Abstract
A significant proportion of healthy seniors report difficulty swallowing, thought to result from age-related decline in muscle bulk/function. Effortful Swallowing (ES) is used both as a compensatory maneuver to improve pharyngeal propulsion/clearance and has been proposed as an exercise to improve pharyngeal strength. This study sought to quantify the immediate kinematic, temporal and functional changes during an ES maneuver to quantify its exercise potential to combat age-related changes in swallowing.
Videofluoroscopy data were collected from 44 healthy seniors (22 male) over 65 years old (mean=76.9, SD=7.1). Each participant swallowed six 5ml boluses of Varibar nectar-thick liquids: three with regular effort and three using ES. Individual swallows (n = 260) were measured on pharyngeal constriction, pharyngeal shortening, laryngeal closure duration, hyoid movement duration, UES opening duration, stage transition duration, pharyngeal transit time, pharyngeal response duration, Normalized Residue Ratio Scale (NRRS) and the Penetration Aspiration Scale (PAS). Non-parametric Wilcoxon Rank Sum for repeated measures tested the effect of ES on each outcome. Exact p-values were calculated based on permutation methods, individual p-values <0.008 was deemed to be significant.
The ES maneuver significantly prolonged all temporal variables. While we found no significant differences for pharyngeal constriction, significantly less (i.e. worse) pharyngeal shortening was observed in ES condition compared with regular effort swallows. Further, significantly worse pyriform sinus residue (NRRSv) was observed in ES condition. No differences between ES and regular effort swallows were noted for pharyngeal constriction, NRRSv or PAS. We speculate these negative manifestations of worse kinematics (less pharyngeal shortening) and function (increase in NRRSp) may be the result of forced volitional manipulation of swallowing in the ES condition in an otherwise normal elderly swallow.
Keywords: Deglutition, dysphagia, aging, effortful swallow, kinematics, temporal, NRRS, pharynx
Introduction
A vital mechanism for safely and efficiently propelling the bolus through the pharynx is through the action of the pharyngeal muscles. The pharyngeal constrictors contract in a rostro-caudal sequence behind the bolus, propelling it toward the esophagus, while the longitudinal muscles of the pharynx facilitate simultaneous pharyngeal shortening, decreasing the distance the bolus must travel [1,2]. Both of these actions (pharyngeal constriction and shortening) play a crucial role in executing an efficient and safe swallow [3–6]. However, the pharyngeal musculature appears to be susceptible to atrophy in the context of aging. Recent research has confirmed significant age-related reductions in pharyngeal muscle thickness as well as increases in pharyngeal lumen volume on axial MRI slices of the neck in a sample of 60 women equally stratified by age [7]. The primary goal of the present study is to establish whether the Effortful Swallow (ES) maneuver immediately improves the action of the pharyngeal muscles, confirming its potential as an exercise to combat age-related pharyngeal atrophy.
The ES was initially described by the Logemann group in the early nineties as a swallow maneuver designed to improve posterior tongue base motion during the pharyngeal swallow [8]. While the ES has also been described as an exercise-based intervention, the focus of this work is to study the immediate effects of an ES on swallowing biomechanics and function. Early work by Pouderoux and Kahrilas [9] confirmed that hard volitional swallowing resulted in significantly higher swallowing pressures in both the oral and pharyngeal cavities. Since then, other studies have investigated the impact of the ES on pharyngeal pressures with high-resolution manometry [10–13], or videofluoroscopy in combination with concurrent manometry (manofluoroscopy) [14–18], or concurrent with other modalities such as EMG or tongue pressure measurement [19–21].
While there are some conflicting findings across studies, two major trends can be extrapolated from this literature. ES improves (increases and/or prolongs) swallowing pressures [10–12,14,22–25] and improves (decreases) pressure in and/or prolongs the opening of the upper esophageal sphincter [19,22,24]. While these studies provide an important base for understanding the impact of this pharyngeal maneuver on swallowing, there are significant gaps in our understanding at the present time. First, the overwhelming majority of these studies come from healthy young (<40) volunteers (see [12,14,18,19] for exceptions). Second, the impact of the ES on the biomechanics of the pharyngeal phase of swallowing are poorly understood, especially in patient and elderly populations. Finally, to our knowledge, the literature investigating the impact of ES on post-swallow residue has been limited to perceptual rating methods for residue.
In the present study, we compared pharyngeal swallowing measures in healthy seniors in regular effort and effortful conditions. Pharyngeal measures include kinematics (pharyngeal constriction and shortening), timing (laryngeal closure duration, UES opening duration, hyoid movement duration, stage transition time, pharyngeal transit time and pharyngeal response duration) and swallow function (residue and penetration-aspiration). Our hypothesis is that compared with regular swallows from the same individual, the ES maneuver will elicit improved pharyngeal constriction and pharyngeal shortening, prolong temporal measures of swallowing and reduced residue (quantified by the Normalized Residue Ratio Scale [26]). Finally, we expect measures of swallowing safety (penetration-aspiration scores) to be unchanged in this healthy dataset.
