Abstract
In this study, electrochemical impedance spectroscopy was used for the first time to study the adsorption of dopamine in carbon fiber microelectrodes. In order to show a proof-of-concept, static and dynamic measurements were taken at potentials ranging from −0.4 to 0.8 V vs Ag|AgCl to demonstrate the versatility of this technique to study dopamine without the need of its oxidation. We used electrochemical impedance spectroscopy and single frequency electrochemical impedance to measure different concentrations of dopamine as low as 1 nM. Moreover, the capacitance of the microelectrodes surface was found to decrease due to dopamine adsorption, which is dependent of its concentration. The effect of dissolved oxygen and electrochemical oxidation of the surface in the detection of dopamine was also studied.
Non-oxidized and oxidized carbon fiber microelectrodes were prepared and characterized by optical microscopy, scanning electron microscopy, cyclic voltammetry, and electrochemical impedance spectroscopy. Optimum working parameters of the electrodes, such as frequency and voltage, were obtained for better measurement. Electrochemical impedance of dopamine was determined at different concentration, voltages, and frequencies. Finally, dynamic experiments were conducted using a flow cell and single frequency impedance in order to study continuous and real-time measurements of dopamine.
Keywords: Dopamine adsorption, Electrochemical Impedance Spectroscopy, Single Frequency Impedance, flow cell, carbon fiber microelectrode
TOC image
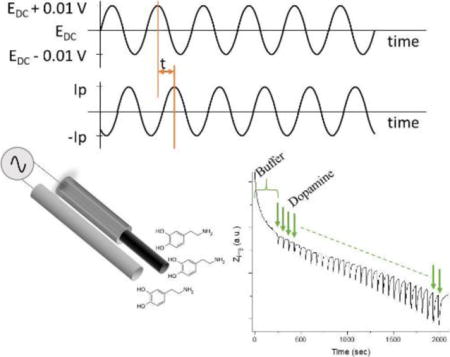
Introduction
In the biochemical field, the necessity of electrochemical techniques that display high sensitivity with high temporal and spatial resolution is of great significance. Many chemical reactions in the body occurred at a fast rate, on a sub-second time scale such as neurotransmission1. Among the different electrochemical methods, fast-scan cyclic voltammetry (FSCV)2,3 has been used for the detection of diverse biomolecules such as neurotransmitters on a fast sampling rate, capable of measuring the dynamic of fast fluctuations4. However, there’s still a lack of technique that gather all of these properties (i.g., high sensitivity, high temporal and spatial resolution) in one with the capacity to measure molecules that are not electroactive or at potentials where no redox reaction happens. In this work, we propose the research community to start developing the use of electrochemical impedance spectroscopy (EIS) for this purpose. Much work remains to be done, but as a starting point we used EIS to measure a model biomolecule that is known to adsorb before oxidation, dopamine, at a range of potentials from −0.4V vs Ag|AgCl, where it does not oxidize, up to 0.8V where it readily oxidizes.
It is well demonstrated that the used of carbon fiber microelectrode (CFME) couple to electrochemical techniques can enhance the sensitivity and the temporal and spatial resolution2. The oxidation of neurotransmitters and neuromodulators, such as dopamine, has been actively studied using CFMEs with electrochemical techniques (e.g., fast scan cyclic voltammetry (FSCV)3,5–7, chronoamperommetry8,9, and different potential waveforms10–12). CFME are one of the most common electrodes used to study neurotransmission, not only because of their properties such as small diameter, biocompatibility, electrochemical properties, and adsorption affinity for cationic biomolecules (e.g., dopamine)13,14, but also because of their compatibility with fast electrochemical methods due to its low currents15. In this study we coupled the use of EIS with CFME in standard stagnant solutions as well as in dynamic measurements where the theoretical temporal resolutions of 10 ms were obtained. This number was calculated taking into account the 100 Hz signal and the fact that we have one impedance value per full wave (100 per second), which gives 10 ms theoretical resolution. The adsorption process of dopamine onto carbon-based material have been well-recognized for many years16 consequently in this work dopamine is used as the model biomolecule for this first work where dynamic processes were measured continuously using EIS. We are not proposing to use EIS to detect dopamine directly on the brain due to the lack of selectivity of EIS at this moment, which must be addressed before in vivo measurements can be carried out. However, EIS has the great advantage that surface modifications can be easily studied to enhance selectivity because of the stability of the carbon surface at potentials where dopamine adsorbs.
EIS is one of the most powerful analytical technique since it is a rapid and non-destructive method with the ability to study the interfacial behavior of a wide range of materials in electrochemical system17. EIS is capable of characterize physicochemical processes in different modification layers, sampling charge transfer at high frequencies and following mass transfer at low frequencies18. In principle, in EIS, a sine wave voltage with a small amplitude (≈10 mV) is applied to the working electrode while the current is measured at different frequencies (1-5 MHz to 1-10 Hz) (Figure 1A). The voltage amplitude must be small enough to have a pseudo-linear behavior of the i-v plot in order to have a current response with the same frequency than the voltage applied, and 10 mV meet this requirement. The current measured at each frequency is used to calculate the impedance by different methods (e.g., Lissajous curve) in order to obtain a model circuit of the chemical processes in the electrode surface (Figure 1C). It is important to clarify that the capacitance of the water/borosilicate/electrode junction is not considered in this model because it is constant with time and does not affect the change in impedance due to dopamine adsorption. EIS can also be used at a wide frequencies range opening the possibility to enhance the temporal resolution and therefore the sensitivity. Unlike to chronoamperometry and cyclic voltammetry, this technique can provide information about the electrode’s surface without large sweeps of potentials or the need to oxidize the electroactive compounds. This allows to measure at a constant applied potential chosen depending on the reaction to study, and measuring the impedance at different frequencies19,20. However, due to the intrinsic difficulty of this technique, most scientific literature limits its use to the Nyquist representation for the detection of molecules.21
Figure 1.
