Abstract
Although great success has been achieved in asymmetric Claisen rearrangement for the synthesis of chiral γ,δ-unsaturated carbonyl compounds bearing vicinal tertiary-quaternary stereocenters, the development of asymmetric versions for stereodivergent construction of adjacent quaternary-quaternary stereocenters remains a formidable challenge because of the high steric hindrance. Here we report a catalytic enantioselective dearomatization Claisen rearrangement of allyl furyl ethers catalyzed by chiral N,N′-dioxide-NiII complex catalysts. A variety of chiral γ,δ-unsaturated carbonyl compounds bearing vicinal quaternary-quaternary stereocenters were obtained with excellent outcomes under mild conditions. Furthermore, we disclosed that by matching the configuration of the catalysts and the alkene unit of the substrates, four stereoisomers of the products could be prepared in excellent yields and stereoselectivities. Finally, the fascination of this strategy was demonstrated by stereodivergent synthesis of bioactive natural products hyperolactones B, C, and their epimers. A possible catalytic model was proposed to explain the origin of the asymmetric induction.
Stereodivergent construction of adjacent quaternary-quaternary stereocenters remains a formidable synthetic challenge. Here, the authors report a nickel-catalyzed enantioselective dearomatization Claisen rearrangement leading to vicinal all-carbon stereocentres and apply it to the stereodivergent synthesis of bioactive hyperolactones.
Introduction
The Claisen rearrangement of allyl vinyl ethers is one of the most reliable and useful methods for synthesizing γ,δ-unsaturated carbonyl compounds, valuable intermediates in natural product construction1–4. On the basis of chiral Lewis acids5–10, Jacobsen’s guanidinium salts11, N-heterocyclic carbenes12, or transition metals13–15 catalysts, various of linear and cyclic vinyl units have been transformed via catalytic asymmetric Claisen rearrangement to afford chiral γ,δ-unsaturated carbonyl compounds bearing vicinal tertiary-quaternary stereocenters (Fig. 1a). However, the direct and catalytic stereoselective assembly of these compounds with two adjacent quaternary-quaternary stereocenters remains scarce, because of the inherent steric congestion in the formation of C–C bond. To obtain vicinal quaternary-quaternary arrays and accomplish the total synthesis of several natural products, chiral starting materials were usually conducted through this type of reaction. Zhai and co-workers reported the total synthesis of (−)-Jiadifenin, in which the key intermediate was acquired on the basis of Ireland–Claisen rearrangement of chiral acetal precursor16. Nakamura developed a stereoselective Ireland–Claisen Rearrangement for the synthesis of the CDE ring system of antitumor and chiral building blocks for oxygenated Terpenoids17,18. In regard of catalytic asymmetric methodology, in 2010, the Jacobsen group reported the only catalytic example to produce the vicinal quaternary-quaternary array19. However, limited substrates were evaluated though good results were obtained, which restricted further application in the total synthesis of natural products. Although great achievement has been made in asymmetric Claisen rearrangement, the direct catalytic stereodivergent accessing to optically pure γ,δ-unsaturated carbonyl compounds bearing two adjacent quaternary stereocenters is yet to be described and would be of great value for the natural product-oriented asymmetric synthesis.
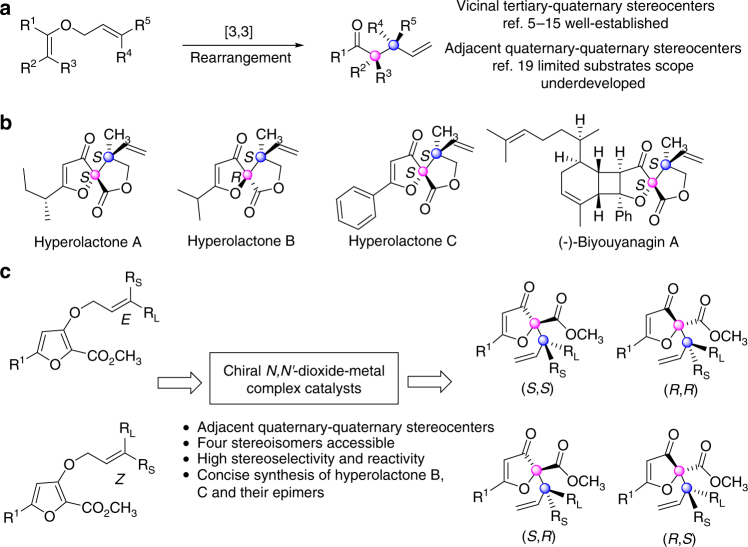
Fig. 1.
