SUMMARY
To achieve simultaneous measurement of multiple cellular events in molecularly defined groups of neurons in vivo, we designed a spectrometer-based fiber photometry system that allows for spectral unmixing of multiple fluorescence signals recorded from deep brain structures in behaving animals. Using green and red Ca2+ indicators differentially expressed in striatal direct- and indirect-pathway neurons, we were able to simultaneously monitor the neural activity in these two pathways in freely moving animals. We found that the activities were highly synchronized between the direct and indirect pathways within one hemisphere, and were desynchronized between the two hemispheres. We further analyzed the relationship between the movement patterns and the magnitude of activation in direct- and indirect-pathway neurons, and found that the striatal direct and indirect pathways coordinately control the dynamics and fate of movement.
eTOC Blurb
Using a new method for simultaneous multi-color fluorescence measurements, Meng et al. show that neural activities are synchronized between two parallel striatal pathways, and the magnitude of activation in these two pathways coordinately determines the dynamics and fate of movement.
INTRODUCTION
Owing to the recent advances in genetically encoded fluorescent sensors (Chen et al., 2013; Dana et al., 2016; Gong et al., 2015; Li and Tsien, 2012), in vivo fiber photometry, a class of optical recording methods that use optical fibers to deliver excitation light and collect fluorescence emission photons, has become an increasingly popular tool for assessing a variety of cellular events from specific types of cells in deep brain circuits of freely moving animals (Cui et al., 2013; Gunaydin et al., 2014; Kim et al., 2016). Simultaneous multi-color fiber photometry using two or more fluorescent sensors is a highly desirable approach, as it provides the capability to monitor multiple cellular and molecular events in action at the same time. However, current fiber photometry systems are not ideal for multi-color measurements, because they either lack a light-dispersing mechanism to capture the fluorescence emission spectrum (Gunaydin et al., 2014; Kim et al., 2016) or are limited in spectral span and spectral resolution (Cui et al., 2014; Cui et al., 2013). Spectral information is pivotal for the application of fiber photometry methods in the measurement of multi-color fluorescence, as it is required for correct signal identification and accurate signal unmixing when using fluorophores with overlapped emission spectra (Figure S1). In this study, we designed a novel spectrally resolved fiber photometry system that can be used for high-speed simultaneous measurement of multiple fluorophores between the UV (350 nm) and IR (1100 nm) range. To demonstrate the functionality of this new system, and to reveal the relationship between the two parallel projection pathways in the basal ganglia in movement control (Albin et al., 1989; Alexander and Crutcher, 1990; Gerfen et al., 1990; Gerfen and Surmeier, 2011), we sought to record the fluorescence from green and red genetically encoded Ca2+ indicators (GECIs) GCaMP6f and jRGECO1a differentially expressed in striatal direct- and indirect-pathway spiny projection neurons (SPNs) in freely moving mice.
RESULTS
Spectrally resolved fiber photometry system for unmixing of multiple fluorescence signals
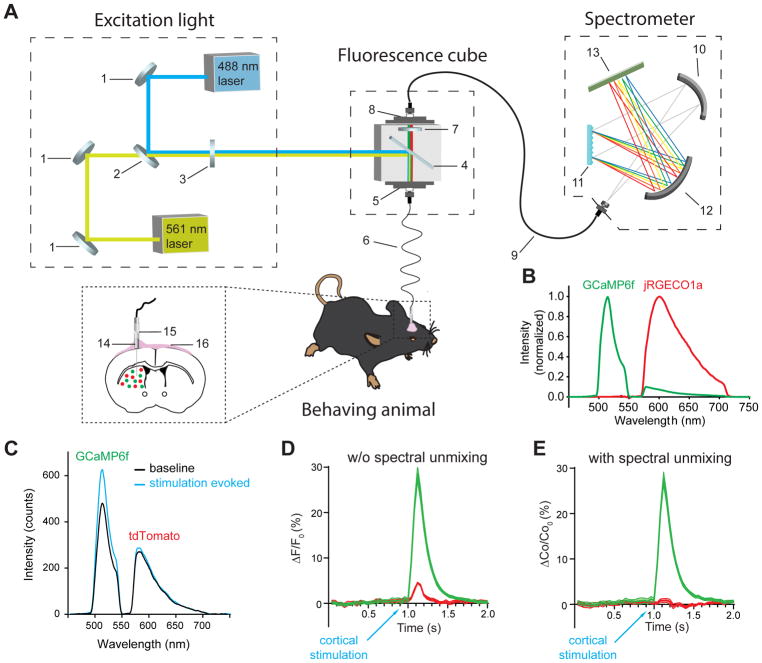
The fiber photometry system consists of three major components: an excitation light source, a fluorescence cube with interchangeable filters and a spectrometer (Figure 1A, Figure S3, see STAR Methods for detailed parts list). To simultaneously measure green and red fluorophores, we used a 488 nm continuous wave (CW) laser and a 561 nm CW laser as the excitation light sources and a set of matching dual band dichroic and emission filters. The high performance spectrometer is equipped with a diffraction grating as the light dispersing device and a cooled back-thinned linear CCD array as the detector.
Figure 1. Spectrally resolved fiber photometry for simultaneous measurement of multiple fluorescence signals.
(A) Schematic illustration of the photometry system used for measuring red and green fluorescent sensors expressed in two populations of neurons in the striatum of a freely moving mouse. Shown in the picture are broadband mirrors (1), laser beam combiner (2), neutral density filter (3), dual-edge dichroic beamsplitter (4), front fiber port (5), 105 μm core multi-mode optical fiber patch cable (6), dual bandpass emission filter (7), rear fiber port (8), 200 μm core multi-mode optical fiber patch cable (9), collimating mirror (10), diffraction grating (11), focusing mirror (12), linear CCD array (13), implanted fiber probe with 1.25 mm OD ceramic ferrule (14), ceramic mating sleeve (15), dental cement (16). (See also Figure S3 and STAR Methods).
(B) Normalized emission spectrum of GCaMP6f (green) and jRGECO1a (red) measured separately from the striatum of a mouse expressing GCaMP6f and a mouse expressing jRGECO1a in SPNs. Note the spectral bleed-through between the two sensors. (See also Figure S1F).
(C–E) Correction for fluorescence bleed-through using linear spectral unmixing.
(C) Emission spectrum of GCaMP6f and tdTomato co-expressed in striatal SPNs measured at 100 ms before (black) and 100 ms after (blue) intracortical electrical stimulation in vivo. The stimulation-induced increase in GCaMP6f fluorescence causes an apparent increase in tdTomato fluorescence owing to the spectral bleed-through.