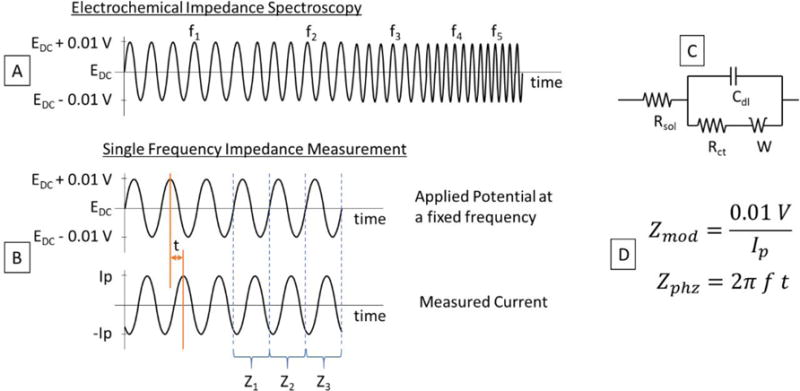
Diagram of the voltage signals used for (A) Electrochemical Impedance Spectroscopy and (B) Single Frequency Impedance Measurement. These experiments provide information to obtain (C) the model circuit used and each parameter needed for the calculation of the electrochemical impedance using the equations in (D).
It is well known that dopamine adsorbs on CFME, therefore EIS can be used to study the impedance of the system due to dopamine adsorption without the hindrance of dopamine desorption due to oxidation. Even though EIS is one of the best technique to study chemical and biological processes, the measurement of impedance over a wide frequency range is time consuming, especially if it involves a low-frequency range17. A novel impedance technique called single frequency impedance (SFI) is emerging as an alternative method to EIS to obtain a fast, continuous and real-time measurements. Unlike to EIS, single frequency impedance measures the impedance at a fixed frequency versus time as shown in Figure 1B, enabling continuous measurements. A single frequency is used as an excited signal instead of wide frequency range sweep to reduce the complexity in signal acquisition and its processing. Similar to EIS, in SFI the frequency can be modulated (1-5 MHz to 1-10 Hz) in order to influence the sensitivity of the system and therefore the temporal resolution. This technique can be used to monitor time-dependent changes in an electrode surface, therefore, it should be possible to control the experiment with repeat time and total time22. SFI not only possess a great ability to detect fast response processes which make it suitable to study real time biological processes, but also can be used to evaluate slow time-dependent changes in a biosensor surface22. SFI method is usually applied for the investigation of molecular interaction occurring between DNA-protein, protein-protein, biomolecules or electrode-molecules23. No experimental evidence demonstrate the used of SFI to study the interaction between dopamine and CFME, for that reason, here we perform continuous and real-time impedance measurements trigger by the adsorption of dopamine onto the electrode.
Experimental Section
Development of Carbon Fiber Microelectrodes
CFME used in this work had a diameter of ≈7 μm and were sealed with the borosilicate glass capillary (1.0 mm × 0.5 mm) using a micropipette puller. A single carbon fiber (Thornel T-300) was aspirated into the glass capillary using mechanical vacuum with a filter to avoid the fibers to reach the motor. Then, microelectrodes were placed in the micropipette puller (Narishige PC-10) with a power of ca. 65.0%, to obtain two electrodes with usually only one of them working correctly due to the mechanics of this specific micropipette puller. After that, the carbon fiber exposed was cut and microscopic observation with water immersion was used to ensure that a seal between the glass and the carbon fiber was present to avoid leaking of the solution into the capillary that would change the electroactive area. The electrical connection was made with silver paint and a silver-plated copper wire that was connected to the potentiostat used for measurements. The silver paint was placed with a silver-plated copper wire within the capillary in direct contact with the carbon fiber, which provides the fastest response as reported by J. T. Cox et al24.
The trimming of the carbon fiber was done using two different methods: electrochemical etching and physical cutting using a razor blade. The electrochemical etching was done applying 6 Vac to the CFME using as a counter electrode a platinum loop with a drop of 5 M H2SO4 and 0.5 mM K2Cr2O7 using a micromanipulator (Narishige MHW-3) to control the final length of the exposed fiber. This method provided a highly-oxidized carbon fiber tip with a bare carbon surface closer to the glass, which resulted in inconsistencies in measurements. In the second method, a razor blade was used to cut the carbon fiber obtaining a homogenous and reproducible bare carbon fiber electrode surface. The fiber carbon was trimmed to a length of 70 – 280 μm25,26. The different carbon fiber lengths used did not give any significant changes in the results, but only showed a minimum change in the background noise. In dynamic measurements using a flow cell, the fiber carbon was trimmed to a 50 – 150μm. Finally, the electrodes were soaked in isopropanol and placed in an ultrasonic bath to clean the debris in their surface. Cylinder microelectrodes were chosen instead of disks to increase the active surface area of the microelectrodes.
Reagents
Electrochemical characterization of CFME was carried out in artificial cerebrospinal fluid (aCSF) at pH 7.4 (150 mM NaCl, 1.4 mM CaCl2, 3 mM KCl, 0.8 mM MgCl2, 0.8 mM Na2HPO4. 7 H2O, 0.17 mM NaH2PO4. 2 H2O, 0.5 M Tris in nanopure water). Dopamine hydrochloride was also diluted in aCSF for the preparation of the standard solutions. The master solutions were prepared fresh daily at concentrations of 1 μM, 10 μM and 100 μM, and they were used to prepare the different standard solutions in the electrochemical cell by addition to the initial 15 mL of aCSF in the electrochemical cell. The detection of dopamine was done using different concentrations by adding dopamine at the end of each run (from 1 nM up to 0.5 μM) to understand the behavior of the microelectrode at different concentration ranges. The electrochemical cell was stirred for 1 minute without disturbing the electrodes after adding each aliquot of dopamine and waited until stagnant after stirring. In the experiments where nitrogen was used, the electrochemical cell was bubbled with dry nitrogen before electrochemical experiments. The cell was kept closed during each nitrogen run and a bed of nitrogen was used at the surface of the electrode to avoid oxygen contamination.