Methods
Participants
Healthy seniors (age >65 years old) were recruited from senior centers (drop-in facilities) in the lower Manhattan region. The average age was 76.9 years old (SD=7.1) and the distribution was nearly balanced between the sexes (21 male, 23 female). Exclusion criteria (confirmed by questionnaire and oral motor sensory exam) were prior history of dysphagia, neurological disease, head and neck cancer, or head and neck surgery (other than routine dental/tonsil/adnoid surgeries). During recruitment, participants were screened for maximal tongue strength. The purpose was to collect a convenience sample of seniors who were categorized as having maximum isometric anterior tongue strength <40kPa or > 40kPa using the Iowa Oral Performance Instrument (IOPI Medical). This was done to ensure an adequate distribution of strong and weak seniors in the sample and to reduce the potential for a volunteer bias. Table 1 summarizes the participant characteristics. This study was approved by the local IRB and all participants signed an IRB-approved consent form prior to participation.
Table 1.
Participant demographics.
| MEN (n=21) | WOMEN (n=23) | |||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Age (years) | 75.3 | 6.6 | 78.3 | 7.5 |
| Height (cm) | 171.5 | 8.0 | 158.8 | 7.5 |
| BMI | 27.1 | 3.3 | 26.0 | 3.7 |
| Mean Anterior Tongue Strength (kPa) | 39.8 | 11.3 | 34.9 | 12.0 |
Data Collection
Participants attended two consecutive days of data collection. Day one tasks (completed by trained research assistants in pairs) included demographics, questionnaires, and data scales. Day two data collection included videofluoroscopy and acoustic pharyngometry. Only the videofluoroscopic data will be described in this manuscript.
This research study represents a secondary question using a dataset collected for a study of pharyngeal atrophy in aging. For the full study, each participant swallowed 12 barium boluses under fluoroscopy. Only the final 6 boluses (3× 5ml nectar-thick barium with and without effort) are used for this analysis. The order of bolus administration was not randomized: 3× nectar regular effort swallow followed by 3× nectar effortful swallow. This choice was based on protocol requirements for the larger study. Participants self-administered boluses in pre-filled medicine cups. Volumes were measured via syringe to contain 1ml more than the target volume to control for residual barium left in the cup [27]. The nectar-thick barium sulfate was 40% w/v ratio with target viscosity of 300 centipoise (Varibar ®, Bracco Imaging). All subjects were given the same cue, “Squeeze really hard with all of your throat muscles, as if you are trying to get down a piece of steak that is stuck in your throat”. Videofluoroscopy was conducted on a GE Advantax digital fluoroscope (GE Healthcare) at a pulse rate of 30 pulses per second and captured at 30 frames per second on a Kay Pentax Digital Swallowing Workstation.
Data Analysis
All individual swallows (n=264) were spliced out of the larger full-length video for blinded, randomized rating by trained research assistants (doctoral- and masters-level speech pathology students) using ImageJ software (NIH). Four effortful swallows were completely excluded from analysis secondary to piecemeal deglutition (n=3) and image quality (n=1). Each individual swallow was analyzed on 1) pharyngeal kinematics: pharyngeal constriction and pharyngeal shortening; 2) pharyngeal timing measures: laryngeal closure duration, stage transition duration, pharyngeal transit time, pharyngeal response duration, hyoid movement duration; and 3) functional swallowing measures: swallowing safety and efficiency. If image quality or participant positioning prevented reliable measurement of a specific variable, it was excluded from analysis. Each measure is described below.
Kinematics
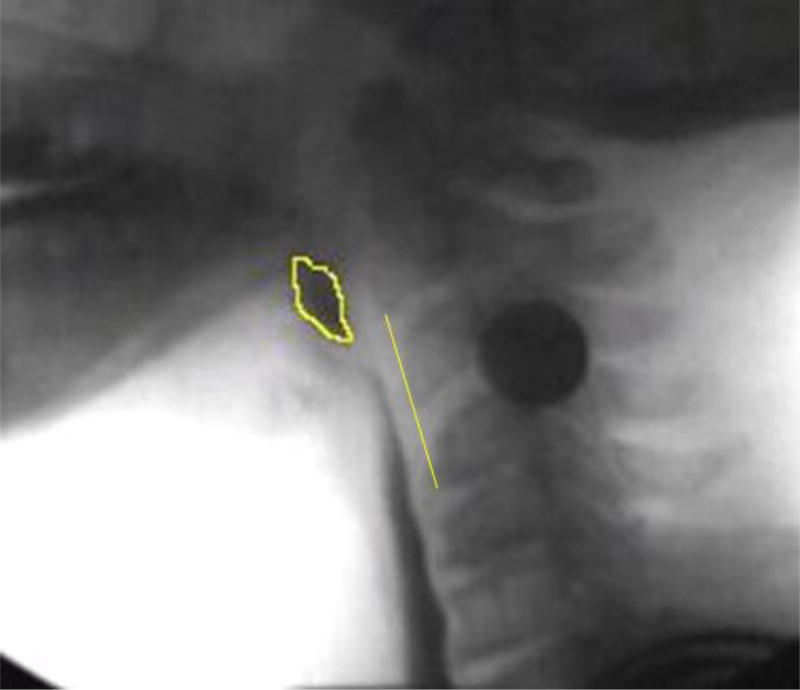
Pharyngeal constriction was measured on the frame of maximal pharyngeal constriction by using the Normalized Maximum Pharyngeal Constriction Area (MPCAN) as originally described by Stokely et al [28]. Using the free-hand tool in ImageJ, the unobliterated pharyngeal space (represented by bolus or air) is outlined. This yields the area in pixels. To normalize this value to the size of the participant, the area was divided by the C2–4 length squared. In the case that the pharynx fully constricts, the value is zero. Note that traditional pharyngeal constriction ratio [3] measures were not feasible given the lack of a 1ml bolus hold frame (PAhold) for measurement comparison.
