Abstract
Hepatoblastoma (HB) is the most common malignant liver tumor in children. Although survival of patients has improved significantly over the last 2 decades, a significant number of patients do not respond to standard chemotherapy. We conducted a pilot study to understand if there was immunophenotypic difference between tumors that respond well to chemotherapy versus that do not. We selected 10 cases of HB from children presenting at our hospital. All patients had initial tissue diagnosis, underwent chemotherapy followed by surgical resection. The cases were divided into 2 groups: aggressive group with 5 cases (all of which had a poor response to chemotherapy); and a good clinical outcome group with 5 cases (all of which responded well to chemotherapy). We excluded the small cell variant of HB from the study because its poor clinical outcome is well known. To be placed in the aggressive group we used the following criteria: < 70% necrosis following chemotherapy or recurrence/distant metastasis following chemotherapy. From tissue obtained before chemotherapy, 1 representative block of formalin-fixed, paraffin-embedded tissue was selected for immunohistochemistry. Following review of published literature, antibodies were selected to detect Survivin, PLK-1, Cytokeratin19 (CK19), N-Myc, Yap, Notch2, Hes1, Hes5, and C-Myc. Our results show that Survivin, CK19, and Yap have a diffuse (> 75%) positive staining of tumor cells in the aggressive tumors compared with good outcome tumors. However, staining for Yap was weak. Interestingly, there was loss of nuclear expression of C-Myc in majority of tumor cells in aggressive tumors, whereas nuclear staining was retained in most tumor cells of good responders. The N-Myc and PLK-1 immunostains did not reveal any significant differences in the 2 groups of HB. The immunostains for Notch2, Hes1, and Hes5 showed weak to moderately strong staining in tumor cells, but there was no obvious difference in the 2 groups. Our pilot study suggests that in nonsmall cell HB, diffusely increased expression of Survivin and CK19, and loss of nuclear expression of C-Myc marks the tumors as having an aggressive course.
Keywords: hepatoblastoma, immunohistochemistry, C-Myc, Survivin, CK19, chemotherapy, aggressive
Hepatoblastoma (HB) is a primary malignant tumor of liver occurring in children. Clinically, majority of the HB present with a liver mass and high serum level of α-fetoprotein. A high pretreatment extent of tumor as determined by imaging studies and either very low levels or very high levels of α-fetoprotein may predict a high-risk tumor. Histologically, HB can present with several patterns and is accordingly classified into several histologic subtypes.1 The pure fetal (PF) subtype has the best prognostic outcome and the small cell variant of HB has the worst prognostic outcome. The other histologic subtypes, which comprise the majority of the HB, do not dictate the prognostic outcome by virtue of their histologic subtype alone. In HB tumors, β-catenin/Wnt and Notch cell-signaling pathways have been noted to be active.2 The differential expression of genes of these cell-signaling pathways may underlie the differences in tumor behavior. Indeed, several gene expression profiling studies have suggested a role for individual genes in aggressive tumor behavior or poor survival.3–5
On the basis of the published literature cited above, we identified a set of genes that have been associated with either aggressive course or poor outcome. Then, we set out to see if immunohistochemical expression of these genes segregates with aggressive group or good clinical outcome group. In this brief report, we describe the results of our pilot study which is unique in using tumor necrosis following chemotherapy as a measure of poor response in HB and correlating with immunohistochemical expression of the selected genes.
MATERIALS AND METHODS
Study Design
We undertook a retrospective study with a convenience sample of 10 subjects who were diagnosed with HB at our hospital. All patients had initial tissue diagnosis, underwent chemotherapy followed by surgical resection. The cases were divided into 2 groups: aggressive and good clinical outcome. All 5 cases in aggressive group had fetal-embryonal epithelial histology. The good clinical outcome group had 5 cases, comprising 3 with fetal-embryonal epithelial, 1 with well-differentiated, low-mitotic activity, PF histology, and 1 with mixed-mesenchymal epithelial histology. From tissue obtained before chemotherapy, 1 representative block of formalin-fixed, paraffin-embedded tissue was selected for immunohistochemistry (IHC). Following review of published literature, 9 antibodies were selected to detect Survivin, PLK-1, Cytokeratin19 (CK19), N-Myc, Yap, Notch2, Hes1, Hes5, and c-Myc. IHC was performed manually (Survivin, CK19, Yap, Notch2, Hes1, and Hes5) or automated (N-Myc, C-Myc, and PLK-1) following antigen retrieval in citrate buffer at pH 6.0. The various antibodies used are detailed in Table 1.
