Abstract
Since the emergence of cyprinid herpes virus 3 (CyHV-3), outbreaks have been devastating to koi and common carp leading to high economic losses. Current diagnostics for detecting CyHV-3 are limited in sensitivity and are further complicated by latency. Here we describe the detection of CyHV-3 by recombinase polymerase amplification (RPA). The RPA assay can detect as low as 10 copies of CyHV-3 genome by an isothermal reaction and yields results in approximately 20 minutes. Using the RPA assay, CyHV-3 genome can be detected in total DNA of white blood cells isolated from koi latently infected with CyHV-3, while less than 10% of the latently infected koi can be detected by a real-time PCR assay in total DNA of white blood cells. In addition, RPA products can be detected in a lateral flow device that is cheap, fast, and can be used outside of the diagnostic lab. The RPA assay and lateral flow device provide for the rapid, sensitive, and specific amplification of CyHV-3 that with future modifications for field use and validation could lead to enhanced surveillance and early diagnosis of CyHV-3 in the laboratory and field.
Introduction
Cyprinid herpes virus 3 (CyHV-3), also known as koi herpesvirus (KHV), is a highly pathogenic and contagious member of Alloherpesviridae (Pikarsky et al. 2004; Waltzek et al. 2009). Other closely related members of the group include carp pox herpesvirus (CyHV-1) and goldfish hematopoietic necrosis virus (CyHV-2). Initial reports describing the virus originated from Israel, the United States and Germany; however, CyHV-3 has since been reported worldwide (Bretzinger et al. 1999; Calle et al. 1999; Hedrick et al. 2000; Choi et al. 2004; Bondad-Reantaso et al. 2005; Grimmett et al. 2006; Garver et al. 2010; Marek et al. 2010; Taylor et al. 2010; Cheng et al. 2011; Avarre et al. 2012; Dong et al. 2013).
Productive infection occurs in several tissues/organs, including but not limited to gills, eyes, skin, kidney, heart, and brain (Gilad et al. 2004; Miyazaki et al. 2008). Based on the severe clinical manifestations of disease, mortality rates have been reported to be 80–100% (Hedrick et al. 2000; Dixon et al. 2009; Bergmann et al. 2010). As is consistent with other known herpesviruses, CyHV-3 can become latent in koi that recover from an initial viral infection (St-Hilaire et al. 2009; Eide et al. 2011). Latency is a hallmark of herpesvirus infection, and is characterized by the persistence of the viral genome within the host cells and the lack of virus particle production. The main site for latency of CyHV-3 has been identified to be white blood cells (WBC), specifically the B lymphocyte (Eide et al. 2011; Reed et al. 2014). Fish with latent CyHV-3 infection can live normally; however, they can shed infectious viruses and succumb to viral disease when they experience latency reactivation under stressful conditions. While the severity and consequences of disease have been devastating to the carp aquaculture industry as well as the ornamental koi trade, latency and reactivation highlight additional risk for subsequent reinfection of surviving populations and the spread to naïve groups of fish (St-Hilaire et al. 2005). Diagnostic methods to detect CyHV-3 must be able to detect the latent infection so that naïve populations are not unknowingly exposed to carriers, which would put them at risk of exposure to this deadly virus. Currently, no test is available that can detect latent infection. CyHV-3 becomes latent in less than 1% of cells in the peripheral white blood cells (Reed et al. 2015). Only a few copies of viral genome are present in the latently infected cells.
Several diagnostic methods have been employed to detect CyHV-3, including ELISA (St-Hilaire et al. 2009), immunohistochemistry based assays (Aoki et al. 2011; Tu et al. 2014), hybridization assays (Monaghan et al. 2015; Saleh and El-Matbouli 2015), numerous PCR assays (Gilad et al. 2002; Bercovier et al. 2005; Ishioka et al. 2005; El-Matbouli et al. 2007; Bergmann et al. 2010; Yuasa et al. 2012), and loop mediated isothermal amplification (Gunimaladevi et al. 2004; Soliman and El-Matbouli 2005, 2010; Yoshino et al. 2009). PCR-based strategies have been the “gold standard” as they are more sensitive than ELISA based methods and are less invasive than strategies requiring invasive tissue sampling, like many hybridization assays. However, these available assays all have limitations in analytical specificity and sensitivity. Currently available diagnostic tests, such as enzyme-linked immunosorbent assay (ELISA) and PCR, often misdiagnose koi that are CyHV-3 latently infected. New tests that are capable of detecting low copy numbers of CyHV-3 genome are needed to diagnose CyHV-3 carriers and therefore prevent the spread of the virus..
Lateral flow (immuno) assay/ immunochromatography is a technique with broad spectrum applications including the detection of nucleic acids (Posthuma-Trumpie et al. 2009). Nucleic acid detection is through primer sets with two different tags recognized in the lateral flow device by antibodies specific to the tags. As the sample is applied to a strip containing a polymeric material, it reacts with areas where specific antibody molecules have been affirmed to the strip resulting in a colorimetric signal. Each lateral flow device (LFD) has at least two lines, a test line where the sample is recognized and a control line to ensure the proper flow of sample through the strip (Posthuma-Trumpie et al. 2009). In this study, a commercially available LFD detects nucleic acid in less than 10 minutes and has a simple and straightforward protocol that can be easily used without extensive technical training.