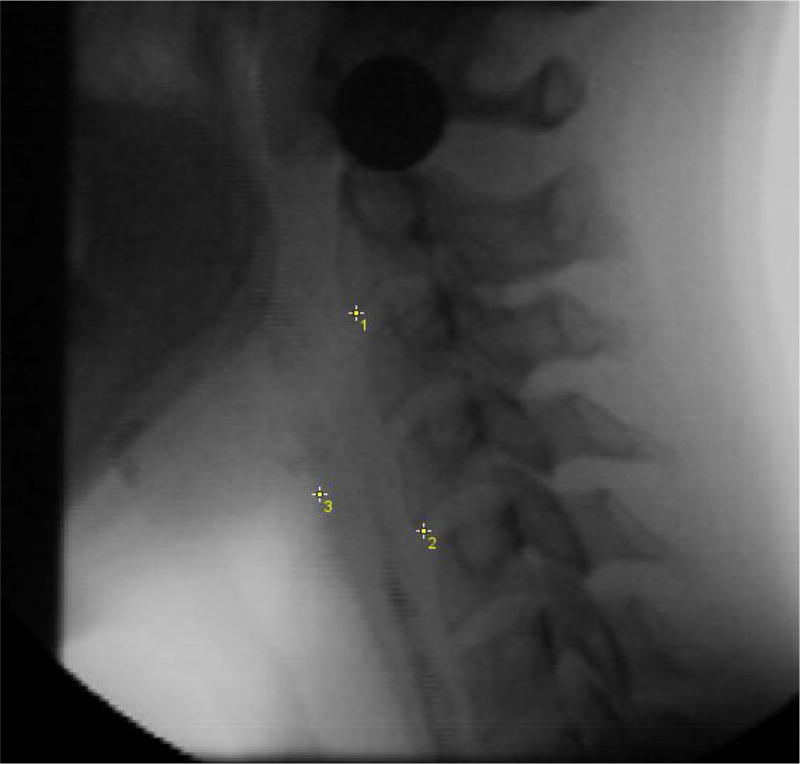
Pharyngeal shortening was captured by measuring the peak superior position of the laryngeal air column (where the vocal folds intersects with the posterior trachea) measured from C4. A vertical line drawn between C2–C4 represented the Y-axis of the Cartesian coordinate system. Peak position was measured in %C2–4 units to control for individual size variation. Note that we first attempted to track the peak position of the pyriform sinuses to measure pharyngeal shortening, however, the bolus obstructed the view at the peak position and we were unable to achieve adequate reliability. Our chosen method builds on techniques whereby laryngeal locations are chosen to serve as proxy for pharyngeal shortening [2,5]. Despite this, we consciously chose to continue to refer to this variable as ‘pharyngeal shortening’ to reflect the broad research question regarding the effect of effortful swallowing on pharyngeal muscle function. All kinematic measures were taken on the initial swallow only (no data from clearing swallows was included).
Temporal Measures
Temporal measures were captured using frame-by-frame advancement of each swallow to identify specific events during swallowing. These events are used to derive measures of interest. Swallowing events included: bolus past mandible, hyoid burst (rapid upward and forward motion), hyoid rest, onset laryngeal closure, offset laryngeal closure, UES opening, and UES closure. Relevant events are subtracted from each other to derive our six temporal measures of interest. Finally, we convert the frames to seconds by dividing by 30 (given that our VF was collected at 30 frames per second). A summary of the temporal variables appears in Table 2. Detailed procedures and operational definitions are described in detail in Molfenter & Steele [27]. If clearing swallows were present, temporal measures were taken from the initial swallow only.
Table 2.
Formulas for calculating temporal variables included in our analysis.
| Temporal Variables | Contributing Events |
|---|---|
| Laryngeal Closure Duration (LCD) | offset laryngeal closure – onset laryngeal closure |
| Hyoid Movement Duration (HMD) | hyoid rest – hyoid burst |
| UES Opening Duration (UESOD) | UES closure – UES opening |
| Stage Transition Duration (STD) | hyoid burst – bolus past mandible |
| Pharyngeal Transit Time (PTT) | UES closure – bolus past mandible |
| Pharyngeal Response Duration (PRD) | UES closure – hyoid burst |
Functional Measures
Swallowing safety was captured using the 8-point penetration aspiration scale (PAS) [29]. In the case that a participant had clearing swallows, PAS was rated on each sub-swallow and the worst PAS score per swallow was used to represent swallowing safety for that particular swallow.