Electrochemical Measurements
All electrochemical measurements were done using a three-electrodes electrochemical cell using the CFME as the working electrode, a platinum wire as counter electrode, and a Ag|AgCl reference electrode (3 M NaCl filled solution, Prod. MF-2052, 0.209 V vs NHE, Bioanalytical Systems, Inc.). A Reference 600+ Gamry potentiostat was used for electrochemical measurements and all data was collected using Gamry Instruments Framework software. All potential in this work are expressed vs. Ag|AgCl. Finally, CFMEs were measured by EIS at different potentials of 0.8 V, 0.4 V, 0.1 V, and 0.0 V with an amplitude of 10 mV for each concentration with an initial frequency of 5 MHz and a final of 10 Hz. A grounded Faraday’s cage was used during the experiments to avoid external electrical noise. All of data was analyzed by Gamry Echem Analyst and Matlab. The obtained electrical current during the dynamic experiments was filtered in Matlab using a bandpass filter of order 10 and 99.3 – 100.7 Hz, to avoid high frequency and 60 Hz noise.
Static measurements were done using an electrochemical cell with a capacity of up to 15 mL (Prod. MF-1084, Bioanalytical Systems, Inc.). Dynamic measurements were carried out in a flow cell made at our machine shop. A 2” rod of Teflon was machined to have a 1.5” dia. × 1” depth reservoir in which a 1/16” PEEK tubing entered from below. On the side, a hole was made so that the solution would overflow to a waste recipient that was placed below the cell. The tip of the microelectrodes was positioned as far inside the PEEK tubing as possible using a micromanipulator. The inlet of the flow cell was connected to a 6-port valve (VICI Cheminert) with a 20 μL loop where dopamine solutions were introduced before being pushed to the cell by the buffer when the valve was actioned. A syringe pump at 1 mL/min was used to introduce the buffer to the flow cell.
Physical Characterization
Scanning Electron Microscopy (JEOL JSM-6010LA) and Optical Microscopy (Nikon Eclipse FN1 with DIC) were used to observe the microelectrode surface. Optical Microscopic observation with water immersion was used to ensure that a seal between the glass and the carbon fiber was present.
Results and Discussion
EIS is an electrochemical technique used to measure a small perturbation at virtually constant applied potential chosen (i.g., without large sweeps of potentials or the need to oxidize the analyte) depending on the reaction to study, and measuring the impedance at different frequencies19,20. EIS allows to modulate frequency in order to influence the sensitivity of the system. In this project, we hypothesized that the use of EIS using CFMEs could be applied for the measurement of biomolecules such as dopamine. Basically, in EIS, a sine wave voltage with a small amplitude (≈10 mV) is applied to the working electrode while the current is measured. This is done at different frequencies as shown in Figure 1A. Typically, frequency ranges from 1-5 MHz to 1-10 Hz, however, these ranges may vary depending on the equipment and application for which EIS is being used. The current measured at each frequency is used to calculate the impedance by different methods such as using the Lissajous curve, which plots the voltage vs current for each cycle and provides the parameters with which the impedance can be calculated. As shown in Figure 1D, the magnitude of the impedance is obtained by dividing the peak voltage and the peak current, and the phase is obtained by multiplying 2π times the frequency times the out-of-phase time between voltage and current.
CFMEs have been prepared without any surface modifications to avoid additional variables that may change the impedance of their surface. The objective of this work is to provide a proof-of-concept for the development of EIS and SFI for the measurement of dopamine concentration using CFMEs. We were able to measure dopamine concentrations as low as 1 nM at potentials as low as 0.0 V vs Ag|AgCl in standard solutions where dopamine is stable electrochemically and has been proven to be adsorbed in the carbon fiber electrode as shown by Bath et al.16 Dynamic measurements were also done, in which we measured the concentrations as low as 1 μM that is typically what is found in excitation experiments in brain slices. These results provide the scientific community with an additional innovative technique for future biomedical studies. This technique has been widely used in the evaluation of electrochemical behavior of electrode interfaces and has been very useful in the interpretation of redox phenomena. Moreover, it has the potential to add an additional dimension (i.e. frequency) to the detection of biomolecules when performed using chronoamperometry since only a small perturbation in voltage is required for EIS.
Carbon Fiber Microelectrodes
CFMEs were prepared with a length of up to 280 μm of exposed carbon fiber, and the seal between the glass and carbon fiber was tested in water. Optical microscopy with differential interference contrast (DIC) was used to observe clearly the glass, which was so thin that was difficult to visualize. In the case that electrodes leaked solution into the capillary, a meniscus was easily visible within the capillary as seen in Figure 2A. Different fabrication techniques were tested in order to shorten the exposed CFME after glass pulling. The electrochemical etching method used to shorten the carbon fiber produced sharp tips (Figure 2A) as well as a high control of the length desired using a hydraulic micromanipulator (Narishige MHW-3); however, the results obtained were not reproducible, which we attributed to the difference in adsorption properties between the functionalized (oxidized) sharp tip and bare carbon fiber neck, which may vary in length between electrodes. On the other hand, using a razor blade and optical microscope to trim the carbon fiber (Figure 2B) produced bare carbon fiber tips with consistency in the surface properties along the length of the exposed fiber, with the detriment of the difficulty to control the size of the exposed fiber as good as with etching. As will be shown later, the bare carbon fibers were compared with and without oxidation of the fiber, resulting in more reproducible results compared to etched fibers.
Figure 2.