Recombinase polymerase amplification (RPA) is an emerging method for the isothermal amplification of nucleic acid (Piepenburg et al. 2006), and has been employed for the detection of various pathogens (Euler et al. 2012a, 2012b; Abd El Wahed et al. 2013a, 2013b; Amer et al. 2013; Boyle et al. 2013, 2014; Ahmed et al. 2014; del Río et al. 2014; Jaroenram and Owens 2014; Krõlov et al. 2014; Mekuria et al. 2014; Tsaloglou et al. 2015; Xia et al. 2014, 2015; Zaghloul and El-Shahat 2014; Zhang et al. 2014). The RPA method utilizes three main enzymes to amplify template DNA: recombinases, single stranded binding protein (SSB), and a strand-displacing polymerase (Piepenburg et al. 2006). Exponential amplification of target amplicons proceeds by the use of two opposing oligonucleotide primers similar to PCR. The length of the RPA primers, >30 bp, is longer than those primers commonly used in traditional PCR; melting temperature differences are inconsequential because thermocycling is unnecessary for the RPA reaction to proceed. Amplification in a RPA reaction starts by primers forming a complex with recombinase enzymes that pair primers to their homologous template sequences (Piepenburg et al. 2006). SSB then bind to displaced template DNA strands and synthesis proceeds with a strand displacing polymerase (Piepenburg et al. 2006). RPA has been used as a rapid and sensitive method for amplifying nucleic acid of pathogens. In this study, we employed RPA to isothermally amplify CyHV-3 DNA from latently infected koi fish and detected the product using a lateral flow device. In addition, we directly compare the use of RPA to amplify CyHV-3 from latently infected koi to a PCR-based assay.
Methods
Koi sampling
Twelve adult koi fish with confirmed exposure to CyHV-3 were used in this study with no clinical signs of CyHV-3 infection within the past two years. One fish with true negative for CyHV-3 was used. We have confirmed the latency by RT-PCR and have been described in detail in our previously published paper (Reed et al 2015). To investigate whether CyHV-3 has any gene expression during latency, all eight open reading frames (ORFs) from the terminal repeat were investigated by RT-PCR (Fig. 1 and Table 1 Reed et al 2015). It was shown that the amplification from ORF1 to -5 and ORF7 to -8 was not observed. However, Only a spliced ORF6 transcript was found to be abundantly expressed in IgM+ WBC from CyHV-3 latently infected koi. All the 12 fish that were positive with RPA assay were true positives and one fish that was negative with RPA was true negative. Koi were maintained at the Oregon State University John L. Fryer Salmon Disease Laboratory (OSU-SDL) and blood samples were collected as previously described (Reed et al. 2014). White blood cells (WBC) were isolated by a Ficoll-Paque PLUS gradient according to the manufacturer’s instructions (GE Healthcare, United Kingdom).
Figure 1.
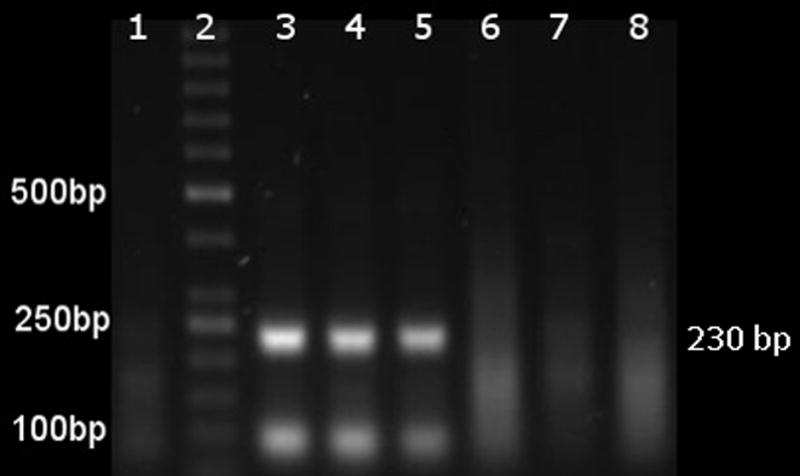
Electrophoresis of RPA amplification products following DNA purification. Products were run at 85 volts for 70 minutes in 1.5% of agarose in TAE buffer. The numbers in the left margin indicate the positions in base pairs for selected bands of the DNA marker (Thermo Scientific™ GeneRuler™ 50 bp DNA Ladder, 50–1000 bp).The number in the right margin indicates the predicted 230 bp size of RPA amplification. Lane 1 is the negative control (water as template), lane 2 is a 50 bp DNA ladder. Lanes 3–5 are RPA products from 100, 50, and 10 copies of CyHV-3 genomes respectively as template. Lane 6 is RPA product from a goldfish skin DNA, lane 7 is RPA product from goldfish WBC DNA, and lane 8 is RPA product using WBC DNA from a fish positive for Cyprinid herpesvirus 2.
Table 1.
RPA and quantitative Taqman PCR primers used for the detection of CyHV-3 genome.
| Name | Sequence (5′-3′) | 5′ modificationa |
|---|---|---|
| RPA | ||
| KHV-MCP2F | TTCTTCAAGCCGGACGCCTTCAACGTGCAGCG | FAM |
| KHV-MCP2R | TTCTCCAGGCGGCTCATGACGCTGGTGTTCTCGG | Biotin |
| Quantitative PCRb | ||
| KHV-86f | GACGCCGGAGACCTTGTG | |
| KHV-163r | CGGGTTCTTATTTTTGTCCTTGTT | |
| KHV-109p | CTTCCTCTGCTCGGCGAGCACG | FAM |
Modified primers were added for product detection via LFD.
Template preparations
The genomic DNA purified from strain CyHV-3 (KHV-U) was used as a positive control in this study. Briefly, Common carp brain cell line (CCB) was infected with KHV-U strain at a moi of 1.0. After characteristic KHV cytopathogenic effect (CPE) is observed, the supernatant was harvested and virus was purified by ultracentrifigation (Eide et al. 2011). Genomic DNA was extracted from the virus with a High Pure PCR Template Preparation Kit according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN, USA). CyHV-3 genomic DNA was diluted to 100, 50, and 10 copies per sample estimated by spectrophotometric assessment by NanoDrop. A minimum of seven replicates was tested for each dilution.
Koi white blood cells (WBC) were isolated on a Ficoll-Paque gradient from 2 ml of whole blood as described previously (Reed et al. 2014). Total DNA was extracted from WBC with the High Pure PCR template preparation kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions; DNA was eluted in nanopurified water. 0.5 μl of total extracted DNA (500 ng-1 mg) was used in the RPA assay as template.