Post-swallow residue was measured using the Normalized Residue Ratio Scale (NRRS) for the valleculae (NRRSv) and pyriforms (NRRSp) [26]. The NRRS expresses the pixels of residue relative to the pixels of the spatial housing (the valleculae or pyriforms) as well as a function of the squared C2–4 distance (to control for subject size). All NRRS measures were taken after initial swallows on the post-swallow rest frames as originally described in Pearson et al [26].
Reliability Analysis
Twenty percent of the full dataset was re-rated by the same rater and also by a second rater for reliability purposes. Reliability was tested using two-way mixed intraclass correlation coefficients (ICCs). Results appear in Table 3. With the exception of one variable, all values achieved ICC > 0.75 which is considered ‘excellent’ [30]. Inter-rater results for NRRSv narrowly missed this cut-off (=0.74) and can be described as having ‘good’ reliability [30].
Table 3.
Reliability results.
| INTRA RATER | INTRA RATER | ||||
|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ||
| Kinematic Measures | Pharyngeal Constriction | 0.82 | (0.73–0.88) | 0.76 | (0.64–0.84) |
| Pharyngeal Shortening | 0.93 | (0.90–0.95) | 0.93 | (0.89–0.95) | |
|
| |||||
| Temporal Measures | Laryngeal Closure Duration | 0.98 | (0.98–0.99) | 0.85 | (0.78–0.90) |
| Hyoid Movement Duration | 0.97 | (0.96–0.98) | 0.94 | (0.87–0.94) | |
| UES Opening Duration | 0.90 | (0.86–0.93) | 0.81 | (0.72–0.87) | |
| Stage Transition Duration | 0.98 | (0.98–0.99) | 0.96 | (0.93–0.97) | |
| Pharyngeal Transit Time | 0.98 | (0.97–0.99) | 0.92 | (0.88–0.95) | |
| Pharyngeal Response Duration | 0.91 | (0.86–0.94) | 0.82 | (0.74–0.88) | |
|
| |||||
| Functional Measures | PAS | 0.88 | (0.83–0.92) | 0.86 | (0.79–0.90) |
| NRRSv | 0.89 | (0.83–0.93) | 0.74 | (0.61–0.82) | |
| NRRSp | 0.97 | (0.96–0.98) | 0.86 | (0.79–0.91) | |
ICC = Intraclass Correlation Coefficient, 95% CI = 95% Confidence Interval
Statistical Analysis
Given that normality assumptions for parametric tests were violated, non-parametric Wilcoxon Rank Sum test for Repeated Measures were therefore used, for each response variable, to test the two-sided hypothesis regarding the effect of effortful swallowing on kinematic, temporal and functional measures of swallowing. Models tested the contribution of age, sex and tongue strength for each variable. Exact p-values are calculated based on permutation methods. No significant trial effect was detected and the order of repeated trials was randomly sampled in the permutation test to account for trial-level variability. This result suggests that there is little bias due to practice when we compare regular swallows followed by effortful swallows. Hommel adjustment was used to correct for multiple hypothesis testing and p<0.008 was deemed to be significant for each individual test in order to maintain an overall type I error at 5%. Cohen’s D was calculated to quantify the effect of significant findings with values <0.2 considered to be negligible effect, 0.2–0.5 were considered to show small effects, 0.5–0.8 to show medium effects, and values > 0.8 to show large effects [31].
Results
Descriptive results for kinematic, temporal and functional measures by swallow type (regular effort vs effortful) appear in Table 4. In addition, the distribution of the PAS scores is presented by swallow type in Table 5. The distribution is, as expected for a healthy population, largely skewed to safe normal PAS scores of 1 and 2. There were 3/132 (2.2 %) regular effort swallows with abnormal PAS scores (all ‘3’) and 10/126 (7.9 %) effortful swallows with abnormal PAS scores (nine scores of ‘3’and one score of ‘5’). No instances of aspiration (scores 6–8) were observed.
Table 4.
Descriptive statistics for kinematic, temporal and functional variables separated by swallow type.
| Regular Effort Swallows 5ml nectar |
Effortful Swallows 5ml nectar |
||||
|---|---|---|---|---|---|
| n | mean (SE) | n | mean (SE) | ||
|
|
|||||
| Kinematic Measures | Pharyngeal Constriction (MPCAN) | 132 | 0.036 (0.058) | 128 | 0.034 (0.052) |
| Pharyngeal Shortening (%C2–4) | 131 | 65.05 (13.07) | 116 | 61.43 (11.51) | |
|
| |||||
| Temporal Measures | Laryngeal Closure Duration (sec) | 132 | 0.411 (0.09) | 126 | 0.792 (0.739) |
| Hyoid Movement Duration (sec) | 130 | 1.154 (0.309) | 125 | 1.701 (0.839) | |
| UES Opening Duration (sec) | 132 | 0.479 (0.096) | 128 | 0.553 (0.172) | |
| Stage Transition Duration (sec) | 130 | 0.151 (0.225) | 125 | 0.219 (0.269) | |
| Pharyngeal Transit Time (sec) | 132 | 0.77 (0.231) | 128 | 0.909 (0.331) | |
| Pharyngeal Response Duration (sec) | 130 | 0.623 (0.114) | 125 | 0.694 (0.23) | |
|
| |||||
| Functional Measures | PAS | 132 | 1.219 (0.041) | 126 | 1.341 (0.061) |
| NRRSv | 131 | 0.027 (0.075) | 126 | 0.033 (0.079) | |
| NRRSp | 131 | 0.042 (0.268) | 126 | 0.049 (0.302) | |
Table 5.
