Abstract
Previous work has demonstrated that a single subcutaneous dose of salmon calcitonin leads to a transient decline in circulating levels of FGF23 in patients with X-linked hypophosphatemia (XLH). Since the calcitonin receptor is expressed on osteocytes, this raises the possibility that interdicting signals through that receptor could modulate circulating levels of FGF23 in XLH. In the present study, 21 subjects with XLH were randomly assigned to receive either placebo nasal spray or 400 IU of nasal salmon calcitonin daily for three months. On the first and last day of the study, serial measurements of FGF23, 1,25-dihydroxyvitamin D, and TmP/GFR were made over 27 h. At the beginning of Visit 2 (the first day of month 2) and the beginning of Visit 3 (the first day of month 3), single, first-morning, fasting measurements of these same parameters were made before the next administered dose of study drug. Following the initial or final dose of study drug, there were no differences in area under the curve, based on treatment assignment, for the three principal outcome variables. Similarly, there were no differences in the fasting measures taken at the beginning of Visit 2 or Visit 3 compared to the fasting values on either day 2 of Visit 1 or the fasting values on day 2 of Visit 4. There were also no significant changes over time in serum phosphorus, serum calcium, circulating levels of PTH, CTx, or P1NP. The reasons why nasal salmon calcitonin did not recapitulate the findings with subcutaneously administered drug may relate to the kinetics of drug delivery, the bioavailability of drug or peak drug dose achieved. It remains possible, however, that other means of altering calcitonin receptor signaling may still provide an opportunity for regulating FGF23 production.
Keywords: Clinical trial, Hypophosphatemia, FGF23, Calcitonin
Introduction
X-linked hypophosphatemia (XLH), caused by overproduction of FGF23 by osteocytes, is the most common inherited form of phosphate wasting [1, 2]. Increased circulating levels of FGF23 in XLH lead to chronic renal phosphate wasting and concomitant suppression of 1,25-dihydroxyvitamin D production [1]. The disease presents as rickets in children, accompanied by short stature and dental abscesses. Adults with XLH suffer insufficiency fractures impaired mobility, hearing loss, and tooth abscesses [3, 4].
Adherence to current therapy for XLH, which includes supplementation with calcitriol and phosphorus, is difficult [5]. A blocking antibody to FGF23, shows therapeutic promise [6, 7], but there are no drugs that correct the overproduction of FGF23 by osteocytes.
In a small clinical trial, a single subcutaneous dose of salmon calcitonin lowered circulating levels of FGF23 by an average of 23% in patients with XLH [8]. The current study was designed to determine whether nasal calcitonin would achieve the same effect and whether that effect could be sustained over 3 months.
Methods
Subjects
Subjects were recruited from the practices of two of the authors (KI, TC) and from a panel of XLH patients who have, or have inquired, about participation in clinical trials.
Inclusion/Exclusion
Inclusion criteria included a family history of XLH, clinical findings, and biochemical features consistent with XLH, age ≥ 18 years; fasting serum levels of calcium ≤ 10.5 mg/dL; PTH ≤ 1.7-fold above the upper limit of normal; and phosphorus ≥ 1.0 mg/dL. Exclusion criteria included a serum phosphorus < 1.0 mg/dL, a serum creatinine > 1.5 mg/dL, or an estimated creatinine clearance of < 60 cc/min; and a serum 25-hydroxyvitamin D < 20 ng/mL. Other exclusion criteria included pregnancy, breast feeding, treatment with a glucocorticoid, or any anti-osteoporotic or anti-seizure medication. Potential study subjects whose serum 25-hydroxyvitamin D was < 20 ng/mL, were supplemented with vitamin D3 to achieve a serum value ≥ 20 ng/mL and then re-screened if they otherwise met inclusion criteria.
Patients taking conventional therapy with calcitriol and phosphorus at screening stopped both agents at least 2 weeks prior to enrolling in the study.
