SUMMARY PARAGRAPH
Fenethylline, also known as Captagon, is a synthetic psychoactive stimulant that has recently been linked to substance use disorder and ‘pharmacoterrorism’ in the Middle East.1–4 Although fenethylline shares a common phenethylamine core with other amphetamine-type stimulants, it additionally incorporates a covalently-linked xanthine moiety into its parent structure.5,6 These independently-active pharmacophores are liberated during metabolism, resulting in a structurally-diverse chemical mixture being deployed to the central nervous system.7–9 Although fenethylline’s psychoactive properties have been reported to differ from other synthetic stimulants, the in vivo chemical complexity it manifests upon ingestion has impeded efforts to unambiguously identify the specific species responsible for these effects.10,11 Here we develop a ‘dissection through vaccination’ approach, called DISSECTIV, to mitigate fenethylline’s psychoactivity and show that its rapid-onset and distinct psychoactive properties are facilitated by functional synergy between theophylline and amphetamine. Our results demonstrate that incremental vaccination against single chemical species within a multi-component mixture can be used to uncover emergent properties arising from polypharmacologic activity. We anticipate that DISSECTIV will be employed to expose unidentified active chemical species and illuminate pharmacodynamic interactions within other chemically complex systems, such as those found in counterfeit or illegal drug preparations, post-metabolic tissue samples, and natural product extracts.
Keywords: vaccine, fenethylline, Captagon, amphetamine, DISSECTIV
MAIN
In the last decade, an increasing number of substance use problems have been caused by synthetic psychoactive drugs (SPD), including amphetamine and methamphetamine.12,13 Despite their predominance in the US, these two drugs are not the most widely abused amphetamine analogues in all countries.14,15 In Saudi Arabia it has been estimated that 40% of drug users between ages 12–22 are addicted to fenethylline.1 While fenethylline addiction has historically been regionally confined, increased production and global trafficking of counterfeit tablets from Syria has been implicated as a source of revenue for militant groups to fund operations.1–3,16,17 Furthermore, as with the use amphetamine and methamphetamine in prior conflicts, fenethylline has been identified as a source of pharmacological morale in battle.3,4,18–20 The ultimate source of fenethylline’s psychoactive effects has historically been a debated subject, with some results supporting the position that the parent compound itself exhibits psychoactive effects distinct from those of other central stimulants, while others posit that it acts as merely an amphetamine prodrug.6–11 Considering the additional effort required to synthesize fenethylline, its continued manufacture and use in resource-strained conflict areas provides support for the hypothesis that this drug exhibits exploitable psychostimulant effects that differ from amphetamine alone.
Therefore, we supposed that any platform developed to block fenethylline’s effects would be most useful if it could also unambiguously identify the chemical sources of this drug’s psychoactivity. A large body of literature supports the overall concept of vaccination for the treatment of drug abuse, where a small molecule hapten-protein conjugate is delivered with the intention of generating antibodies that sequester the target drug in the periphery, preventing induction of central nervous system (CNS) mediated effects.21 A ‘dynamic’ variant of this general approach was recently developed to blunt heroin self-administration, wherein a single chemically-labile hapten was used to induce antibodies directed against multiple opioid metabolites.22 These foundational studies inspired us to pursue a generalizable ‘dissection through vaccination’ method that could isolate the effects of single compounds within a structurally- and pharmacologically-diverse chemical system, such as that generated by fenethylline.7–9 We termed the resulting approach “Determining the Identities of Species Supporting Expression of CNS-activity Through Incremental Vaccination”, or DISSECTIV (Extended Data Figure 1).
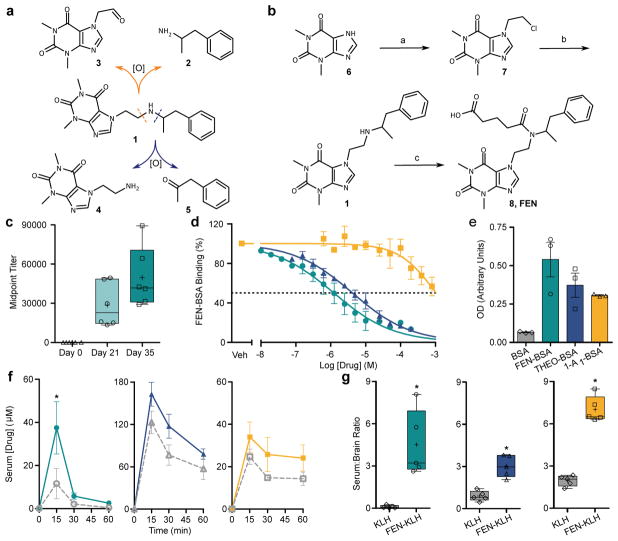
Our chemical strategy for synthesizing the parent fenethylline hapten was informed by the knowledge that fenethylline (1) liberates metabolites 2–5, including amphetamine (2) and substituted-theophylline metabolites, (which are further processed to theophylline (6)) following oxidative metabolism by cytochrome P450 (CYP450) enzymes (Figure 1A). We therefore installed our linker on the amphetamine-associated nitrogen that separates the two carbons where oxidation produces metabolites 2–5. Starting from 6, we first performed an N-alkylation with 1-chloro-2-iodoethane to generate 7, which was subjected to an in situ Finkelstein reaction and a second N-alkylation with 2 to generate 1. The final hapten species (FEN, 8) was generated from the condensation of 1 with glutaric anhydride in 24% overall yield (Figure 1B and Supplementary Methods). This hapten was conjugated to Keyhole Limpet Hemocyanin (KLH) using sulfo-N-hydroxysuccinimide in phosphate buffered saline (PBS) to generate the immunogenic species for vaccination, termed FEN-KLH. A separate conjugation to bovine serum albumin (BSA) was carried out to generate FEN-BSA for in vitro analyses (Extended Data Figure 2).
Figure 1.
