Abstract
Dedicated breast computed tomography (CT) is the latest in a long history of breast imaging techniques dating back to the 1960s. Breast Imaging is performed both for cancer screening as well as for diagnostic evaluation of symptomatic patients. Dedicated breast CT received United States Food and Drug Administration (US FDA) approval for diagnostic use in 2015 and is slowly gaining recognition for its value in diagnostic 3D imaging of the breast, and also for injected contrast-enhanced imaging applications. Conventional mammography has known limitations in sensitivity and specificity, especially in dense breasts. Breast Tomosynthesis was US FDA approved in 2011 and is now widely used. Dedicated breast CT is the next technological advance, combining real 3D imaging with the ease of contrast administration. The lack of painful compression and manipulation of the breasts also makes dedicated breast CT much more acceptable for the patients.
Historical perspective
X-ray imaging of the breast for the diagnosis of cancer started in the early 1930s and it gradually evolved to today’s digital mammography (DM) and breast tomosynthesis. Breast tomosynthesis is also called “three-dimensional (3D)” mammography but in this approach, the breast is not imaged in a multi-planar mode in three-dimensional and isotropic resolution. Fully 3D imaging of the breast can be performed by conventional multi-detector computed tomography (CT). However, this will require axial acquisitions through the chest and this is clearly undesirable because of unnecessary radiation dose to the patient. An experimental CT scanner for imaging the breast was developed in the late 1970s by General Electric and clinical feasibility trials with human subjects were conducted with this system between 1976 and 1981. The single-slice fan-beam CT system used prone patient positioning with the pendant breast surrounded by a water bath. Patient positioning and rotation of the x-ray tube and detector assembly was similar to current stereotactic breast biopsy tables. The reconstructed images were 1 cm in slice width and had in-plane voxel size of 1.56 × 1.56 mm. In a study of 1625 subjects, of which 71 patients had 78 histology confirmed cancers, breast CT with intravenously administered contrast media detected 94% of cancers compared to 77% by mammography.1 In spite of encouraging results, the technology and image reconstruction techniques available at that time could not meet the spatial resolution and radiation dose requirements for breast imaging. These early investigators deserve credit for their vision and concept but they were about 40 years before technology evolved to meet the challenge of breast imaging. Advances in CT technology over subsequent decades renewed interest in dedicated breast CT2, 3, with one key study demonstrating the potential for performing dedicated breast CT at radiation dose comparable to mammography3. Development of flat panel x-ray detectors and improvements in image reconstruction techniques have enabled high-resolution cone beam computed tomography (CBCT), an approach which was more amenable for adapatation to dedicated breast CT.
The technique
In dedicated breast CT, the subject lies prone with the breast pendant through an aperture. The primary x-ray beam is limited to only image the breast. Broadly, breast CT can be classified into those where the projection data are acquired by helical motion of the x-ray tube and detector assembly similar to multi-detector CT (MDCT) systems or cone-beam breast CT (CBBCT) systems where the detector is large enough to cover the entire breast in each projection and the projection data are acquired in a single circular scan. At present, most dedicated breast CT systems are CBBCT systems4, 5, however, development of a helical or spiral scanning dedicated breast CT system has been reported6. This helical CT approach uses photon counting x-ray detectors instead of charge integrating flat panel detectors. At the time of this writing, in vivo clinical studies with helical scanning breast CT system is yet to be reported and hence the rest of this article focuses on CBBCT systems. During a typical scan with dedicated CBBCT, typically 300 to 500 projections of the breast are acquired over a 360° rotation of the x-ray tube and detector assembly. The acquired projection data are reconstructed mostly using variants of the filtered-backprojection algorithm appropriate for the acquisition technique. The reconstructed images can be viewed in any orientation including standard coronal, axial and sagittal planes along with 3D volume rendering. This precludes the need for multiple acquistions common to digital mammography and breast tomosynthesis to view the breast in the desired orientation. Several publications describe the design aspects and clinical feasibility of dedicated CBBCT7–13. In 2015, the FDA approved one dedicated CBBCT system, supported by a large reader study that showed improved sensitivity of non-contrast CBBCT over mammography for diagnostic imaging14. The FDA approval restricts its use for non-contrast diagnostic imaging of the breast. The potential role of dedicated breast CT in screening has yet to be established.
