CP or underlying these race differences.
In summary, these findings support the idea that signal spill-in from the CP impacts HC FTP measurements, sensitivity to detect associations between HC FTP and memory and to detect race differences in the association between Aβ burden and tau pathology. These observations add complexity to correctly interpreting the HC signal in older individuals, and caution is warranted when investigating this region in FTP images.
Supplementary Material
Acknowledgments
The authors wish to thank the investigators and staff of the Harvard Aging Brain Study and the individual research participants. This study was supported by the NIH National Institute on Aging [R01 AG046396 to K.A.J.; P01 AG036694 to R.A.S. and K.A.J.; P50 AG00513421 to K.A.J. and R.A.S.; AG005134 and AG036694 to T.G.], Fidelity Biosciences, Harvard Neurodiscovery Center, Alzheimer’s Association, Alzheimer Nederland [WE.15-2014-06 to H.I.L.J], and the ASISA Foundation, Madrid, Spain [to M.M.]
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-0840r1).
Footnotes
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170840.
References
- 1.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 2.Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwim-mer HD, Rabinovici GD, Jagust WJ. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shcherbinin S, Schwarz AJ, Joshi A, Navitsky M, Flitter M, Shankle WR, Devous MD, Sr, Mintun MA. Kinetics of the Tau PET tracer 18F-AV-1451 (T807) in subjects with normal cognitive function, mild cognitive impairment, and Alzheimer disease. J Nucl Med. 2016;57:1535–1542. doi: 10.2967/jnumed.115.170027. [DOI] [PubMed] [Google Scholar]
- 4.Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, Lyoo CH, Ryu YH, Lee MS. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87:375–383. doi: 10.1212/WNL.0000000000002892. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, Kantarci K, Boeve BF, Pandey MK, Bruinsma T, Knopman DS, Jones DT, Petrucelli L, Cook CN, Graff-Radford NR, Dickson DW, Petersen RC, Jack CR, Jr, Murray ME. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquié M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, Klunk WE, Mathis CA, Ikonomovic MD, Debnath M, Bien EA, Vanderburg CR, Costantino I, Makaretz S, DeVos SL, Oakley DH, Gomperts SN, Growdon JH, Domoto-Reilly K, Lucente D, Dicker-son BC, Frosch MP, Hyman BT, Johnson KA, Gomez-Isla T. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol. 2017;81:117–128. doi: 10.1002/ana.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML, Vasdev N, Dickerson BC, Gomperts SN, Growdon JH, Johnson KA, Frosch MP, Hyman BT, Gomez-Isla T. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, Schott JM, Revesz T, Fox NC, Arstad E. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other demen-tias. Alzheimers Dement. 2016;12:1116–1124. doi: 10.1016/j.jalz.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Hostetler ED, Walji AM, Zeng Z, Miller P, Bennacef I, Sali-nas C, Connolly B, Gantert L, Haley H, Holahan M, Purcell M, Riffel K, Lohith TG, Coleman P, Soriano A, Ogawa A, Xu S, Zhang X, Joshi E, Della Rocca J, Hesk D, Schenk DJ, Evelhoch JL. Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J Nucl Med. 2016;57:1599–1606. doi: 10.2967/jnumed.115.171678. [DOI] [PubMed] [Google Scholar]
- 11.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimers disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Wolburg H, Paulus W. Choroid plexus: Biology and pathology. Acta Neuropathol. 2010;119:75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- 13.Puri D. Textbook of Medical Biochemistry. Elsevier; New Delhi: 2006. [Google Scholar]
- 14.Johanson C, McMillan P, Tavares R, Spangenberger A, Dun-can J, Silverberg G, Stopa E. Homeostatic capabilities of the choroid plexus epithelium in Alzheimer’s disease. Cerebrospinal Fluid Res. 2004;1:3. doi: 10.1186/1743-8454-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miklossy J, Kraftsik R, Pillevuit O, Lepori D, Genton C, Bosman FT. Curly fiber and tangle-like inclusions in the ependyma and choroid plexus–a pathogenetic relationship with the cortical Alzheimer-type changes? J Neuropathol Exp Neurol. 1998;57:1202–1212. doi: 10.1097/00005072-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Wen GY, Wisniewski HM, Kascsak RJ. Biondi ring tangles in the choroid plexus of Alzheimer’s disease and normal aging brains: A quantitative study. Brain Res. 1999;832:40–46. doi: 10.1016/s0006-8993(99)01466-3. [DOI] [PubMed] [Google Scholar]