(D) Normalized fluorescence change of GCaMP6f (calculated by the total number of photons between 500–540 nm) and tdTomato (calculated by the total number of photons between 575–650 nm) over time. The stimulation-induced apparent increase in tdTomato fluorescence is an artifact caused by spectral bleed-through of GCaMP6f.
(E) After applying a linear spectral unmixing algorithm (see STAR Methods for details), the normalized coefficients of GCaMP6f and tdTomato extracted from the mixed spectra are plotted over time. The bleed-through induced artifact in tdTomato fluorescence is corrected.
To address the concern that the overlapped portion of the GCaMP6f and jRGECO1a spectra may confound the explanation of the obtained results (Figure 1B), and to demonstrate the advantage of spectral unmixing in multi-fluorescence measurements, we co-expressed GCaMP6f and control red fluorescent protein tdTomato, whose emission spectrum largely overlaps with jRGECO1a (tdTomato emission peak: 584 nm, jRGECO1a emission peak: 600 nm, measured in vivo, Figure S1F), in striatal SPNs, and measured intracortical stimulation-evoked striatal responses in anesthetized mice. We found that intracortical electrical stimulation produced a large increase in GCaMP6f fluorescence, and, at the same time, also caused an apparent increase in the tdTomato fluorescence due to the spectral bleed-through (Figure 1C, D). To correct for the artifact in the tdTomato fluorescence change (and therefore the potential red sensors to be used), we applied a linear unmixing algorithm (see STAR Methods for details) and used the coefficients of GCaMP6f and tdTomato produced by the model to represent the changes in their fluorescence intensity, which effectively corrected the artifact caused by the spectral bleed-through (Figure 1E). Therefore, we used the unmixed coefficients over time to represent the dynamic fluorescence intensity changes of individual fluorophores throughout the rest of the study.
Validation of GCaMP6f and jRGECO1a as neural activity reporters in striatal SPNs
To test if the GCaMP6f and jRGECO1a fluorescence signals recorded by the photometry system can faithfully report the neural activity in freely moving animals, we performed in vivo recordings when GCaMP6f was co-expressed with tdTomato (Figure 2A–C), and when jRGECO1a was co-expressed with eGFP (Figure 2E–G, Figure S1F) in striatal SPNs. We observed frequent and transient increases only in GCaMP6f and jRGECO1a fluorescence but not in the corresponding tdTomato and eGFP fluorescence (Figure 2D, H), suggesting that the fluorescence transients in GCaMP6f and jRGECO1a were caused by Ca2+ influx associated with neural activity and were not movement related artifacts.
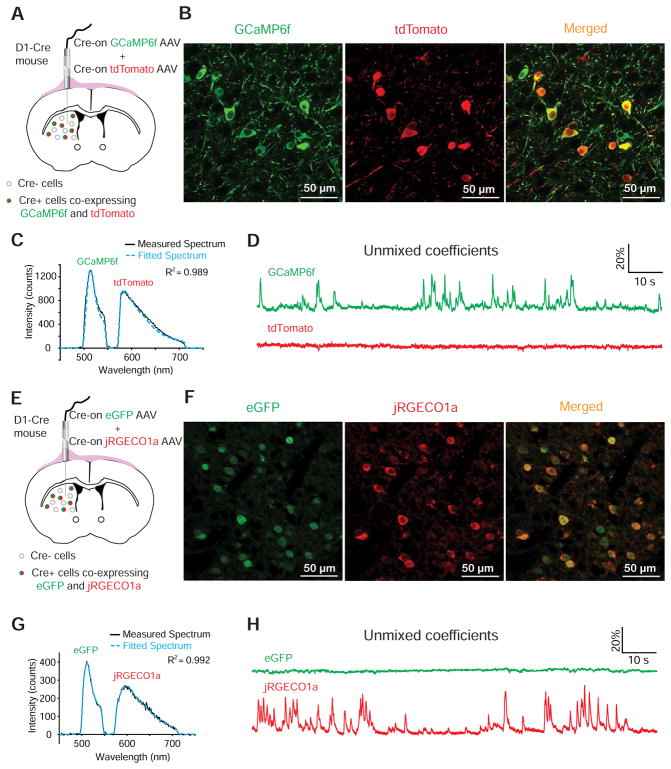
Figure 2. Simultaneous dual-color recordings of green Ca2+ sensor GCaMP6f co-expressed with tdTomato and red Ca2+ sensor jRGECO1a co-expressed with eGFP.
(A and B) Co-expression of GCaMP6f and tdTomato in direct-pathway striatal neurons by intrastriatal infusion of Cre-on AAVs in D1-Cre mice.
(C) A representative frame of emission spectrum of GCaMP6f and tdTomato captured at 50 Hz (black) and the corresponding best fitted spectrum (blue dashed) generated by a linear unmixing algorithm. (See also Figure S1F).
(D) The normalized coefficients of GCaMP6f and tdTomato calculated from the fitted spectrum plotted over time.
(E and F) Co-expression of jRGECO1a and eGFP in direct-pathway striatal neurons by intrastriatal infusion of Cre-on AAVs in D1-Cre mice.
(G) A representative frame of emission spectrum of jRGECO1a and eGFP captured at 50 Hz (black) and the corresponding best fitted spectrum (blue dashed) generated by a linear unmixing algorithm. (See also Figure S1F).
(H) The normalized coefficients of jRGECO1a and eGFP calculated from the fitted spectrum plotted over time.
Since GCaMP6f and jRGECO1a have been shown to have different sensitivity, dynamic range and kinetics in detecting action potential (AP) associated cytosolic Ca2+ increases (Chen et al., 2013; Dana et al., 2016), we further tested if GCaMP6f and jRGECO1a can report neural activity with comparable temporal profiles and magnitude changes by co-expressing them in the same population of striatal SPNs (Figure 3A–C). We found that GCaMP6f and jRGECO1a signals showed high temporal correlation (Figure 3D, E), and that the normalized changes in the magnitude of these two sensors, expressed either as the percent change of coefficient (Figure 3F) or the Z scores (Figure 3G), displayed a linear relationship in freely moving animals. These results built the foundation for us to use the GCaMP6f and jRGECO1a together to report the neural activities in striatal direct- and indirect-pathway SPNs. Since the slope of the fitting curve is closer to 1 when Z score is used to represent the normalized magnitude than when percent change is used, which could partially compensate for the innate differences in the dynamic range of GCaMP6f and jRGECO1a, we chose to use the Z scores to represent the magnitude of normalized fluorescence transients when GCaMP6f and jRGECO1a are used together.
Figure 3. Comparison between GCaMP6f and jRGECO1a signals when they are co-expressed in the same population of striatal SPNs.
(A) Illustration of optical recordings from the striatum of a D1-Cre mouse when GCaMP6f and jRGECO1a are co-expressed in direct-pathway SPNs using Cre-on DIO-GCaMP6f and DIO-jRGECO1a viral vectors.