TABLE 1.
Primary Antibodies Showing Clonality, Dilution, and Supplier
| Antibodies | Dilution | Manufacturer/Supplier |
|---|---|---|
| Survivin monoclonal | 1:400 | Cell signaling |
| PLK-1 monoclonal | 1:50 | Cell signaling |
| CK19 polyclonal | 1:100 | Proteintech |
| N-Myc polyclonal | 1:200 | Proteintech |
| Yap polyclonal | 1:100 | Cell signaling |
| Notch2 polyclonal | 1:500 | Abcam |
| Hes1 monoclonal | 1:100 | Origene |
| Hes5 monoclonal | 1:50 | Abcam |
| C-Myc monoclonal | 1:100 | Santa Cruz |
Criteria for Aggressive Group Cases
The tumors following chemotherapy had < 70% necrosis, either showed recurrence following chemotherapy or distant metastasis.
Scoring
If > 5% of tumor cells were positive, then the case was designated positive. Positive cases were designated as (1) diffusely positive if > 50% tumor cells were positive for the immunostain and (2) focally positive, when positive cells were present in clusters and accounted for < 50% tumor cells.
RESULTS
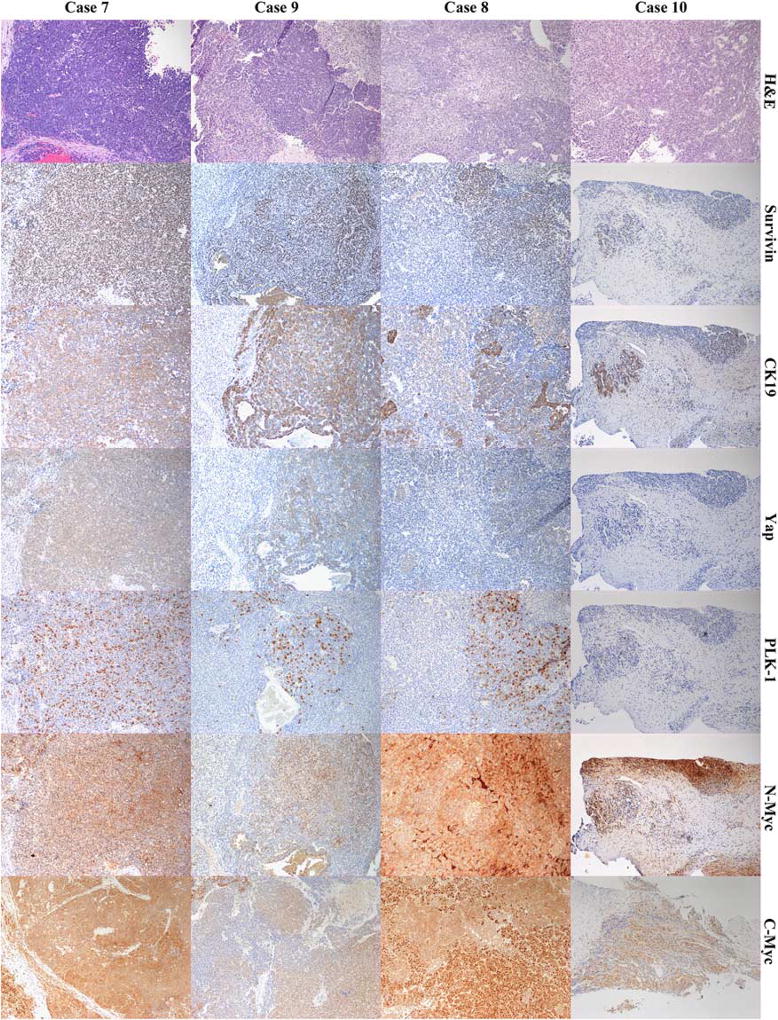
In our pilot study of 10 HB tumor samples, all of the tumors excepting the PF, had a heterogenous appearance with areas of well-differentiated fetal HB tumor cells, foci of crowded fetal HB, foci of embryonal HB, foci of macrotrabecular areas, and pleomorphic fetal HB. The clinical data of all the patients is shown in Table 2. IHC for Survivin was significant for its sharp nuclear staining, and the pattern as focal or clustered in good outcome cases and diffuse in aggressive tumors. The CK19 immunostain was very strongly positive in bile ducts in the nontumor tissue; but moderately strong in some of the tumor cells. In particular, the aggressive tumors had a more diffuse expression of CK19, whereas good outcome cases had focal presence of CK19. Immunostaining of Yap was in general weak, but showed cytoplasmic positivity and focal nuclear positivity in embryonal tumor cells. The PLK-1 immunostaining was strong, cytoplasmic, and seemed to be more frequently present in embryonal cells. However, foci of increased labeling were present in both good outcome and the aggressive groups. The N-Myc immunostain had