Fifty-six individuals were screened by phone, of whom 23 agreed to participate. Two of these declined to enroll after reviewing the consent, leaving 21 individuals in the final analysis. Of these 21 individuals, two did not complete Visit 3, but were included in the analysis.
Supplemental Fig. 1 summarizes the recruitment flowchart. Table 1 summarizes the demographics of the 21 individuals who participated in this study. There were 16 women and 5 men. The mean age was 47. All but two of these individuals had been previously exposed to treatment with calcitriol and phosphorus. Eleven of these individuals were on treatment within the year prior to enrollment.
Table 1.
Demographics and baseline biochemistries in the study volunteers
| Treatment Arm | SI units | Placebo | SI units | |
|---|---|---|---|---|
| Age | 47 ± 5 | 47 ± 3 | ||
| Gender M/F (% F) | 1/9 (90%) | 4/7 (64%) | ||
| On treatment within the last year for XLH | 5 | 6 | ||
| Wt (kg) | 71.6 ± 5.3 | 70.4 ± 6.4 | ||
| BMI | 33 ± 3 | 33 ± 3 | ||
| PTH nLEq/mL, (pmol/L) | 42 ± 5 | (94 ± 12) | 47 ± 5 | (105 ± 12) |
| Serum calcium mg/dL (mmol/L) | 8.6 ± 0.1 | (2.2 ± 0.03) | 8.7 ± 0.1 | (2.2 ± 0.03) |
| Serum phosphorus mg/dL (mmol/L) | 1.8 ± 0.1 | (0.58 ± 0.03) | 1.7 ± 0.1 | (0.55 ± 0.03) |
| Serum creatinine mg/dL (μmol/L) | 0.8 ± 0.1 | (70.7 ± 8.8) | 0.8 ± 0.1 | (70.7 ± 8.8) |
| TmP/GFR (mg/100 mL GF) | 1.3 ± 0.1 | 1.2 ± 0.1 | ||
| 25 (OH) Vitamin D ng/mL (nmol/L) | 35 ± 4 | (87 ± 10) | 31 ± 2 | (77 ± 5) |
| FGF23 (pg/mL) | 97 ± 12 | 138 ± 24 | ||
| CTx (ng/mL) | 1.14 ± 0.75 | 0.99 ± 0.73 | ||
| P1NP ng/mL (nmol/L) | 89.6 ± 41.3 | (2.56 ± 1.18) | 78.3 ± 49.7 | (2.24 ± 1.42) |
Biochemical Measurements
Serum and urine creatinine and phosphorus, serum calcium, PTH, 1,25-dihydroxyvitamin D, and 25-hydroxyvitamin D were measured as previously reported [8–11]. TmP/GFR was calculated using a nomogram [12]. Serum intact FGF23 levels were measured by ELISA (Kainos Laboratories, Inc., Tokyo, Japan).
Study Protocol
This study was approved by the Institutional Review Board at Yale University School of Medicine and registered with http://www.clinicaltrials.gov (NCT01652573). This was a prospective, randomized double-blind placebo-controlled trial. Randomization was conducted by the Yale Investigational Pharmacy, using a random numbers generator. All studies were conducted on the Hospital Research Center at Yale New Haven Hospital, which is supported by the Yale CTSA Award. Study enrollment began in 2012 and ended in 2015.
The placebo and nasal salmon calcitonin were prepared by the Yale Investigational Pharmacy using identical vials that dispensed an identical volume of spray. The vehicle control was normal saline. Nasal calcitonin was purchased from Novartis® (East Hanover, NJ) and transferred to the vials. The nasal calcitonin contained 200 IU/activation. Two nasal sprays represented 400 IU of calcitonin.
