Abstract
BACKGROUND
brown adipose tissue (BAT) has a great relevance in metabolic diseases and has been shown to be reduced in obesity and insulin resistance patients. Currently, Dixon MRI is used to calculate fat-water fraction (FWF) and differentiate BAT from white adipose tissue (WAT). However, it may fail in areas of phase wrapping and introduce fat-water swapping artifacts.
PURPOSE
to investigate the capacity of the Z-Spectrum Imaging (ZSI) for the identification of BAT in vivo.
STUDY TYPE
retrospective study.
SPECIMENS
WAT, BAT and lean tissue from healthy mice. ANIMALS: Four C57BL/6 healthy mice. POPULATION: 5 healthy volunteers.
FIELD STRENGTH
9.4T, 3T for volunteers.
SEQUENCE
Z-Spectra data were fitted to a model with three Lorentzian peaks reflecting the direct saturation of tissue water (W) and methylene fat (F), and the magnetization transfer from the semi-solid tissues. The peak amplitudes of water and fat were used to map the FWF. The novel FWF metric was calibrated with an oil and water mixture phantom and validated in specimens, mice and human subjects.
ASSESSMEMT
FWF distribution was compared to published work and values compared to Dixon’s MRI results.
STATISTICAL ANALYSIS
Comparisons were performed by t-tests.
RESULTS
ZSI clearly differentiated WAT, BAT, and lean tissues by having FWF=1, 0.5 and 0 respectively. Calibration with oil mixture phantoms revealed a linear relationship between FWF and the actual fat fraction (R2=0.98). In vivo experiments in mice confirmed in vitro results by showing FWF=0.6 in BAT. FWF maps of human subjects showed the same FWF distribution as Dixon’s MRI (p>0.07). ZSI is independent from B0 field inhomogeneity and fat-water swapping since both lipid and water frequency offsets are determined simultaneously during Z-Spectral fitting.
DATA CONCLUSION
ZSI can derive artifact-free FWF maps, which can be used to identify BAT distribution in vivo non-invasively.
Keywords: Brown Adipose Tissue, Fat Fraction, Z-Spectrum, CEST MRI, Obesity
INTRODUCTION
Over the recent years the study of fat has attracted increasing interest of clinicians and researchers as it grew from being considered a simple energy storage tissue to a much more faceted organ secreting hormones involved in critical functions, from metabolism regulation to motivation and depression(1,2). In particular, the last ten years have seen the resurgence of the interest in brown adipose tissue (BAT). This peculiar kind of fat has a great significance in whole-body energy balance, given to its property of converting triglycerides and glucose into heat, through the non-shivering thermogenesis process(3–6). Moreover, BAT has a great biological relevance in metabolic diseases and has been shown to be reduced in obesity and insulin resistance subjects(7–10). Localization and quantification of BAT reservoirs is therefore key to study the disrupted metabolic homeostasis and investigate potential treatment strategies.
Currently, 18FDG-PET/CT is the standard method to detect BAT activation by measuring the increased glucose uptake due to BAT metabolism. However, it has been shown that 18F-FDG uptake can be fully maintained even when oxygen consumption and BAT thermogenesis are diminished, suggesting that increased BAT 18F-FDG uptake can occur independently of thermal function(11). Also, PET radiation exposure limits its application to longitudinal studies necessary for treatment assessment, and can only detect active BAT function, while it is unusable for measuring the resting-state BAT mass(12).
Conventional and novel MRI-based techniques have been used as well, in particular to target the structural difference in relative water and lipid content between brown and white adipose tissues (WAT). The latter in fact is characterized by large lipid droplets taking up most of the intracellular space, while BAT shares the space between smaller lipid droplets and other organelles immersed in the cytoplasm(12–14). The cell structural difference leads to a different balance of fat and water within the cells, providing a marker for MRI.
Dixon multiecho MRI has an established track record in the study of fat and can differentiate white and brown adipose reservoirs, providing a measure of the relative amount of fat contained in the tissue, i.e. fat-water fraction(14,15). However, despite the valuable contribution of reconstruction algorithm like IDEAL (Iterative Decomposition of water and fat with Echo Asymmetry and Least square estimation), the technique still has some pitfalls, like the ever-present sensitivity to phase wrapping and B0 field inhomogeneity artifacts(16–19). On the other hand, innovative and promising approaches under development, such as multiple quantum coherence(20) and hyperpolarized Xe gas imaging(21), face challenges in signal to noise ratio and the requirement of exogenous contrast agents and/or special instruments that are not readily available in most of the clinical scanners.