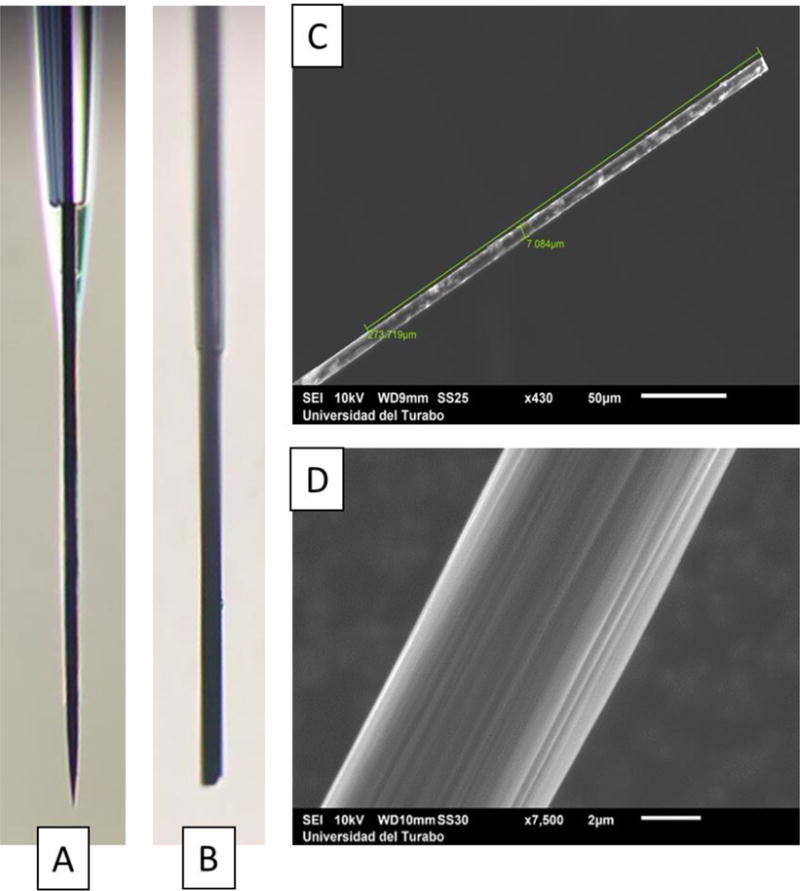
Optical microscopy of (A) electrochemically etched and (B) physically trimmed carbon fiber microelectrodes. Scanning Electron Microscopy images of (C) the exposed carbon fiber and (D) higher magnification showing the surface structure.
Scanning Electron Microscopy (SEM) was used to assess the length and integrity of the exposed carbon fiber (Figure 2C) as well as their surface structure (Figure 2D). Figure 2C shows a carbon fiber trimmed using a razor blade with the length and diameter of the exposed carbon fiber labeled with the glass seal seen at the lower-left corner. Figure 2D shows a better image of the structure of the surface of the carbon fiber where ridges are seen due to the extrusion process during the fabrication of the fiber. While SEM images showed a good seal between the carbon fiber and the glass, we continued to find solution leaking into the capillary when testing with the uncut (longer) fiber. This leakage was stopped when the fibers were shortened before testing leaks, and the problem was attributed to the surface tension of the water that made the fibers to bend, opening the tip of the glass capillary allowing a small amount to enter and providing the false appearance of a leakage.
Electrochemical Characterization
Electrochemical characterization of CFMEs was carried out in artificial cerebrospinal fluid (aCSF) at pH 7.4. Dopamine HCl was also diluted in aCSF for the preparation of the standard solutions, which were done fresh daily. EIS was done potentiostatically at 0.8 V, 0.4 V, 0.1 V, and 0.0 V for each concentration from 10 Hz to 5 MHz. These potentials were chosen to understand the differences in electrochemical impedance at potentials where dopamine does not oxidize (0.0 V), close to the equilibrium potential (0.1 V)16,27, and at potentials where dopamine readily oxidizes at different rates (0.4 V and 0.8 V). EIS was analyzed using Bode diagrams to separate the effects seen in the modulus and phase with respect to frequency. The frequency range was limited to 10 Hz which is the lowest frequency normally used FSCV and 5 MHz which is the limit of the potentiostats used. The frequency range was tested by connecting the potentiostat between the silver-plated copper wire (working electrode lead) and the carbon fiber (reference and counter electrode leads). The data obtained between 1 and 5 MHz was constant regardless of the system, and it was attributed to the physical properties of the electrical connection within the microelectrodes or the intrinsic error of the potentiostats, and independent of the electrochemical properties of the cell. The data showed up to 5 MHz is for the sake of completion, but no conclusion was obtained using frequencies between 1 and 5 MHz due to the high resistivity of carbon fibers.
The fabrication of the microelectrode is very important when using EIS and it was found to be a strong limitation of the frequency range from which data could be extracted. In order to understand the implications of the electrical connection between the microelectrodes and the potentiostat, we varied the length of the carbon fiber where the electrical connection was made. We fabricated CFMEs with the silver-plated copper wired connected to the fiber using silver paint at the middle of the capillary and other microelectrodes where the connection was closest to the tip. As seen in Figure S1, this change in length tripled the impedance at high frequency, which was expected due to the high resistance of the carbon fiber; however, the useful frequency range was also changed in the upper frequency at which the capacitance of the electrode surface started to be measured from 50 kHz to 10 kHz. In this way, data was lost by means of the electrode fabrication that can translate into slower measurements in the development of a microelectrode.
To understand better the mechanisms of the reaction under study, and to predict with more certainty the potential of EIS to be used in biological systems, the importance of the dissolved oxygen on the solution needed to be clarified. Therefore, we compared the electrochemical reaction with and without the presence of dissolved oxygen by using dry nitrogen gas. The electrochemical cell was bubbled with nitrogen before each run and the impedance was measured afterwards. Figure S2 shows the Bode plot of the impedance in aCSF with dopamine at different potentials. No significant change was found between both systems with or without nitrogen but only a small difference in the phase of the impedance between ca. 100 Hz and 50 kHz. This small change was consistent in all the experiments, meaning that the oxygen may only have a small effect on the results, which did not affect the measurement of dopamine. This is a very positive result toward the use of these microelectrodes in-vivo since biological fluids have oxygen dissolved and cannot be avoided.
Dopamine Measurement using EIS in standard solutions
The measurement of dopamine concentrations was done by subtracting the impedance obtained from the aCSF from the spectra of each concentration to account for the electrolyte28. In order to analyze the measurements of dopamine, we used -w*Zimag, being w the radial frequency and Zimag the imaginary component of the impedance. -w*Zimag is correlated to the capacitance of the system and it was found to be directly associated to the different concentrations of dopamine. Therefore, we decided to use this parameter for the measurement of dopamine.