Vaccination with FEN-KLH hapten generates antibodies against fenethylline and its
active metabolites. a, Oxidation by CYP450 enzymes liberates active
metabolites from fenethylline. b, Synthetic route to FEN
(8). Conditions: a - ICH2CH2Cl,
K2CO3, Dioxanes, 50 °C, 1 h, microwave heating
(μw); b – Amphetamine, KI, K2CO3, DMF, 150
°C, 20 min, μw; c – glutaric anhydride,
K2CO3, CHCl3, 2 h. c, Midpoint
titers, n=6, two replicates. d, Binding of FEN-KLH serum to
FEN-BSA with competing fenethylline (  ), theophylline (
), theophylline (  ), or amphetamine (
), or amphetamine (  ), pooled from n = 12, two replicates.
e, Serum cross-reactivity to BSA-hapten conjugates, optical
density (OD), pooled from n=12, three replicates. f, Serum
time courses in KLH (open) and FEN-KLH (solid) vaccinated animals for
fenethylline (
), pooled from n = 12, two replicates.
e, Serum cross-reactivity to BSA-hapten conjugates, optical
density (OD), pooled from n=12, three replicates. f, Serum
time courses in KLH (open) and FEN-KLH (solid) vaccinated animals for
fenethylline (  ,
,  ,20 mg/kg; [p,
interaction=0.0478; F(3,24)=3.05;
*-p<0.01, Bonferroni), theophylline (
,20 mg/kg; [p,
interaction=0.0478; F(3,24)=3.05;
*-p<0.01, Bonferroni), theophylline (  ,
,  ,8 mg/kg; [p, vaccine=0.0602; F(1,8)=4.78]),
and amphetamine (
,8 mg/kg; [p, vaccine=0.0602; F(1,8)=4.78]),
and amphetamine (  ,
,  ,8 mg/kg; [p, vaccine=0.0488;
F(1,8)=5.39]), n=5, repeated measures two-way ANOVA.
g, Serum: Brain ratios at 15 min for fenethylline (
,8 mg/kg; [p, vaccine=0.0488;
F(1,8)=5.39]), n=5, repeated measures two-way ANOVA.
g, Serum: Brain ratios at 15 min for fenethylline (
 ,
,  ,20 mg/kg;
*p=0.0136 vs KLH, t-test, Welch’s
correction, df=4), theophylline (
,20 mg/kg;
*p=0.0136 vs KLH, t-test, Welch’s
correction, df=4), theophylline (  ,
,  ,8 mg/kg; *-p<0.0005 vs KLH, t-test, df=8),
and amphetamine (
,8 mg/kg; *-p<0.0005 vs KLH, t-test, df=8),
and amphetamine (  ,
,  ,8 mg/kg; *-p<0.0001 vs
KLH, t-test, df=8) in KLH and FEN-KLH vaccinated animals, n=5.
c,g, Data shown as median with quartiles ±
10–90% CI (+=mean).
d–f, Data shown as mean ± SEM.
,8 mg/kg; *-p<0.0001 vs
KLH, t-test, df=8) in KLH and FEN-KLH vaccinated animals, n=5.
c,g, Data shown as median with quartiles ±
10–90% CI (+=mean).
d–f, Data shown as mean ± SEM.
To prepare the vaccine formulation, FEN-KLH was combined with two adjuvants, alum and CpG 1826. Intraperitoneal (IP) administration of the vaccine to Swiss Webster mice on days 0, 14, and 28 generated robust antibody midpoint titers (Figure 1C). Competitive surface plasmon resonance (SPR) was then used to measure the relative binding strength of antibodies generated from FEN-KLH vaccination for fenethylline and its active metabolites. In these assay conditions, the binding of fenethylline was strongest, followed by theophylline, then amphetamine (Figure 1D). Complementary use of an enzyme-linked immunosorbent assay (ELISA) to assess antibody specificity confirmed that antibodies within FEN-KLH serum were able to recognize the general structure of all three compounds (Figure 1E). To further explore the functional antibody binding profile of FEN-KLH in an immediately relevant model, we measured whether vaccination could alter the pharmacokinetics of fenethylline, theophylline, and amphetamine in vivo, using LC/MS analysis to quantify drug concentrations (Extended Data Figure 3). Satisfyingly, when the effect of vaccination on drug disposition was assessed over time following IP administration, it was seen that FEN-KLH binding was indeed sufficient to increase peak fenethylline, theophylline, and amphetamine sequestration in the periphery due to the binding of drug-specific antibodies (Figure 1F). As anticipated, this peripheral binding ultimately led to reductions in the rapid distribution of these compounds into the CNS (Figure 1G).
We also assessed whether endophenotypes relevant to fenethylline abuse could be blocked by FEN-KLH in a battery of animal models. When control animals were given fenethylline in an open field hyperlocomotor activity assay, a clear dose-dependent increase in locomotor activity was observed, and this effect was significantly diminished in FEN-KLH vaccinated animals (Figure 2A–B). Furthermore, we used an elevated plus maze (EPM) assay to measure anxiety-related behaviors following drug administration, as recent reports of fenethylline use in Syria have noted its apparent ability to induce hypervigilance, a behavior that is associated with persistent anxiety states in humans.23,24 In this experiment, fenethylline reduced the time that control animals spent in the open arms of the apparatus, while there was no such alteration in animals that had been vaccinated with FEN-KLH (Figure 2C). Additionally, because fenethylline demonstrates rewarding effects in human users, we assessed whether it could induce a conditioned place preference (CPP). We found that training with fenethylline could indeed induce CPP and observed that FEN-KLH demonstrated a trend toward blockade of this effect, although efficacy was variable (Figure 2D). Importantly, vaccination with FEN-KLH was able to decrease the pharmacodynamic (PD) effects of amphetamine and methamphetamine alone, which are often found alongside theophylline and caffeine in counterfeit tablets (Extended Data Figure 4).17
Figure 2.