The Radiation dose
The radiation dose from CBBCT depends on the intended use (e.g., screening or diagnostic evaluation) and if the study also involves contrast administration. A preliminary study with CBBCT targeting radiation dose similar to screening mammography has shown improved conspicuity of soft tissue lesions with CBBCT compared to mammography4. For diagnostic imaging, the radiation dose from CBBCT is well within the range for diagnostic mammography15, 16. Independent studies have shown that for diagnostic evaluation with CBBCT, the average mean glandular dose (MGD) ranges between 7 and 13.9 mGy15–18, with more recent studies reporting an average MGD of 7 to 7.2 mGy17, 18. The range for diagnostic mammography was much wider, with the maximum as high as 31.6 to 36 mGy15, 16 due to multiple views including magnification views.
The need for dedicated breast CT
Simply stated, there is a need to detect breast cancer at the earliest possible stage, ideally before it becomes invasive. Recent national benchmarks19 show that 76.9% of cancers detected by screening digital mammography are stage 0 or 1, indicating that one-fourth of the cancers are not detected early enough. Imaging of the breast needs a high-resolution, high-contrast technology capable of both detecting calcifications as small as a few hundred microns, as well as subtle density differences to be able to detect early cancers (Figure 1). This requires moving beyond mammography and recognizing that it is also important to plan for contrast administration. When contrast-enhanced examination is indicated, it needs to be performed at an acceptable radiation dose.
Fig. 1. Ductal Carcinoma In-situ.
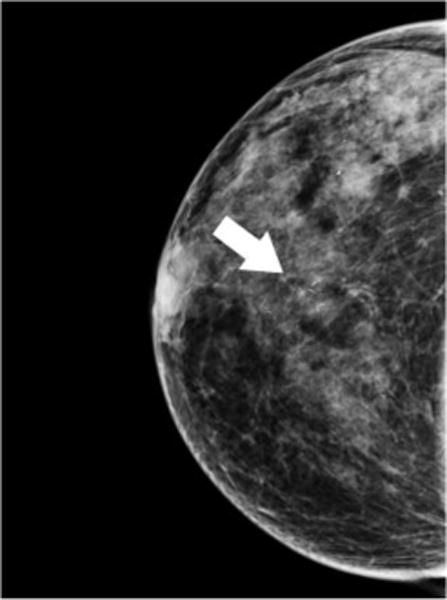


64 year-old woman presents for screening mammogram. Mammographic CC (A) and MLO (B) views demonstrate grouping of pleomorphic calcifications in the left breast at the 12 o’clock position (arrows). Reformatted non-enhanced CBCT images at 0.273 mm standard resolution (C) and 0.122 mm high-resolution (D) clearly depict grouping of calcifications; morphology of calcifications are more clearly depicted in the higher resolution images. Lesion diagnosed as DCIS at time of biopsy.
Since all of imaging exists in a continuum, it can be understood as to how breast imaging exemplifies this concept. From the earliest experiences in breast imaging in the 1960s, imaging of the breast has evolved through xeromammography, screen-film mammography, digital mammography and now breast tomosynthesis. Breast imaging has become more sensitive and specific with each technological advance. Each technology ultimately reaches a performance plateau, and subsequent technological advances are needed such as with digital mammography gradually giving way to breast tomosynthesis. At this time, breast tomosynthesis is the closest mammography can come to 3D imaging. It is an excellent technique, allowing low dose slices of the breast to be reconstructed in planes parallel to the detector20. However, it still has many of the limitations of two-dimensional (2D) mammography such as compression and substantial tissue overlap between successive slices, particularly in dense breasts. Cone-beam breast CT (CBBCT) is the next technical development, allowing for true 3D imaging of the uncompressed breast.
Need 3D imaging for a 3D structure
Mammography is an excellent technology and is responsible for early detection of numerous breast cancers. This has allowed earlier and better treatment resulting in a decrease in breast cancer mortality of at least 30% in the past 40 years21. If we were to start designing an imaging system today for early detection of breast cancer, the current method of compression mammography would almost certainly not be the first choice. Requirements would include low radiation dose, some form of 3D imaging that would not require vigorous and often painful compression of each breast twice for the initial screening exam, and easy administration of contrast media, if indicated.