(B) Immunostaining for GCaMP6f and jRGECO1a shows their co-localization in the same cells.
(C) A representative frame of emission spectrum of GCaMP6f and jRGECO1a co-expressed in direct-pathway SPNs. The black trace shows the measured spectrum; the blue dashed trace shows the corresponding best fitted spectrum generated by a linear unmixing algorithm. (See also Figure S6B).
(D) The Z scores of normalized coefficients of GCaMP6f and jRGECO1a calculated from the fitted spectrum plotted over time. (See also Figure S6C–F).
(E) Auto-correlation of the Z scores of GCaMP6f (green) and cross-correlation of the Z scores of jRGECO1a to the Z scores of GCaMP6f (red) during a 2-min recording in a freely moving mouse.
(F and G) Linear regression of the amplitude of the corresponding fluorescence transients in GCaMP6f and jRGECO1a normalized as percent change of coefficients (F) and Z scores (G).
Neural activities are highly synchronized between direct- and indirect-pathway SPNs
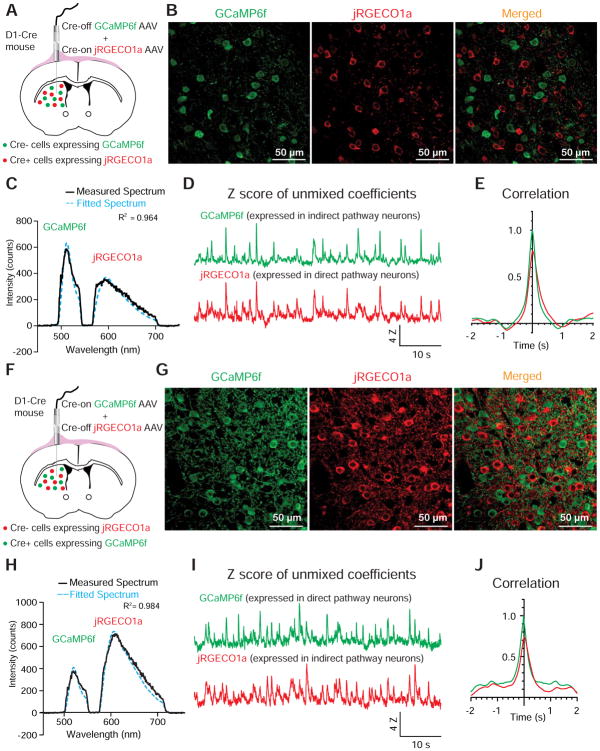
To differentially express GCaMP6f and jRGECO1a in striatal direct- and indirect-pathway SPNs, we infused Cre-off GCaMP6f (FAS-GCaMP6f) and Cre-on jRGECO1a (DIO-jRGECO1a) viral vectors into the striatum of D1-Cre mice, so that GCaMP6f would be expressed by indirect-pathway SPNs while jRGECO1a would be expressed by direct-pathway SPNs (Gerfen et al., 2013; Gong et al., 2007; Saunders et al., 2012) (Figure 4A). Immunostainings for GCaMP6f and jRGECO1a confirmed clear separation of these two sensors in different populations of striatal neurons (Figure 4B). The nearly exclusive expression of GCaMP6f and jRGECO1a by projection neurons within the two striatal pathways was further shown by their absence in the cholinergic interneurons (Figure S2A–C). In freely moving mice, we found that the fluorescence of GCaMP6f in indirect-pathway SPNs and the jRGECO1a in direct-pathway SPNs showed highly synchronized changes (Figure 4C–E). This same observation was also made when we swapped sensors between the two pathways by using Cre-on GCaMP6f (DIO-GCaMP6f) and Cre-off jRGECO1a (FAS-jRGECO1a) viral vectors in D1-Cre mice (Figure 4F–J), and when we used the Cre-on and Cre-off viral vectors in A2A-Cre mice to direct the expression of the two sensors (Figure S2D–M).
Figure 4. Simultaneous dual-sensor recording from striatal direct- and indirect-pathway SPNs reveals highly correlated neural activities between the two pathways.
(A and F) Illustration of optical recordings from the striatum of a D1-Cre mouse when jRGECO1a is expressed in direct-pathway SPNs while GCaMP6f is expressed in indirect-pathway SPNs using Cre-on DIO-jRGECO1a and Cre-off FAS-GCaMP6f viral vectors (A), and when GCaMP6f is expressed in direct-pathway SPNs while jRGECO1a is expressed in indirect-pathway SPNs using Cre-on DIO-GCaMP6f and Cre-off FAS-jRGECO1a viral vectors (F).
(B and G) Immunostaining for GCaMP6f and jRGECO1a to show their differential expression when using Cre-on and Cre-off viral vectors. (See also Figure S2B, C).
(C and H) A representative frame of emission spectrum of GCaMP6f and jRGECO1a differentially expressed in indirect-pathway and direct-pathway SPNs, respectively (C), and in direct-pathway and indirect-pathway SPNs, respectively (H). The black trace shows the measured spectrum; the blue dashed trace shows the corresponding best fitted spectrum generated by a linear unmixing algorithm.
(D and I) The Z scores of normalized coefficients of GCaMP6f and jRGECO1a calculated from the fitted spectrum plotted over time.
(E and J) Auto-correlation of the Z scores of normalized GCaMP6f coefficient (green), and cross-correlation of the Z scores of normalized jRGECO1a coefficient to the Z scores of normalized GCaMP6f coefficient (red) during a 2-min recording in freely moving mice.
Interestingly, when we used two fiber photometry systems or a modified dual-spectrometer system (Figure S3) to simultaneously measure the striatal direct- and indirect-pathway neural activities bilaterally (Figure 5A), we found that, although the neural activities were highly synchronized between the direct- and indirect-pathway SPNs in the same hemisphere, the activation in one striatum was always accompanied by a lack of activation in the contralateral striatum (Figure 5B–F), consistent with previous studies showing that the striatal neural activity is associated with contraversive but not ipsiversive movements in rodents (Cui et al., 2013; Tecuapetla et al., 2014).
Figure 5. Simultaneous bilateral dual-sensor recording from striatal direct- and indirect- pathway SPNs reveals desynchronized striatal neural activities between two hemispheres.
(A) Illustration of bilateral striatal recording from an A2A-cre mouse expressing GCaMP6f in direct-pathway SPNs and jRGECO1a in indirect-pathway SPNs.