Subjects received one activation of spray in each nostril each morning at approximately 8:00 a.m. except on Study Visit Days when it was given at 10:00 a.m. after blood and urine collections were complete. There were four Study Visits over 3 months (Supplemental Table 1). Visit 1 occurred over two consecutive inpatient days on the Hospital Research Unit. After baseline measurements (detailed in Supplemental Table 1) were collected between 8:00 and 10:00 a.m. on Day 1 of Visit 1, the patient was administered study drug at 10 am, and subsequent blood and urine studies were collected over the next 26 h. On Visit 2, spot fasting blood and urine measurements were made one month after enrollment, which were repeated two months after their enrollment, at Visit 3 (Supplemental Table 1). Visit 4 occurred at the end of month three, and was identical to Visit 1. TmP/GFR was calculated using mid-point measurements of blood creatinine and phosphorus during a of 2-hour urine collection for creatinine and phosphorus.
Compliance was assessed by weighing vials of study drug at the time they were dispensed and again upon their return. The difference in weight was compared to the expected amount of drug the study subject should have taken had he/she been 100% compliant.
Statistical Analyses
Baseline characteristics of the patients were generated and compared between treatment groups by using descriptive statistics. Repeated-measures analyses were performed to evaluate the group difference on outcomes, accounting for the correlation among repeated measurements made on the same individuals. Baseline measurements for each outcome, treatment group, time, and interaction between treatment and time, were entered into the model as fixed effects. Group differences at each time point and area under the curve (AUC) were generated by using linear contrast. All analyses were conducted using SAS System for Windows (Version 9.4). A p value less than 0.05 was considered as statistically significant. The primary outcome variable in this trial was the AUC for FGF23. Secondary outcome variables included AUC for TmP/GFR and 1,25-dihydroxyvitamin D. We reported that a single parenteral dose of calcitonin caused serum FGF23 levels to decline by 23% [8]. The SD for this value is 18%. Using these data as an estimate of effect size, n = 10 provides > 90% power with p = 0.05 (one-sided). One-sided is appropriate in this case because there was a priori evidence that calcitonin lowers FGF23 levels in XLH [8].
Results
Figure 1 summarizes the AUC for FGF23, TmP/GFR, and 1,25-dihydroxyvitamin D after adjusting for differences in baseline values during Visit 1 and Visit 4. These data summarize changes over 24 h following administration of the first and final daily dose of either calcitonin or placebo nasal spray. The Visit 1 data represent the changes induced in treatment-naïve individuals and the Visit 4 data represent the changes following three months of daily nasal calcitonin administration. One study subject had mean serum FGF23 value during Visit 1 of 1929 ± 236, which is 5–10 times higher the mean value in any other study subject, so the data from this study subject were excluded from the Visit 1 analyses.
Fig. 1.
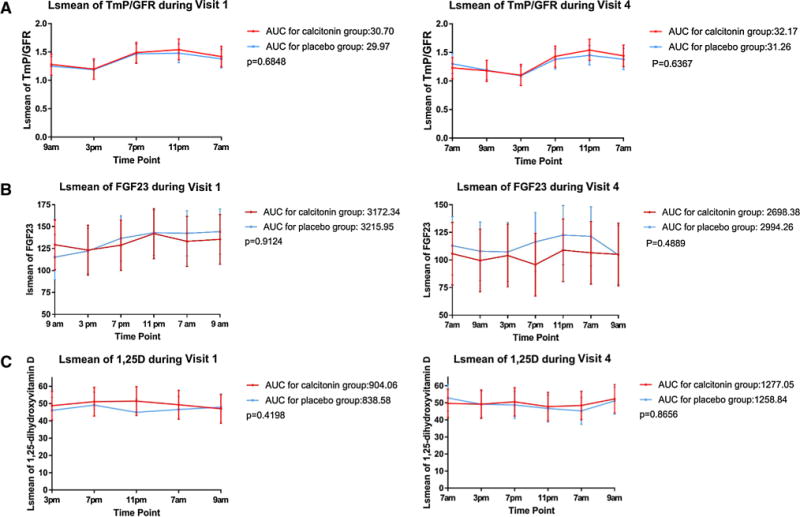
A Area under curve for TmP/GFR during Visit 1 (left panel) and Visit 4, for patients treated with nasal calcitonin (red line) or placebo nasal spray (blue line). B Area under the curve for FGF23 during Visit 1 (left panel) or Visit 4 (right panel), for patients treated with nasal calcitonin (red line) or placebo nasal spray (blue line). C Area under the curve for 1,25 dihydroxyvitamin D during Visit 1 (left panel) or Visit 4 (right panel) for patients treated with nasal calcitonin (red line) or placebo (blue line). In all panels, data are presented as area under the curve for Least-Squares Means. (Color figure online)
There was no significant difference in the AUC for FGF23 during each of these visits. Similarly, TmP/GFR and 1,25-dihydroxyvitamin D were not significantly influenced by the administration of nasal calcitonin, either in treatment-naïve individuals or in patients who had been exposed to drug for 3 months. Detailed time point data for each one of the outcomes are provided in Supplemental Table 2.