The breast is a 3D structure which is ideally imaged in its natural form. It is very rare in modern imaging to distort an organ or body part from its natural state prior to imaging. There are 3D imaging modalities such as whole breast ultrasound and MRI, but both have limitations. Neither US nor MRI can detect the microcalcifications that most often lead to the diagnosis of ductal carcinoma in situ (DCIS), which is believed to be an obligate precursor of invasive ductal carcinoma, the most common form of breast cancer. MRI is also inherently much more costly to perform compared to mammography, tomosynthesis or breast CT, is much more time consuming examination for the patient, and requires intravenous gadolinium contrast administration.
CBCT is potentially better in dense breasts
The sensitivity of conventional mammography ranges between 50–85%, depending on breast density22. In dense breasts, it is 50% or even less23. Tissue superposition is a major reason, which results in both false positives resulting in unnecessary call backs for additional imaging and also false negatives by masking true lesions. Breast tomosynthesis was developed to partially overcome tissue superposition (Figure 2). Breast tomosynthesis is referred to as 3D mammography, but in reality the slice separation is limited24. While breast tomosynthesis reduces call back rates compared to digital mammography for both dense and non-dense breasts, the call back rate for dense breasts is higher than that for non-dense breasts25. Dedicated cone beam breast CT is true isotropic 3D imaging, meaning that the images are equally sharp and accurate in all planes. This could clarify potential false positive findings due to overlapping dense tissue, at the same time reducing the potential for missing subtle abnormalities.
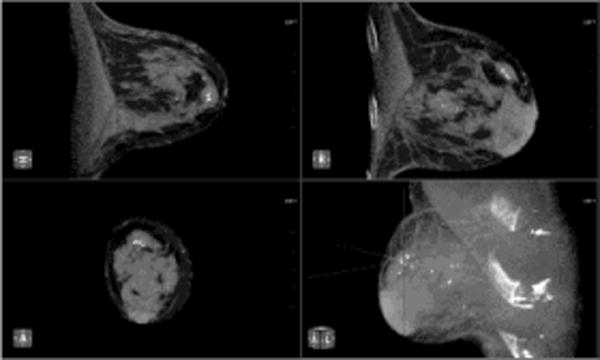
Fig. 2. Small Invasive Ductal Carcinoma.


55 year-old woman presents for screening mammogram. Mammographic CC (A) and MLO (B) views demonstrate heterogeneously dense breast tissue with ill-defined focal asymmetry in the deep right breast at the 12 o’clock position (white circles). Contrast enhanced CBCT demonstrates more clearly a subcentimeter irregular mass (crosshairs) at the 12 o’clock location (C). Lesion diagnosed as invasive ductal carcinoma at time of biopsy.
It is time for contrast
In diagnostic imaging of cancers in other organs, the use of contrast media is routine. For example, if one considers CT imaging for cancer of the liver, kidney or brain, it is unimaginable to perform these examinations without administration of contrast media. There is no clinical or scientific argument as to why this should be different for breast cancers. Among clinically used modalities, contrast-enhanced breast MRI has shown the highest sensitivity, but with variable specificity26. However, breast MRI is currently recommended only for adjunctive screening of women with lifetime risk greater than 20–25%, for extent-of-disease evaluation of verified cancers, when there is concern for residual or recurrent cancer and on rare occasion for diagnostic evaluation of equivocal findings from other modalities. While contrast enhanced MRI is a powerful tool, it is not suitable for some women (e.g., for women with pacemakers, implantable devices, and claustrophobia). Cost and limited availability are also important concerns with MRI. Recent experience with contrast enhanced CBCT (CE-CBCT) has shown great promise as a diagnostic imaging tool and as a potential substitute for breast MRI17, 27 (Figure 3).