(B) Left: Simultaneously captured emission spectra of GCaMP6f and jRGECO1a from the left (top) and the right striatum (bottom). The black trace is the measured spectrum; the blue dashed trace is the corresponding best fitted spectrum generated by a linear unmixing algorithm. Right: Z score of normalized coefficients of GCaMP6f (expressed in direct-pathway SPNs) and jRGECO1a (expressed in indirect-pathway SPNs) simultaneously recorded from the left (top) and the right striatum (bottom). Grey and yellow vertical boxes highlight a few examples where GCaMP6f and jRGECO1a transients are seen in one hemisphere but are absent in the contralateral hemisphere.
(C) Auto-correlation of the normalized GCaMP6f coefficient over time (solid and dashed green) and the cross-correlation of normalized jRGECO1a coefficient to normalized GCaMP6f coefficient (solid and dashed red) during a 2-min recording from the left and the right striatum in freely moving mice. Note that the jRGECO1a signal is highly correlated to the GCaMP6f signal on the same side.
(D) Auto-correlation of normalized GCaMP6f coefficient recorded from the left striatum (solid green) and cross-correlation of normalized coefficients of the left striatal jRGECO1a (solid red), the right striatal GCaMP6f (dashed green) and the right striatal jRGECO1a (dashed red) to the left striatal GCaMP6f.
(E) The Z scores of normalized coefficients of GCaMP6f (direct pathway) and jRGECO1a (indirect pathway) measured at the peaks of the GCaMP6f transients that occurred in the left hemisphere (**** P< 0.0001, one-way ANOVA followed by Tukey’s multiple comparisons test, n=36).
(F) The Z scores of normalized coefficients of GCaMP6f (direct pathway) and jRGECO1a (indirect pathway) measured at the peaks of the GCaMP6f transients that occurred in the right hemisphere (**** P< 0.0001, one-way ANOVA followed by Tukey’s multiple comparisons test, n=21).
The magnitude of activation in direct- and indirect-pathway SPNs collaboratively determines the movement patterns
Although the neural activities in direct- and indirect-pathway SPNs were temporally synchronized, the magnitude of activation at each activation event was often different between the two pathways. To reveal the relationship between the animal’s locomotor behavior and the magnitude of the activation in direct- and indirect-pathway SPNs, we used the x-y coordinates of the animal’s head and body to create a vector to represent the animal’s location and orientation at a given time point, and used the cumulative angle between the vectors to describe the animal’s turning behavior (Figure 6A). To test if different magnitudes of activation in direct- and indirect-pathway SPNs lead to different movement patterns, we examined the events where one pathway showed larger magnitude of activation than the other and plotted the cumulative angle 0.5 sec before and 1.0 sec after the onset of the selected event (Figure 6B). The onset of an event was defined by a Z score value that is larger than mean plus 3 times the standard deviation of the Z scores of recorded fluorescence during the previous 0.5 sec period. We found that in the events when the direct-pathway SPNs showed strong activation and the indirect-pathway SPNs showed weak activation, the animal was able to initiate and complete large contraversive turning movements (events b, c, d in Figure 6B, C, Movie S1). When the direct-pathway SPNs showed weak activation and the indirect-pathway SPNs showed strong activation, the animal was also able to initiate contraversive turning movements; however, the movements were terminated immediately after the initiation (events a and e in Figure 6B, C, Movie S2).
Figure 6. The magnitude of activation in striatal direct and indirect pathways coordinately determines the dynamics and fate of movement.
(A) An example trace of dual-sensor recording from direct- and indirect-pathway neurons plotted together with movement tracking over time. Cumulative degrees are used to describe the turning behavior with upward changes representing contralateral turnings. Five events with different magnitudes of activation in direct- and indirect-pathway neurons (events a–e) are highlighted.
(B) The five selected events in (A) are replotted in 1.5 sec windows using the 0.5 sec before the onset of the neuronal activation as the new baseline.
(C) Movement path heat maps overlaid with the initial positions of the animal for the events shown in (A) and (B). Warmer color represents longer time spent at each location along the path.
(D) A scatter plot showing the relationship between the magnitude of activation in direct- (x-axis) and indirect-pathway (y-axis) neurons and the animal’s movement behavior (cumulative contraversive turning degrees, color coded). 52 events in which the magnitude of activation in direct-pathway neurons is at least 20% larger than the indirect-pathway neurons, and 51 events in which the magnitude of activation in indirect-pathway neurons is at least 20% larger than the direct-pathway neurons are included in the analysis. The data are pooled from 5 mice with GCaMP6f expressed in the direct pathway and jRGECO1a in the indirect pathway, and 3 mice with GCaMP6f expressed in the indirect pathway and jRGECO1a in the direct pathway.
(E–G) The animals displayed ‘start and go’ type of movement when strong activation in direct-pathway was paired with weak activation in the indirect-pathway. (E and F) Summary of the events (represented as mean ± S.E.M.) in which the magnitude of activation in direct-pathway was at least 20% larger than the indirect-pathway when GCaMP6f is expressed in direct-pathway SPNs while jRGECO1a is expressed in indirect-pathway SPNs (E, n=34), and when GCaMP6f is expressed in indirect-pathway SPNs while jRGECO1a is expressed in direct-pathway SPNs (F, n=18). (See also Movie S1).
(H–J) The animals displayed ‘start and stop’ type of movement when strong activation in indirect-pathway was paired with weak activation in the direct-pathway. (H and I) Summary of the events (represented as mean ± S.E.M.) in which the magnitude of activation in indirect-pathway was at least 20% larger than the direct-pathway when GCaMP6f is expressed in direct-pathway SPNs while jRGECO1a is expressed in indirect-pathway SPNs (H, n=18), and when GCaMP6f is expressed in indirect-pathway SPNs while jRGECO1a is expressed in direct-pathway SPNs (I, n=33). (See also Movie S2).
Since GCaMP6f and jRGECO1a have different dynamic ranges in detecting neural activity related Ca2+ fluctuations, we counterbalanced the way these two sensors were expressed in direct- and indirect-pathway SPNs. When we pooled the data from eight mice (5 mice with GCaMP6f expressed in the direct pathway and jRGECO1a in the indirect pathway, and 3 mice with the reversed order) and compared the animal’s turning behavior, we found that the cumulative contraversive turning degrees measured at 1.5 s after the onset of the fluorescence transients were significantly larger in the events when strong activation in direct-pathway neurons was paired with weak activation in indirect-pathway neurons (cum-degrees=96.24±4.62, n=52) than in the events when weak activation in direct-pathway neurons was paired with strong activation in indirect-pathway neurons (cum-degrees=37.22±2.36, n=51) (Figure 6D, P<0.0001, unpaired t-test). We further analyzed the results separately based on which pathways the sensors were expressed in. When GCaMP6f was expressed in direct-pathway SPNs and jRGECO1a expressed in indirect-pathway SPNs, the cumulative turning degrees were significantly larger in the events with strong direct pathway activation and weak indirect pathway activation (cum-degrees=95.28±7.02, GCaMP6f Z score=4.359±0.372, jRGECO1a Z score=2.502±0.268, n=34, Figure 6E) than in the events with weak direct pathway activation and strong indirect pathway activation (cum-degrees=46.42±8.56, GCaMP6f Z score=1.915±0.261, jRGECO1a Z score=3.651±0.368, n=18, P<0.0001, unpaired t-test between cum-degrees, Figure 6H). Similar results were also seen when the sensors were reversed in these two pathways. The cumulative turning degrees were larger in the events with strong direct pathway activation and weak indirect pathway activation (cum-degrees=100.7±8.06, jRGECO1a Z score=3.311±0.303, GCaMP6f Z score=1.621±0.255, n=18, Figure 6F) than in the events with weak direct pathway activation and strong indirect pathway activation (cum-degrees=24.99±7.46, jRGECO1a Z score=1.455±0.204, GCaMP6f Z score=3.705±0.456, n=33, P<0.0001, unpaired t-test between cum-degrees, Figure 6I).