To investigate the analytical specificity of the RPA assay samples known to be negative for CyHV-3 were used as control. Total DNA was extracted from a closely related cyprinid species, the goldfish (Carrasius auratus) which included WBC and skin samples. Additionally, a negative control sample of total DNA from a goldfish with confirmed Cyprinid herpesvirus 2 (CyHV-2) infection was also used as negative control.
Primer design
Primers suitable to RPA amplification were designed based on the CyHV-3 reference sequence (NCBI accession no. NC_009127.1). The primers were selected based on 100% conserved sequence of major capsid protein gene of all the Cyprinid herpesvirus 3 strains: reference (NC_009127), KHV-GZ11, TUMST1, KHV-U, KHV-I, Israel and FL BAC that are available from NCBI gene bank. The major capsid gene is highly conserved among the CyHV-3 strains and this was the basis for selection of this gene for primer sequence. The forward primer (32bp long) matched 12 and 13 nucleotide bases with CyHV-1 (NG-J1 strain) and CyHV-2 (ST-J1 strain) and the reverse primer (34bp long) matched 11 and 13 bases with CyHV-1 (NG-J1 strain) and CyHV-2 (ST-J1 strain), respectively. RPA primers were designed to detect a 230 bp region of the major capsid protein of CyHV-3 (Table 1). The primers for quantitative TaqMan PCR were selected as previously described (Table 1) (Gilad et al. 2004). For product detection on a lateral flow device, a 5′ modification of either FAM or biotin was added to each original RPA primer. The modified primers were synthesized by Thermo Fisher Scientific (Waltham, MA) and unmodified primers were synthesized by Eurofins MWG Operon (Huntsville, AL).
Quantitative PCR
To detect and quantify CyHV-3 genome from each koi, ~500 ng of total DNA extracted from WBC was used as template in quantitative PCR with primers KHV-86f and KHV-163r and fluorescently labeled TaqMan probe KHV-109p as previously described (Gilad et al. 2004; Reed et al. 2014). Briefly, amplification was performed on the Bio-Rad CFX96 thermocycler (Bio-Rad Laboratories, Hercules, CA) using a 25-μl reaction mixture consisting of 12.5 μl Platinum quantitative PCR (qPCR) Supermix-UDG with ROX (Invitrogen, Carlsbad, CA), 0.5 μl of each primer (20 nM) and probe (10 mM), and 5 μl (approximately 1 μg total) of DNA template; the reaction mixture was subjected to the following steps: (i) 50°C for 2 min; (ii) 95°C for 2 min; (iii) 60 cycles, with 1 cycle consisting of 95°C for 15 s and 60°C for 60 s (a slower ramp time was adjusted to 2°C/s to facilitate higher sensitivity). Genome copy numbers from real-time PCR were extrapolated from a standard curve established by using 10-fold serial dilutions of CyHV-3 DNA from 109 to 105 genome copies. Samples without the template was used as a negative control. Data analysis was performed using the associated CFX Management Software suite (Bio-Rad Laboratories, Hercules, CA).
RPA assay
To detect CyHV-3 genome in koi WBC using RPA, the TwistAmp Basic kit (TwistDx, LTd., Cambridge, United Kingdom) with the RPA specific primers (Table 1) were utilized. The RPA assay contained a lyophilized pellet premeasured and distributed by the manufacturer that was dissolved using a mix of 29.5 μl rehydration buffer, 0.5 μL DNA template, 3 μl primers (300 nM each) and 14.5 μl water to a final reaction volume of 50 μl. Magnesium acetate (2.5 μl, 280 nM) was used to initiate the RPA reaction as described in the manufacturer’s instructions. A negative control (water with no DNA) sample was included in each run along with a positive control for assay runs involving koi WBC DNA samples, which were run in duplicate. The reaction was conducted at 37°C (unless otherwise specified) on a heat block for 20 minutes and purified using a GeneJET DNA purification kit (Thermo Fisher Scientific, Waltham, MA) for samples observed by gel electrophoresis.
To confirm the correct DNA product was amplified, Sanger sequencing was performed at the Center for Genome Research and Biocomputing (CGRB) at Oregon State University. Following gel electrophoresis, selected bands of the approximated product size were visualized on an LED blue light transilluminator and were excised using a razor blade. DNA was purified using a GeneJet kit eluted using diH2O. Sequencing results were aligned to the predicted CyHV-3 sequence by pairwise alignment tool. Amplification products were detected by either visualization by gel electrophoresis on a 1.5% agarose gel stained with SYBR Safe (Life Technologies, Carlsbad, CA) following DNA purification or by lateral flow strip detection (LFD).
Lateral Flow Device application
For rapid detection of CyHV-3 genome, RPA product was visualized using a lateral flow device. Briefly, 5′ modified primers (Table 1) were substituted for original RPA primers in the RPA assay, and end products now labeled with both FAM and biotin were diluted with supplied buffer and the diluted product was applied onto the PCRD-2 strips (Forsite Diagnostics, United Kingdom). Results were assessed by visual detection of colorimetric signal on the lateral flow strip which detects the combination of the 5′ primer modifications, FAM and biotin, by anti-FAM and anti-biotin antibodies that are immobilized together at the test line (T-line). A control line (C-line) is incorporated as a flow check control. Negative results are indicated on the strip by a single line at the C-line position and no color detected on the T-line. Positive results are indicated by lines at both the C- and T-line positions. Strips that do not develop a line on the C-line are discarded as faulty.
Results
Detection of CyHV-3 using the RPA assay
To test the ability of the RPA assay to detect CyHV-3 DNA, DNA purified from the KHV-U strain at 100, 50, and 10 copies was used as template in the RPA assay. As shown in Figure 1, an approximately ~230 bp product was detected in reaction with templates at 100, 50, and 10 copies of CyHV-3 genomic DNA (Figure 1, lanes 3, 4, and 5 respectively). No band was seen from reactions without CyHV-3 DNA (nanopure water) when ran in parallel to CyHV-3 DNA samples (Figure 1 lane 1, Table 2). This result was consistent with all replicates for each of the dilutions tested. To confirm the amplification is specific for the CyHV-3 genome, RPA products were examined by Sanger sequencing. Based on sequence data, all the RPA products showed 100% sequence identity to the CyHV-3 reference DNA. To test the RPA analytical specificity, templates from CyHV-2 and total DNA of goldfish (Carrasius auratus), which is known to be CyHV-3 free, were also evaluated (Figure 1, Table 2). As shown in Figure 1, lanes 7–8, no product was amplified from any of the negative control samples, which suggests the primers selected for CyHV-3 is specific for the virus. Our results demonstrated that CyHV-3 RPA is specific and capable of detecting viral genome copy number as low as 10 copies.