The concentration of dopamine was measured using CFMEs taking advantage of its adsorption to the surface of the microelectrodes at potentials where dopamine does not oxidize but only adsorbs. This adsorption changes the electrochemical properties of the electrode surface, which is where EIS thrives. Figure 3 shows the measurement of dopamine from 1 to 500 nM concentrations at different potentials, after the subtraction of the impedance obtained in aCSF (0 nM dopamine concentration). The measurement of dopamine at potentials below 0.1 V showed that the adsorption of dopamine in the electrode surface was the responsible for this detection and not its oxidation since the oxidation starts above 0.1 V. It is important to emphasize that the X axis is in logarithmic scale showing a better resolution at lower concentrations.
Figure 3.
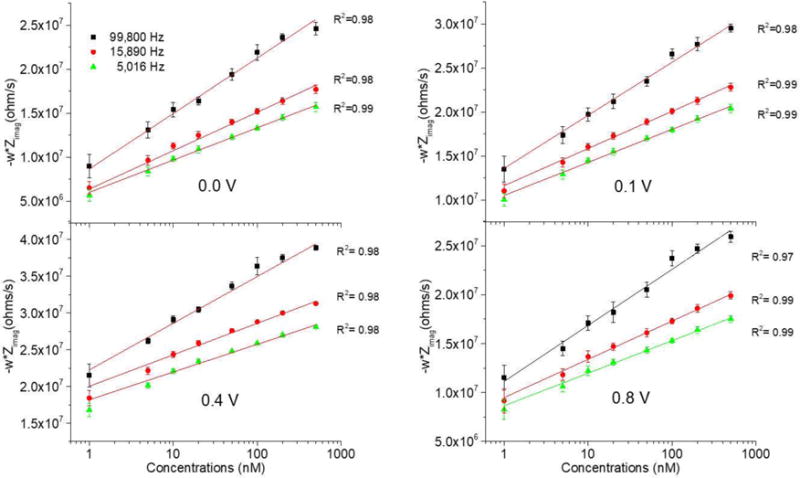
Calibration curves for the detection of dopamine using Electrochemical Impedance Spectroscopy at different frequencies and potentials performing 6 measurements using the same microelectrode.
Oxidation of the Microelectrode Surface
It has been reported that the oxidation of microelectrodes improves the sensitivity of the detection of dopamine29,30. Among the different methods of oxidation, the application of an electrochemical pre-treatment consisting of a triangular waveform potential applied to the CFMEs increases the sensitivity, which has been explained by the change in the adsorption of dopamine to the electrode surface given by the oxidation of the carbon fiber29. Therefore, a treatment with a triangular wave between −0.4 and 1.3 V was applied during 10 min at a frequency of 50 Hz before the electrochemical analysis in order to study whether this pre-treatment enhanced the measurements as seen in the scientific literature. In this way, we studied whether this also applies to the measurement of dopamine using EIS.
Figure 4 shows the comparison between the measurement of dopamine without (left column) and with (right column) the electrochemical pre-treatment. In this case, it also increases the sensitivity as it can be observed that the detection of dopamine at lower concentrations is constant after the pre-treatment at different frequencies. Moreover, more consistent results are seen in terms of a more constant -w*Zimag value below 50 kHz up to lower concentrations of dopamine when the pre-treatment was applied. Between 1 and 100 kHz, non-oxidized CFMEs showed a bigger difference in capacitance between the different concentrations of dopamine than oxidized fibers (Figure S3); however, in the dynamic experiments conducted, the change in impedance was not significantly seen in non-oxidized fibers, which we attributed to the slow adsorption rate of dopamine to without the presence of carbon-oxygen moieties. As can be seen in Figure 4, there are two specific regions in the plot delimited at ca. 50 kHz. At higher frequencies, the results were constrained by the limits of our potentiostats and the physical properties of the microelectrodes as explained above, while at lower frequencies, the separation between each concentration is clearly visualized. At 60 Hz, an erratic measurement was seen due to the electric line, which was unavoidable without applying a filter that would modify the data obtained.
Figure 4.
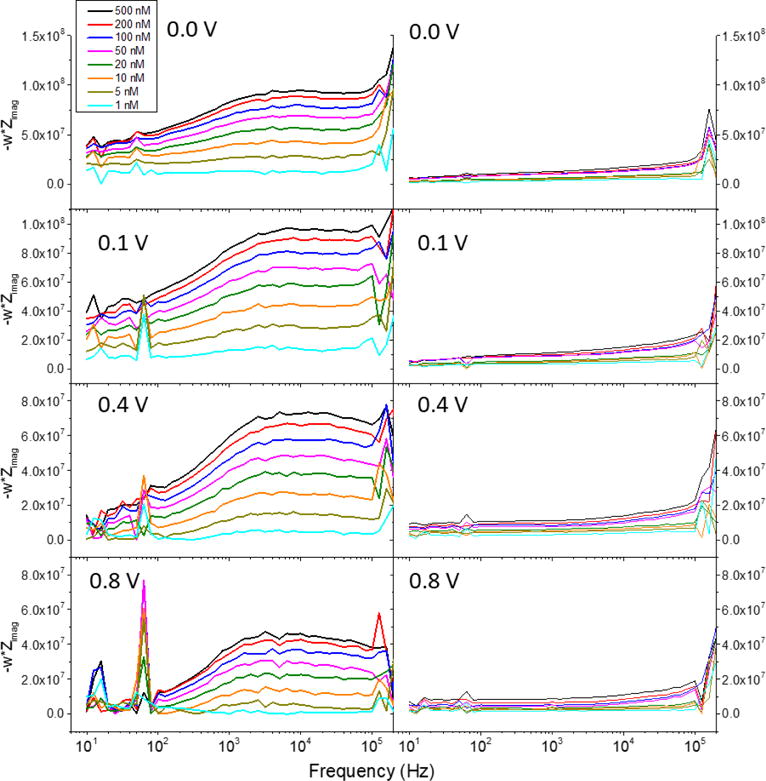
Electrochemical Impedance Spectroscopy (-w*Zimag) of different dopamine concentrations at 0.0, 0.1, 0.4, and 0.8 V vs Ag|AgCl. Left and right columns show the data obtained before and after the electrochemical pre-treatment was applied to the carbon fiber, respectively.