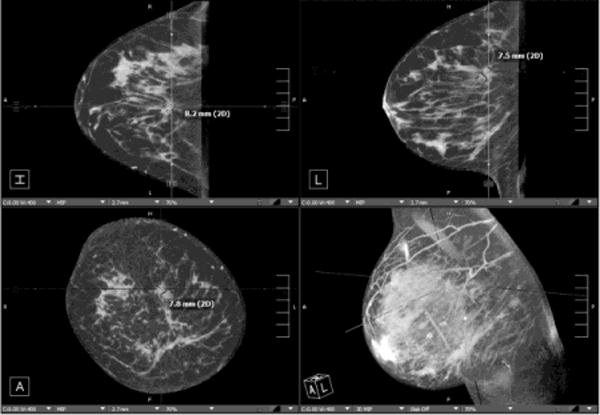
Fig. 3. Assessment of index lesion and extent of disease.


Patient presents with palpable mass in the right breast. Mammographic CC (A) and MLO (B) views demonstrate large irregular mass at the 12 o’clock position consistent with BIRADS-5:highly suspicious (white arrows). (c) Contrast enhanced CBCT was performed to evaluate extent of disease, which demonstrates the index lesion in exquisite detail (crosshairs). No satellite lesions or axillary adenopathy is identified.
Considering the historical progression from digital mammography, to digital breast tomosynthesis, to dedicated breast CT, it is essential to plan for contrast administration with dedicated breast CT as the next step to achieve higher sensitivity and specificity. Uncompressed dedicated breast CT is ideally suited for contrast administration to avoid the confounding effects of compression on contrast agent uptake and may facilitate quantitative evaluation of contrast enhancement. After morphological imaging has been optimized with true 3D imaging, there is still limitation due to the inability to differentiate asymmetric breast tissue from pathological processes. Even after detection of a mass, the distinction between benign and malignant conditions often can not be made without biopsy. In these circumstances, contrast enhancement can be valuable for diagnostic evaluation. Many masses can be interpreted as benign with a high degree of certainty and these subjects can be returned to routine screening. If considered to be “probably benign” i.e., <2% chance of malignancy they can be undergo short-interval follow up, usually in 6 months. Some will still need biopsy for tissue sampling and full pathological evaluation. A preliminary study showed that quantitative analysis of contrast enhanced CBBCT (CE-CBBCT) had an accuracy of 0.876 in discriminating benign and malignant lesions27. Similar to contrast enhancement breast MRI, indications for CE-CBBCT can be for high-risk screening, extent-of-disease evaluation, and evaluation of equivocal findings. CE-CBCT has also been shown to demonstrate extensive DCIS27. Since overdiagosis and overtreatment of DCIS is a great source of controversy, perhaps CE-CBBCT as an indicator of biologic activity and vascular flow may help separate indolent DCIS that can be followed more conservatively from the more aggressive sub types that need aggressive treatment.
Added value from CBCT: Downgrade the BIRADS
Recalls and false positives leading to unnecessary biopsies are an unavoidable downside to high quality screening, when the aim is to find ever smaller cancers to allow the earliest possibe diagnosis. Just as breast tomosynthesis reduces callbacks and finds additional cancers compared to mammography, breast CT has the capability to do the same. When screening mammography results in a call back (BIRADS 0) because aof asymmetries, or possible masses, a single non contrast CBBCT can be helpful. Asymmetries are displayed as real findings and can distinguish from superimposed glandular tissue resulting in a false positive mammogram.
Every BI-RADS situation that can be safely downgraded is a benefit to the patient and a reduction in cost to the healthcare system, both in terms of additional imaging and/or biospy. In addition, lower BI-RADS assessment categories decrease patient anxiety. In other diagnostic situations such as palpable masses or pain, the CBBCT is also helpful. For masses, the margins are displayed without overlap and for pain, there may be no focal finding, and the patient may then procede to US (Figure 4). For women with pain, this is a more acceptable diagnostic workup than multiple mammographic compression views.
Fig. 4. Benign lesion and potential for BI-RADS downgrade.