Table 2.
Sensitivity and Specificity of RPA assay for the detection of CyHV-3
| Sample | Detection of CyHV3 DNA by RPA assay |
|---|---|
| CyHV-3 genome: 100 copies | Positive |
| CyHV-3 genome: 50 copies | Positive |
| CyHV-3 genome: 10 copies | Positive |
| Water (no DNA) | Negative |
| Goldfish skin | Negative |
| Goldfish WBC | Negative |
| CyHV-2 positive goldfish WBC | Negative |
Evaluation of temperature effects on RPA amplification of CyHV-3
To investigate the range of temperatures in which the RPA assay can be performed, positive control DNA was diluted to 10 copies per reaction and the RPA assay was run at temperatures of 41°C, 39°C, 35°C, 33°C, as well as at the manufacturer’s recommendation standard of 37°C . As seen in Figure 2, product was detected at all temperatures, confirming that the RPA amplification of CyHV-3 DNA was able to amplify across the temperature range of 33–41°C.
Figure 2.
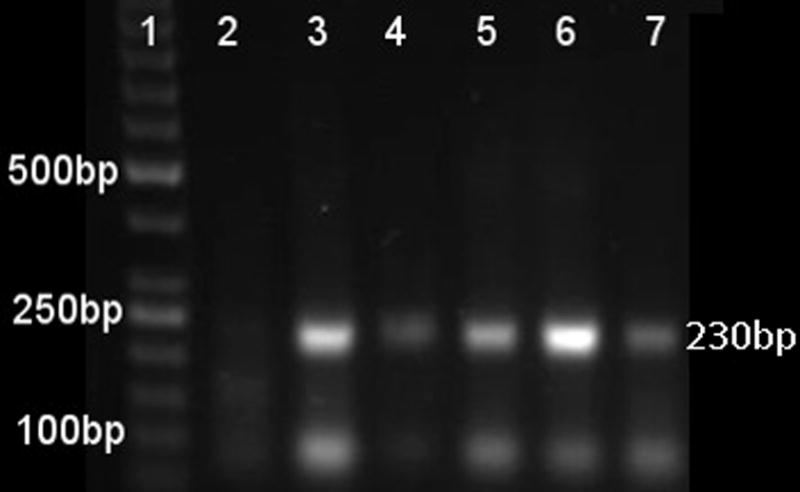
Reaction temperature range of RPA amplification of CyHV-3. The numbers in the left margin indicate selected bands of the DNA marker, and the estimated amplified product size is indicated at 230 bp in the right margin. Lane 1 is a 50bp DNA ladder (Thermo Scientific™ GeneRuler™ 50 bp DNA Ladder, 50–1000 bp), lane 2 is the negative control (water as template). Lanes 3–7 represent RPA amplification products from 10 copies of CyHV-3 genome per reaction at temperatures 41°C, 39°C, 37°C, 35°C, and 33°C respectively.
Detection of CyHV-3 by RPA assay in latently infected koi
To determine the ability of the RPA assay to detect latent CyHV-3 infection in koi, total DNA of WBC from latently infected koi was evaluated by the RPA assay. As shown in Figure 3, CyHV-3 DNA was amplified from 12 of the 12 true positive samples in all replicates by the RPA assay. All fish samples were tested by the RPA assay with a minimum of two replicates using total DNA (500ng-1mg) in each reaction. The same samples of DNA from each of the fish were also analyzed by quantitative TaqMan PCR, where only one sample out of 12 positive samples was tested positive. One out of 13 fish, which was true negative, gave negative result with both RPA and qPCR. Amplified products from three samples were selected for further confirmation of RPA results by Sanger sequencing. As confirmed by sequencing, all RPA products from three different koi have DNA sequence with 100% identity to the CyHV-3 genome (NCBI accession no. NC_009127.1). This result demonstrated that the RPA assay is capable of detecting CyHV-3 DNA in latently infected fish and has a greater analytical sensitivity than the quantitative PCR as described previously (Gilad et al. 2002).
Figure 3.
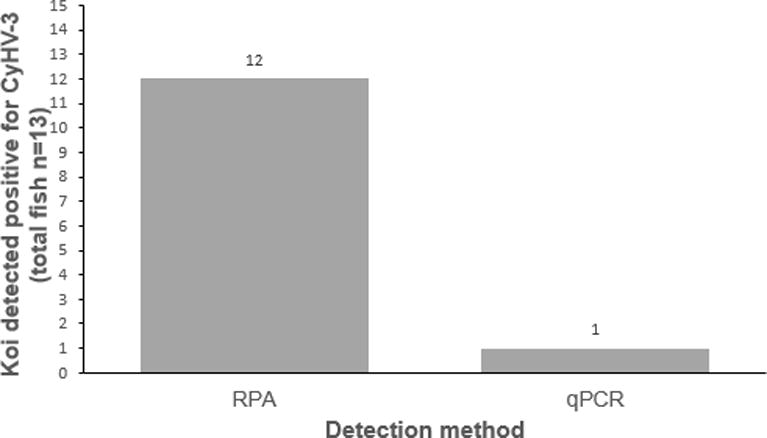
Summary of positive CyHV-3 detection from latently infected koi. Detection of DNA extracted from WBC samples (n=13) from exposed or latently infected koi using RPA and quantitative PCR (qPCR).