Asymmetric Claisen rearrangement of allyl vinyl ethers and representative natural products containing adjacent quaternary stereocenters. a Previous work for the catalytic asymmetric Claisen rearrangement of allyl vinyl ethers. b The structures of natural products hyperolactones A–C and (−)-biyouyanagin A. c Our catalytic asymmetric Claisen rearrangement strategy for stereodivergent construction of adjacent quaternary stereocenters
The hyperolactones A–C20,21 and (−)-biyouyanagin A22 are a family of spirolactone natural products isolated from Hypericum chinese L, which possess significant activity against HIV replication (Fig. 1b). These compounds contain a mutual spirolactone fragment with two chiral vicinal quaternary carbon centers. Besides, the configuration of hyperolactones A, C, and (−)-biyouyanagin A is (S,S), while the configuration of hyperolactone B is (R,S), which varies from the spirocyclic center. Because of their anti-HIV biological activities and unique structures, synthetic chemists have developed many methodologies for their total syntheses22–32. Particularly, Kraus utilized a tandem Claisen rearrangement to realize the synthesis of racemic hyperolactones C33. Though, the yield was only 25% even heating the reaction mixture in a sealed tube at 130 °C for 15 h, which indicated the challenge of the construction of adjacent quaternary stereocenters. Motivated by these initial results, we try to establish a general and efficient asymmetric Claisen rearrangement methodology for the synthesis of the family compounds of hyperolactone. Besides, we envision that by matching the configuration of the chiral catalysts and the substrates, all of the four stereoisomers might be accessible (Fig. 1c).
Herein, we report the application of chiral N,N′-dioxide-NiII complex catalysts in the catalytic asymmetric dearomatization Claisen rearrangement of allyl furyl ethers. A series of γ,δ-unsaturated carbonyl compounds bearing two adjacent quaternary stereocenters were obtained in up to 99% yield, 19:1 dr, and 99% ee under mild reaction conditions. Moreover, the catalytic reaction shows a broad substrate scope, which may lead to the discovery of new bioactive molecules. Furthermore, four stereoisomers could be obtained and stereodivergent synthesis34–48 of natural products hyperolactone B, C could be achieved as well.
Results
Optimization of reaction conditions
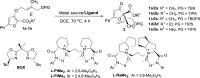
Initially, we selected compound 1a with E-configuration as the model substrate to optimize the reaction conditions. The classical catalysts of Cu(OTf)2/BOX and Ni(OTf)2/BOX yielded trace amount of the targeted product 2a even at 70 °C (Table 1, entries 1 and 2)5. Next, a range of Lewis acids were examined by complexing with N,N′-dioxide ligand (L-PiMe2) derived from L-pipecolic acid in DCE at 70 °C (For details, see Supplementary Table 1). The reaction proceeded sluggishly in the presence of Yb(OTf)3 (Table 1, entriy 3). Fortunately, Ni(OTf)2 showed excellent catalytic ability and the reaction could complete within 4 h to afford the desired product 2a in 85% yield with 5:1 dr and 98% ee (Table 1, entry 4). Inspired by these results, other N,N′-dioxide ligands were evaluated, however, no better results were obtained (Table 1, entries 5 and 6). Our further attempts to improve the yield and diastereoselectivity focused on the investigation of different silyl protecting group of the substrate 1. Substrates 1b and 1c were synthesized to inspect the reaction (entries 7 and 8). The substrate 1b with bulky TIPS group resulted in the formation of the corresponding product 2b in 90% yield, 8:1 dr with a comparable ee value (Table 1, entry 7). After variation of the metal counter ion, Ni(BF4)2·6H2O was found to improve the yield to 96% (Table 1, entry 9). Furthermore, we discovered that the reaction temperature even could be reduced to 35 °C by prolonging the reaction time to 4 days and the desired product 2b could be obtained in 96% yield, 10:1 dr and 99% ee (Table 1, entry 10). But further decreasing the reaction temperature led to low conversion. Variation of ester group on furyl unit of the substrate was also explored in the reaction. However, the yield and enantioselectivity were excellent but the dr was moderate (Table 1, entries 11 and 12).
Table 1.
