The orbitofrontal cortex (OFC) is centrally involved in high-level cognitive functions. For instance, it has long been associated with decision-making (Wallis, 2012), with traditional views focusing on its role in response inhibition (Dias, Robbins, & Roberts, 1996), and more recent studies emphasizing valuation or outcome prediction (Rudebeck & Murray, 2014; Schoenbaum, Roesch, Stalnaker, & Takahashi, 2009). The OFC is also implicated in emotional processing, evidenced by OFC patients having difficulties attending to emotional stimuli (Hartikainen & Knight, 2003), and recognizing emotional expressions (Hornak et al., 2003). These studies suggest that the OFC subserves high-level functions; however, its role in lower-level functions of sensory processing remains largely unknown.
The OFC’s involvement in sensory response draws support from animal and human research. Functional data has shown the OFC receives information from the auditory sensory modality in monkeys (Zald & Kim, 1996). Structural studies have demonstrated connections between the OFC and the superior temporal gyrus in monkeys (Barbas, Ghashghaei, Dombrowski, & Rempel-Clower, 1999; Petrides & Pandya, 2007) and Heschl’s gyrus in humans (Cammoun et al., 2015), areas critical for auditory sensory processing. The sensory response to auditory input is commonly indexed by the N1 event-related potential (ERP) component, which is primarily generated in the supratemporal plane in humans, with additional contributions from lateral temporal regions and the parietal cortex (Knight, Hillyard, Woods, & Neville, 1980; Nååtånen & Picton, 1987). Top-down attentional control has been shown to modulate this auditory evoked potential (Knight, Hillyard, Woods, & Neville, 1981; Waldorff et al., 1993). Previous studies implicating the OFC in the aforementioned high-level cognitive functions indicate that the OFC is capable of exerting top-down control. Together, these studies suggest the OFC has the neuroanatomical connections and the functional capacity to exert top-down attentional control to modulate auditory sensory processing. To our knowledge, this has not been directly tested in humans. We used a combined electrophysiological and neuropsychological approach to determine whether human OFC is a critical brain region for regulating sensory processing of auditory stimuli.
We recruited nine patients with lesions in the OFC, and nine matched healthy controls to participate in the experiment. All controls were tested at the University of California, Berkeley, while patients were tested at two sites: University of California, Berkeley (n = 5) and the University of Oslo (n = 4). Fig. 1a shows the lesion overlap for OFC patients. Subjects’ demographic information and patient characteristics are presented in Supplementary Information. All subjects provided written informed consent, and were reimbursed for their participation. This study was approved by the institutional review boards at the University of California, Berkeley, the Oslo University Hospital, and the Norwegian Regional Committee for Medical Research Ethics, Region South.
Fig. 1.
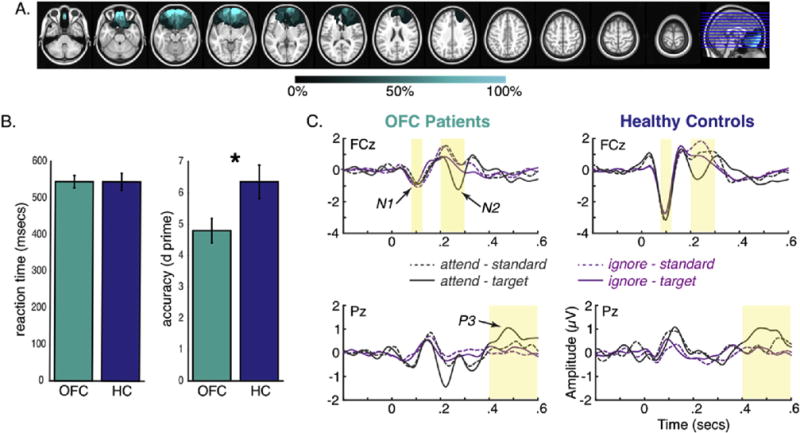
A) Lesion overlap for OFC patients. Each horizontal line on the far right image reflects the location of each slice shown on the left. Color map indicates percentage of overlap across patients. B) Behavioral performance for OFC patients (shown in teal) and healthy controls (shown in blue). OFC patients showed reduced d prime compared to healthy controls. C) ERP waveforms for OFC patients (left) and healthy controls (right) for electrodes FCz (top) and Pz (bottom). Time windows of analyses for N1 (.08–.12 sec), N2 (.2–.3 sec), and P3b (.4–.6 sec) are highlighted in yellow.
Subjects were presented with a series of standard tones (800 Hz) and target tones (1000 Hz), one at a time in a random order with probabilities of .8 and .2, respectively. The duration of each tone was 200 msec, and the inter-trial interval was randomly jittered between 800 and 1200 msec. In the attend condition, subjects were instructed to focus on the tones and press a button only when the target tone is heard. Mean reaction time and d prime were computed for each subject. D prime is an accuracy measure that takes into consideration both hit rate and false alarm rate, and was calculated as follows: d’ = Z (hit rate) – Z (false alarm rate). In the ignore condition, they were instructed to refrain from paying explicit attention to the tones. We recorded subjects’ EEG from 64 active electrodes. EEG signals were bandpass-filtered at 1–15 Hz for Event-Related Potential (ERP) analysis. The N1 and N2 peak amplitudes were measured over fronto-central midline sites, whereas the P3b mean amplitude was measured over parietal sites. ERP responses to standard and target tones in the attend and ignore conditions were averaged separately across subjects within each group. See Supplementary Information for EEG data preprocessing and analysis details.