LSM data for FGF23, serum calcium, phosphorus, PTH, 1,25-dihydroxyvitamin D, and TmP/GFR, for each time point where measurements were made 24 h after the previous dose of study drug, were also analyzed. There were five such time points in the study all at 9:00 a.m.: Visit 1, Day 2; Visit 2; Visit 3; Visit 4, Day 1 and Visit 4, Day 2. (Supplemental Table 3). When compared to placebo-treated individuals, subjects receiving nasal calcitonin showed no significant difference in any of these six parameters over the five time points measured.
LSM data for serum CTx and P1NP are presented in Supplemental Table 4. These turnover markers were measured fasting at 9:00 a.m. once, at the beginning of each visit. There was no significant effect of treatment assignment, time or time by treatment assignment for either of these two parameters.
In the 18 individuals for whom compliance data were available, compliance was 96 ± 2%.
Discussion
We found no effect of nasal calcitonin on circulating levels of FGF23 or measures of phosphate metabolism in patients with X-linked hypophosphatemia. In contrast, we previously found that a single subcutaneous dose of 200 IU of salmon calcitonin did lower circulating levels of FGF 23 and caused a transient rise in serum phosphate in subjects with XLH. The reasons for this difference in outcome are unclear. The rapid rise in serum calcitonin seen with parenteral administration is not achieved when the drug is administered as a nasal preparation which may explain this difference [13]. Nonetheless, 400 IU of nasal preparation has been reported in some studies to result in a more prolonged increase in serum levels of the drug compared to parenteral administration, [14]. It is possible that there is a threshold serum concentration of drug required to suppress FGF23 levels, which could not be achieved with the dose of nasal calcitonin we used. However, a lower dose of nasal calcitonin (200 IU/d) was shown to reduce vertebral fracture risk [15]. While tachyphylaxis might explain a loss of efficacy over time, no effect was observed even with the first dose of the drug. Our sample size was relatively small, but since there was an effect with subcutaneous calcitonin, while there was no trend in our current study, it is unlikely that a larger sample size would have altered our results.
Although calcitonin may not be a viable therapy for XLH, the fact that this drug suppresses FGF23 production when given subcutaneously remains an important observation. Presumably, activation of the calcitonin receptor, which is known to be expressed by osteocytes [16], modulates FGF23 production in these cells. Understanding that metabolic pathway may provide important new therapeutic targets for this serious disease.
Supplementary Material
Acknowledgments
The intact FGF23 assay kits were a generous gift from Kyowa Hakko Kirin Co. Ltd. This work was supported by the Yale Bone Center. It was also supported by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s0022 3-017-0382-0) contains supplementary material, which is available to authorized users.
Author Contributions Study design: KI and AA. Data collection: KI, TC, AA, CS, RS, EO. Data analysis: YD, CC, RS, KI, AA, CS. Data interpretation: KI, RS, YD and CC. Drafting manuscript: RS, KI. Approving final version of manuscript: RS, KI, TC, AA, CS, YD, and CC. KI assumes responsibility for the integrity of the data analysis.
Compliance with Ethical Standards
Conflict of Interest Rebecca Sullivan, Alice Abraham, Christine Simpson, Elizabeth Olear, Thomas Carpenter, Yanhong Deng, Chuqing Chen, Karl L. Insogna declare that they have no conflicts of interest relevant to this study.