Distribution of the penetration-aspiration scale (PAS) scores by swallow type.
| Normal | Penetration | Aspiration | total N | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Regular Effort | 106 | 23 | 3 | 0 | 0 | 0 | 0 | 0 | 132 |
| Effortful | 95 | 21 | 9 | 0 | 1 | 0 | 0 | 0 | 126 |
Inferential statistics appear in Table 6. Wilcoxon Rank Sum tests for repeated measures were run for each variable. There was no significant influence of age, sex and/or tongue strength for any of the variables tested. Interestingly, a significant worsening of pharyngeal shortening (smaller values indicate better constriction). The magnitude of this finding is considered a small effect (D 0.2–0.5). There was no significant difference noted for pharyngeal constriction. All temporal variables are significantly prolonged in the effortful condition, with medium to strong effects for the duration measures (LCD, HMD, UESOD) and small effects for all three interval measures (STD, PTT, PRD). Finally, the use of effortful swallowing appears to have resulted in significantly worse NRRSp residue scores. NRRSp and PAS scores are significantly not different between the two conditions.
Table 6.
Results of the comparison of regular effort and effortful swallows.
| Average Treatment Effect |
SE | p-value | Cohen’s D |
||
|---|---|---|---|---|---|
|
|
|||||
| Kinematic Measures | Pharyngeal Constriction (MPCAN) | −0.0005 | 0.0029 | 0.8900 | -- |
| Pharyngeal Shortening (%C2–4) | −2.629 | 1.0669 | 0.0033*** | 0.29 | |
|
| |||||
| Temporal Measures | Laryngeal Closure Duration (sec) | 0.3800 | 0.0543 | <0.0001*** | 0.72 |
| Hyoid Movement Duration (sec) | 0.5446 | 0.0676 | <0.0001*** | 0.86 | |
| UES Opening Duration (sec) | 0.0739 | 0.0164 | <0.0001*** | 0.53 | |
| Stage Transition Duration (sec) | 0.0687 | 0.0249 | <0.0001*** | 0.27 | |
| Pharyngeal Transit Time (sec) | 0.1396 | 0.0285 | <0.0001*** | 0.49 | |
| Pharyngeal Response Duration (sec) | 0.0713 | 0.0214 | 0.0007*** | 0.39 | |
|
| |||||
| Functional Measures | PAS | 0.1214 | 0.0694 | 0.0713 | -- |
| NRRSv | 0.0073 | 0.0068 | 0.2467 | -- | |
| NRRSp | 0.0072 | 0.0155 | 0.0020*** | 0.02 | |
Discussion
In this study, we quantified the immediate within-subject changes to pharyngeal kinematics, timing and function as the result of executing the effortful swallow maneuver in a sample of 44 healthy, community-dwelling seniors over the age of 65. Consistent with previous findings [19] and with our hypothesis, we found significant prolongation of all pharyngeal temporal variables in the effortful condition. To our knowledge, these data contribute novel normative references using nectar barium stimuli in healthy older adults.
A recent publication by Kim [32] reports improved pharyngeal constriction in a series of 19 patients who completed a 4-week intervention of ES during resistive electrical stimulation. While this is a promising finding, there is no way to tease apart the effect of the ES from the stimulation given the lack of a control group/condition. Our analysis failed to detect an immediate significant improvement in pharyngeal constriction as the result of employing the ES maneuver. We acknowledge the limitations of 2D lateral videofluoroscopy in answering this question. Indeed, an improvement in pharyngeal constriction as the result of effortful swallowing cannot be captured if full pharyngeal obliteration is achieved in the normal condition. A post-hoc exploration revealed that 36/132 of the regular effort swallows were measured to have a MPCAN of 0 indicating a ceiling effect for 27% of the data for the pharyngeal constriction variable. This limitation may have obscured a difference in pharyngeal constriction by swallow condition in this healthy population but warrants further investigation in patients with dysphagia.
In 2015, Stokely et al [28] reported pharyngeal constriction data (using the MPCAN) for swallows with no residue (mean: 0.02, 95% CI: 0.015–0.022), swallows with significant vallecular residue (mean: 0.12, 95% CI: 0.10–0.14) and swallows with significant pyriform sinus residue (mean: 0.15, 95% CI: 0.12–0.18). The data for that study came from 20 healthy young individuals and 40 individuals with dysphagia. Our findings for healthy seniors, predictably fall between their functional (no residue) data and their impaired (significant residue) findings for pharyngeal constriction. Thus, we believe this study fills a gap in the literature regarding normative pharyngeal constriction values in the context of healthy aging.