The subtraction of the parameter -w*Zimag measured at aCSF to the solutions of dopamine in aCSF resulted in an almost constant -w*Zimag value at frequencies between 10 Hz and ca. 50 kHz. Therefore, the surface capacitance was constant during this frequency range with regard to dopamine. If, for example, dopamine was changing its interaction with the surface at different frequencies in this range, -w*Zimag would not have stayed constant after the subtraction. These changes in the plateau found in a wide range of frequencies demonstrates that the concentration of dopamine changes the surface capacitance of the electrode. In simple systems where only one electrochemical reaction exists, -w*Zimag = 1/C where C is the surface capacitance. After subtracting the electrolyte, it was found that the increase of dopamine concentrations decreases the capacitance of the electrode surface, which was seen by the increase in the plateaus in Figure 4. The electrochemical pre-treatment, however, showed an increase in the capacitance of the carbon fiber surface seen by the lower plateaus for the same concentrations at each potential, which was consistent with measurements done by cyclic voltammetry in other reports27. Therefore, we contrasted our findings by using cyclic voltammetry.
It is known that the triangular waveform pre-treatment oxidizes the carbon fiber surface adding at the same time a more selective and effective dopamine detection27. This change in the electrochemical properties of the surface is well observed when using cyclic voltammetry at different scanning speeds, 500 mV/s, 200 mV/s, 100 mV/s, and 50 mV/s, in both, aCSF (left) and dopamine solutions (right, 500 nM dopamine), as shown in Figure 5. The oxidation of the electrode surface increases the electrical current seen in the voltammograms as well as the capacitance of the surface observed by the width of the cyclic voltammetry, which was consistent with our findings with EIS.
Figure 5.
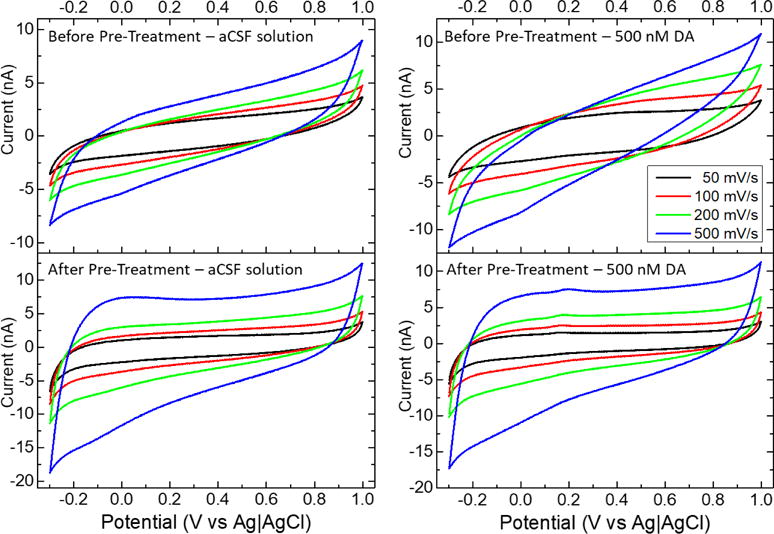
Cyclic voltammetry of carbon fiber microelectrodes after (up) and before (down) electrochemical pre-treatment in presence of 500 nM dopamine at different scan rates.
Dynamic Measurement of Dopamine
The impedance obtained during the experiments where standard solutions were used was provided by the potentiostat software (Gamry Framework) which applies filters internally to obtain the cleanest signal. On the other hand, the dynamic measurements performed with the flow cells were not possible to be run using the same software due to time limitations. Therefore, we used the software Virtual Front Panel 600 (VPF600) which provides continuous online measurements of any signal. In this way, we obtained real time measurements of the voltage applied and current measured with the limitation of 4,000 points per second due to the USB connection speed between the potentiostat and computer, faster than that occasioned randomly lost points that rendered the calculation of phase impossible. Due to this limitation, we decided to use 100 Hz (40 points per full wave) to make sure that we had enough information to perform accurate calculations for this proof-of-concept. It is important to mention that theoretically from the information gathered in Figure 4, frequencies higher than 1 kHz would provide a higher difference between concentrations when non-oxidized CFMEs are used, due to the separation in impedance at each dopamine concentration. However, practically small to no change was seen when using non-oxidized CFMEs in dynamic measurements where faster adsorption times are important.”
In flow cell experiments, the voltage and current obtained were analyzed using Fast Fourier Transform (FFT) where we found many small peaks (<1%) at different frequencies (Figure S4) that were associated with noise even though a grounded Faraday’s cage was used for the experiments. We understand that these noises come from the AC line. While these peaks were very small, there were many of them, causing the signal to have too much noise to be analyzed as obtained. Therefore, a passband filter was used to clean the signal before further impedance calculations were done. Then, the voltage and current was split into each sine wave (100 per second), fitted, and used to calculate the impedance. The magnitude and phase were transformed to the real and imaginary components of the impedance. Using the standard solutions, we found that dopamine was best correlated with the imaginary part of the impedance, which was plot vs time in the flow cell experiments.
The data obtained using standard solutions showed a higher change in impedance at different concentrations of dopamine when the CFMEs were not oxidized (Figure 4) as mentioned before. However, in flow cell experiments, we were only able to measure 1 μM concentration of dopamine using the oxidized CFMEs. As shown in Figure 6, 1 μM concentration was easily measured when using a DC voltage of −0.4 V vs Ag|AgCl while smaller peaks were found at higher potentials. Moreover, at potentials higher than the oxidation potential of dopamine, the change in Zimag was to less negative values and markedly higher but only at higher concentrations of dopamine. The disappearance of the peaks at lower concentrations of dopamine may be due to the oxidation of dopamine at those potentials and the appearance of dopamine-o-quinone. If the concentration of dopamine is low enough that its oxidation is faster than one voltage cycle, being each cycle 0.01 ms, the impedance cannot see the dopamine. However, as soon as the concentration of dopamine is high enough that its oxidation cannot be done in less than one voltage cycles, the impedance changes due to the adsorption of the excess of dopamine that is awaiting oxidation.
Figure 6.