Optimization of the reaction conditionsa
| |||||
|---|---|---|---|---|---|
| Entry | 1 | Metal source/L | Yield (%)b | drc | eed |
| 1 | 1a | Cu(OTf)2/BOX | trace | – | – |
| 2 | 1a | Ni(OTf)2/BOX | trace | – | – |
| 3 | 1a | Yb(OTf)3/L-PiMe2 | 47 | 3:1 | 33 |
| 4 | 1a | Ni(OTf)2/L-PiMe2 | 85 | 5:1 | 98 |
| 5 | 1a | Ni(OTf)2/L-PiMe3 | 85 | 5:1 | 98 |
| 6 | 1a | Ni(OTf)2/L-RaMe2 | 90 | 2:1 | 94 |
| 7 | 1b | Ni(OTf)2/L-PiMe2 | 90 | 8:1 | 98 |
| 8 | 1c | Ni(OTf)2/L-PiMe2 | 90 | 5:1 | 98 |
| 9 | 1b | Ni(BF4)2·6H2O/L-PiMe2 | 96 | 8:1 | 98 |
| 10e | 1b | Ni(BF4)2·6H2O/L-PiMe2 | 96 | 10:1 | 99 |
| 11e | 1d | Ni(BF4)2·6H2O/L-PiMe2 | 94 | 5:1 | 99 |
| 12e | 1e | Ni(BF4)2·6H2O/L-PiMe2 | 90 | 1.5:1 | 99 |
aUnless otherwise noted, the reactions were performed with 1 (0.1 mmol), and metal source/Ligand (1:1, 10 mol%), in DCE (1.0 mL) at 70 °C for 4 h
bIsolated yield
cThe dr value was determined by 1H NMR and HPLC analysis
dDetermined by HPLC analysis on chiral stationary phases
eAt 35 °C for 4 days
Substrate scope
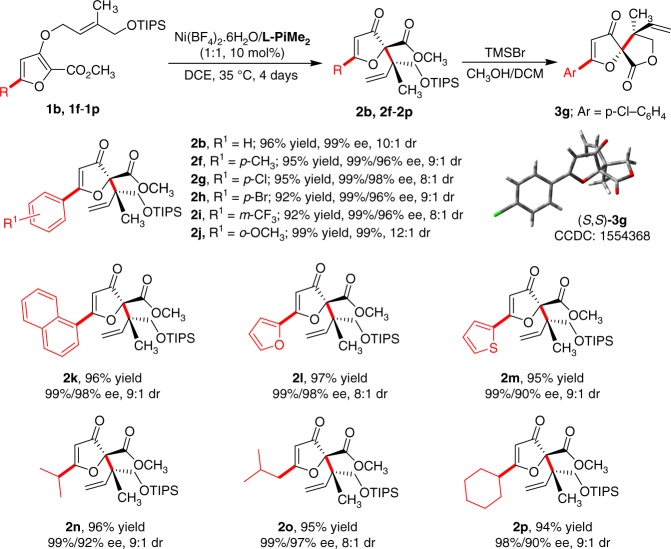
With the optimized reaction conditions identified, a wide range of substrates with varied substituents on furan unit were investigated. In all cases, the reaction proceeded smoothly with excellent yields and stereoselectivities (Fig. 2). Either electron-withdrawing (1g–1i) or electron-donating (1f and 1j) substituents on the 5-phenyl ring of the furan unit were tolerated in the reaction, furnishing the corresponding products (2f–2j) in excellent yields, diastereo- and enantioselectivities (up to 99% yield, 12:1 dr and 99% ee). 1-Naphthyl (1k) and hetero-aromatic (1l and 1m) substituted furan substrates performed the reaction well, giving the related products (2k–2m) in 94–96% yields, 8:1–9:1 dr and 99% ee. Besides, when the 5-alkyl substituted furan substrates (1n–1p) were conducted in the reaction, the products (2n–2p) could also be obtained in excellent results (up to 96% yield, 9:1 dr and 99% ee) after prolonging the reaction time. To demonstrate the utility of this Claisen rearrangement, preparative-scale synthesis of the products 2b and 2k was carried out. Delightedly, the reactivity and stereoselectivity were maintained, which indicated that this method could tolerate the gram-scale chemical production. Furthermore, the absolute configuration of 2g was determined to be (S,S) by the X-ray diffraction of the related spirolactone derivative 3g (See Supplementary Fig. 76) (CCDC 1554368 (3g)). The compounds 2b and 2n exhibited a similar Cotton effect in their circular dichroism (CD) spectra (See Supplementary Figs. 64–75).
Fig. 2.