These results suggest that strong direct-pathway activation combined with weak indirect-pathway activation leads to ‘start and go’ type of contraversive movements featured with large turning angles (Figure 6G). In contrast, weak direct-pathway activation with strong indirect-pathway activation leads to ‘start and stop’ type of movements featured by an abrupt termination of the contraversive movements (Figure 6J).
DISCUSSION
A unique feature of the fiber photometry system that we have described in this study is its capability of obtaining high resolution emission spectrum from multiple fluorescent sensors or proteins. This feature is crucial for verifying that the emission photons collected through each band of the emission filter are indeed from the expressed fluorescent sensors or proteins (Figure S1). It is also required for subsequent mathematical extraction of each spectral component from the measured mixed spectrum, which is essential for eliminating the signal cross-contaminations caused by spectral bleed-through of multiple fluorescent sensors and proteins (Figure 1).
Another feature of the system is its high versatility. By choosing different light sources and appropriate fluorescence filter sets, variable versions can be derived from the same core design to measure different fluorophores of interest and to manage the total cost of the system. For example, LEDs and mercury arc lamps can be used as alternative excitation light sources to significantly reduce the cost. A large selection of commercial multiband filter sets is available for simultaneous measurement of multiple fluorophores with different colors. The system is easy to assemble from all commercially available parts by a person with a basic knowledge of optics. It can be broadly applied in studying a variety of other cellular and molecular processes, such as intracellular signal transduction, cell metabolism and redox state (Klarenbeek et al., 2015; Lukyanov and Belousov, 2014; Tao et al., 2017).
An important prerequisite for achieving multi-sensor measurements in different populations of neurons is to have the proper molecular tools to deliver the fluorescent sensors into specific types of neurons. In this study, we adopted the Cre-on and Cre-off AAV vector strategy to target striatal direct- and indirect-pathway neurons, because these projection neurons constitute 90–95% of total neuronal populations in the striatum (Saunders et al., 2012). This targeting strategy is only applicable for brain regions that are dominated by two molecularly distinct neuronal populations. A more specific strategy that can be broadly applied to all brain regions is to combine promoter-specific Cre and Flpo mouse driver lines with DIO and fDIO AAV vectors when they have become more widely available (Fenno et al., 2014; Madisen et al., 2015).
A potential problem that may limit the use of the fluorescent sensors and confound the explanation of the results is the fading of fluorescence during long periods of illumination. To test whether the method described in this study is suitable for longer experiments, we carried out additional experiments in mice expressing GCaMP6f and jRGECO1a in two striatal pathways. We used intracortical electrical stimulations delivered every minute to evoke striatal responses, and compared the magnitude of evoked responses in GCaMP6f and jRGECO1a fluorescence over a period of 40 minutes. We found that despite the decay of fluorescence intensity in both GCaMP6f and jRGECO1a (more severe in jRGECO1a) over the 40-min recording session (Figure S4A, B), the magnitude (Z score) of the stimulation-evoked fluorescence transients in GCaMP6f and jRGECO1a remained constant (Figure S4C, D), suggesting that the baseline fluorescence drop did not cause severe distortion of the reported values when using the dynamic fluorescence change of these Ca2+ sensors to report the neural activity. Interestingly, we also found that the faded fluorescence could fully recover to the initial intensity when measured the next day (Figure S4A), suggesting that the fluorescence fading we observed was mainly caused by photoswitching (Dana et al., 2016; Shaner et al., 2008).
The role of striatal direct and indirect pathways in movement control has recently been examined using opto- and chemo-genetic manipulations and optogenetic recordings (Barbera et al., 2016; Cui et al., 2013; Ferguson et al., 2011; Kravitz et al.). However, the temporal profile and the magnitude of activation in these two pathways have never been directly compared in the same animals. Using the spectrally resolved fiber photometry method with differentially expressed GCaMP6f and jRGECO1a, our study provides the first set of evidence to reveal that the activities in direct and indirect pathways are highly synchronized within each hemisphere (Figure 4, Figure S2D–M), and that the striatal neural activities are desynchronized between the two hemispheres (Figure 5). These results are consistent with previous findings that both direct- and indirect-pathway neurons show strong activation when the animals turn contraversively but not ipsiversively (Cui et al., 2013). The observed synchrony between the direct and indirect pathways is not surprising, because the corticostriatal inputs that innervate direct- and indirect-pathway neurons are highly overlapped (Kress et al., 2013). Since the information that flows through the indirect pathway has one or two more synaptic delays at the level of globus pallidus external segment and the subthalamic nucleus than the direct pathway, concurrent activation of these two pathways at the striatum level will produce an inherent temporal delay when these two pathways converge at the output nuclei of the basal ganglia. This temporal delay makes it impossible for the indirect-pathway neurons to directly antagonize the effect of direct pathway activation during action initiation.
The most important finding revealed by our method is that the magnitude of activation in direct- and indirect-pathway neurons collaboratively determines the movement dynamics and fate. In the events when the direct-pathway neurons showed strong activation paired with a weak activation in the indirect-pathway neurons, the animals were able to complete a large contraversive turn and move to a distant location (Figure 6, Movie S1). However, in the events when the indirect-pathway neurons showed strong activation, the initiated contraversive movements were abruptly terminated, and the animals would either pause in the same location or initiate an ipsiversive movement (Figure 6, Movie S2). Based on these observations, we hypothesize that the direct pathway activation serves as a movement start signal, and its magnitude determines the vigor of a movement. Meanwhile, the concurrently activated indirect pathway serves as a scalable stop signal that determines whether the initiated movement will continue or be terminated. Our hypothesis is based on correlational observations described in this study. The causal relationship between the neural activity and behavior needs to be further examined by interventional studies where direct- and indirect-pathway SPNs are independently controlled to recapitulate different activation scenarios in vivo.