Z-Spectrum Imaging (ZSI), based on the acquisition of multiple images following a saturation pulse swept over a short range of frequencies(22,23), shows both water and fat dips due to the direct saturation at their resonance frequencies. With these signals, fat-water fraction may be quantified and used to differentiate BAT from WAT and lean tissues. This technique is intrinsically immune from phase heterogeneity given that information on water and fat resonance and ΔB0 can all be determined from the Z-Spectrum. In this study, we aimed to address the efficacy of ZSI in fat-water fraction quantification and therefore BAT detection.
METHODS
The procedure was first tested on ex vivo tissue samples and calibrated on phantoms with known mixture of oil and water. It was then applied in vivo on healthy mice and finally translated to human subjects. All animals and human studies were performed according to protocols approved by the Institutional Animal Care and Use Committee and Institutional Review Board. Signed informed consent was obtained by all volunteers.
Ex vivo studies
Specimens of fat and lean tissues were extracted from 3 healthy C57BL/6 mice (male, 7 weeks old). Lean muscle tissue from the flank, WAT from the visceral area and BAT from the interscapular depot were resected from freshly deceased animals and pressed into separate NMR tubes (5 mm in diameter, New Era Enterprises, Inc., Vineland, NJ, USA). For calibration purpose, a cylinder phantom (1cm diameter) was prepared containing water and peanut oil, chosen to mimic human triglycerides(24,25). In order to provide different fat / water ratios, the imaging slice (2.5 mm thick) was placed at an angle (56° was found to be optimal for this set up) crossing the oil-water interface such as to have a linear gradient of fat-water fraction in the image plane. The actual fat-water fraction was estimated geometrically from the sagittal T2 images as the fraction of oil volume covered within the slice depth at any position, resulting in partial volumes overlap of oil and water.
MRI was carried out at an Agilent Varian 9.4T preclinical scanner with a 39 mm proton volume coil. A CEST sequence was used to acquire Z-Spectra with a square saturation pulse of 3.5 μT for 1s and frequency offsets ranging from −5 to 5 ppm at intervals of 0.25 ppm, together with ±10, ±20, ±100 ppm offsets. The saturation pulse was followed by single-slice fast spin echo (FSE) readout with parameters: FOV = 40 x 40 mm2, matrix size = 128 x 128, echo train length (ETL) = 16, effective TE = 4.9 ms, T1 delay time, i.e. the time between successive repetitions = 3 s. The acquisition time for the entire Z-Spectrum was about 26 min. A WAter Saturation Shift Referencing (WASSR) scheme was adopted for correction of B0 inhomogeneity as previously reported(26). The sequence had a low-power pulse of 0.4 μT for 200 ms and frequency range from −1 to +1 ppm and same readout parameters as the Z-Spectrum Imaging.
In vivo mouse study
Four C57BL/6 male mice, 7 weeks old, were used in this study. As a general procedure for all the mice, anesthesia was induced by 2 % isoflurane, later maintained at 1–1.5 % in 100 % O2 via a nose cone with spontaneous respiration throughout the experiment. A rectal probe was used to monitor the body temperature, which was maintained at 33±0.5 °C via regulating the warm air flow into the scanner bore. Temperature and respiration rate were monitored using an MRI- compatible physiological monitoring system (Model 1025, SA Instruments Inc., Stony Brook, NY, USA). The interscapular region containing the largest known BAT depot in mice was placed in the center of the RF coil. First and second order shimming was performed in this area prior to the acquisition. T2-weighted images of the region were obtained for anatomical reference. Single slice Z-Spectrum was acquired through the center regions of interscapular BAT depot using the same protocol as in the ex vivo studies.
In vivo human study
The ZSI concept was then tested on healthy human subjects (n=5, male, age 30–40, BMI 21–27) at a clinical 3T MRI scanner (GE750, GE Healthcare, Waukesha, WI) with a 32 channels cardiac coil. The saturation pulse consisted of a train of 10 Hanning windowed saturation pulses 98 ms long with a 2 ms inter-pulse delay, resulting in a excitation of 3.5 μT for 1 s. Saturation was followed by a single shot FLASH readout with centric phase encoding order with parameters: slice thickness = 10 mm, flip angle = 10°, shot TR = 6 s, TE = 3.2 ms, field of view = 48 × 48 cm2, matrix size = 128 × 128, and in-plane resolution = 3.75×3.75 mm2. CEST images were collected at 16 frequencies, specifically from −4.75 to 4.75 ppm with 0.64 ppm intervals and a 100 ppm image for referencing. The acquisition time for collecting the partial Z-Spectrum was about 3.5 min. In addition, a Dixon 6-points sequence with IDEAL reconstruction was acquired in the same session to quantify the fat fraction distribution. The sequence is the GE commercial version (IDEAL IQ), with a model including 6 fat peaks and 6 echoes: TE1 = 1.3 ms, ΔTE=2.0 ms, TR = 7.3s, matrix size = 256x256(27,28).