significant background staining and seemed to be mostly cytoplasmic with nuclear staining noted in foci of embryonal tumor cells. Remarkably, the PF HB tumor was negative for Survivin, PLK-1, and CK19. The C-Myc immunostain had a strong and sharp nuclear staining and weak cytoplasmic staining in the PF HB similar to normal hepatocytes. Significantly, this pattern of nuclear and cytoplasmic staining of C-Myc was remarkably retained in the foci of fetal HB cells in all samples and was more diffuse and widespread in good outcome cases. In contradistinction, the aggressive group tumors showed fewer foci of HB cells with nuclear C-Myc but numerous tumor cells in nests and groups showing loss of nuclear staining of C-Myc and stronger cytoplasmic staining of C-Myc. Interestingly, biliary epithelial cells lacked nuclear staining of C-Myc and had weak cytoplasmic expression. Representative images of all of the above-mentioned immunostains are shown in Figure 1. The immunostains for Notch2, Hes1, and Hes5 showed weak to moderately strong staining in a focal distribution, but there was no obvious difference in the 2 groups.
TABLE 2.
Clinical Characteristics of Patients in the 2 Groups
| Aggressive Group | Good Clinical Outcome Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Case 1 | Case 3 | Case 5 | Case 7 | Case 9 | Case 2 | Case 4 | Case 6 | Case 8 | Case 10 | |
| Age (y) | 9 | 1 | 9 | 2 | 2 | 1 | 1 | 1 | 1 | 2 |
| Serum AFP | 600,000 | 28,060,000 | 184,603 | 75,802 | 114,098 | 237,622 | 106,737 | 55,560 | 189,168 | 93,383 |
| Other | Recurrence postchemotherapy | Portal vein invasion | Portal vein invasion | MME | Pure fetal | |||||
AFP indicates α-fetoprotein; MME, mixed-mesenchymal epithelial.
FIGURE 1.
Composite image of 4 cases of hepatoblastoma, fetal-embryonal epithelial type. First row labeled as H&E shows hematoxylin and eosin-stained sections of aggressive tumors (case 7 and 9) and good clinical outcome tumors (case 8 and 10). Shown in the rows accordingly labeled are immunohistochemical stains for Survivin, CK19, Yap, PLK-1, N-Myc, and C-Myc for the 4 cases. All images have a final magnification of × 100.