Vaccination with FEN-KLH blunts the behavioral effects of fenethylline.
a, Total hyperlocomotion (90 min) due to fenethylline in KLH (
 ) and FEN-KLH (
) and FEN-KLH (  ) vaccinated animals, n=5,
repeated-measures two-way ANOVA ([p, vaccine=0.0048;
F(1,8)=14.88]; *-p<0.01 vs KLH,
Bonferroni). b,c, Fenethylline locomotor behavior at 0
mg/kg (p, interaction=0.8803; F(30,240)=0.70) and 20 mg/kg (p,
interaction<0.0001; F(30,240)=5.51];
*-p<0.001 vs KLH, Bonferroni), n=5,
repeated-measures two-way ANOVA, one outlier removed for KLH.
d,e, EPM for fenethylline (20 mg/kg), (
) vaccinated animals, n=5,
repeated-measures two-way ANOVA ([p, vaccine=0.0048;
F(1,8)=14.88]; *-p<0.01 vs KLH,
Bonferroni). b,c, Fenethylline locomotor behavior at 0
mg/kg (p, interaction=0.8803; F(30,240)=0.70) and 20 mg/kg (p,
interaction<0.0001; F(30,240)=5.51];
*-p<0.001 vs KLH, Bonferroni), n=5,
repeated-measures two-way ANOVA, one outlier removed for KLH.
d,e, EPM for fenethylline (20 mg/kg), (
 , KLH, n=12;
*p=0.0137 vs saline, df=11), (
, KLH, n=12;
*p=0.0137 vs saline, df=11), (
 , FEN-KLH, n=11;
p=0.7893 vs saline, df=10), paired t-test, one outlier removed
for FEN-KLH. f, CPP for fenethylline (20 mg/kg), (
, FEN-KLH, n=11;
p=0.7893 vs saline, df=10), paired t-test, one outlier removed
for FEN-KLH. f, CPP for fenethylline (20 mg/kg), (  , KLH, n=11;
, KLH, n=11;  , FEN-KLH, n=10;t-test, Welch’s
correction(p =0.3995, df=12)).
a–c, Data shown as mean ± SEM.
d–f, Data shown as median with quartiles
± 10–90% CI (+=mean).
, FEN-KLH, n=10;t-test, Welch’s
correction(p =0.3995, df=12)).
a–c, Data shown as mean ± SEM.
d–f, Data shown as median with quartiles
± 10–90% CI (+=mean).
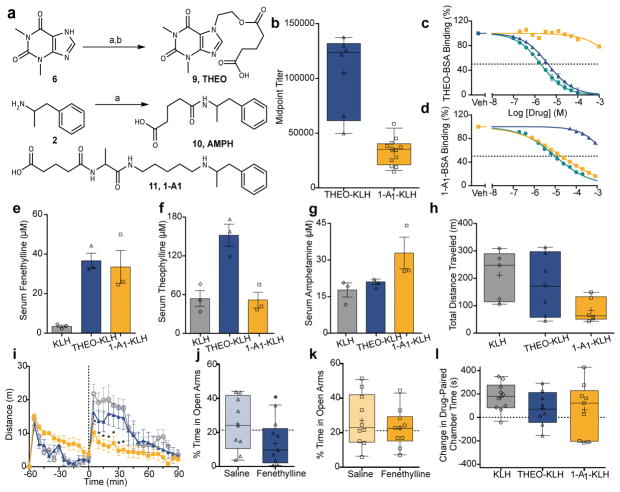
Because FEN-KLH demonstrated binding to fenethylline and its active metabolites, its blockade of stimulant effects could not be attributed to perturbation of one specific chemical species. Therefore, we employed our DISSECTIV approach to identify the compound(s) supporting fenethylline’s overall psychoactivity. The presence of fenethylline’s distinct psychoactive effects, as compared to amphetamine alone, imply that either the parent drug has unique activity or that the combined activities of the metabolites act together to produce an altered response.9,10 Compellingly, the fenethylline structure itself demonstrated little binding at 10 μM across a suite of 31 CNS targets, so we prioritized exploration of how fenethylline’s active metabolites contribute to its unique psychoactive profile (Extended Data Table 1). Thus, a theophylline hapten (THEO, 9) and two amphetamine haptens (AMPH, 10; 1-A1, 11) were generated (Figure 3A and Supplementary Methods).25 Following formulation and immunization with these haptens, robust titers were observed for THEO-KLH and 1-A1-KLH (Figure 3B). While THEO-KLH serum readily bound theophylline and fenethylline but not amphetamine, 1-A1-KLH bound amphetamine and fenethylline but not theophylline (Figure 3C). The same pattern of binding activity was observed for in vivo serum measurements (Figure 3E–G). In contrast, AMPH-KLH generated ineffective antibodies, despite its structural similarity to the previously reported hapten SMA-KLH (Extended Data Figure 5).26 While THEO-KLH had a relatively minor impact on total distance traveled in the hyperlocomotor assay, 1-A1-KLH vaccination substantially decreased fenethylline-induced activity (Figure 3H–I). This evidence indicated that amphetamine was a major component of fenethylline’s stimulant behavior. Similarly, fenethylline’s activity in the EPM assays was found to be substantially blunted by 1-A1-KLH, but not THEO-KLH (Figure 3E). The CPP data again showed a trend toward 1-A1-KLH being somewhat more effective than THEO-KLH, although efficacy was variable, as with FEN-KLH (Figure 3F). Though the impact of vaccination with 1-A1-KLH was more obvious across the entire battery of behavioral testing, vaccination with THEO-KLH did appear to weakly blunt fenethylline’s behavioral effects overall, implying that theophylline has a supportive, rather than antagonistic, role in modulating these amphetamine-driven effects of fenethylline
Figure 3.
Vaccination with THEO-KLH and 1-A1-KLH haptens reveals dominant
activity for amphetamine. a, Synthetic route to THEO
(9). Conditions: a – BrCH2CH2OH,
NaH, DMF, 150 °C, 1 h, μw; b – glutaric anhydride,
4-DMAP, THF, 90 °C, 16 h. Route to AMPH (10). Conditions: a
– glutaric anhydride, THF, 90 °C, 1 h. Structure of
1-A1 (11). b, Midpoint titers, day 35 (
 , THEO-KLH, n=6), (
, THEO-KLH, n=6), (
 ,1-A1-KLH,
n=12), two replicates. c,d, THEO-KLH or
1-A1-KLH serum binding to THEO-BSA or 1-A1-BSA in the
presence of fenethylline (
,1-A1-KLH,
n=12), two replicates. c,d, THEO-KLH or
1-A1-KLH serum binding to THEO-BSA or 1-A1-BSA in the
presence of fenethylline (  ),
theophylline (
),
theophylline (  ), or amphetamine (
), or amphetamine (
 ), pooled serum from
n=12. e,f,g, Serum concentrations
at 15 minutes in KLH (
), pooled serum from
n=12. e,f,g, Serum concentrations
at 15 minutes in KLH (  ), THEO-KLH
(
), THEO-KLH
(  ), and 1-A1-KLH (
), and 1-A1-KLH (
 ) vaccinated animals for
fenethylline (20 mg/kg), theophylline (8 mg/kg), and amphetamine (8 mg/kg),
n=3. h, Total hyperlocomotion (90 min) due to fenethylline
(20 mg/kg), n=6, one-way ANOVA (p=0.0772; F(2,14)=3.09).