Detection of CyHV-3 RPA products on a Lateral Flow Device
To test the application of this CyHV-3 RPA assay as a rapid detection assay, RPA products were applied to a lateral flow device. The RPA products from six koi WBC DNA samples were added to a LFD containing PCRD-2 strips as well as negative control (RPA product amplified with no DNA template) and positive control (RPA product amplified from 50 copies KHV-U DNA) products. As seen in Figure 4, a positive colorimetric signal from the RPA reaction was detected on a LFD from all latently infected koi and not from the negative control sample. The same RPA products showed a positive band by gel electrophoresis for all latent WBC samples. This data demonstrates detection of purified RPA products by LFD as an alternative to visualization by gel electrophoresis.
Figure 4.
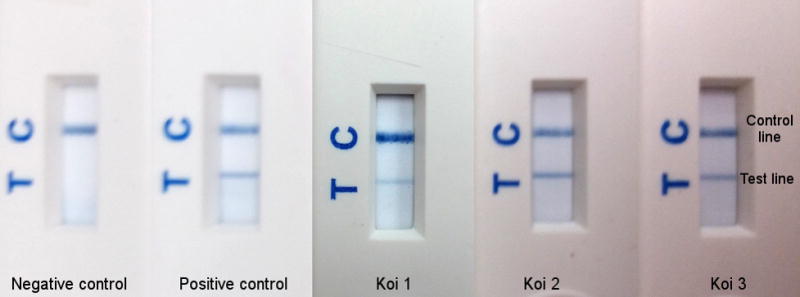
Detection of CyHV-3 product from RPA assay in lateral flow detection strips. The control line is labeled as C which indicates the strip is working as predicted. The test line is labeled as T which indicates the presence of doubly–labeled (with FAM and biotin) amplified product. Samples from left to right: Negative control: water as template, positive control: 50 copies of KHV-U, Koi 1–3: three representative products amplified from total DNA of WBC DNA from latently infected koi.
Discussion
The need for a highly sensitive assay capable of detecting latent infections is paramount as undiagnosed latent fish could be introduced to naïve populations leading to subsequent infection, loss of fish, and monetary losses. The RPA assay we report herein is a rapid and robust alternative test for detecting CyHV-3. This RPA assay is specific to CyHV-3 and is capable of detecting as little as 10 copies of CyHV-3 genome (Figure 1, Table 2). The RPA assay consistently and repeatedly amplified CyHV-3 genome, while negative control reactions remained unamplified. The assay successfully detected CyHV-3 DNA across the temperature range of 33–41°C, suggesting that the RPA assay can be performed at any one temperature without a thermocycler (Figure 2). Using the RPA assay, CyHV-3 was detected in all previously confirmed latently infected fish, and provided a more sensitive detection of latently infected koi as directly compared to quantitative PCR (Figure 3). In addition, we illustrated the ability of the end products to be visualized by LFD detection (Figure 4). The data we present demonstrated the advantage and resilience of using RPA in conjunction with LFD to detect CyHV-3.
RPA assay identified all 12 latently infected fish. A true negative fish was negative with Taqman PCR, qPCR and RPA methods. While the 12 confirmed latently infected fish used in this study had previously been positively identified by PCR strategies, in this study, only one was identified as positive by detection with quantitative PCR. This can be explained in several ways as the quality of DNA extraction could have varied from previous samples tested or the circulating genomic copies may fluctuate over time. The discrepancy in the detection of latent fish between qPCR and RPA methods may have been due to inhibitors in the DNA prep. RPA has been shown to be highly resistant to crude samples in comparison to PCR and in addition, RPA works on a wide range of temperatures. We believe PCR is more sensitive to inhibitors as compared to RPA and this may have affected the results. However, the direct comparison of RPA with quantitative PCR demonstrates that RPA is a much more sensitive assay for detecting latent CyHV-3 infections.
RPA is an isothermal method of DNA amplification; therefore, it can be performed at one constant temperature and expensive machinery necessary to perform thermocyling is not required. RPA produces reliable results at a wide range of temperatures, further demonstrating its robust nature and highlighting its flexibility over traditional PCR strategies. The optimum reaction temperature of the RPA enzymes is near 37°C and although our assay is successful at a range of temperatures, running at 37°C will increase the chance of optimum enzyme performance. Ideally a diagnostic assay could be performed by minimally trained technicians in a patient-side manner and produce results as quickly as possible to prevent translocation of potentially infected fish and subsequent transmission of the disease.
We have shown that LFD technology can be used in conjunction with our RPA assay demonstrating the potential development of RPA as a field assay. Detection by LFD adds less than 10 minutes to the already rapid assay time and omits the use of expensive quantitative machinery associated with real time detection strategies and gel electrophoresis equipment otherwise necessary to visualize the amplified product. As an alternative to lateral flow device, the dynamic nature of the RPA assay allows for the addition of a fluorescent probe to detect amplified results in real time. The speed, analytical sensitivity, and overall dynamic nature of the RPA assay highlight a viable option for future development of a CyHV-3 specific rapid diagnostic test capable of detection in an onsite manner. In future developments we would like to improve upon the handling and DNA extraction process for a more accessible protocol which would decrease detection time and further eliminate technical equipment needed to adequately employ this assay in the field.
RPA detection of CyHV-3 has many advantages when compared to other detection methods. An ELISA based detection assay has been reported to be less sensitive than TaqMan PCR (Eide et al. 2011)); ELISA detects whether the fish has been exposed to the infection but may not detect the carrier or latent infection. in a direct comparison, this RPA assay is more sensitive than quantitative PCR assay as latent CyHV-3 was detected in more fish using RPA than qPCR. In addition to analytical sensitivity, RPA compared to other genomic detection strategies has additional advantages. RPA is faster (amplification in about 20 minutes), and ease of performance is equal or greater than PCR, with less expensive and sophisticated equipment required. Fluctuations in temperature have little effect on the results and a wide range of temperatures provides flexibility and will lead to more successful application of the diagnostic test. RPA has true promise for further development of a rapid, affordable, easy, and sensitive diagnostic test to detect CyHV-3.
Acknowledgments
Aimee Reed was supported by the Office of the Director of the National Institutes of Health T32 training grant RR023917 and T32OD011020. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Ronald Hedrick (University of California, Davis) for providing the KHV-U strain used in this study.