Per Logemann [8], the effortful swallow should improve pharyngeal clearance of post-swallow residue, yet the support for this in the literature is mixed. Lazarus and colleagues [14] examined the effect of voluntary swallow maneuvers in a series of three patients who were treated for head and neck cancer. In addition to prolonged duration of tongue base contact and increase of BOT to PPW pressure, they described a reduction in percent of residue in effortful condition compared to regular swallows. Their findings are descriptive only, given the small, heterogeneous sample. In 2001, Hind and colleagues [19] investigated the impact of effortful swallowing in healthy adults across various parameters. With respect to residue (captured using a 3-point perceptual rating scale: no residue/coating of residue/pooling of residue), they found no significant differences in any of the locations that residue was examined (oral cavity, vallecular, posterior pharyngeal wall, pyriform sinuses and UES). Bülow and colleagues [16] reported no significant improvement in post-swallow residue (on a 4-point perceptual rating scale: none/mild/moderate/severe) for effortful swallowing compared to regular swallows at the group level (eight patients with dysphagia). Interestingly, however, the individual data reveal that two of the eight participants in the study experienced worse residue in the effortful condition. The present study is the first, to our knowledge, to use a quantitative pixel-based method (NRRS) to reliably quantify post-swallow residue in the context of effortful swallowing. We found no significant difference in vallecular residue between swallowing conditions (consistent with Hind [19]). Interestingly, contrary to our hypothesis, the data pointed to significantly worse pyriform sinus residue in the effortful swallow condition. This result is to be interpreted with caution given the extremely small effect indicated by the Cohen’s D value. Yet, it certainly warrants further investigation.
The worse pyriform sinus residue in ES condition may be explained, at least in part, by a second finding that contradicted our hypotheses: the ES condition was associated with less (i.e. worse) pharyngeal shortening compared to the regular swallowing condition. In their study of physiological abnormalities related to pharyngeal retention, Olsson and colleagues confirm a negative relationship between pharyngeal shortening (using laryngeal elevation as a proxy) and post-swallow residue [5]. Foundational work conducted by Kahrilas and colleagues [2] emphasizes the importance of pharyngeal shortening to minimize post-swallow residue but also advocates that the ‘clinical assessment of pharyngeal function must include determination of effective shortening, timing of shortening relative to bolus transit and finally the characteristics of the propagated posterior pharyngeal wall contraction itself” (p 135). Thus, we conducted a post-hoc exploration of the difference in latency for effortful vs regular swallows between the peak frame of pharyngeal shortening and three swallowing events: bolus past mandible, UES open, and UES close. Paired t-tests (Table 7) revealed that latency between peak pharyngeal shortening and bolus passing the ramus of the mandible occurred significantly later (5.1 frames or 0.17 seconds, on average) in the effortful condition. Yet, the time that lapses between the peak pharyngeal shortening and UES closure is almost identical. Taken together, it appears that ES prolongs the amount of time the bolus is in the pharynx, but not the amount of time for the bolus to be swept through the UES; a phenomenon which may contribute to the manifestation of pyriform sinus residue.
Table 7.
Post-hoc exploration of the latency of swallow events from peak pharyngeal shortening (PS) measured in frames.
| Regular Effort | Effortful | t | p | |||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||
| Peak PS - BPM (frames) | 9.54 | 15.17 | 14.69 | 15.91 | −2.84 | 0.005 |
| Peak PS - UES open (frames) | 0.66 | 13.93 | 3.47 | 13.97 | −1.67 | 0.097 |
| Peak PS - UES close (frames) | −13.80 | 13.86 | −13.17 | 15.50 | −0.37 | 0.713 |
Penetration-aspiration scale scores were heavily skewed toward normal in our study and this situation mirrors the distribution reported by others for healthy individuals. For example, Allen et al [33] report penetration on 2.9% of swallows in healthy adults; we observed 2.2% (on regular effort swallows). While the penetration-aspiration scale scores did not change significantly in the context of effortful swallowing, we observed a slightly greater proportion of unsafe swallows in the effortful condition (7.9%). This finding stands in contrast to work by Bülow and colleagues [16] which demonstrated improvements in swallowing safety as a result of effortful swallowing.
We speculate that the observed negative manifestations (worse pyriform sinus residue and worse pharyngeal shortening) may be the result of forced volitional manipulation of swallowing in the ES condition in an otherwise normal elderly swallow. It may be worth noting that we are not the first to identify maladaptive effects of the effortful swallow. Garcia, Hakel and Lazarus [34] reported a case study in which a patient with severe pharyngeal dysphagia who developed severe nasal backflow in the context of therapeutic effortful swallowing, apparently related to premature tongue base contact to the posterior pharyngeal wall during effortful swallowing. Taken together, these findings underscore the importance of physiologically-targeted swallowing interventions based on careful instrumental evaluation.