i, Fenethylline (20 mg/kg) locomotor behavior in animals
vaccinated with KLH (
) vaccinated animals for
fenethylline (20 mg/kg), theophylline (8 mg/kg), and amphetamine (8 mg/kg),
n=3. h, Total hyperlocomotion (90 min) due to fenethylline
(20 mg/kg), n=6, one-way ANOVA (p=0.0772; F(2,14)=3.09).
i, Fenethylline (20 mg/kg) locomotor behavior in animals
vaccinated with KLH (  ,
n=5), THEO-KLH (
,
n=5), THEO-KLH (  ,
n=9) or 1-A1-KLH (
,
n=9) or 1-A1-KLH (  , n=6), repeated-measures two-way
ANOVA([p, interaction<0.0001; F(60,420)=4.68];
*-p<0.01 vs KLH, Bonferroni).
j,k, EPM for fenethylline (20 mg/kg). Dotted line
is KLH + saline mean (
, n=6), repeated-measures two-way
ANOVA([p, interaction<0.0001; F(60,420)=4.68];
*-p<0.01 vs KLH, Bonferroni).
j,k, EPM for fenethylline (20 mg/kg). Dotted line
is KLH + saline mean (  ,
THEO-KLH, n=9; *-p=0.0094 vs saline,
df=8), (
,
THEO-KLH, n=9; *-p=0.0094 vs saline,
df=8), (  ,1-A1-KLH, n=10; p=0.4508 vs saline, df=9),
paired t-test. l, CPP for fenethylline (20 mg/kg), (KLH,
n=10; THEO-KLH, n=9; 1-A1-KLH, n=8;
Kruskal-Wallis (p=0.4264).
b,h,j–l, Data
shown as median with quartiles ± 10–90% CI
(+=mean).
c-g,i, Data shown as mean ±
SEM.
,1-A1-KLH, n=10; p=0.4508 vs saline, df=9),
paired t-test. l, CPP for fenethylline (20 mg/kg), (KLH,
n=10; THEO-KLH, n=9; 1-A1-KLH, n=8;
Kruskal-Wallis (p=0.4264).
b,h,j–l, Data
shown as median with quartiles ± 10–90% CI
(+=mean).
c-g,i, Data shown as mean ±
SEM.
Interestingly, during further analysis of the hyperlocomotor behavior induced by fenethylline, the onset of its stimulant activity was found to be more rapid than that of amphetamine alone (Figure 4A). However, the penetration of amphetamine into brain tissue following liberation from fenethylline lags behind that of directly administered amphetamine (Figure 4B). This disparity further implicated a potential polypharmacologic basis for stimulant activity, and indeed, when otherwise ineffective doses of theophylline and amphetamine were administered together, the total locomotor response was potentiated (Figure 4C). Furthermore, this synergistic stimulant effect was observed for other xanthine/phenethylamine combinations, opposing the earlier concept that co-liberation of these two classes of drugs would uniformly improve the risk/benefit profile of fenethylline (Figure 4D). These findings instead support the emergence of an altered subjective stimulant experience generated by coincident alteration of adenosinergic and dopaminergic signaling, thus providing a plausible explanation for why militant groups are motivated to expend additional effort to incorporate xanthines into counterfeit products, instead of amphetamine-type stimulants alone.27,28
Figure 4.

Synergistic stimulant effects of theophylline and fenethylline support
fenethylline’s activity. a, Hyperlocomotor activity for
fenethylline (  ,20 mg/kg) or
amphetamine (
,20 mg/kg) or
amphetamine (  ,2 mg/kg),
n=10, repeated-measures two-way ANOVA ([p,
interaction=0.2281; F(4,32)=1.49]). b, Time
course of brain concentrations for amphetamine release due to fenethylline (
,2 mg/kg),
n=10, repeated-measures two-way ANOVA ([p,
interaction=0.2281; F(4,32)=1.49]). b, Time
course of brain concentrations for amphetamine release due to fenethylline (
 ,50 mg/kg) or amphetamine (
,50 mg/kg) or amphetamine (
 ,5 mg/kg) administration,
n=5, two-way ANOVA ([p, interaction<0.0001;
F(4,10)=41.77]; *-p<0.001 vs amphetamine,
Bonferroni). c, Total hyperlocomotion (90 min) for saline
(n=10), amphetamine (1 mg/kg, n=10), theophylline (8 mg/kg,
n=11), or amphetamine + theophylline (A+T,1 mg/kg
+ 8 mg/kg, n=11) Kruskal-Wallis (p=0.0014;
***-p<0.001 vs A+T,
*-p<0.05 vs A+T,
**-p<0.01 vs A+T, Dunn’s).
d, Total hyperlocomotion (90 min) due to saline (n=10),
methamphetamine (1 mg/kg, n=5), caffeine (8 mg/kg, n=5),
methamphetamine + theophylline (M+T,1 mg/kg + 8 mg/kg,
n=5), or methamphetamine + caffeine (M+C,1 mg/kg
+ 8 mg/kg, n=5), one-way ANOVA (p=0.1177;
F(4,25)=2.051; *-p<0.05 vs M+T, Bonferroni).
a,b Data shown as mean ± SEM.
c,d, Data shown as median with quartiles ±
10–90% CI (+=mean).
,5 mg/kg) administration,
n=5, two-way ANOVA ([p, interaction<0.0001;
F(4,10)=41.77]; *-p<0.001 vs amphetamine,
Bonferroni). c, Total hyperlocomotion (90 min) for saline
(n=10), amphetamine (1 mg/kg, n=10), theophylline (8 mg/kg,
n=11), or amphetamine + theophylline (A+T,1 mg/kg
+ 8 mg/kg, n=11) Kruskal-Wallis (p=0.0014;
***-p<0.001 vs A+T,
*-p<0.05 vs A+T,
**-p<0.01 vs A+T, Dunn’s).
d, Total hyperlocomotion (90 min) due to saline (n=10),
methamphetamine (1 mg/kg, n=5), caffeine (8 mg/kg, n=5),
methamphetamine + theophylline (M+T,1 mg/kg + 8 mg/kg,
n=5), or methamphetamine + caffeine (M+C,1 mg/kg
+ 8 mg/kg, n=5), one-way ANOVA (p=0.1177;
F(4,25)=2.051; *-p<0.05 vs M+T, Bonferroni).
a,b Data shown as mean ± SEM.
c,d, Data shown as median with quartiles ±
10–90% CI (+=mean).