In conclusion, we have developed a novel spectrometer-based fiber photometry method for simultaneous multi-color fluorescent signal measurement and unmixing from deep brain structures in vivo. Using this method, we show for the first time that the neural activities of two parallel striatal projection pathways are highly synchronized, and the magnitude of activation in these two pathways collaboratively determines the dynamics and fate of movement.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents or analysis code may be directed to and will be fulfilled by the Lead Contact, Guohong Cui (cuig@mail.nih.gov).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All animal protocols were approved by the US National Institute of Environmental Health Sciences Animal Care and Use Committee. Experiments were carried out using 2 to 12-month-old male and female mice. D1-Cre (MMRRC_036916-UCD) and A2A-Cre (MMRRC_036158-UCD) mice were obtained from the Mutant Mouse Resource Research Centers (MMRRC).
All mice housed under reverse light cycle conditions and had access to food and water ad libitum. Mice were housed two to five per cage before optical probe implantation surgery, and singly housed after surgery. Experimental D1-cre and A2A-cre mice were produced by breeding the D1-cre and A2A-cre hemizygous BAC transgenic mice with C57BL/6J mice.
METHOD DETAILS
Viral vectors
All viral vectors were recovered in house by the NIEHS Viral Vector Core and had titers of 1.3×1013 to 2×1014 genome copies per ml, except for AAV9-Syn-DIO-EGFP-WPRE-hGH (AV-9-PV2629, a.k.a. DIO-EGFP) and AAV9-CAG-Flex-tdTomato-WPRE-bGH (AV-9-ALL864, a.k.a. DIO-tdTomato), which were obtained from the University of Pennsylvania Viral Vector Core and had titers of ~1×1013 genome copies per ml. For AAV vectors made in house: pAAV-hSyn-DIO-GCaMP6f-WPRE (a.k.a DIO-GCaMP6f) was constructed from pAAV-hSyn-DIO-mCherry (a gift from Bryan Roth, Addgene 50459) and pGP-CMV-GCaMP6f (Chen et al., 2013) (a gift from Douglas Kim, Addgene 40755). pAAV-hSyn-DIO-NES-jRGECO1a-WPRE (a.k.a. DIO-jRGECO1a) was constructed from plasmids pAAV-hSyn-DIO-mCherry and pGP-CMV-NES-jRGECO1a (Dana et al., 2016) (a gift from Douglas Kim, Addgene 61563). Restriction sites AscI and NheI were utilized and cloning was performed using the In-Fusion HD Cloning kit (Clontech). pAAV-Ef1a-FAS-NES-jRGECO1a-WPRE (a.k.a. FAS-jRGECO1a) was constructed using the same method from pAAV-Ef1a-FAS-EGFP-WPRE-pA (Saunders et al., 2012) (a gift from Bernardo Sabatini, Addgene 37091) and pGP-CMV-NES-jRGECO1a, as was pAAV-Ef1a-FAS-GCaMP6f-WPRE (a.k.a. FAS-GCaMP6f) using Addgene plasmids 37091 and 40755. All in-house AAVs were packaged with AAV9 capsid.
Viral expression of GECIs and control fluorescent proteins
Viral vectors were micro-injected into the left and right dorsal striatum by standard stereotaxic procedures with animals under isoflurane anesthesia (Cui et al., 2014). The coordinates used for targeting the dorsal striatum were AP +0.50 mm, ML ±1.8 mm from Bregma; and DV −2.75 mm from the brain surface. A total volume of 1.0 μl of AAV vectors per site was injected at the rate of 0.1 μl per min through a Hamilton Neuros syringe with a 30-gauge needle. The needle was left in place for five more minutes before withdrawal. The volume ratios of viral vectors in the mixtures for injections were 5:1 for DIO-GCaMP6f and DIO-tdTomato, 9:1 for DIO-jRGECO1a and DIO-EGFP, 2:1 for DIO-jRGECO1a and DIO-GCaMP6f, 5:1 for DIO-jRGECO1a and FAS-GCaMP6f, and 5:1 for FAS-jRGECO1a and DIO-GCaMP6f.
Spectrally resolved fiber photometry system
The single fiber photometry system illustrated in Figure 1A consists of three functional units: a dual-wavelength excitation light source, a fluorescence cube and a spectrometer (Figure 1A). The laser beams from a 488 nm 60 mW continuous wave (CW) laser (OBIS 488LS-60, Coherent, Inc.) and a 561 nm 60 mW CW laser (OBIS 561LS-60, Coherent, Inc.) are aligned and combined using broadband dielectric mirrors (BB1-E02, Thorlabs, Inc.) and a long-pass dichroic mirror (ZT488rdc, Chroma Technology Corp) before they are launched into the fluorescence cube (DFM1, Thorlabs, Inc.). (Note that the actual power output requirement for photometry is in the range of 10–100 μW, thus lower power CW lasers such as 20 mW versions at lower costs are sufficient for this application.) Neutral density filters (NEK01, Thorlabs, Inc.) are placed between the laser combiner and the fluorescence cube to provide additional adjustment for the final laser power that is launched into the cube. The combined 488 and 561 nm laser beams are reflected by a dichroic mirror (ZT488/561rpc, Chroma Technology Corp) and launched into the core of a 105/125 μm core/cladding multimode optical fiber patch cable (2.5 m long, lab modified from part # M43L02, Thorlabs) by an achromatic fiber port (PAFA-X-4-A, Thorlabs). The 1.25 mm OD ceramic ferrule at the distal end of the patch cable is connected to an implantable optical fiber probe (lab made using a 105/125 μm core/cladding multimode optical fiber and a 1.25 mm OD ceramic ferrule) by a ceramic spilt sleeve (SM-CS125S, Precision Fiber products Inc). The emission fluorescence collected from the fiber probe travels back along the patch cable, through the dichroic mirror, an emission filter (ZET488/561m, Chroma Technology Corp) in the fluorescence cube, and is launched into the core of an AR-coated 200/230 μm core/cladding multi-mode patch cable (M200L02S-A, Thorlabs) by an aspheric fiber port (PAF-SMA-11-A, Thorlabs) mounted on the fluorescence cube. The other end of the AR-coated multi-mode patch cable is connected to the entrance port of a spectrometer (QE Pro-FL, Ocean Optics, Inc). Inside the spectrometer, the emission light is collimated by a collimating mirror, dispersed by a diffraction grating, and refocused onto a linear CCD array by a focusing mirror. Spectral data were acquired by the software OceanView (Ocean Optics, Inc). During the revision of this study, a low cost, high speed (10 μs minimum integration time), high sensitivity CMOS spectrometer Ocean FX (Ocean Optics, Inc) became available. We compared the performance of Ocean FX with QE Pro-FL in our in vivo tests, and found that both spectrometers performed equally well at 25 and 50 Hz acquisition rates (Figure S5). However, Ocean FX outperformed QE Pro-FL in the maximum acquisition rate, and could obtain high quality spectra at 100 Hz and even 1 kHz acquisition rate, making it a better choice for working with fast fluorescence sensors.