Image processing
To separately quantify the direct saturation of fat and water and the semi-solid MT effect, Z-Spectral data were first normalized to the reference signal at ±100 ppm and then fitted to a model that consists of three Lorentzian functions representing the three different compartments (Fig. 1). The amplitudes of the water (W) and methylene-fat (F) peaks were used to compute the fat-water fraction as:
Fig. 1.
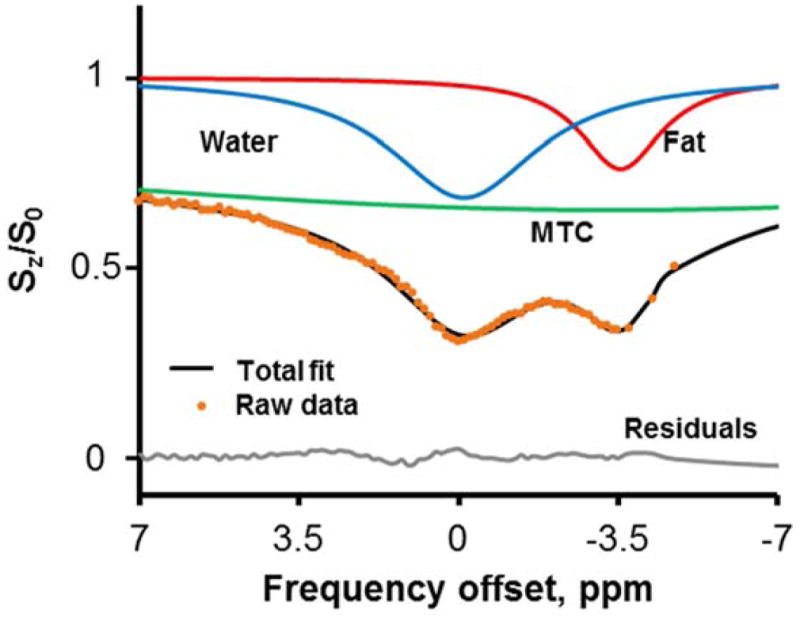
Z-Spectrum Imaging data are fitted to a multi-Lorentzian model including the peaks corresponding to water (cyan), fat (red) and MTC (green) pools.
Total fit performance is good (R2>0.98) with residuals < 2%.
The pixelwise FWF value was used to produce color-coded FWF maps and overlapped onto T2-weighted images for anatomical reference. To produce quantitative maps, a calibration was performed assuming FWF=0.9 in areas of pure fat and FWF=0 in lean tissue.
Statistical analysis
The FWF maps were evaluated based on a priori knowledge of BAT depots distribution and values were presented as mean ± standard deviation and compared to results from previous published works.
Fit performance was assessed by both the correlation coefficient and the residuals between the fit and the raw data. Average values from regions of interest in subcutaneous white and interscapular or supraclavicular brown fat were presented. Differences between tissue types both in vitro and in vivo were assessed by Student’s t-test and significance level was set to 0.05. Also, comparison to Dixon IDEAL fat water fraction was evaluated by t-tests in the human subjects.
RESULTS
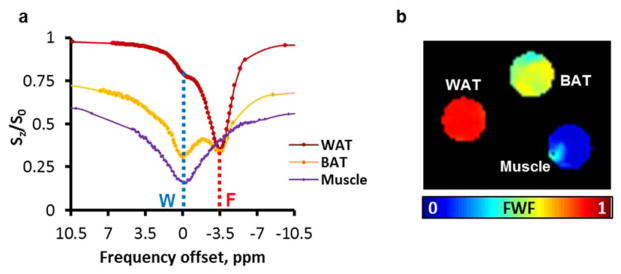
The Lorentzian fitting of a representative Z-Spectrum from ex vivo BAT tissue is shown in Fig. 1 with three peaks corresponding to fat, water and MTC. Z-Spectra were well fitted with an average R2>0.981 and residues < 2%. Fig. 2a shows representative Z-Spectra from BAT, WAT, and lean tissue (muscle). Z-Spectra from the extracted tissues were characterized by two major peaks corresponding to the water and lipid direct saturation. Samples containing white fat showed predominantly the peak originating from the methylene protons at −3.5 ppm from the water resonance (set at 0 ppm), while lean tissue samples showed only the water peak. Interscapular BAT samples showed apparently larger water dip than WAT. Maps of fat-water fraction were obtained for the three samples, clearly showing the difference in relative fat content (Fig. 2b).