Overall, by employing FEN-KLH, THEO-KLH, and 1-A1-KLH as chemical neuroscience tools in our DISSECTIV approach, we have reconciled previously conflicting data regarding the underlying pharmacology driving the psychoactive profile of fenethylline. Its prominent stimulant features can be attributed to amphetamine, with synergistic support from theophylline, and no direct contributions from the parent drug molecule (Extended Data Table 2). These results have direct relevance for the study of other poorly understood, yet clinically-applied covalent theophylline conjugates, such as cafedrine and theodrenaline.29 Furthermore, our findings implicate the use of amphetamine and theophylline binding haptens, including FEN and 1-A1, as efficacious and potentially complementary vaccination strategies to blunt the pharmacodynamic effects of fenethylline, a synthetic drug whose abuse is undergoing conflict-driven global expansion. Perhaps most importantly, we expect that this ‘dissection through vaccination’ strategy can be broadly applied to numerous other complex chemical systems—including natural product extracts, SPD’s, and post-metabolic tissue samples—in order to illuminate unexpected polypharmacologic interactions and identify the exact chemical origin for each component within a multi-faceted pharmacodynamic profile.
METHODS
Animals
Animal studies were approved by TSRI’s Institutional Care and Use Committee and carried out according to NIH guidelines. Male Swiss Webster mice (Taconic Biosciences, 6–8 weeks) were used for all studies, and housed four per cage in a temperature-controlled (22 °C) vivarium on a reversed 12-h light cycle (9 PM – 9 AM) with ad libitum access to food and water. Animals were randomly assigned to their experimental groups with stratification to use littermates as controls. The experimenter was blinded to group identity during data processing, and behavioral results were automatically scored using AnyMAZE v.4.99 (Stoelting Co).
Drugs
Theophylline, amphetamine hemisulfate, and methamphetamine hydrochloride (>95%, Sigma Aldrich) were obtained commercially. Fenethylline (>95%) was synthesized and purified at TSRI. Amphetamine-d11, Fenethylline-d3 (Lipomed) and Theophylline-d6 (CDN Isotopes) were obtained commercially for use as LC/MS internal standards (IS). For in vivo studies, all drugs were dosed IP at a volume load of 10 mL/kg in bacteriostatic saline (0.9% w/v).
Chemical Analysis
Details of chemical synthesis, characterization, and relevant spectra are included in the Supplementary Methods.
Hapten Conjugation and Vaccination
Conjugation of haptens 8 – 11 to carrier proteins was performed as previously reported.30 Each hapten-KLH conjugate was combined with alum (Invivogen) and CpG 1826 (Eurofins Genomics) and shaken for 20 minutes prior to injection. Animals were immunized IP on study days 0, 14, and 28 with a vaccine formulation containing 50 μg of hapten, 1 mg of Alum, and 50 μg of CpG ODN 1826 in 125 μL of sterile-filtered PBS (pH 7.4).
Antibody Binding
ELISA analyses were carried out as previously described using horseradish peroxidase-donkey-anti-mouse IgG (Jackson Immunoresearch Catalog # 715-035-151).26,31 SPR was conducted on a Biacore 3000 (GE Healthcare) equipped with a research-grade CM4 sensor chip. BSA and the hapten-BSA conjugates were immobilized using NHS/EDC coupling chemistry and dilution or anti-sera was performed to normalize baseline binding across samples. Serum was pre-incubated with compound at room temperature for 30 minutes, the mixture was injected over the flow cell for 5 minutes, and dissociated for 2.5 minutes in running buffer (HBS-EP+ buffer, pH7.4, GE Healthcare) before the surface was regenerated with Gly-HCl, pH1.5.
Behavioral Analysis
Vaccinated animals were tested in behavioral models on days 36–42 in a dedicated 4.6 × 4.6 m room. Each individual animal received all drug treatments in a random order over the course of the experiment, with 24–72 hour washout periods between. For open field hyperlocomotion tests, the animals were acclimated for one hour in a plastic box (267 × 483 × 203 mm) with a clear lid. Mice were then injected and immediately returned to the cage to be recorded for 90 min (45–50 lux). For EPM tests, animals were injected, then immediately placed in the center of a plastic plus maze (500 mm height) with two open arms (50 × 300 × 3 mm wall) and two closed arms (50 × 300 × 150 mm wall) and tracked for 5 minutes (120 lux [center], 85 lux [ends]). The percentage of time spent in the open arms was calculated by: (open arm time/total time)x100. For the CPP test, all animals were placed in a plastic box (267 × 483 × 203 mm) divided by color (black vs white walls) and texture (smooth vs textured floor), and tracked for 20 min (1 PM; 45–50 lux). On the next day, a wall was placed in between the two chambers. Animals then received saline and were placed in the more-preferred chamber for 20 min (8 AM). Later, each animal received drug and was placed in the less-preferred chamber for 20 min (6 PM). This training was repeated for three days. Following training, the dividing wall was removed and the animals were tracked for 20 min (1 PM). The percent preference for the drug-paired chamber before and after training was calculated by: (chamber time post training– chamber time pre-training).