Human and Animal Rights and Informed Consent This study was approved by the Yale Human Research Protection Program. All study participants provided written informed consent before study entry.
References
- 1.Goldsweig BK, Carpenter TO. Hypophosphatemic rickets: lessons from disrupted FGF23 control of phosphorus homeostasis. Curr Osteoporos Rep. 2015;13:88–97. doi: 10.1007/s11914-015-0259-y. [DOI] [PubMed] [Google Scholar]
- 2.Francis F, Hennig S, Korn B, Reinhardt R, De Jong P, Poustka A, Lehrach H, Rowe PS, Goulding JN, Summerfield T, Mountford R. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 3.Connor J, Olear EA, Insogna KL, Katz L, Baker S, Kaur R, Simpson CA, Sterpka J, Dubrow R, Zhang JH, Carpenter TO. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab. 2015;100:3625–3632. doi: 10.1210/JC.2015-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, Olear E, Gundberg CM. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab. 2010;95:E352–E357. doi: 10.1210/jc.2010-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26:1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, Insogna KL, Peacock M. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Investig. 2014;124:1587–1597. doi: 10.1172/JCI72829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imel EA, Zhang X, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey JS, Glorieux FH, Portale AA, Insogna K, Peacock M, Carpenter TO. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab. 2015;100:2565–2573. doi: 10.1210/jc.2015-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu ES, Carpenter TO, Gundberg CM, Simpson CA, Insogna KL. Calcitonin administration in X-linked hypophosphatemia. N Engl J Med. 2011;364:1678–1680. doi: 10.1056/NEJMc1010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerstetter JE, Caseria DM, Mitnick ME, Ellison AF, Gay LF, Liskov TA, Carpenter TO, Insogna KL. Increased circulating concentrations of parathyroid hormone in healthy, young women consuming a protein-restricted diet. Am J Clin Nutr. 1997;66:1188–1196. doi: 10.1093/ajcn/66.5.1188. [DOI] [PubMed] [Google Scholar]
- 10.Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, Kleppinger A, Reynolds J, Dziura J, Kenny AM, Insogna KL. The effect of a whey protein supplement on bone mass in older caucasian adults. J Clin Endocrinol Metab. 2015;100:2214–2222. doi: 10.1210/jc.2014-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson C, Maria Cusano A, Bihuniak J, Walker J, Insogna K. Effect of 25(OH) vitamin D reference method procedure (RMP) alignment on clinical measurements obtained with the IDS-iSYS chemiluminescent-based automated analyzer. J Steroid Biochem Mol Biol. 2014;148:41–46. doi: 10.1016/j.jsbmb.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Bijvoet O. Kidney function in calcium and phosphate metabolism. In: Avioli LV, Krane SM, editors. Metabolic bone disease. Academic Press; New York: 1977. pp. 48–140. [Google Scholar]
- 13.Overgaard K, Agnusdei D, Hansen MA, Maioli E, Christiansen C, Gennari C. Dose-response bioactivity and bioavailability of salmon calcitonin in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 1991;72:344–349. doi: 10.1210/jcem-72-2-344. [DOI] [PubMed] [Google Scholar]
- 14.Torring O, Bucht E, Sjostedt U, Sjoberg HE. Salmon calcitonin treatment by nasal spray in primary hyperparathyroidism. Bone. 1991;12:311–316. doi: 10.1016/8756-3282(91)90016-c. [DOI] [PubMed] [Google Scholar]
- 15.Chesnut CH, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, LeBoff M, Maricic M, Miller P, Moniz C, Peacock M, Richardson P, Watts N, Baylink D. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. Am J Med. 2000;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 16.Gooi J, Pompolo S, Karsdal M, Kulkarni N, Kalajzic I, McAhren S, Han B, Onyia J, Ho P, Gillespie M, Walsh N, Chia L, Quinn J, Martin T, Sims N. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone. 2010;46:1486–1497. doi: 10.1016/j.bone.2010.02.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