Spectral linear unmixing algorithm
At time point n, the linear regression model used in this study is given by Yn = A + Co1*G + Co2*R + en, where Yn is the observed mixed spectrum, G and R are the normalized reference emission spectra of green and red fluorophores, respectively. In the model, A is the unknown constant, Co1 and Co2 are the unknown regression coefficients corresponding to the green and red channels, respectively. The random error associated with the model is denoted by en. Using the lm() function in the R/stats package, we estimated A, Co1, and Co2 at each time point.
Fiber photometry measurement of Alexa Fluor Dye mixtures
1 μl Alexa Fluor 488 (A-11039, Thermo Fisher Scientific Inc) and 1 μl Alexa Fluor 514 (A-31555 Thermo Fisher Scientific Inc) were added in a centrifuge tube containing 1 ml deionized water. A measurement was taken to obtain the emission spectrum with the volume ratio of Alexa Fluor 488: Alexa Fluor 514 = 1:1. Then the measurement was repeated three more times after 0.5 μl, 0.5 μl and 1 μl Alexa Fluor 488 were added to the tube to achieve the volume ratio of Alexa Fluor 488: Alexa Fluor 514 = 1.5:1, 2:1 and 3:1. The laser power measured at the tip of the optical fiber was 120 μW. The integration time was 50 ms for each spectrum. Five spectra were obtained for each concentration of the dye mixture.
Optical fiber probe implantation for in vivo fiber photometry recordings
One month after the AAV injection, mice underwent the second stereotaxic surgery to receive fiber probe implantation. One or two bur holes were drilled though the skull to target the dorsal striatum unilaterally or bilaterally (AP −0.5 mm, ML ±2.0 mm from Bregma) using a #1/2 (0.027″ Diameter) drill bit. Another pair of bur holes for anchoring screws were drilled bilaterally above the parietal lobes. After the anchoring screws were in place, the optical-fiber probe was slowly lowered onto the surface of the cortex through the burr hole, then further lowered towards the dorsal striatum at approximately 200 μm per step until the spectrum was detected. The probe was then lowered at 50 μm per step until the fluorescence intensity reached a plateau. The final tip location was approximately 1.8–2.2 mm below the brain surface. The probe was then fixed in place with a generous amount of dental acrylic (Jet, Lang Dental Mfg. Co., Inc.). The animals were allowed to recover for 2 weeks before experiments proceeded.
Fiber photometry measurement of GECI fluorescence in freely moving mice
The in vivo recordings in awake behaving mice were carried out in an open-top mouse operant chamber (21.6 × 17.8 × 12.7 cm, Med-Associates, Inc.) housed in a sound attenuating box. Fluorescence spectra were acquired using 11 ms integration time and were triggered by 50 Hz TTL pulses or using 19 ms integration time and were triggered by 25 Hz TTL pulses sent from a digital output module (DIG-726TTL, Med-associates, Inc.). A digital video camera (Grasshopper3 GS3-U3-23S6M-C, FLIR Integrated Imaging Solutions, Inc.), frame by frame triggered by the same TTL pulses, was used to capture the animal’s behavior. The output power of the 488 nm and 561 nm lasers measured at the end of patch cable was first set at 50 μW for each laser, then adjusted as needed based on the intensity of each emission spectrum. The final output power was never exceeding 120 μW. Under these conditions, we found that the jRGECO1a fluorescence showed significant fading during the recordings, likely due to reported photo-switching behavior of jRGECO1a and its parent protein mApple (Dana et al., 2016; Shaner et al., 2008). To correct for the fluorescence fading, we first applied single or double decay non-linear regression to fit the unmixed coefficients plotted over time, and then used the fitted curve as the theoretical baseline (Co0) to calculate ΔCo/Co0%. Z scores were calculated by z = (x − μ)/σ, where x is the ΔCo/Co0% at a given time, μ is the mean of ΔCo/Co0% values in a single recording session, and σ is the standard deviation of all ΔCo/Co0% values in the same recording session. The whole data processing procedure is described in Figure S6.
Intracortical electrical stimulation in anesthetized mice
Mice were anesthetized with 1–5% isoflurane and secured to a stereotaxic frame. An optical fiber probe was implanted into dorsal lateral striatum at: AP −0.5 mm, ML ±2.5 mm from Bregma, DV 2.4 mm from the brain surface. A twisted bipolar stainless-steel electrode (MS303/3-B/SPC, Plastics one) was implanted in the motor cortex (AP +1.2 mm, ML ±1.5 mm from Bregma, DV 1.1 mm from the brain surface) at 15-degree angle. Isoflurane was reduced to 0.5% for 10 min before the experiments began. A train of stimulating pulses (5 pulses at 50 Hz, 100–200 μs pulse duration, 0.5–1mA current intensity) were delivered every minute using a constant current isolated stimulator (DS3, Digitimer, Ltd) controlled by a waveform generator (SDG1025, Siglent technologies co.). The power of both 488 nm and 561nm lasers for photometry recording was set around 50 μW.
Behavior tracking and movement analysis
The animal’s behavior was captured by a digital video camera (Grasshopper3 GS3-U3-23S6M-C, FLIR Integrated Imaging Solutions, Inc.), which was triggered by the same TTL pulses that triggered the spectrometer. Recorded videos were opened with ImageJ (1.50b, National Institutes of Health, USA). The ‘multi-point’ tool was used to select and track the x-y coordinates of a “head point” and a ‘body point’ at each video frame. The bright spot (caused by laser light) at the junction between the patch cable and implanted ferrule on animal’s head was selected as the “head point”. The midpoint between two posterior limbs on the back was selected as the ‘body point’. The coordinates were given by the ‘measure’ function with pixels as the unit. To calculate the turning angle between frame ‘n−1’ and frame ‘n’, we defined u as the vector in frame ‘n−1’, v as the vector in frame ‘n’, α as the turning angle between frame ‘n−1’ and frame ‘n’.
sin (α) = u × v/(|u| * |v|). Positive sin (α) values indicate rightward turning, negative sin (α) values indicate leftward turning. Movement path heat maps were generated using EthoVision XT (Noldus Information Technology Inc).