Fig. 2.
Z-Spectra from extracted mouse tissues. Data are centered to water frequency. WAT and Muscle are characterized by either the fat or the water peak, while BAT shows a mixed contribution of both (a). Fat-water fraction can be derived from the fitted amplitudes in every pixel to produce colored maps (b). W: Water; F: Methylene-Fat.
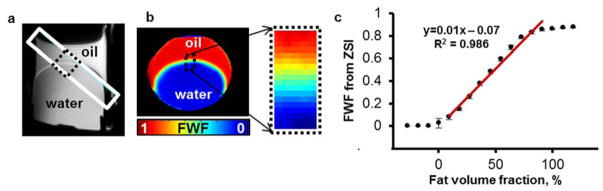
In the calibration phantom, the oblique placement of the imaging plane resulted in a linear gradient of oil and water composition as shown on the T2-weighted images (Fig. 3a). As expected, FWF map derived from the Z-Spectral fitting showed the gradient of oil to water ratio across the oil-water interface (Fig. 3b, c). The measured FWF values from the pixels crossing the oil-water interface were found to be linearly correlated to the actual fat water fraction (R2=0.986).
Fig. 3.
FWF calibration in phantom containing a mix of peanut oil and water. (a) T2-weighted image displaying the oblique slice placement. The interface area shows a gradual mix of fat and water within the slice. (b) FWF map of the slice shows a linear gradient across the interface (dotted black box). (c) FWF scales linearly with the estimated fat volume fraction in the interface area (R2 = 0.986).
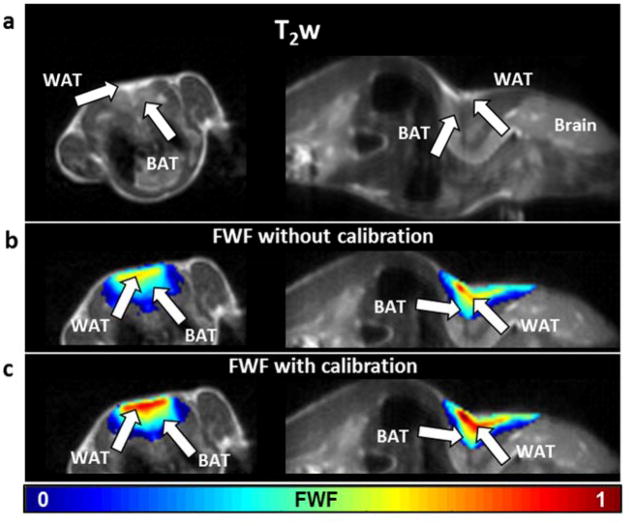
In vivo data from healthy young mice confirmed the in vitro results. From the sagittal and axial T2-weighted images covering the central region of the fat depot, we can identify the triangular shaped subcutaneous WAT and the two underlying lobes of BAT, the largest known BAT depots in the interscapular region. The FWF maps computed from this area showed that BAT has lower levels of fat-water fraction than WAT. The fraction values were found comparable to what previously reported(14,29,30), ranging between 0.4 and 0.6 (0.54 ± 0.08) in the BAT lobes (Fig. 4b). The subcutaneous WAT identified on both T2-weighted and CEST images, instead, showed FWF values ranging from 0.7 to 0.9 (0.74 ± 0.06). Areas with predominantly lean tissues showed very low FWF (< 0.3). After calibration, values were corrected to 0.62± 0.10 and 0.82 ± 0.04 for BAT and WAT respectively (Fig. 4c). As expected, t-tests revealed that FWF in BAT was significantly different (p<0.004) from WAT in all animals and in the specimens sets.
Fig. 4.
T2-weighted images from healthy male mice, showing location of the interscapular BAT depot with respect to the WAT layer, in axial (left) and sagittal (right) orientation (a). Fat-water fraction maps superimposed to the same anatomical references before (b) and after (c) calibration.