We acknowledge that our study has several limitations. First, this is a study of effortful swallowing in healthy older individuals and more work is required before we can extrapolate these findings to individuals with dysphagia. That being said, our sample was stratified by tongue strength in order to capture a wide (and representative) range of oropharyngeal strength in seniors. The results can serve as normative data for comparisons with older individuals with dysphagia. Second, the order of swallowing condition (effortful vs regular) was not randomized. The data collection protocol was chosen based on our primary research questions (not discussed in this manuscript) which required the task order to be restricted. However, our statistical approach confirmed no effect of trial order, a finding that has been confirmed by others [18]. Third, comparison of our findings to others in the literature may be limited by the variation across studies regarding the instructions given to participants for executing the effortful swallow (see an excellent summary in Lenius et al.[18]). Our participants were instructed to focus on pharyngeal effort. Previous research by Steele and Huckabee [25] has established healthy young participants demonstrated significantly prolonged time between the peak amplitude of submental sEMG data and peak upper pharyngeal (manometric) pressure when effortful swallows were conducted with an emphasis on tongue pressure generation. Finally, these data are restricted to 5ml boluses of nectar-thick barium stimuli and future work should expand the analysis to larger bolus sizes and a wider range of viscosities.
Conclusion
In conclusion, we set out to document the immediate, within-subject effects of the effortful swallow maneuver on temporal, kinematic and functional measures of swallowing from lateral view videofluoroscopy in healthy seniors over 65. Our goal was to determine whether the effortful swallow immediately improves the action of the pharyngeal musculature and function to establish whether it may serve as a useful exercise to target age-related pharyngeal atrophy. While, our results support previous research that documents increased temporal durations during effortful swallowing, we found no significant differences for pharyngeal constriction and worse pharyngeal shortening in the context of effortful swallowing. Worse pharyngeal shortening appears to have manifested in the functional consequence of significantly greater pyriform sinus residue. We found no significant differences in vallecular residue or penetration-aspiration scores in the context of effortful swallowing compared with regular effort swallows. Replication in dysphagic populations is warranted, especially to clarify the true potential of the effortful swallow maneuver to reduce of post-swallow residue.
Figure 1.
Pharyngeal constriction measurement example. Pixels of unobliterated pharyngeal space at maximal pharyngeal constriction are expressed as a function of the C2–4 distance squared.
Figure 2.
Pharyngeal shortening measurement example. Peak laryngeal position (point 3) from C4 (point 2) in a participant-defined coordinate system (Y axis through points 1 and 2).
Acknowledgments
The authors would like to thank Charles Lenell, Erica Herzberg, Danielle Brates, Mehak Noorani, Emily Ottinger, Shelby Norman, Il Young Jung, Julie Bancroft, Wendy Liang, Chelsea Sandler, Marina Casale and Katrin Gabriel for their assistance during data collection and data analysis.
Funding: This study was funded by NIH National Institute on Deafness and Other Communication Disorders 1R21DC015067.
Footnotes
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
This work has been accepted for oral presentation at the 2017 European Society for Swallowing Disorders (ESSD).
No other conflicts of interest to disclose.
Contributor Information
Sonja M Molfenter, Department of Communicative Sciences & Disorders, NYU Steinhardt.
Chuan-Ya Hsu, Department of Applied Statistics, Social Science & Humanities, NYU Steinhardt.
Ying Lu, Department of Applied Statistics, Social Science & Humanities, NYU Steinhardt.
Cathy L Lazarus, Department of Otolaryngology Head & Neck Surgery, Mount Sinai Beth Israel.
References
- 1.Leonard R, Kendall KA, McKenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19(2):133–141. doi: 10.1007/s00455-003-0508-6. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: A combined manometric and videofluoroscopic study. Gastroenterology. 1992;103(1):128–136. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 3.Leonard R, Rees CJ, Belafsky P, Allen J. Fluoroscopic Surrogate for Pharyngeal Strength: The Pharyngeal Constriction Ratio (PCR) Dysphagia. 2011;26(1):13–17. doi: 10.1007/s00455-009-9258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer JB, Tanaka E, Ensrud E. Motions of the posterior pharyngeal wall in human swallowing: A quantitative videofluorographic study. Arch Phys Med Rehabil. 2000;81(11):1520–1526. doi: 10.1053/apmr.2000.17829. [DOI] [PubMed] [Google Scholar]
- 5.Olsson R, Castell J, Johnston B, Ekberg O, Castell DO. Combined videomanometric identification of abnormalities related to pharyngeal retention. Acad Radiol. 1997;4(5):349–354. doi: 10.1016/s1076-6332(97)80116-x. [DOI] [PubMed] [Google Scholar]
- 6.Ekberg O, Nylander G. Pharyngeal constrictor paresis in patients with dysphagia: a cineradiographic study. Clin Radiol. 1982;33(3):253–258. doi: 10.1016/s0009-9260(82)80253-5. [DOI] [PubMed] [Google Scholar]