- 17.Mesquita SD, Ferreira AC, Sousa JC, Santos NC, Correia-Neves M, Sousa N, Palha JA, Marques F. Modulation of iron metabolism in aging and in Alzheimer’s disease: Relevance of the choroid plexus. Front Cell Neurosci. 2012;6:25. doi: 10.3389/fncel.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeiren C, Mercier J, Viot D, Mairet-Coello G, Hannes-tad J, Courade J-P, Citron M, Gillard M. T807, a reported selective tau tracer, binds with nanomolar affinity to monoamine oxidase a. Alzheimers Dement. 2015;11:283. [Google Scholar]
- 19.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schnei-der JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delacourte A, Sergeant N, Wattez A, Maurage CA, Lebert F, Pasquier F, David JP. Tau aggregation in the hip-pocampal formation: An ageing or a pathological process? Exp Gerontol. 2002;37:1291–1296. doi: 10.1016/s0531-5565(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 21.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, Johnson KA, Sperling RA. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, Becker JA, Svarer C, Knudsen GM, Sperling RA, Johnson KA. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–343. doi: 10.1016/j.neuroimage.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the selective reminding test. J Clin Exp Neuropsychol. 1989;11:615–630. doi: 10.1080/01688638908400920. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler DS. Wechsler Memory Scale-Revised. Psychological-Corp; San Antonio, TX: 1987. [Google Scholar]
- 29.Wechsler DS. WAIS-III, Wechsler Adult Intelligence Scale—Third Edition, Administration and Scoring Manual. The Psychological Corporation; New York, NY: 1997. [Google Scholar]
- 30.Reitan RM. Manual for administration of neuropsy-chological test batteries for adults and children. Reitan Neuropsychology Laboratories; Tuscon, AZ: 1979. [Google Scholar]
- 31.Benton AL, Sivan AB, Hamsher KD, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. Oxford University Press; New York, NY: 1983. [Google Scholar]
- 32.Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, McConathy J, Cairns NJ, Fagan AM, Morris JC, Ances BM. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. JAMA Neurol. 2016;73:1070–1077. doi: 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleisher AS, Joshi AD, Sundell KL, Chen YF, Kollack-Walker S, Lu M, Chen S, Devous MD, Sr, Seibyl J, Marek K, Siemers ER, Mintun MA. Use of white matter reference regions for detection of change in florbetapir positron emission tomography from completed phase 3 solanezumab trials. Alzheimers Dement. 2017;13:1117–1124. doi: 10.1016/j.jalz.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neu-ropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol. 2006;65:1143–1148. doi: 10.1097/01.jnen.0000248548.20799.a3. [DOI] [PubMed] [Google Scholar]
- 35.Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiol Aging. 2001;22:169–175. doi: 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 36.Marquié M. Human Amyloid Imaging. Miami, FL: 2017. Neuropathology and biochemical correlations of [F-18]AV-1451 and [C-11]PiB PET imaging in a subject with Alzheimer’s disease. [Google Scholar]
- 37.van Nieuwpoort F, Smit NP, Kolb R, van der Meulen H, Koerten H, Pavel S. Tyrosine-induced melanogenesis shows differences in morphologic and melanogenic preferences of melanosomes from light and dark skin types. J Invest Dermatol. 2004;122:1251–1255. doi: 10.1111/j.0022-202X.2004.22533.x. [DOI] [PubMed] [Google Scholar]
- 38.Ikonomovic MD, Abrahamson EE, Price JC, Mathis CA, Klunk WE. [F-18]AV-1451 positron emission tomography retention in choroid plexus: More than “off-target” binding. Ann Neurol. 2016;80:307–308. doi: 10.1002/ana.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller FD, Hicks SP, D’Amato CJ, Landis JR. A descriptive study of neuritic plaques and neurofibrillary tangles in an autopsy population. Am J Epidemiol. 1984;120:331–341. doi: 10.1093/oxfordjournals.aje.a113897. [DOI] [PubMed] [Google Scholar]
- 40.Gottesman RF, Schneider AL, Zhou Y, Chen X, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH., Jr The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87:473–480. doi: 10.1212/WNL.0000000000002914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- 42.Matts PJ, Dykes PJ, Marks R. The distribution of melanin in skin determined in vivo. Br J Dermatol. 2007;156:620–628. doi: 10.1111/j.1365-2133.2006.07706.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