Pharmacokinetic Analysis
On day 49 animals were injected IP with test compounds. Blood was taken via the retro-orbital route, brain tissue was flash frozen using acetone: CO2(s). Tissues were stored at -80 °C until analysis. For serum samples, 20 μL were added to 80 μL of methanol + 200 ng IS. This was centrifuged for 10 min at 10,000 rpm, and 60 μL were transferred to a LC/MS vial. Fenethylline brain samples were diluted into four volumes of H2O, homogenized, and 1 mL was placed into 1 mL dichloromethane (DCM) + 200 ng IS. This was stirred for 2 hours, the DCM was collected, centrifuged as above, dried under vacuum, and then resuspended in 60 μL methanol. Theophylline brain tissue was homogenized in four volumes of H2O, and 1 mL + 200 ng IS was stirred for 2 hours, centrifuged, dried, and resuspended as above. Amphetamine brain tissue was homogenized in four volumes of 10 M NaOH, 1 mL was then placed into 1 mL hexane + 200 ng IS, stirred for 2 hours, and the hexane fraction was counter-extracted into 200 μL of 0.1 M HCl, dried, and resuspended as above. Analysis occurred on an Agilent 1100 LC/MS system with a Poroshell 120 SB-C8 column using H2O/ACN (with 0.1% Formic Acid) as the mobile phase (5–95% ACN, 10 min gradient). Using the ratio of drug to IS integration values, the unknown tissue concentrations were determined using a standard curve for the drug in question.
Statistical Analysis
Sample sizes were calculated to give > 80% power using means and standard deviations from our previous results for small molecule vaccines. Where ‘n=‘ is listed, it represents the number of animals used for analysis. Where ‘x replicates’ is listed, it represents the number of times the experiment was repeated in the laboratory. Outliers were detected at a predefined level of p <0.05 using Grubb’s Outlier test and excluded from analysis. Data were graphed and analyzed using Prism 5.02 (GraphPad Software), setting p <0.05 as the critical value. Groups were analyzed for similarity of variance using Bartlett’s Test, and non-parametric tests were employed where the data was found to be non-normally distributed. All statistical tests were performed using two-tailed analysis.
Data Availability
Data supporting the findings of this study are linked to the appropriate figures in the online version of the paper at www.nature.com. Additional information can be supplied by the authors upon reasonable request.
Extended Data
Extended Data Figure 1.
General schematic demonstrating the concept behind using DISSECTIV to isolate central nervous system activity of single chemical species within a complex chemical mixture that elicits psychoactive effects. Mixtures of known (solid) and unknown (dashed) chemical species are frequently observed due to active metabolite liberation from a parent compound, within natural products, toxins, or their extracts, and in counterfeit or clandestine production of synthetic drugs. In DISSECTIV, incremental vaccination against individual known chemical species can identify effects due to the actions of those species (A/B), effects emerging from the interaction of multiple known species (C), effects due to actions of unknown species (D), or effects emerging from the interaction of known and unknown species (E). Furthermore, while five discrete ‘all or nothing’ effects are listed here for clarity, the approach can also be used to determine the impact of each compound in altering the magnitude of each discrete effect.
Extended Data Figure 2.

Conjugation of FEN, THEO, 1-A1 and AMPH haptens to BSA. a, BSA (m/z 66134 – 66284; ESI+ m/z 66431). b, FEN-BSA (m/z 70031 – 70106). c, THEO-BSA (m/z 69424 – 71275). d, 1-A1-BSA (m/z 70727 - 71058). e, AMPH-BSA (m/z 69563). Representative data shown for BSA, FEN-BSA, THEO-BSA, and 1-A1-BSA, two replicates.
Extended Data Figure 3.
Standard curves used to quantify the concentrations of drugs in
biological tissue samples. a,b, Standard curves
for fenethylline (  ) at low
(slope=44941 ± 1026; r2=0.9806) and high
(slope=45929 ± 143.1; r2=0.9998)
concentrations in the presence of
fenethylline-d3 (
) at low
(slope=44941 ± 1026; r2=0.9806) and high
(slope=45929 ± 143.1; r2=0.9998)
concentrations in the presence of
fenethylline-d3 (  ) as an internal standard.
c,d, Standard curve for theophylline (
) as an internal standard.
c,d, Standard curve for theophylline (
 ) at low
(slope=93380 ± 844.4; r2=0.9970) and high
(slope=83746 ± 1406; r2=0.9954)
concentrations in the presence of
theophylline-d6 (
) at low
(slope=93380 ± 844.4; r2=0.9970) and high
(slope=83746 ± 1406; r2=0.9954)
concentrations in the presence of
theophylline-d6 (  ) as an internal standard.
e,f, Standard curve for amphetamine (
) as an internal standard.
e,f, Standard curve for amphetamine (
 ) at low
(slope=299394 ± 1980; r2=0.9995) and high
(slope=254747 ± 16197; r2=0.9898)
concentrations in the presence of
amphetamine-d11 (
) at low
(slope=299394 ± 1980; r2=0.9995) and high
(slope=254747 ± 16197; r2=0.9898)
concentrations in the presence of
amphetamine-d11 (  ) as an internal standard. All lines fit
through the origin.
) as an internal standard. All lines fit
through the origin.
Extended Data Figure 4.

Vaccination with FEN-KLH blunts the behavioral effects of
amphetamine and methamphetamine. a, Amphetamine (2 mg/kg)
locomotor behavior in animals vaccinated with KLH (  ) or FEN-KLH (
) or FEN-KLH (  , [p, interaction<0.0001;
F(30,240)=3.70]; *-p<0.01 vs KLH, Bonferroni),
n=5, repeated-measures two-way ANOVA. b,
Methamphetamine (2 mg/kg) locomotor behavior in animals vaccinated with KLH
(
, [p, interaction<0.0001;
F(30,240)=3.70]; *-p<0.01 vs KLH, Bonferroni),
n=5, repeated-measures two-way ANOVA. b,
Methamphetamine (2 mg/kg) locomotor behavior in animals vaccinated with KLH
(  , n=9) or FEN-KLH (
, n=9) or FEN-KLH (
 , n=11, [p,
interaction=0.0385; F(30,540)=1.52];
*-p<0.01 vs KLH, Bonferroni), repeated-measures two-way ANOVA.
c, EPM in KLH vaccinated animals following saline
(n=9), amphetamine (2 mg/kg, n=10), or methamphetamine (2
mg/kg, n=10) administration, one-way ANOVA (p=0.1325;
F(2,26)=2.187). d, EPM in FEN-KLH vaccinated animals
following saline (n=9), amphetamine (2 mg/kg, n=10), or
methamphetamine (2 mg/kg, n=10) administration, one-way ANOVA
(p=0.9588; F(2,26)=0.042). a,b,
Data presented as mean ± SEM. c,d, Data
shown as median with quartiles ± 10–90% CI
(+=mean).