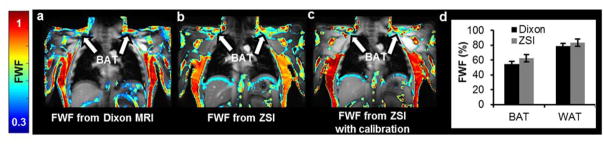
The human study further demonstrated the capability of ZSI to characterize BAT. FWF map of the supraclavicular area, site of active BAT depots in adult humans, showed a heterogeneous FWF distribution compared to the homogeneous subcutaneous fat in the torso, with FWF values of 0.56 ± 0.09, compared to 0.72 ± 0.04 of WAT. In addition, we found a high agreement in FWF spatial distributions between values derived from Z-Spectrum data and those produced by the Dixon’s method (Fig. 5a,b). The agreement is further improved by the calibration performed using WAT from the torso and muscles as reference points (Fig. 5c) resulting in no significant difference between the values evaluated in the ROIs (p>0.07). Fat-water fractions were 0.63 ± 0.07 for BAT and 0.83 ± 0.05 for WAT as evaluated by ZSI, while 0.55 ± 0.04 and 0.79 ± 0.03 as given by the Dixon MRI (Fig. 5d). As expected, Dixon’s method occasionally rendered fat-water switch artifacts caused by phase wrapping during the IDEAL reconstruction. The artifact can be visualized in the whole neck region (Fig. 6a, arrow), where a high fat fraction value is assigned to areas containing lean tissue. No such errors were observed in the colormap derived from the ZSI method (Fig. 6b).
Fig. 5.
The protocol was tested at a clinical 3T scanner on healthy volunteers. FWF maps derived from Dixon MRI (a) and ZSI (b) show similar patterns. Similarity is further improved after calibration (c). Supraclavicular BAT depots (a–c, arrows) have lower fat content than the subcutaneous WAT. (d) No significant difference is assessed between the results from the two techniques in both brown and subcutaneous white fat.
Fig. 6.
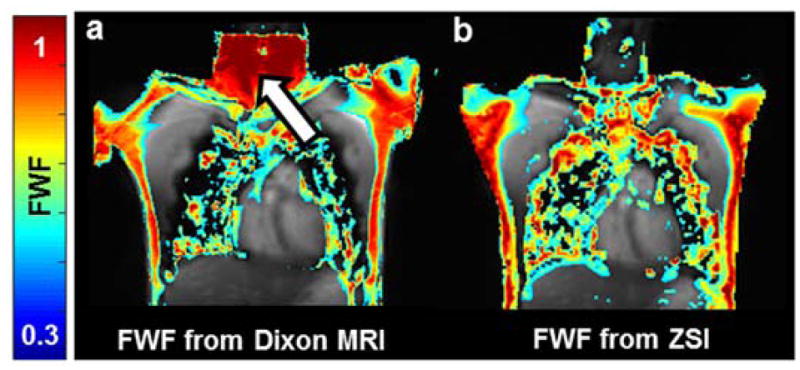
(a) Dixon MRI is sensitive to fat-water swapping artifacts and can produce erroneous values in sensitive areas like the neck (arrow). (b) No errors are detected in the colormaps derived from ZSI.
DISCUSSION
We demonstrated in this study with in vitro and in vivo experiments that Z-Spectral imaging can measure the fat-water fraction in tissues based on the direct saturation of both water and lipid signals, providing a tool to quantify brown adipose tissue mass.
Multi-point Dixon sequences with reconstruction algorithms like GE’s IDEAL have been often used to measure fat and water signals for BAT detection and differentiation from white fat. However, up to 10% of routine scans are affected by fat-water swapping artifacts, due to algorithm’s convergence to local minima in areas where phase is wrapped(16,17,31,32). The novel ZSI method is inherently insensitive to phase wrapping and field inhomogeneity issues. Z-Spectrum Imaging can provide the signal of each component alone through fitting, with no prior knowledge about the chemical shifts of either pool, producing B0-insensitive fat-water fraction maps. For the same reason, ZSI is also independent from chemical shifts induced by environmental factors, like local susceptibility and temperature variations. Conventional multiecho sequences instead, rely on the assumption that the chemical shift between water and fat is constant and do not take into account such confounding factors(16,18).
It is also worthwhile to note that chemical shift encoded techniques (such as the Dixon’s method) can suffer from T2* biases(29). In fact, the different signal losses between the in- and out-of-phase echoes due to varying TEs and hence T2* decays of water and fat can erroneously be attributed to phase cancellation and lead to an inaccurate quantification of FWF. Currently this effect has been reported only in cases of very short T2*, like in fatty livers with excessive iron(29), and the T2* impact on high vascularized BAT tissues remains to be explored. In any case, ZSI is carried out at the same TE for each frequency offset and is immune to T2* decay issues.