OFC patients had reduced d prime [t (16) = −2.35, p = .032; Fig. 1b] compared with controls, whereas mean reaction time to attended targets did not differ between groups (p = .99). ERP measures were compared between groups using an ANOVA that included within-subject factors of condition (attend vs ignore) and tone (standard vs target), and a between-subject factor of group. Bonferroni correction was applied to account for the three ANOVAs performed for each ERP component, yielding a critical α of .017. Results are shown in Fig. 1c. A main effect of group revealed that OFC patients showed reduced N1 peak amplitude compared with the controls (patients: −1.15 μV; controls: −3.19 μV; p = .001). A similar pattern was observed for the preceding P1 ERP component; however, the effect only approached significance. Details regarding this post-hoc analysis are reported in the Supplementary Information. No other effects involving attention or group for the N1 were significant (p = .524–.887). There was a condition by tone interaction for the N2 (p = .006), driven by larger N2 peak amplitudes for target relative to standard tones during the attend (p = .002), but not ignore condition (p = .211). The P3b showed effects of condition (p = .001) and tone (p < .001) that were modified by a condition by tone interaction (p = .004). This was driven by larger P3b mean amplitudes for target relative to standard tones during the attend (p < .001), but not ignore condition (p = .195). The main and interaction effects involving group for both the N2 and P3b were not significant (p = .174–.926). Interestingly, the N1 peak amplitude for target tones in the attend condition negatively correlated with d prime (rs (18) = −.49, p = .040; Fig. S2), with larger N1 amplitude predicting higher accuracy. Lesion volume had no effects on any ERP or behavioral measures (see Supplementary Information).
These results indicate that OFC damage dampens the N1 sensory response primarily generated in human auditory cortices (Knight et al., 1980; Nååtånen & Picton, 1987). The implications are two-fold. First, this result provides insight into the functional role of structural connections between the OFC and superior temporal gyrus previously reported in monkeys (Barbas et al., 1999; Petrides & Pandya, 2007). Another study in mice also showed OFC stimulation modulates sensory-evoked activity in the primary auditory cortex (Winkowski, Bandyopadhyay, Shamma, & Kanold, 2013). These studies converge on the OFC’s capacity for top-down sensory modulation of the auditory cortex across species.
Second, given the OFC’s structural connections to sensory cortices (Petrides & Pandya, 2007) and its importance for assigning value to incoming signals (Rudebeck & Murray, 2014), our findings may reflect a general reduction in values assigned to sensory inputs following OFC damage. Despite the robust attenuation in sensory response however, subsequent stages of detecting target tones were not impaired, suggesting these processes do not require the OFC. Specifically, OFC patients not only showed comparable magnitude of the N2 and P3b responses as the controls, but they also showed the same attention effect with target tones eliciting a larger N2 and P3b than the standard tones during the attend condition. This indicates that OFC lesions do not impair post-sensory level response to these emotionally neutral stimuli, consistent with previous studies reporting intact attentional orienting in OFC patients (Funderud et al., 2013).
The observation that OFC patients showed reduced sensory response but normal target detection highlights an important distinction in the OFC’s role between sensory level processing and subsequent stages of processing. While the literature primarily implicates the OFC in post-sensory, high-level cognitive functions, our data shows it also has the capacity to exert top-down control to selectively regulate initial stages of auditory processing. The critical role of OFC in top-down modulation of auditory processing suggests that impairments in early sensory processing may contribute to emotional and reward processing deficits observed in these patients.
Supplementary Material
Acknowledgments
We appreciate our subjects for taking the time to participate in our study. This work was supported by the Natural Sciences and Engineering Research Council and the James S. MacDonald Foundation for JWYK, Research Council of Norway 240389/F20 and Internal Funding from the University of Oslo for AKS, TE, and TRM, and NINDS R3721135 and NIMH grant PO1 MH109420-01 to RTK.
Footnotes
Declaration of interest
None.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cortex.2017.12.023.
References
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. Journal of Computational Neurology. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cammoun L, Thiran JP, Griffa A, Meuli R, Hagmann P, Clarke S. Intrahemispheric cortico-cortical connections of the human auditory cortex. Brain Structure and Function. 2015;220:3537–3553. doi: 10.1007/s00429-014-0872-z. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Funderud I, Løvstad M, Lindgren M, Endestad T, Due-Tønnessen P, Meling TR, et al. Preparatory attention after lesions to the lateral or orbital prefrontal cortex – an event-related potentials study. Brain Research. 2013;1527:174–188. doi: 10.1016/j.brainres.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen KM, Knight RT. Lateral and orbital prefrontal cortex contributions to attention. In: Polich J, editor. Detection of change: Event-related potential and fMRI findings. Norwell, MA: Kluwer Academic; 2003. pp. 99–116. [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Knight RT, Hillyard SA, Woods DL, Neville HJ. The effects of frontal and temporal-parietal lesions on the auditory evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1980;50:112–126. doi: 10.1016/0013-4694(80)90328-4. [DOI] [PubMed] [Google Scholar]
- Knight RT, Hillyard SA, Woods DL, Neville HJ. The effects of frontal cortex lesions on event-related potentials during auditory selective attention. Electroencephalography and Clinical Neurophysiology. 1981;52:571–582. doi: 10.1016/0013-4694(81)91431-0. [DOI] [PubMed] [Google Scholar]
- Nååtånen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. Journal of Neuroscience. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. The orbitofrontal oracle: Cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014;84:1143–1156. doi: 10.1016/j.neuron.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new Perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Review Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, et al. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proceedings of National Academy of Science. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nature Neuroscience. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Bandyopadhyay S, Shamma SA, Kanold PO. Frontal cortex activation causes rapid plasticity of auditory cortical processing. Journal of Neuroscience. 2013;33:18134–18148. doi: 10.1523/JNEUROSCI.0180-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex, I: Anatomy, neurocircuitry and obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 1996;8:125–138. doi: 10.1176/jnp.8.2.125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