References
- Abd El Wahed A, El-Deeb A, El-Tholoth M, Abd El Kader H, Ahmed A, Hassan S, Hoffmann B, Haas B, Shalaby MA, Hufert FT, Weidmann M. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS One. 2013;8:e71642. doi: 10.1371/journal.pone.0071642. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23977101 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El Wahed A, Patel P, Heidenreich D, Hufert FT, Weidmann M. Reverse transcription recombinase polymerase amplification assay for the detection of middle East respiratory syndrome coronavirus. PLoS Currents. 2013;5 doi: 10.1371/currents.outbreaks.62df1c7c75ffc96cd59034531e2e8364. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24459611 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, van der Linden H, Hartskeerl RA. Development of a recombinase polymerase amplification assay for the detection of pathogenic Leptospira. International Journal of Environmental Rersearch and Public Health. 2014;11:4953–4964. doi: 10.3390/ijerph110504953. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24814943 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer HM, Abd El Wahed A, Shalaby MA, Almajhdi FN, Hufert FT, Weidmann M. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. Journal of Virological Methods. 2013;193:337–340. doi: 10.1016/j.jviromet.2013.06.027. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23811231 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Takano T, Unajak S, Takagi M, Kim YR, Park SB, Kondo H, Hirono I, Saito-Taki T, Hikima J, Jung TS. Generation of monoclonal antibodies specific for ORF68 of koi herpesvirus. Comparative Immunology. Microbiology and Infectious Diseases. 2011;34:209–216. doi: 10.1016/j.cimid.2010.11.004. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/21134694 >. [DOI] [PubMed] [Google Scholar]
- Avarre JC, Santika A, Bentenni A, Zainun Z, Madeira JP, Maskur M, Bigarré L, Caruso D. Spatio-temporal analysis of cyprinid herpesvirus 3 genetic diversity at a local scale. Journal of Fish Diseases. 2012;35:767–774. doi: 10.1111/j.1365-2761.2012.01404.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/22805046 >. [DOI] [PubMed] [Google Scholar]
- Bercovier H, Fishman Y, Nahary R, Sinai S, Zlotkin A, Eyngor M, Gilad O, Eldar A, Hedrick RP. Cloning of the koi herpesvirus (KHV) gene encoding thymidine kinase and its use for a highly sensitive PCR based diagnosis. BMC Microbiology. 2005;5:13. doi: 10.1186/1471-2180-5-13. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15774009 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann SM, Riechardt M, Fichtner D, Lee P, Kempter J. Investigation on the diagnostic sensitivity of molecular tools used for detection of koi herpesvirus. Journal of Virological Methods. 2010;163:229–233. doi: 10.1016/j.jviromet.2009.09.025. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19819263 >. [DOI] [PubMed] [Google Scholar]
- Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M. Disease and health management in Asian aquaculture. Veterinary Parasitology. 2005;132:249–272. doi: 10.1016/j.vetpar.2005.07.005. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16099592 >. [DOI] [PubMed] [Google Scholar]
- Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, Parker M, Piepenburg O, Overbaugh J. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. MBio. 2013:4. doi: 10.1128/mBio.00135-13. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23549916 >. [DOI] [PMC free article] [PubMed]
- Boyle DS, McNerney R, Teng Low H, Leader BT, Pérez-Osorio AC, Meyer JC, O’Sullivan DM, Brooks DG, Piepenburg O, Forrest MS. Rapid detection of Mycobacterium tuberculosis by recombinase polymerase amplification. PLoS One. 2014;9:e103091. doi: 10.1371/journal.pone.0103091. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25118698 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretzinger A, fischer-Scherl T, Oumouma M, Hoffmann R, Truyen U. Mass mortalities in koi, Cyprinus carpio, associated with gill and skin disease. Bulletin of the European Association of Fish Pathologists. 1999;19:182–185. [Google Scholar]
- Calle PP, McNamara T, Kress Y. Herpesvirus-associated papillomas in koi carp (Cyprinus carpio) Journal of Zoo and Wildlife Medicine. 1999;30:165–169. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/10367660 >. [PubMed] [Google Scholar]
- Cheng L, Chen CY, Tsai MA, Wang PC, Hsu JP, Chern RS, Chen SC. Koi herpesvirus epizootic in cultured carp and koi, Cyprinus carpio L., in Taiwan. Journal of Fish Diseases. 2011;34:547–554. doi: 10.1111/j.1365-2761.2011.01266.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/21675996 >. [DOI] [PubMed] [Google Scholar]
- Choi DL, Sohn SG, Bang JD, Do JW, Park MS. Ultrastructural identification of a herpes-like virus infection in common carp Cyprinus carpio in Korea. Diseases of Aquatic Organisms. 2004;61:165–168. doi: 10.3354/dao061165. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15584424 >. [DOI] [PubMed] [Google Scholar]
- del Río JS, Yehia Adly N, Acero-Sánchez JL, Henry OY, O’Sullivan CK. Electrochemical detection of Francisella tularensis genomic DNA using solid-phase recombinase polymerase amplification. Biosensors and Bioelectronics. 2014;54:674–678. doi: 10.1016/j.bios.2013.11.035. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24334283 >. [DOI] [PubMed] [Google Scholar]
- Dixon PF, Joiner CL, Way K, Reese RA, Jeney G, Jeney Z. Comparison of the resistance of selected families of common carp, Cyprinus carpio L., to koi herpesvirus: preliminary study. Journal of Fish Diseases. 2009;32:1035–1039. doi: 10.1111/j.1365-2761.2009.01081.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19602097 >. [DOI] [PubMed] [Google Scholar]
- Dong C, Li X, Weng S, Xie S, He J. Emergence of fatal European genotype CyHV-3/KHV in mainland China. Veterinary Microbiology. 2013;162:239–244. doi: 10.1016/j.vetmic.2012.10.024. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23146169 >. [DOI] [PubMed] [Google Scholar]