- 7.Molfenter SM, Amin M, Branski RC, Brumm J, Hagiwara M, Roof S, et al. Age-related changes in pharyngeal lumen size: A retrospective MRI analysis. Dysphagia. 2015;30(3):321–327. doi: 10.1007/s00455-015-9602-9. [DOI] [PubMed] [Google Scholar]
- 8.Logemann JA. Evaluation and treatment of swallowing disorders. 2. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- 9.Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108(5):1418–1426. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- 10.Takasaki K, Umeki H, Hara M, Kumagami H, Takahashi H. Influence of effortful swallow on pharyngeal pressure: evaluation using a high-resolution manometry. Otolaryngol Head Neck Surg. 2011 Jan;144(1):16–20. doi: 10.1177/0194599810390885. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman MR, Mielens JD, Ciucci MR, Jones CA, Jiang JJ, McCulloch TM. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012;27(3):418–426. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nativ-Zeltzer N, Logemann JA, Zecker SG, Kahrilas PJ. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography-a normative study of younger and older adults. Neurogastroenterol Motil. 2016;28(5):721–731. doi: 10.1111/nmo.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamvik K, Macrae P, Doeltgen S, Collings A, Huckabee M. Normative data for pharyngeal pressure generation during saliva, bolus, and effortful saliva swallowing across age and gender. Speech, Language and Hearing. 2014;17(4):210–215. [Google Scholar]
- 14.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002 Jul-Aug;54(4):171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 15.Bülow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14(2):67–72. doi: 10.1007/PL00009589. [DOI] [PubMed] [Google Scholar]
- 16.Bülow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia. 2001;16(3):190–195. doi: 10.1007/s00455-001-0065-9. [DOI] [PubMed] [Google Scholar]
- 17.Bülow M, Olsson R, Ekberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17(3):197–201. doi: 10.1007/s00455-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 18.Lenius K, Stierwalt J, LaPointe LL, Bourgeois M, Carnaby G, Crary M. Effects of lingual effort on swallow pressures following radiation treatment. Journal of Speech, Language, and Hearing Research. 2015;58(3):687–697. doi: 10.1044/2015_JSLHR-S-14-0210. [DOI] [PubMed] [Google Scholar]
- 19.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Arch Phys Med Rehabil. 2001;82(12):1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 20.Yeates EM, Steele CM, Pelletier CA. Tongue pressure and submental surface electromyography measures during noneffortful and effortful saliva swallows in healthy women. American Journal of Speech-Language Pathology. 2010;19(3):274–281. doi: 10.1044/1058-0360(2010/09-0040). [DOI] [PubMed] [Google Scholar]
- 21.Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. Journal of Speech, Language, and Hearing Research. 2008;51(5):1072–1087. doi: 10.1044/1092-4388(2008/07-0016). [DOI] [PubMed] [Google Scholar]
- 22.Hiss SG, Huckabee ML. Timing of pharyngeal and upper esophageal sphincter pressures as a function of normal and effortful swallowing in young healthy adults. Dysphagia. 2005;20(2):149–156. doi: 10.1007/s00455-005-0008-y. [DOI] [PubMed] [Google Scholar]
- 23.Fritz M, Cerrati E, Fang Y, Verma A, Achlatis S, Lazarus C, et al. Magnetic resonance imaging of the effortful swallow. Ann Otol Rhinol Laryngol. 2014 Nov;123(11):786–790. doi: 10.1177/0003489414538607. [DOI] [PubMed] [Google Scholar]
- 24.Witte U, Huckabee M, Doeltgen SH, Gumbley F, Robb M. The effect of effortful swallow on pharyngeal manometric measurements during saliva and water swallowing in healthy participants. Arch Phys Med Rehabil. 2008;89(5):822–828. doi: 10.1016/j.apmr.2007.08.167. [DOI] [PubMed] [Google Scholar]
- 25.Steele CM, Huckabee ML. The influence of orolingual pressure on the timing of pharyngeal pressure events. Dysphagia. 2007;22(1):30–36. doi: 10.1007/s00455-006-9037-4. [DOI] [PubMed] [Google Scholar]
- 26.Pearson WG, Jr, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: The Normalized Residue Ratio Scale. Dysphagia. 2013;28(2):167–177. doi: 10.1007/s00455-012-9426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molfenter SM, Steele CM. Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia. 2013;28(2):226–233. doi: 10.1007/s00455-012-9437-6. [DOI] [PubMed] [Google Scholar]
- 28.Stokely SL, Peladeau-Pigeon M, Leigh C, Molfenter SM, Steele CM. The relationship between pharyngeal constriction and post-swallow residue. Dysphagia. 2015;30:349–356. doi: 10.1007/s00455-015-9606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 30.Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- 31.Kotrlik J, Williams H. The incorporation of effect size in information technology, learning, and performance research. Inform Technol Learn Perform. 2003;21(1):1–7. [Google Scholar]
- 32.Kim H, Park J, Nam K. Effortful swallow with resistive electrical stimulation training improves pharyngeal constriction in patients post-stroke with dysphagia. J Oral Rehabil. 2017 doi: 10.1111/joor.12538. [DOI] [PubMed] [Google Scholar]
- 33.Allen JE, White CJ, Leonard RJ, Belafsky PC. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Dysphagia. 2010;25(4):347–348. doi: 10.1016/j.otohns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Garcia JM, Hakel M, Lazarus C. Unexpected consequence of effortful swallowing: case study report. Journal of Medical Speech-Language Pathology. 2004;12(2):59–67. [Google Scholar]