51 year-old woman presents with palpable lump in the left breast and refused conventional work-up using compression mammography because of discomfort. Following initial evaluation with ultrasound and ultrasound-guided biopsy results consistent with benign fibroadenoma, the patient presented for post-clip imaging using CBCT as a more comfortable alternative to compression mammography. Nonenhanced CBCT clearly demonstrates benign features and biopsy clip in place (crosshairs). Notably, this lesion would been initially categorized as BIRADS-3:probably benign by CBCT appearance, with a recommendation for short interval follow-up imaging – clearly a preferable alternative to ultrasound-guided biopsy in a patient concerned about breast discomfort.
Other applications
In addition to imaging of women in diagnostic situations and selected screening situations in the future, there are other useful applications for dedicated breast CT. CBCT, with or without contrast, is effective in assessing response to therapy in cases of known malignancy (Figure 5). Another application is in the evaluation of the post surgical breast, for example, for assessment of implant integrity and other post operative indications including infection, scarring, and fat necrosis. And,although male breast cancer is only 1% of all breast cancers diagnosed,men also present with other masses requiring imaging. Breast CT is much more comfortable and acceptable in these sutuations.
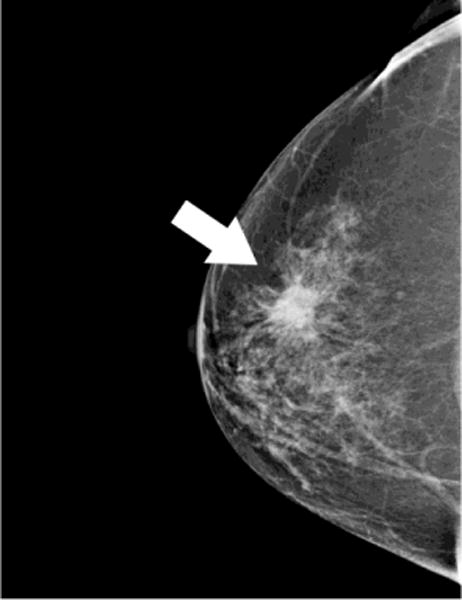
Fig. 5. Evaluation of response to therapy.

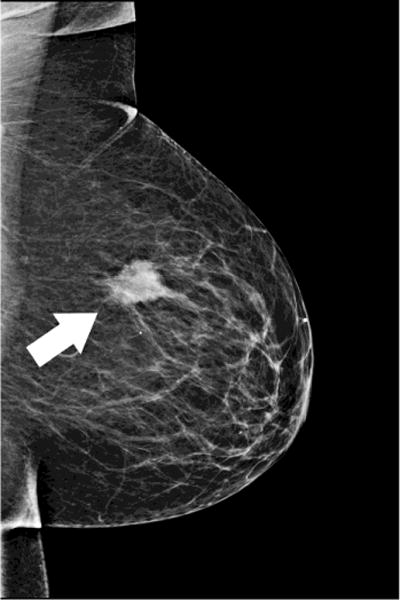

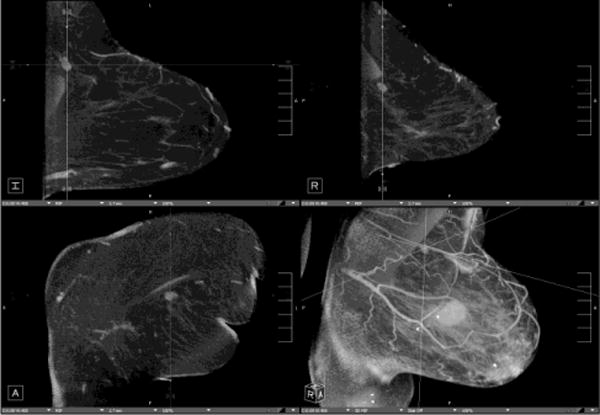

66 year-old woman presents with palpable lump in the left breast. Mammographic CC (A)and MLO (B) views demonstrate large irregular mass in the upper outer breast (white arrows). Nonenhanced CBCT (C) demonstrates known mass (crosshairs). In addition, an abnormal-appearing lymph node (D, crosshairs) is clearly seen in the axilla, not evident in the standard mammographic views. Repeat nonenhanced CBCT 6 months later following neoadjuvant chemotherapy (E) demonstrates interval enlargement of index lesion (with biopsy clip in place), consistent with lack of therapeutic response.