The calibration of the ZSI technique with oil and water phantoms revealed a linear relationship between the computed and actual FWF, allowing for a straightforward interpretation of quantitative results.
In the images, the transition area between the fluids appears curvilinear. This is likely caused by the fact that the oblique orientation of the imaging plane cuts through the interface in a cylindrical probe, but it is also due to the curvature induced by the different superficial tension in the two fluids at the interface. Nevertheless, the area where the transition is linear is clearly visible and extends for more than 10 pixels along the whole curve profile.
The same phantom design we used in the calibration was previously adopted by other influential studies(33). Such design provides a continuous range of fat water fraction from 0 to 100%, artificially created from the partial volume effect of oil present in an imaging slice obliquely crossing the oil-water interface. Given its starkly hydrophobic nature, oil doesn’t mix with water naturally and it’s challenging to obtain homogeneous mixtures with controlled ratios of the two species. Other groups addressed this limitation by using emulsifiers to bind oil molecules to water, and agar or other gelling agents to trap the emulsion in a stable phase(24,34). Although this approach can deliver satisfactory compounds at low fat fractions, it fails almost completely to produce stable mixture at increasing fat content (from FWF>50%)(24,35). Moreover, the mixing procedure during the gel cooling phase always entails the risk of creating air bubbles and inhomogeneous density distributions. Aware of this practical limitation, we opted for this imaging method relying on partial volume effect.
It must be noted, though, that the imaging device we produced is simply a simulation of the partial volume effect, not exactly a replica of the BAT structure at the cellular scale, which is characterized by a mixed composition of water and fat droplets within the cell. Nevertheless, this method allows us to validate and calibrate our technology for the quantification of FWF. Also, the calibration performed in vitro doesn’t translate directly to the in vivo quantification given that the calibrated slope may change with tissue relaxation times and imaging parameters. Calibrations can nevertheless be easily established for any in vivo applications with only two required reference data points, for example by setting the FWF of subcutaneous WAT to 0.9 and muscle to 0, according to published results(35,36).
Similarly to the present study, other works used MR spectroscopy or spectroscopic imaging to measure FWF difference between BAT and WAT(35,37). Although the basic principles are similar, our approach has a number of advantages by providing higher spatial resolution and taking shorter acquisition time. While shorter than MRS methods, the acquisition time in ZSI is still the limiting factor when compared to Dixon MRI. However, fast imaging technologies are being increasingly applied to CEST techniques(38,39), and we foresee that reasonable scanning time will bridge 3D ZSI to wide clinical applications in the near future.
Another potential limitation is the use of a single peak to model the lipid compartment, which has been described by up to 9 small resonant groups(18,32,39). Even though the fitting of the methylene peak alone provided a reliable FWF quantification in this study, the inclusion of the smaller contributions in a Z-Spectrum acquired with a lower and shorter saturation pulse may reveal intriguing results about tissue lipid composition.
Other players can also contribute to the Z-Spectrum, like CEST effects from exchanging protons and Nuclear Overhauser Enhancement (NOE) effects from dipole-dipole interactions between semi-solid components and water. However, such contributions are expected to be small compared to the dominant direct saturation on water and fat signals. In fact, the current fitting is accurate enough to render negligible errors (<2%) across the entire Z-spectrum. Further investigation of these smaller components may be used to study the metabolism of fat tissues, which will be our future work.
The spatial resolution used in this study is not high and can be improved by increasing the matrix size during the signal short acquisition, without notably increasing the acquisition time. However, due to the sparse nature of BAT distribution, high resolution imaging is definitely beneficial while not critically important for the quantification of fat water fraction.
Finally, we decided not to study the correlation with a widespread technique like 18FDG-PET. In fact, we already mentioned how PET may have biases in the detection of active BAT and is virtually insensitive to inactive BAT(11,12). In addition, we feel that the comparison of FWF data with fat fractions derived geometrically (in phantoms) or from Dixon MRI (in vivo) sufficiently validates the ZSI strategy. Nevertheless, we plan to further characterize the technique by including the aforementioned correlations and by increasing the sample size in future studies.
In conclusion, Z-Spectrum Imaging has been demonstrated in this study to be a potentially valuable tool in the measurement of brown fat mass in vivo in preclinical and clinical settings. Unlike other chemical shift based imaging methods, this novel technique is insensitive to imaging phase issues and field inhomogeneity. ZSI for the quantification of BAT may therefore serve as an important novel tool for the diagnosis and treatment of metabolic diseases.
Acknowledgments
Grant Support
This research is supported by NIH under grant # R21 EB023516 (Cai).