Immunohistochemistry
Mouse brains were first fixed by transcardial perfusion of 4% PFA, and then stored in 4% PFA at 4°C overnight. Coronal or sagittal slices (35 μm) were cut using a vibratome (VT1200S, Leica) and blocked with 10% normal goat serum (Vector Labs) and 0.2% TritonX-100 (Sigma) for 1 hour at room temperature, followed by incubation in primary antibodies at 4°C overnight. The primary antibodies used were chicken anti-GFP (1:1000, ab13970, Abcam) for detecting GCaMP6f and eGFP, rabbit anti-RFP (1:1000, ab62341, Abcam) for detecting tdTomato and jRGECO1a, and rabbit anti-ChAT (1:1000, ab178850, Abcam). After washing out excessive primary antibodies, the slices were incubated in the secondary antibodies for 2 h at room temperature. The secondary antibodies used were Alexa Fluor 488 conjugated goat anti-chicken (1:500, A-11039, Invitrogen), Alexa Fluor 568 conjugated goat anti-rabbit (1:500, A-11036, Invitrogen) and goat anti rabbit Alexa Fluor 633 (1:1000, cat#1387814, Life Technologies). After thoroughly washing, the slices were mounted on slides and imaged on a Zeiss laser-scanning inverted confocal microscope (LSM 880) equipped with a multiline argon laser (458, 488 and 514nm) and two HeNe lasers (594 and 633nm) using an oil-immersion 40x objective (numerical aperture 1.3) and 20x objective (numerical aperture 0.8). The images were acquired and processed using Zen 2012 Black software (Carl Zeiss).
QUANTIFICATION AND STATISTICAL ANALYSIS
The auto-correlation and cross-correlation analysis was carried out using NeuroExplorer 5 (Nex Technologies) and GraphPad Prism 7 (GraphPad Software). Spectral linear unmixing was carried out using a customized program written in R. Linear, non-linear regression (single and double exponential decay for photo bleaching correction), one-way ANOVA followed by multiple comprisions test and t-test were carried out using GraphPad Prism 7 (GraphPad Software). Statistical data including means, sample numbers and significance values are indicated in either the text or figure legends. All error bars in the figures are SEM.
DATA AND SOFTWARE AVAILABILITY
Software
The customized spectral linear unmixing algorithm script written in R is available at https://www.niehs.nih.gov/research/atniehs/labs/ln/pi/iv/tools/index.cfm. Data analyses and software guidance are available upon request.
Supplementary Material
The mouse initiated a rightward movement and continued to move to the right. The corresponding fiber photometry recording of direct- and indirect-pathway neural activity from the left striatum is shown as ‘event b’ in Figure 6A–C.
The mouse initiated a rightward movement but stopped immediately and moved slightly back to the left. The corresponding fiber photometry recording of direct- and indirect-pathway neural activity from the left striatum is shown as ‘event a’ in Figure 6A–C.
Highlights.
Spectral unmixing of multi-color fluorescence signals in vivo
Versatile, inexpensive and easy to assemble from off-the-shelf parts
Dual-sensor recordings reveal synchronized activity between dual striatal pathways
Direct and indirect pathways coordinately control the dynamics and fate of movement
Acknowledgments
We thank Douglas Kim and the GENIE project of the Janelia Farm Research Campus of the Howard Hughes Medical Institute for the gifts of GCaMP6f and jRGECO1a plasmids, Bryan Roth for the gift of pAAV-hSyn-DIO vector plasmid, Bernardo Sabatini for the gift of lox-FAS plasmid, Bernd Gloss and NIEHS Viral Vector Core for packaging AAVs, Pattie Lamb for sharing AAV constructs, Jeff Tucker and Erica Scappini of NIEHS Fluorescence Microscopy and Imaging Center for assistance on fluorescence microscopy, Mingshan Xue for helpful discussions, David Armstrong and Rui Costa for comments on the manuscript. This work is supported by the Intramural Research Program of the NIH/NIEHS of the United States.
Footnotes
AUTHOR CONTRIBUTIONS
C.M. and J.Z. designed and carried out the experiments and analyzed data with equal contribution. A.P. made most of the viral constructs used in this study. T.P. wrote the program for applying the linear unmixing algorithm. T.P. and K.X. made FAS-GCaMP6f and FAS-jRGECO1a viral constructs with A.P.’s help. G.C. conceived and supervised the project. All authors contributed to the writing of the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Barbera G, Liang B, Zhang L, Gerfen CR, Culurciello E, Chen R, Li Y, Lin DT. Spatially Compact Neural Clusters in the Dorsal Striatum Encode Locomotion Relevant Information. Neuron. 2016;92:202–213. doi: 10.1016/j.neuron.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Luo G, Pham MD, Lovinger DM, Vogel SS, Costa RM. Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nature protocols. 2014;9:1213–1228. doi: 10.1038/nprot.2014.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, et al. Sensitive red protein calcium indicators for imaging neural activity. eLife. 2016;5 doi: 10.7554/eLife.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nature methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature neuroscience. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Huang C, Li JZ, Grewe BF, Zhang Y, Eismann S, Schnitzer MJ. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 2015;350:1361–1366. doi: 10.1126/science.aab0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN, Berndt A, Lee SY, Ramakrishnan C, et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nature methods. 2016;13:325–328. doi: 10.1038/nmeth.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PloS one. 2015;10:e0122513. doi: 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Yamawaki N, Wokosin DL, Wickersham IR, Shepherd GM, Surmeier DJ. Convergent cortical innervation of striatal projection neurons. Nature neuroscience. 2013;16:665–667. doi: 10.1038/nn.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tsien RW. pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nature neuroscience. 2012;15:1047–1053. doi: 10.1038/nn.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanov KA, Belousov VV. Genetically encoded fluorescent redox sensors. Biochimica et biophysica acta. 2014;1840:745–756. doi: 10.1016/j.bbagen.2013.05.030. [DOI] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Johnson CA, Sabatini BL. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Frontiers in neural circuits. 2012;6:47. doi: 10.3389/fncir.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Lin MZ, McKeown MR, Steinbach PA, Hazelwood KL, Davidson MW, Tsien RY. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nature methods. 2008;5:545–551. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Zhao Y, Chu H, Wang A, Zhu J, Chen X, Zou Y, Shi M, Liu R, Su N, et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nature methods. 2017;14:720–728. doi: 10.1038/nmeth.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Matias S, Dugue GP, Mainen ZF, Costa RM. Balanced activity in basal ganglia projection pathways is critical for contraversive movements. Nature communications. 2014;5:4315. doi: 10.1038/ncomms5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The mouse initiated a rightward movement and continued to move to the right. The corresponding fiber photometry recording of direct- and indirect-pathway neural activity from the left striatum is shown as ‘event b’ in Figure 6A–C.
The mouse initiated a rightward movement but stopped immediately and moved slightly back to the left. The corresponding fiber photometry recording of direct- and indirect-pathway neural activity from the left striatum is shown as ‘event a’ in Figure 6A–C.