Summary: The top 10 reasons we need CBCT.
It is the next technical improvement after mammography and tomosynthesis have reached their limits.
One “view” only per breast, then we can “manipulate the image not the patient”.
It is better for dense breasts (eliminates overlap).
Reduces false positives and false negatives.
Better resolution than MRI (CBCT can visualize calcifications).
Ease of contrast administration.
Less costly to perform.
Shorter time to peform than diagnostic mammography and MRI.
Improved patient comfort-no compression.
More acceptable to many patients for cultural and privacy reasons.
Acknowledgments
This work was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) grants R21 CA134128, R01 CA195512 and R01 CA199044. The contents are solely the responsibility of the authors and do not represent the official views of the NIH or NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Avice M. O’Connell, Professor, Department of Imaging Sciences, Director of Women’s Imaging, University of Rochester Medical Center, Rochester, NY.
Andrew Karellas, Professor, Department of Medical Imaging, Director of Biomedical Imaging Innovation/Clinical Translation in Next-Gen CT, Vice Chair of Faculty Development, Department of Medical Imaging, University of Arizona College of Medicine, Banner University Medical Center.
Srinivasan Vedantham, Professor, Department of Medical Imaging (DMI), Associate Director, Biomedical Imaging Innovation/Clinical Translation in Next-Gen CT, Director, Office for Project Statistical and Design Support - DMI, University of Arizona College of Medicine, Banner University Medical Center.
Daniel T. Kawakyu-O’Connor, Assistant Professor, Department of Imaging Sciences, Section Chief, Section of Emergency Imaging, University of Rochester Medical Center, Rochester, NY.
References
- 1.Chang CH, Sibala JL, Fritz SL, Dwyer SJ, 3rd, Templeton AW, Lin F, et al. Computed tomography in detection and diagnosis of breast cancer. Cancer. 1980;46(4 Suppl):939–46. doi: 10.1002/1097-0142(19800815)46:4+<939::aid-cncr2820461315>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Raptopoulos V, Baum JK, Hochman M, Karellas A, Houlihan MJ, D’Orsi CJ. High resolution CT mammography of surgical biopsy specimens. J Comput Assist Tomogr. 1996;20(2):179–84. doi: 10.1097/00004728-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Boone JM, Nelson TR, Lindfors KK, Seibert JA. Dedicated breast CT: radiation dose and image quality evaluation. Radiology. 2001;221(3):657–67. doi: 10.1148/radiol.2213010334. Epub 2001/11/24. [DOI] [PubMed] [Google Scholar]
- 4.Lindfors KK, Boone JM, Nelson TR, Yang K, Kwan AL, Miller DF. Dedicated breast CT: initial clinical experience. Radiology. 2008;246(3):725–33. doi: 10.1148/radiol.2463070410. Epub 2008/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connell A, Conover DL, Zhang Y, Seifert P, Logan-Young W, Lin CF, et al. Cone-beam CT for breast imaging: Radiation dose, breast coverage, and image quality. AJR Am J Roentgenol. 2010;195(2):496–509. doi: 10.2214/AJR.08.1017. Epub 2010/07/24. [DOI] [PubMed] [Google Scholar]
- 6.Rossler AC, Kalender W, Kolditz D, Steiding C, Ruth V, Preuss C, et al. Performance of Photon-Counting Breast Computed Tomography, Digital Mammography, and Digital Breast Tomosynthesis in Evaluating Breast Specimens. Acad Radiol. 2017;24(2):184–90. doi: 10.1016/j.acra.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Vedantham S, Shi L, Karellas A, O’Connell AM, Conover D. Dedicated breast CT: anatomic power spectrum; The Second International Conference on Image Formation in X-ray Computed Tomography; June 24-27; Fort Douglas/Salt Lake City, UT. 2012. pp. 70–3. [Google Scholar]