We are grateful for the research supports provided by the Department of Radiology and the Center for MR Research at the University of Illinois at Chicago.
References
- 1.Ruegsegger GN, Booth FW. Running from disease: Molecular mechanisms associating dopamine and leptin signaling in the brain with physical inactivity, obesity, and type 2 diabetes. Front Endocrinol (Lausanne) 2017;8(MAY):1–8. doi: 10.3389/fendo.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajan T, Menon V. Psychiatric disorders and obesity: A review of association studies. J Postgrad Med. 2017;63(3):182. doi: 10.4103/jpgm.JPGM_712_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrero L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 2016;5(2):98–118. doi: 10.1080/21623945.2015.1122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Pan R, Pfeifer A. Fat tissues, the brite and the dark sides. Pflügers Arch - Eur J Physiol. 2016:1803–7. doi: 10.1007/s00424-016-1884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls DG. The hunt for the molecular mechanism of brown fat thermogenesis. Biochimie. 2016:1–10. doi: 10.1016/j.biochi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown Fat in Humans: Consensus Points and Experimental Guidelines. Cell Metab. 2015;20(3):408–15. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beijer E, Schoenmakers J, Vijgen G, Kessels F, Dingemans A, Schrauwen P, et al. A role of active brown adipose tissue in cancer cachexia? Oncol Rev. 2012;6:88–94. doi: 10.4081/oncol.2012.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trayhurn P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2016 doi: 10.1016/j.biochi.2016.09.007. [DOI] [PubMed]
- 9.Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, et al. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016;3(1):19. doi: 10.1186/s40658-016-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristina T, Bargut L, Aguila MB, Mandarim-de-lacerda CA. Tissue and Cell Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell. 2016;48(5):452–60. doi: 10.1016/j.tice.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Abreu-vieira G, Hagberg CE, Spalding KL, Cannon B, Nedergaard J. Adrenergically stimulated blood flow in brown adipose tissue is not dependent on thermogenesis. Am J Physiol Endocrinol Metab. 2015:822–9. doi: 10.1152/ajpendo.00494.2014. [DOI] [PubMed] [Google Scholar]
- 12.Gifford A, Towse TF, Walker RC, Avison MJ, Welch EB, Gifford A, et al. Characterizing active and inactive brown adipose tissue in adult humans using PET-CT and MR imaging. Am J Physiol Endocrinol Metab. 2016;i(2):95–104. doi: 10.1152/ajpendo.00482.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Pan R, Pfeifer A. Fat tissues, the brite and the dark sides. Pflügers Arch - Eur J Physiol. 2016:1803–7. doi: 10.1007/s00424-016-1884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu HH, Jr, DLS, Nayak KS, Goran MI, Nagy TR. Identification of Brown Adipose Tissue in Mice with Fat-Water IDEAL-MRI. J Magn Reson Imaging. 2010;31(5):1195–202. doi: 10.1002/jmri.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton G, Jr, DLS, Bydder M, Nayak KS, Hu HH. Magnetic resonance properties of brown and white adipose tissues. J Magn Reson Imaging. 2012;34(2):468–73. doi: 10.1002/jmri.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bley TA, Wieben O, Francois CJ, Brittain JH, Reeder SB. Fat and water magnetic resonance imaging. J Magn Reson Imaging. 2010;31(1):4–18. doi: 10.1002/jmri.21895. [DOI] [PubMed] [Google Scholar]
- 17.Ladefoged CN, Hansen AE, Keller SH, Holm S, Law I, Beyer T, et al. Impact of incorrect tissue classification in Dixon-based MR-AC: fat-water tissue inversion. EJNMMI Phys. 2014;1(1):101. doi: 10.1186/s40658-014-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Shimakawa A, Hines CDG, Mckenzie CA, Hamilton G, Sirlin CB, et al. Combination of Complex-Based and Magnitude-Based Multiecho Water-Fat Separation for Accurate Quantification of Fat-Fraction. MRM. 2012;66(1):199–206. doi: 10.1002/mrm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glover GH. Multipoint Dixon Technique for Water and Fat Proton and Susceptibility Imaging. JMRI. 1991;1:521–30. doi: 10.1002/jmri.1880010504. [DOI] [PubMed] [Google Scholar]