DISCUSSION
The treatment of patients with HB is slightly different in Europe compared with the United States. Although all HB cases undergo chemotherapy up front in Europe, a few low-risk tumors such as well-differentiated HB with low-mitotic activity may be treated exclusively by surgery in the United States. The standard risk HB patients receive a chemotherapy combination of Cisplatin, 5-fluorouracil, and Vincristine (C5V), whereas the high-risk HB patients receive C5V and Doxorubicin (C5VD). A great majority of HB patients respond well to the chemotherapy; but a significant number have progressive disease, relapse, or have recurrence. In 1 recent study 28% patients had either progressive disease, relapse, or have recurrence.6 For our study we chose the cutoff of 70% tumor necrosis because in their study assessing tumor necrosis following chemotherapy, Venkatramani et al6 reported a median of 70% necrosis of tumor in their cohort of patients. As the treatment of this tumor is heavily dependent on chemotherapy, we undertook this pilot study to assess the immunophenotype of aggressive tumors that did not have optimal response to chemotherapy.
The low numbers of cases in our pilot study preclude from drawing statistically significant conclusions. However, several clinically relevant observations were noted that could be tested on a larger sample in future studies. The good outcome cases did not show diffuse expression of Survivin and CK19, whereas the aggressive tumors had diffuse expression. So, we infer that the presence of diffusely positive staining for Survivin and CK19 in HB may be a marker of aggressive clinical course. Another noteworthy observation was the protein expression pattern of C-Myc which was different in good outcome and aggressive tumors. The aggressive tumors had multiple nests of cells that lacked nuclear staining of C-Myc. Earlier, a study of hepatocellular carcinomas (HCC) reported nuclear expression of C-Myc in the HCC tumors.7 Thus, the C-Myc expression pattern in aggressive HB is different from that of HCC as well.
The presence of CK19 and Yap staining in overlapping clusters of tumor cells suggest that the tumor cells in these foci are pluripotent progenitor hepatoblasts. It is known that hepatic progenitor cells coexpress CK19 and Yap proteins.8 Notch2, Hes1, and Hes5 are proteins in the signaling pathway associated with Yap and their expression was not obviously different in the 2 groups of our cohort. The solitary case of PF (well-differentiated) HB in our cohort lacked expression of Survivin, CK19, and PLK-1.
In summary, our study has identified an immunohistochemical phenotype of aggressive HB tumors that comprise of a loss of nuclear staining of C-Myc and diffuse expression of CK19 and Survivin in a majority of tumor cells. In contrast, the majority of cells in the good outcome group show an immunohistochemical phenotype that comprises of nuclear expression of C-Myc and only focal staining of CK19 and Survivin. The results of our study have important clinical implications as they predict the response to chemotherapy in HB tumors. However, these results need to be validated by a larger sample size.
Acknowledgments
The authors thank Joan Whiting for technical assistance with the automated immunohistochemical stains.
The funding for the research project was provided by Midwest Cancer Alliance. The study was conducted after seeking approval by Institutional Review Board of Children’s Mercy Hospital.
Footnotes
The authors declare no conflict of interest.
References
- 1.Czauderna P, Lopez-Terrada D, Hiyama E, et al. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26:19–28. doi: 10.1097/MOP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Terrada D, Gunaratne PH, Adesina AM, et al. Histologic subtypes of hepatoblastoma are characterized by differential canonical Wnt and Notch pathway activation in DLK + precursors. Hum Pathol. 2009;40:783–794. doi: 10.1016/j.humpath.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Cairo S, Armengol C, De Reynies A, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14:471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Yamada S, Ohira M, Horie H, et al. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23:5901–5911. doi: 10.1038/sj.onc.1207782. [DOI] [PubMed] [Google Scholar]
- 5.Adesina AM, Lopez-Terrada D, Wong KK, et al. Gene expression profiling reveals signatures characterizing histologic subtypes of hepatoblastoma and global deregulation in cell growth and survival pathways. Hum Pathol. 2009;40:843–853. doi: 10.1016/j.humpath.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatramani R, Wang L, Malvar J, et al. Tumor necrosis predicts survival following neo-adjuvant chemotherapy for hepatoblastoma. Pediatr Blood Cancer. 2012;59:493–498. doi: 10.1002/pbc.24038. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Jin R, Zhang X, et al. Oncogenic activation of glypican3 by cMyc in human hepatocellular carcinoma. Hepatology. 2012;56:1380–1390. doi: 10.1002/hep.25891. [DOI] [PubMed] [Google Scholar]
- 8.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]