- 8.Vedantham S, Shi L, Karellas A, O’Connell AM. Dedicated breast CT: fibroglandular volume measurements in a diagnostic population. Med Phys. 2012;39(12):7317–28. doi: 10.1118/1.4765050. Epub 2012/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L, Vedantham S, Karellas A, O’Connell AM. Technical note: Skin thickness measurements using high-resolution flat-panel cone-beam dedicated breast CT. Med Phys. 2013;40(3):031913. doi: 10.1118/1.4793257. Epub 2013/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell AM, Karellas A, Vedantham S. The potential role of dedicated 3D breast CT as a diagnostic tool: review and early clinical examples. Breast J. 2014;20(6):592–605. doi: 10.1111/tbj.12327. Epub 2014/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vedantham S, O’Connell AM, Shi L, Karellas A, Huston AJ, Skinner KA. Dedicated Breast CT: Feasibility for Monitoring Neoadjuvant Chemotherapy Treatment. J Clin Imaging Sci. 2014;4:64. doi: 10.4103/2156-7514.145867. Epub 2015/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazi PM, Yang K, Burkett GW, Jr, Aminololama-Shakeri S, Seibert JA, Boone JM. Evolution of spatial resolution in breast CT at UC Davis. Med Phys. 2015;42(4):1973–81. doi: 10.1118/1.4915079. Epub 2015/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vedantham S, Karellas A, Emmons MM, Moss LJ, Hussain S, Baker SP. Dedicated breast CT: geometric design considerations to maximize posterior breast coverage. Phys Med Biol. 2013;58(12):4099–118. doi: 10.1088/0031-9155/58/12/4099. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole EB, Campbell AS, Vedantham S, Pisano ED, Karellas A. Clinical Performance of Dedicated Breast Computed Tomography in Comparison to Diagnostic Digital Mammography [abstract # SSA01-09]; 101st Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA 2015); 2015 November 29 – December 4; Chicago, IL: Radiology Society of North America; [Google Scholar]
- 15.Vedantham S, Shi L, Karellas A, O’Connell AM, Conover DL. Personalized estimates of radiation dose from dedicated breast CT in a diagnostic population and comparison with diagnostic mammography. Phys Med Biol. 2013;58(22):7921–36. doi: 10.1088/0031-9155/58/22/7921. Epub 2013/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell AM, Kawakyu-O’Connor D. Dedicated Cone-beam Breast Computed Tomography and Diagnostic Mammography: Comparison of Radiation Dose, Patient Comfort, And Qualitative Review of Imaging Findings in BI-RADS 4 and 5 Lesions. J Clin Imaging Sci. 2012;2:7. doi: 10.4103/2156-7514.93274. Epub 2012/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He N, Wu YP, Kong Y, Lv N, Huang ZM, Li S, et al. The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: A prospective study with 212 patients. Eur J Radiol. 2016;85(2):392–403. doi: 10.1016/j.ejrad.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Wienbeck S, Uhlig J, Luftner-Nagel S, Zapf A, Surov A, von Fintel E, et al. The role of cone-beam breast-CT for breast cancer detection relative to breast density. Eur Radiol. 2017 doi: 10.1007/s00330-017-4911-z. [DOI] [PubMed] [Google Scholar]
- 19.Lehman CD, Arao RF, Sprague BL, Lee JM, Buist DS, Kerlikowske K, et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58. doi: 10.1148/radiol.2016161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vedantham S, Karellas A, Vijayaraghavan GR, Kopans DB. Digital Breast Tomosynthesis: State of the Art. Radiology. 2015;277(3):663–84. doi: 10.1148/radiol.2015141303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 22.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. Epub 2003/02/01. [DOI] [PubMed] [Google Scholar]
- 23.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225(1):165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 24.Nosratieh A, Yang K, Aminololama-Shakeri S, Boone JM. Comprehensive assessment of the slice sensitivity profiles in breast tomosynthesis and breast CT. Med Phys. 2012;39(12):7254–61. doi: 10.1118/1.4764908. Epub 2012/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–6. doi: 10.1001/jama.2016.1708. [DOI] [PubMed] [Google Scholar]
- 26.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116–24. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 27.Prionas ND, Lindfors KK, Ray S, Huang SY, Beckett LA, Monsky WL, et al. Contrast-enhanced dedicated breast CT: initial clinical experience. Radiology. 2010;256(3):714–23. doi: 10.1148/radiol.10092311. Epub 2010/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]