, n=11, [p,
interaction=0.0385; F(30,540)=1.52];
*-p<0.01 vs KLH, Bonferroni), repeated-measures two-way ANOVA.
c, EPM in KLH vaccinated animals following saline
(n=9), amphetamine (2 mg/kg, n=10), or methamphetamine (2
mg/kg, n=10) administration, one-way ANOVA (p=0.1325;
F(2,26)=2.187). d, EPM in FEN-KLH vaccinated animals
following saline (n=9), amphetamine (2 mg/kg, n=10), or
methamphetamine (2 mg/kg, n=10) administration, one-way ANOVA
(p=0.9588; F(2,26)=0.042). a,b,
Data presented as mean ± SEM. c,d, Data
shown as median with quartiles ± 10–90% CI
(+=mean).
Extended Data Figure 5.
Vaccination with AMPH-KLH generates ineffective antibodies.
a, Midpoint titers, day 35. Dotted line is mean
1-A1-KLH titer (n=6). b, AMPH-KLH serum
binding to AMPH-BSA with competing fenethylline (  ) or amphetamine (
) or amphetamine (  ), pooled from n=6, two
replicates. Dotted line is mean 1-A1-KLH + amphetamine
binding. c, Total hyperlocomotion (90 min) due to fenethylline
(20 mg/kg) in KLH (
), pooled from n=6, two
replicates. Dotted line is mean 1-A1-KLH + amphetamine
binding. c, Total hyperlocomotion (90 min) due to fenethylline
(20 mg/kg) in KLH (  ,
n=6) and AMPH-KLH (
,
n=6) and AMPH-KLH (  ,
n=6) vaccinated animals. Dotted line is mean 1-A1-KLH
+ fenethylline 20 mg/kg locomotor response, t-test
(p=0.3584, df=9). a,c, Data shown
as median with quartiles ± 10–90% CI
(+=mean). b, Data presented as
mean ± SEM.
,
n=6) vaccinated animals. Dotted line is mean 1-A1-KLH
+ fenethylline 20 mg/kg locomotor response, t-test
(p=0.3584, df=9). a,c, Data shown
as median with quartiles ± 10–90% CI
(+=mean). b, Data presented as
mean ± SEM.
Extended Data Table 1.
Fenethylline binding to CNS targets.
| Target | Inhib. (%)* | Target | Inhib. (%)* |
|---|---|---|---|
| AdenosineA2A | −5 | Muscarinic M2 | −6 |
| Adenosine A1 | 0 | Muscarinic M3 | 2 |
| Adrenergic α2A | 16 | Nicotinic Ach | 2 |
| Adrenergic α1A | 27 | Nicotinic Ach α1 | 9 |
| Adrenergic α1B | 16 | Mu Opiate | 7 |
| Adrenergic β2 | 3 | Phorbol Ester | 9 |
| Adrenergic β1 | 9 | K ATP Channel | 21 |
| L-Type Ca Channel | 6 | hERG K Channel | −4 |
| Cannabinoid CB1 | 16 | Prostanoid EP4 | 3 |
| Dopamine D2S | 1 | Rolipram | 9 |
| Dopamine D1 | 8 | Serotonin 5-HT2B | 23 |
| GABAA – Site 1 | 0 | Sigma σ1 | 7 |
| GABAA – Site 2 | 5 | Na Channel – Site 2 | −4 |
| Glutamate – NMDA | 10 | Norepi. Transporter | 10 |
| Histamine H1 | 12 | Dop. Transporter | 9 |
| Imidazoline I2 | 4 |
Radioligand displacement measured in the presence of 10 μM fenethylline using the method of Eurofins Cerep Panlabs. Data shown as the average of two replicates.
Extended Data Table 2.
Binding and behavioral activity summary for DISSECTIV results.
| Measurement | KLH | FEN-KLH | THEO-KLH | 1-A1-KLH |
|---|---|---|---|---|
| Fenethylline pIC50 | >3.00 | 5.912 ± 0.079 | 5.727 ± 0.021 | 5.000 ± 0.022 |
| Theophylline pIC50 | >3.00 | 5.378 ± 0.079 | 5.385 ± 0.022 | >3.00 |
| Amphetamine pIC50 | >3.00 | >3.00* | >3.00 | 4.707 ± 0.012 |
| Methamphetamine pIC50 | >3.00 | >3.00* | >3.00 | 3.682 ± 0.031 |
| Cocaine pIC50 | >3.00 | >3.00 | >3.00 | >3.00 |
| FEN-BSA Binding(OD) | 0.076 ± 0.013 | 0.539 ± 0.112 | 0.321 ± 0.107 | 0.064 ± 0.054* |
| THEO-BSA Binding(OD) | 0.066 ± 0.003 | 0.372 ± 0.033 | 1.226 ± 0.192 | 0.062 ± 0.056 |
| 1-A1-BSA Binding(OD) | 0.070 ±0.051 | 0.306 ± 0.005 | 0.071 ± 0.067 | 2.590 ± 0.023 |
| Serum Fenethylline (μM)† | 15.76 ±6.21 | 43.40 ±10.90 | 36.70 ±3.81 | 33.53 ±8.25 |
| Serum Theophylline (μM)† | 90.80 ±12.68 | 127.20±22.10 | 151.20±16.87 | 51.50 ±12.12 |
| Serum Amphetamine (μM)† | 18.79 ±2.51 | 30.07 ±4.26 | 20.40 ±1.08 | 31.73 ±6.18 |
| Locomotor Activity‡ | 326.1 ±62.88 | 95.86 ± 24.73 | 270.70± 73.89 | 128.30± 27.64 |
| Plus Maze Activity‡ | 51.31 ±17.32 | 108.80± 22.99 | 62.46 ± 19.00 | 106.20± 16.17 |
| Place Preference Activity‡ | 100.0±22.60 | 53.60 ± 48.29 | 57.79± 31.84 | 62.00± 44.84 |
Binding verified in alternative assay.
Concentration measurements taken at 15 minutes post-injection.
Percent of baseline (20 mg/kg fenethylline). Data shown as mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported by a generous grant from NIH (DA024705-06). The authors thank M. Gooyit for supplying reagents, B. Ellis for assistance with tissue collection, and M. Taffe for providing DEA licensing (RT0485537). This is manuscript # 29481 from The Scripps Research Institute.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com.