- 20.Branca RT, Zhang L, Warren WS, Auerbach E, Khanna A, Degan S, et al. In Vivo Noninvasive Detection of Brown Adipose Tissue through Intermolecular Zero-Quantum MRI. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branca RT, He T, Zhang L, Floyd CS, Freeman M, White C, et al. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proc Natl Acad Sci. 2014;111(50):18001–6. doi: 10.1073/pnas.1403697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grad J, Bryant RG. Nuclear magnetic cross-relaxation spectroscopy. J Magn Reson. 1990;90(1):1–8. doi: 10.1002/mrm.1910170216. [DOI] [PubMed] [Google Scholar]
- 23.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 24.Holmes JH, Johnson KM, Hernando D, Reeder SB, Samsonov A. Magnetization transfer ratio ( MTR ) imaging in the presence of fat. Intl Soc Mag Reson Med. 2015:100651. [Google Scholar]
- 25.Lunati E, Farace P, Nicolato E, Righetti C, Marzola P, Sbarbati A, et al. Polyunsaturated Fatty Acids Mapping by 1 H MR-Chemical Shift Imaging. Magn Reson Med. 2001;883:879–83. doi: 10.1002/mrm.1272. [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Gillen J, Landman BA, Zhou J, Peter CM. WAter Saturation Shift Referencing (WASSR) for chemical Exchange Saturation Transfer Experiments. 2010;61(6):1441–50. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilburn DML, Cooper AS, Murphy P, Sinclair CDJ, Semple SI, Janiczek RL. Initial experience using Magnetization Transfer with Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation ( MT-IDEAL ) in the abdomen. Intl Soc Mag Reson Med. 2015:1744. [Google Scholar]
- 28.Healthcare GE. Tech data. 2009. Discovery TM MR750, a 3.0T system. [Google Scholar]
- 29.Hu HH, Hines CDG, Smith DL, Jr, Reeder SB. Variations in T2* and Fat Content of Murine Brown and White Adipose Tissues by Chemical-Shift MRI. MRM. 2013;30(3):323–9. doi: 10.1016/j.mri.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakash KNB, Srour H, Velan SS, Hsiang-Kai C. A method for the automatic segmentation of brown adipose tissue. Magn Reson Mater Physics, Biol Med. 2016;29(2):287–99. doi: 10.1007/s10334-015-0517-0. [DOI] [PubMed] [Google Scholar]
- 31.Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, et al. Multicoil Dixon Chemical Species Separation With an Iterative Least-Squares Estimation Method. Magn Reson Med. 2004 Jul;45:35–45. doi: 10.1002/mrm.10675. 2003. [DOI] [PubMed] [Google Scholar]
- 32.Ma J. Dixon Techniques for Water and Fat Imaging. JMRI. 2008;558:543–58. doi: 10.1002/jmri.21492. [DOI] [PubMed] [Google Scholar]
- 33.Fuller S, Reeder S, Shimakawa A, Yu H, Johnson J, Beaulieu C, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fat spin-echo imaging of the ankle: Initial clinical experience. Am J Roentgenol. 2006;187(6):1442–7. doi: 10.2214/AJR.05.0930. [DOI] [PubMed] [Google Scholar]
- 34.Qiang G, Kong HW, Xu S, Pham HA, Parlee SD, Burr AA, et al. Lipodystrophy and severe metabolic dysfunction in mice with adipose tissue-specific insulin receptor ablation. Mol Metab. 2016;5(7):480–90. doi: 10.1016/j.molmet.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng X, Ju S, Fang F, Wang Y, Fang K, Cui X, et al. Comparison of brown and white adipose tissue fat fractions in ob, seipin, and Fsp27 gene knockout mice by chemical shift-selective imaging and 1 H-MR spectroscopy. Am J Physiol Endocrinol Metab. 2013;(30):160–7. doi: 10.1152/ajpendo.00401.2012. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Kuang H, Chen C, Yan J, Do-umehara HC, Dada L, et al. Highly-accelerated CEST Measurements in Three Dimensions with Linear Algebraic Modeling. Proc Intl Soc Mag Reson Med. 2015;16(5):458–66. [PMC free article] [PubMed] [Google Scholar]
- 37.Boesch C, Machann J, Vermathen P, Schick F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006;(2):968–88. doi: 10.1002/nbm.1096. [DOI] [PubMed] [Google Scholar]
- 38.Dixon WT, Hancu I, Ratnakar SJ, Sherry AD, Robert E, Alsop DC. A Multislice Gradient Echo Pulse Sequence for CEST Imaging. Magn Reson Med. 2010;63(1):253–6. doi: 10.1002/mrm.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hines CDG, Yu H, Shimakawa A, McKenzie CA, Brittain JH, Reeder SB. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: Validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30(5):1215–22. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]