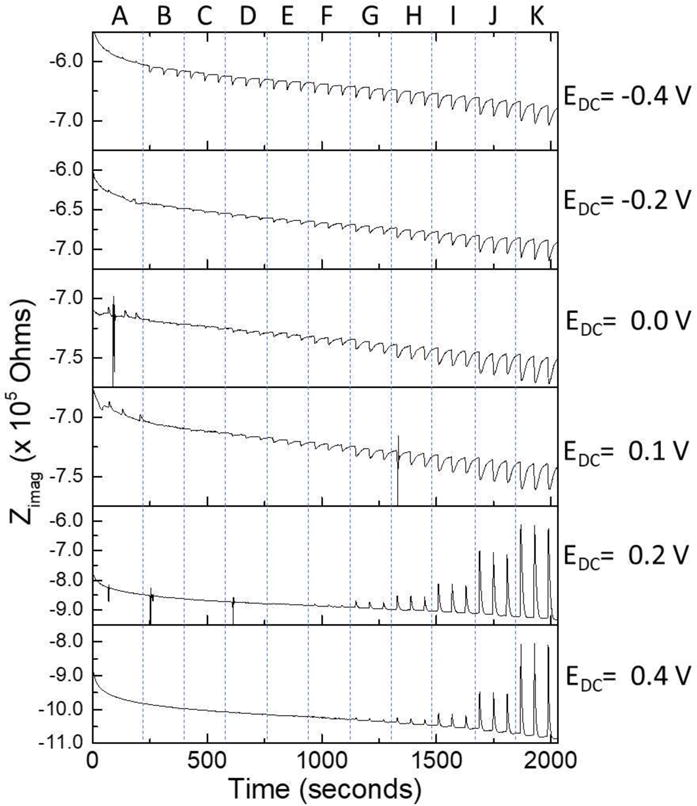
Electrochemical impedance (imaginary part) measured dynamically at different DC potentials. Three measurements were performed at each concentration of dopamine. The concentrations measured were as follows: (A) aCSF buffer, (B) 1 μM, (C) 2 μM, (D) 5 μM, (E) 10 μM, (F) 20 μM, (G) 50 μM, (H) 100 μM, (I) 200 μM, (J) 500 μM, and (K) 1 mM.
The dynamic measurements of dopamine presented with a completely different set of challenges than standard solutions when measuring dopamine adsorption. This is because these dynamic measurements are much more dependent on the adsorption rate than the standard solutions, in which time is not that important because we wait for a steady state. Using a 20 μL loop with a flow of 1 mL/min, it is obvious by the peak-shaped changes in impedance in Figure 6 that a steady state was not reached at each dopamine addition. Therefore, the impedance change should be higher to reach the impedance measured in standard solutions. Moreover, the loop was changed to a 500 μL loop and while the change in impedance was higher, none of the concentrations reached a steady state. This implies that the changes in impedance seen in dynamic measurements will not be as big as in standard solutions, and are dependent on the adsorption rate of the dopamine to the CFME. Figure 7 shows the calibration curve plotted in log-log for the dynamic measurement of dopamine adsorption at −0.4 V vs Ag|AgCl obtained using a 100 Hz sine wave. The curve followed a Freundlich adsorption isotherm model due to the rough microelectrode surface.
Figure 7.
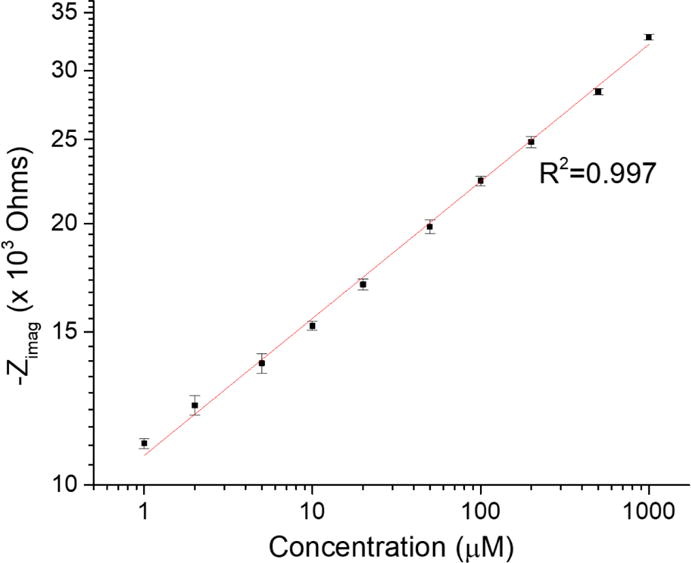
Log-log linear plot of the imaginary impedance and concentration following a Freundlich adsorption isotherm.
Comparing microelectrodes before and after oxidation of their surface, oxidized electrodes provided a much better measurement of dopamine, which we think is related to the adsorption rate. The biggest differences were seen in dynamic measurements where non-oxidized carbon fiber microelectrodes have very small changes in impedance due to dopamine in time. As mentioned before, non-oxidized microelectrodes showed bigger differences in impedance at different dopamine concentrations, but the oxidation of the surface of the microelectrodes provides a negative charged surface that binds faster with dopamine. This adsorption rate was imperative in the measurement of dopamine when dynamic conditions were presented. This was also supported by the differences seen at each DC potential, were the lower the voltage applied to the electrode, the higher the change in impedance at lower concentrations of dopamine. Therefore, it can be easily predicted that surface modifications that increase the negative charge in the carbon fiber will provide better measurements using this technique.
Conclusions
EIS was successfully used to measure dopamine concentrations in standard solutions as well as in dynamic measurements using a flow cell. The measurement of dopamine was better at mid to higher frequencies from the information gathered in standard solutions. Moreover, at these frequencies (ca. 10 Hz to 50 kHz), the currents were very low, from 0.1 nA up to 60 nA, which allows the potential use of two electrode cells instead of three electrodes. This will make this technique an easy addition to the tools used currently in the biochemical field. It is also expected that the time resolution may be different at different potentials because at high potentials where dopamine is oxidized, dopamine must adsorb to the surface in order to be measured again. At low potentials where dopamine is electrochemically stable, it does not desorb from the surface but stays in equilibrium with the solution, which allows for faster measurements. Since the oxidation desorbs dopamine from the surface, techniques such as FSCV are limited by the adsorption time to measure dopamine while EIS and SFI are not limited to measure the capacitance at low potentials. In this work, we have shown that dopamine concentration can be measured using EIS at potentials where there is no oxidation with frequencies up to 50 kHz.