Author Contributions CJW designed and carried out the chemistry, ELISA, and in vivo behavioral experiments, analyzed and interpreted the data, and prepared the manuscript. BZ carried out and analyzed the SPR experiments. KDJ oversaw design of the experiments, interpretation of the data, and preparation of the manuscript.
The authors declare no competing financial interests.
References
- 1.Katselou M, et al. Fenethylline (Captagon) Abuse – Local Problems from an Old Drug Become Universal. Basic Clin Pharmacol Toxicol. 2016;119:133–40. doi: 10.1111/bcpt.12584. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hemiary NJ, Al-Diwan JK, Hasson AL, Rawson RA. Drug and alcohol use in Iraq: findings of the inaugural Iraqi Community Epidemiological Workgroup. Subst Use Misuse. 2014;49:1759–1763. doi: 10.3109/10826084.2014.913633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Hout MC, Wells J. Is Captagon (fenethylline) helping to fuel the Syrian conflict? Addiction. 2016;111:748–749. doi: 10.1111/add.13262. [DOI] [PubMed] [Google Scholar]
- 4.Fond G, Howes O. Pharmacoterrorism: the potential role of psychoactive drugs in the Paris and Tunisian attacks. Psychopharmacology (Berl) 2016;233:933–935. doi: 10.1007/s00213-016-4204-2. [DOI] [PubMed] [Google Scholar]
- 5.Klingler KH. Theophylline derivatives. 3245994. US Patent. 1966
- 6.Kristen G, Schaefer A, von Schlichtegroll A. Fenetylline: therapeutic use, misuse and/or abuse. Drug Alcohol Depend. 1986;17:259–271. doi: 10.1016/0376-8716(86)90012-8. [DOI] [PubMed] [Google Scholar]
- 7.Ellison T, Levy L, Bolger JW, Okun R. The metabolic fate of 3H-fenetylline in man. Eur J Pharmacol. 1970;13:123–128. doi: 10.1016/0014-2999(70)90192-5. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura H, Yoshimitsu T, Yamada H, Koga N, Oguri K. Metabolic fate of fenetylline in rat and man. Xenobiotica. 1988;18:929–940. doi: 10.3109/00498258809167516. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer T, Maurer HH. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24:277–289. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Nickel B, Niebch G, Peter G, Von Schlichtegroll A, Tibes U. Fenetylline: New results on pharmacology, metabolism and kinetics. Drug Alcohol Depend. 1986;17:235–257. doi: 10.1016/0376-8716(86)90011-6. [DOI] [PubMed] [Google Scholar]
- 11.Dimpfel W, Spuler M, Nickel B, Tibes U. “Fingerprints” of central stimulatory drug effects by means of quantitative radioelectroencephalography in the rat (tele-stereo-EEG) Neuropsychobiology. 1986;15:101–108. doi: 10.1159/000118250. [DOI] [PubMed] [Google Scholar]
- 12.Nelson ME, Bryant SM, Aks SE. Emerging drugs of abuse. Emerg Med Clin North Am. 2014;32:1–28. doi: 10.1016/j.emc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Christophersen AS. Amphetamine designer drugs – an overview and epidemiology. Toxicol Lett. 2000;112–113:127–131. doi: 10.1016/s0378-4274(99)00205-2. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Office on Drugs and Crime. World drug report. Trends Organ Crime. 1997;3:11–14. [Google Scholar]
- 15.Keup W. Use, indications and distribution in different countries of the stimulant and hallucinogenic amphetamine derivatives under consideration by WHO. Drug Alcohol Depend. 1986;17:169–192. doi: 10.1016/0376-8716(86)90007-4. [DOI] [PubMed] [Google Scholar]
- 16.Al-Imam A, et al. Captagon: use and trade in the Middle East. Hum Psychopharmacol. 2016 doi: 10.1002/hup.2548. [DOI] [PubMed] [Google Scholar]
- 17.Alabdalla MA. Chemical characterization of counterfeit captagon tablets seized in Jordan. Forensic Sci Int. 2005;152:185–188. doi: 10.1016/j.forsciint.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shufman E, Dickman M. Fenethyllin psychosis: description of two cases. Isr J Psychiatry Relat Sci. 1999;36:129–131. [PubMed] [Google Scholar]
- 20.Rasmussen N. Medical Science and the Military: The Allies’ Use of Amphetamine during World War II. J Interdiscip Hist. 2011;42:205–233. doi: 10.1162/jinh_a_00212. [DOI] [PubMed] [Google Scholar]
- 21.Kinsey B. Vaccines against drugs of abuse: where are we now? Ther Adv Vaccines. 2014;2:106–117. doi: 10.1177/2051013614537818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlosburg JE, et al. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110:9036–9041. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biala G, Kruk M. Amphetamine-induced anxiety-related behavior in animal models. Pharmacol Rep. 2007;59:636–644. [PubMed] [Google Scholar]
- 24.Richards HJ, Benson V, Donnelly N, Hadwin JA. Exploring the function of selective attention and hypervigilance for threat in anxiety. Clin Psychol Rev. 2014;34:1–13. doi: 10.1016/j.cpr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Gooyit M, Miranda PO, Wenthur CJ, Ducime A, Janda KD. Influencing Antibody-Mediated Attenuation of Methamphetamine CNS Distribution through Vaccine Linker Design. ACS Chem Neurosci. 2017;8:468–472. doi: 10.1021/acschemneuro.6b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haile CN, et al. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am J Addict. 2015;24:748–755. doi: 10.1111/ajad.12307. [DOI] [PubMed] [Google Scholar]
- 27.Justinova Z, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- 28.Franco R, et al. Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology. 2000;23:S50–9. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 29.Heller AR, Heger J, Gama de Abreu M, Muller MP. Cafedrine/theodrenaline in anaesthesia: influencing factors in restoring arterial blood pressure. Anaesthesist. 2015;64:190–196. doi: 10.1007/s00101-015-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimishima A, Wenthur CJ, Zhou B, Janda KD. An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chem Biol. 2017;12:36–40. doi: 10.1021/acschembio.6b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are linked to the appropriate figures in the online version of the paper at www.nature.com. Additional information can be supplied by the authors upon reasonable request.