- Eide KE, Miller-Morgan T, Heidel JR, Kent ML, Bildfell RJ, Lapatra S, Watson G, Jin L. Investigation of koi herpesvirus latency in koi. Journal of Virology. 2011;85:4954–4962. doi: 10.1128/JVI.01384-10. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/21389134 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Matbouli M, Rucker U, Soliman H. Detection of Cyprinid herpesvirus-3 (CyHV-3) DNA in infected fish tissues by nested polymerase chain reaction. Diseases of Aquatic Organisms. 2007;78:23–28. doi: 10.3354/dao01858. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/18159669 >. [DOI] [PubMed] [Google Scholar]
- Euler M, Wang Y, Nentwich O, Piepenburg O, Hufert FT, Weidmann M. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. Journal of Clinical Virology. 2012;54:308–312. doi: 10.1016/j.jcv.2012.05.006. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/22683006 >. [DOI] [PubMed] [Google Scholar]
- Euler M, Wang Y, Otto P, Tomaso H, Escudero R, Anda P, Hufert FT, Weidmann M. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. Journal of Clinical Microbiology. 2012;50:2234–2238. doi: 10.1128/JCM.06504-11. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/22518861 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver KA, Al-Hussinee L, Hawley LM, Schroeder T, Edes S, LePage V, Contador E, Russell S, Lord S, Stevenson RM, Souter B, Wright E, Lumsden JS. Mass mortality associated with koi herpesvirus in wild common carp in Canada. Journal of Wildlife Diseases. 2010;46:1242–1251. doi: 10.7589/0090-3558-46.4.1242. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/20966274 >. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Andree KB, Adkison MA, Zlotkin A, Bercovier H, Eldar A, Hedrick RP. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Diseases of Aquatic Organisms. 2002;48:101–108. doi: 10.3354/dao048101. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/12005231 >. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Andree KB, Adkison MA, Zlotkin A, Bercovier H, Eldar A, Hedrick RP. Concentrations of a Koi herpesvirus (KHV) in tissues of experimentally infected Cyprinus carpio koi as assessed by real-time TaqMan PCR. Diseases of Aquatic Organisms. 2004;60:179–187. doi: 10.3354/dao060179. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15521316 >. [DOI] [PubMed] [Google Scholar]
- Grimmett SG, Warg JV, Getchell RG, Johnson DJ, Bowser PR. An unusual koi herpesvirus associated with a mortality event of common carp Cyprinus carpio in New York State, USA. Journal of Wildlife Diseases. 2006;42:658–662. doi: 10.7589/0090-3558-42.3.658. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/17092898 >. [DOI] [PubMed] [Google Scholar]
- Gunimaladevi I, Kono T, Venugopal MN, Sakai M. Detection of koi herpesvirus in common carp, Cyprinus carpio L., by loop-mediated isothermal amplification. Journal of Fish Diseases. 2004;27:583–589. doi: 10.1111/j.1365-2761.2004.00578.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15482423 >. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, Yun Gilad O, Spangenberg JVS, Marty GD, Nordhausen RW, Kebus MJ, Bercovier H, Eldar A. A Herpesvirus Associated with Mass Mortality of Juvenile and Adult Koi, a Strain of Common Carp. Journal of Aquatic Animal Health. 2000;12:44–57. doi: 10.1577/1548-8667(2000)012<0044:AHAWMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ishioka T, Yoshizumi M, Izumi S, Suzuki K, Suzuki H, Kozawa K, Arai M, Nobusawa K, Morita Y, Kato M, Hoshino T, Iida T, Kosuge K, Kimura H. Detection and sequence analysis of DNA polymerase and major envelope protein genes in koi herpesviruses derived from Cyprinus carpio in Gunma prefecture, Japan. Veterinary Microbiology. 2005;110:27–33. doi: 10.1016/j.vetmic.2005.07.002. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16125879 >. [DOI] [PubMed] [Google Scholar]
- Jaroenram W, Owens L. Recombinase polymerase amplification combined with a lateral flow dipstick for discriminating between infectious Penaeus stylirostris densovirus and virus-related sequences in shrimp genome. Journal of Virological Methods. 2014;208:144–151. doi: 10.1016/j.jviromet.2014.08.006. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25152528 >. [DOI] [PubMed] [Google Scholar]
- Krõlov K, Frolova J, Tudoran O, Suhorutsenko J, Lehto T, Sibul H, Mäger I, Laanpere M, Tulp I, Langel Ü. Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples. The Journal of Molecular Diagnostics. 2014;16:127–135. doi: 10.1016/j.jmoldx.2013.08.003. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24331366 >. [DOI] [PubMed] [Google Scholar]
- Marek A, Schachner O, Bilic I, Hess M. Characterization of Austrian koi herpesvirus samples based on the ORF40 region. Diseases of Aquatic Organisms. 2010;88:267–270. doi: 10.3354/dao02170. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/20377015 >. [DOI] [PubMed] [Google Scholar]
- Mekuria TA, Zhang S, Eastwell KC. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. Journal of Virological Methods. 2014;205C:24–30. doi: 10.1016/j.jviromet.2014.04.015. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24797461 >. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kuzuya Y, Yasumoto S, Yasuda M, Kobayashi T. Histopathological and ultrastructural features of Koi herpesvirus (KHV)-infected carp Cyprinus carpio, and the morphology and morphogenesis of KHV. Diseases of Aquatic Organisms. 2008;80:1–11. doi: 10.3354/dao01929. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/18714678 >. [DOI] [PubMed] [Google Scholar]
- Monaghan SJ, Thompson KD, Adams A, Kempter J, Bergmann SM. Examination of the early infection stages of koi herpesvirus (KHV) in experimentally infected carp, Cyprinus carpio L. using in situ hybridization. Journal of Fish Diseases. 2015;38:477–489. doi: 10.1111/jfd.12260. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24925228 >. [DOI] [PubMed] [Google Scholar]
- Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biology. 2006;4:e204. doi: 10.1371/journal.pbio.0040204. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16756388 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikarsky E, Ronen A, Abramowitz J, Levavi-Sivan B, Hutoran M, Shapira Y, Steinitz M, Perelberg A, Soffer D, Kotler M. Pathogenesis of acute viral disease induced in fish by carp interstitial nephritis and gill necrosis virus. Journal of Virology. 2004;78:9544–9551. doi: 10.1128/JVI.78.17.9544-9551.2004. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15308746 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Analytical Bioanalytical Chemistry. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/18696055 >. [DOI] [PubMed] [Google Scholar]
- Reed AN, Izume S, Dolan BP, LaPatra S, Kent M, Dong J, Jin L. Identification of B cells as a major site for cyprinid herpesvirus 3 latency. Journal of Virology. 2014;88:9297–9309. doi: 10.1128/JVI.00990-14. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24899202 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AN, Izume S, Dolan BP, LaPatra S, Kent M, Dong J, Jin L. Application of a nanoflare probe specific to a latency associated transcript for isolation of KHV latently infected cells. Virus Research. 2015;208:129–135. doi: 10.1016/j.virusres.2015.06.003. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/26087404 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, El-Matbouli M. Rapid detection of Cyprinid herpesvirus-3 (CyHV-3) using a gold nanoparticle-based hybridization assay. Journal of Virological Methods. 2015;217:50–54. doi: 10.1016/j.jviromet.2015.02.021. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25738211 >. [DOI] [PubMed] [Google Scholar]
- Soliman H, El-Matbouli M. An inexpensive and rapid diagnostic method of Koi Herpesvirus (KHV) infection by loop-mediated isothermal amplification. Virology Journal. 2005;2:83. doi: 10.1186/1743-422X-2-83. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16216123 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman H, El-Matbouli M. Loop mediated isothermal amplification combined with nucleic acid lateral flow strip for diagnosis of cyprinid herpes virus-3. Molecular and Cellular Probes. 2010;24:38–43. doi: 10.1016/j.mcp.2009.09.002. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19781627 >. [DOI] [PubMed] [Google Scholar]
- St-Hilaire S, Beevers N, Joiner C, Hedrick RP, Way K. Antibody response of two populations of common carp, Cyprinus carpio L., exposed to koi herpesvirus. Journal of Fish Diseases. 2009;32:311–320. doi: 10.1111/j.1365-2761.2008.00993.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19236553 >. [DOI] [PubMed] [Google Scholar]
- St-Hilaire S, Beevers N, Way K, Le Deuff RM, Martin P, Joiner C. Reactivation of koi herpesvirus infections in common carp Cyprinus carpio. Diseases of Aquatic Organisms. 2005;67:15–23. doi: 10.3354/dao067015. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16385803 >. [DOI] [PubMed] [Google Scholar]
- Taylor NG, Dixon PF, Jeffery KR, Peeler EJ, Denham KL, Way K. Koi herpesvirus: distribution and prospects for control in England and Wales. Journal of Fish Diseases. 2010;33:221–230. doi: 10.1111/j.1365-2761.2009.01111.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19878413 >. [DOI] [PubMed] [Google Scholar]
- Tsaloglou MN, Watson RJ, Rushworth CM, Zhao Y, Niu X, Sutton JM, Morgan H. Real-time microfluidic recombinase polymerase amplification for the toxin B gene of Clostridium difficile on a SlipChip platform. Analyst. 2015;140:258–264. doi: 10.1039/c4an01683a. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25371968 >. [DOI] [PubMed] [Google Scholar]
- Tu C, Lu YP, Hsieh CY, Huang SM, Chang SK, Chen MM. Production of monoclonal antibody against ORF72 of koi herpesvirus isolated in Taiwan. Folia Microbioligica (Praha) 2014;59:159–165. doi: 10.1007/s12223-013-0261-7. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24046233 >. [DOI] [PubMed] [Google Scholar]
- Waltzek TB, Kelley GO, Alfaro ME, Kurobe T, Davison AJ, Hedrick RP. Phylogenetic relationships in the family Alloherpesviridae. Diseases of Aquatic Organisms. 2009;84:179–194. doi: 10.3354/dao02023. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19565695 >. [DOI] [PubMed] [Google Scholar]
- Xia X, Yu Y, Hu L, Weidmann M, Pan Y, Yan S, Wang Y. 2015. Rapid detection of infectious hypodermal and hematopoietic necrosis virus IHHNV by real-time, isothermal recombinase polymerase amplification assay. Archives of Virology. 2015;160:987–994. doi: 10.1007/s00705-015-2357-7. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25655264 >. [DOI] [PubMed] [Google Scholar]
- Xia X, Yu Y, Weidmann M, Pan Y, Yan S, Wang Y. Rapid detection of shrimp white spot syndrome virus by real time, isothermal recombinase polymerase amplification assay. PLoS One. 2014;9:e104667. doi: 10.1371/journal.pone.0104667. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25121957 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M, Watari H, Kojima T, Ikedo M, Kurita J. Rapid, sensitive and simple detection method for koi herpesvirus using loop-mediated isothermal amplification. Microbiology and Immunology. 2009;53:375–383. doi: 10.1111/j.1348-0421.2009.00145.x. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19563396 >. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Kurita J, Kawana M, Kiryu I, Oseko N, Sano M. Development of mRNA-specific RT-PCR for the detection of koi herpesvirus (KHV) replication stage. Diseases of Aquatic Organisms. 2012;100:11–18. doi: 10.3354/dao02499. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/22885509 >. [DOI] [PubMed] [Google Scholar]
- Zaghloul H, El-Shahat M. Recombinase polymerase amplification as a promising tool in hepatitis C virus diagnosis. World Journal of Hepatology. 2014;6:916–922. doi: 10.4254/wjh.v6.i12.916. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25544878 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ravelonandro M, Russell P, McOwen N, Briard P, Bohannon S, Vrient A. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP(®) using reverse transcription-recombinase polymerase amplification. Journal of Virological Methods. 2014;207:114–120. doi: 10.1016/j.jviromet.2014.06.026. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/25010790 >. [DOI] [PubMed] [Google Scholar]