Acknowledgments
This project was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103475-14 and the Puerto Rico Science, Technology and Research Trust under agreement number 2016-00068. This content is only the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, and the Puerto Rico Science, Technology and Research Trust. We are very grateful to Dr. Leslie Sombers and Christie Lee from the Chemistry Department at North Carolina State University for their extraordinary help with microelectrode fabrication. The authors acknowledge the Puerto Rico Energy Center at Universidad del Turabo and Ian Gutierrez for the use of the Scanning Electron Microscopy facilities.
Footnotes
Supporting Information.
Filename: Supporting Information - Analytical Chemistry 19-12-2017.pdf
Impedance comparison between different electrical connection lengths; effect of nitrogen gas in the CFMEs impedance; capacitance comparison between non-oxidized and oxidized carbon fibers; typical FFT plots of 100 Hz dynamic impedance data.
References
- 1.Kile BM, Walsh PL, McElligott ZA, Bucher ES, Guillot TS, Salahpour A, Caron MG, Wightman RM. ACS Chem Neurosci. 2012;3:285–292. doi: 10.1021/cn200119u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG, Venton BJ. Anal Chem. 2014;86:5721–5727. doi: 10.1021/ac404050t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heien ML, Johnson MA, Wightman RM. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 4.Makos MA, Han KA, Heien ML, Ewing AG. ACS Chem Neurosci. 2010;1:74–83. doi: 10.1021/cn900017w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodeberg NT, Johnson JA, Bucher ES, Wightman RM. ACS Chem Neurosci. 2016;7:1508–1518. doi: 10.1021/acschemneuro.6b00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyakurel P, Privman Champaloux E, Venton BJ. ACS Chem Neurosci. 2016;7:1112–1119. doi: 10.1021/acschemneuro.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamah-Biassi EB, Almonte AG, Blagovechtchenski E, Grinevich VP, Weiner JL, Bonin KD, Budygin EA. Journal of neuroscience methods. 2015;256:56–62. doi: 10.1016/j.jneumeth.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhardt GA, Hoffman AF. Journal of neuroscience methods. 2001;109:13–21. doi: 10.1016/s0165-0270(01)00396-x. [DOI] [PubMed] [Google Scholar]
- 9.Berglund EC, Makos MA, Keighron JD, Phan N, Heien ML, Ewing AG. ACS Chem Neurosci. 2013;4:566–574. doi: 10.1021/cn3002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang DA, Rand E, Marsh M, Andrews RJ, Lee KH, Meyyappan M, Koehne JE. Mol Neurobiol. 2013;48:380–385. doi: 10.1007/s12035-013-8531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt AC, Dunaway LE, Roberts JG, McCarty GS, Sombers LA. Anal Chem. 2014;86:7806–7812. doi: 10.1021/ac501725u. [DOI] [PubMed] [Google Scholar]
- 12.Burrell MH, Atcherley CW, Heien ML, Lipski J. ACS Chem Neurosci. 2015;6:1802–1812. doi: 10.1021/acschemneuro.5b00120. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Koseoglu S, Manning BM, Meyer AF, Haynes CL. Anal Chem. 2011;83:7242–7249. doi: 10.1021/ac200666c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. Biol Psychiatry. 2012;71:327–334. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang L, Yu P, Hao J, Zhang M, Zhu L, Dai L, Mao L. Anal Chem. 2014;86:3909–3914. doi: 10.1021/ac404232h. [DOI] [PubMed] [Google Scholar]
- 16.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Z, Shi L, Feng H, Zhou HS. Bioelectrochemistry. 2015;101:153–158. doi: 10.1016/j.bioelechem.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Di J, Ma J, Ma Z. Electrochim Acta. 2012;61:179–184. [Google Scholar]
- 19.Lisdat F, Schafer D. Anal Bioanal Chem. 2008;391:1555–1567. doi: 10.1007/s00216-008-1970-7. [DOI] [PubMed] [Google Scholar]
- 20.Randviir EP, Banks CE. Anal Methods. 2013;5:1098. doi: 10.1039/d2ay00970f. [DOI] [PubMed] [Google Scholar]
- 21.Cunci L, Vargas MM, Cunci R, Gomez-Moreno R, Perez I, Baerga-Ortiz A, Gonzalez CI, Cabrera CR. RSC Adv. 2014;4:52357–52365. doi: 10.1039/C4RA09689D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozcan B, Demirbakan B, Yesiller G, Sezginturk MK. Talanta. 2014;125:7–13. doi: 10.1016/j.talanta.2014.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Dervisevic M, Senel M, Cevik E. Mater Sci Eng C Mater Biol Appl. 2017;72:641–649. doi: 10.1016/j.msec.2016.11.127. [DOI] [PubMed] [Google Scholar]
- 24.Cox JT, Gunderson CG, Zhang B. Electroanalysis. 2013;25:2151–2158. doi: 10.1002/elan.201300255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson DL, Venton BJ, Heien ML, Wightman RM. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 26.Maina FK, Mathews TA. ACS Chem Neurosci. 2010;1:450–462. doi: 10.1021/cn100003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 28.de Sanoit J, Vanhove E, Mailley P, Bergonzo P. Electrochim Acta. 2009;54:5688–5693. [Google Scholar]
- 29.Roberts JG, Toups JV, Eyualem E, McCarty GS, Sombers LA. Anal Chem. 2013;85:11568–11575. doi: 10.1021/ac402884n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Trikantzopoulos E, Nguyen MD, Jacobs CB, Wang Y, Mahjouri-Samani M, Ivanov IN, Venton BJ. ACS Sens. 2016;1:508–515. doi: 10.1021/acssensors.6b00021. [DOI] [PMC free article] [PubMed] [Google Scholar]