Substrate scope with variations at the furan unit. Unless otherwise noted, the reactions were performed with 1, Ni(BF4)2·6H2O/L-PiMe2 (1:1, 10 mol%), in DCE at 35 °C for 4 days. Isolated yield. The dr value was determined by 1H NMR and HPLC analysis. The ee value was determined by HPLC analysis on chiral stationary phases. The reaction of 2b and 2k were scaled up to 1.0 mmol. The reaction of 2n-2p were performed at 35 °C for 8 days
The substrates with variation at the alkene unit were also examined (Fig. 3). Under the optimized reaction conditions, E-ethyl substituted substrate 1q and E-isobutyl substituted substrate 1r were transformed into the desired products 2q and 2r in excellent yields and enantioselectivities (Fig. 3a, up to 85% yield and 99% ee). The latter showed relatively lower diastereoselectivity (2q/2r,13:1/5:1dr), which is probably due to the larger steric congestion that affects the stereoselective formation of C–C bond16,19. When E-phenyl substituted alkene substrate (E-1s) was treated in the reaction, no reaction was detected even raising the reaction temperature to 80 °C, which might be caused by the disfavored 1,3-repulsion interaction between aryl group and furan group in the reaction model (See Supplementary Fig. 80). Inversely, Z-aryl substituted alkene substrates could afford the corresponding diastereo-isomers 2′ as the major ones. We found the ligand L-PiMe3 was superior to L-PiMe2 in terms of reactivity and stereoselectivity, and the use of Ni(BF4)2/ent-L-PiMe3 catalyst for the reaction of Z-1s′ resulted in better formation of the related isomer 2s′ compared with Ni(BF4)2/L-PiMe2 catalyst (89% yield, 19:1 dr, 83% ee vs. 75% yield, 9:1 dr, 80% ee). With Ni(BF4)2/ent-L-PiMe3 catalyst, the substrates Z-1s′ to Z-1x′ could participate in the Claisen rearrangement to afford the desired products 2s′–2x′ in excellent yields, stereoselectivities (Fig. 3b, up to 95% yield, 19:1 dr and 93% ee). Halogen substitution on the phenyl ring (Z-1t′–Z-1v′) made a significant increase of the enantioselectivity. However, electron-donating functional group on the phenyl ring (Z-1w′) decreased the enantioselectivity to 62% ee. In addition, the 2-naphthyl substituent alkene E-1x′ was found suitable for this reaction, delivering the product 2x′ in 82% yield with 8:1 dr and 83% ee. Furthermore, terminal mono-phenyl substituted alkene substrate E-1y could afford the desired product 2y in 97% yield with 95% ee and 12:1 dr in the presence of Ni(BF4)2/L-PiMe2 catalyst. Both E-1z and Z-1z′ with terminal disubstituent were suitable in this reaction to afford the corresponding products 2z and 2z′ in excellent results (Fig. 3c, up to 96% yield, 99% ee and 9:1 dr).
Fig. 3.

Substrate scope with variations at alkene unit. a Substrate scope of E-alkyl substituted alkene. b Substrate scope of Z-aryl substituted alkene; c Substrate scope of mono- and disubstituted alkene. The reactions were performed with 1, Ligand/Ni(BF4)2·6H2O (1:1, 10 mol%), in DCE at 35 °C for indicated time. Isolated yield. The dr value was determined by 1H NMR and HPLC analysis. The ee value was determined by HPLC analysis on chiral stationary phases
Asymmetric synthesis of four stereoisomers
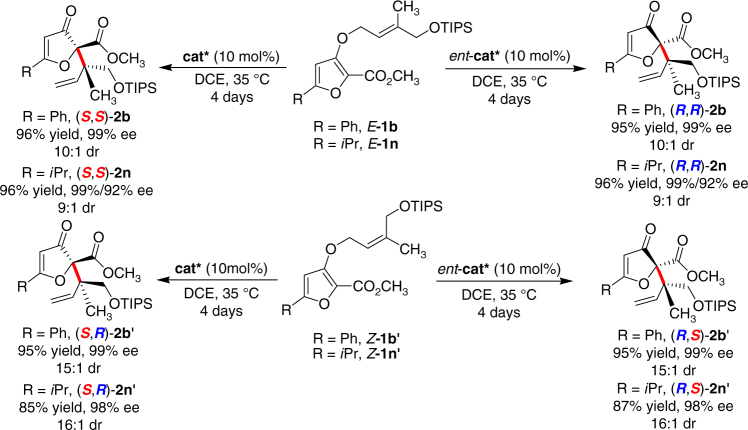
To examine our initial stereodivergent assumption and the purpose of synthesizing natural products hyperolactones B and C, we prepared the E-1b, 1n and Z-1b′, 1n′ and treated them under the optimal reaction conditions (Fig. 4). As expected, the E-substrates preferably afforded (S,S)-configuration products 2b and 2n, while, the Z-substrates yielded the (S,R)-configuration products 2b′ and 2n′ (The configuration of the products were confirmed by CD spectra and NOE spectra analysis of hyperolactones, see Supplementary Figs. 64–79). Besides, by alternating the absolute configuration of N,N′-dioxide ligand L-PiMe2, the corresponding ent-products were available in comparable results. Thus, all four stereoisomers of such γ,δ-unsaturated carbonyl compounds containing vicinal quaternary-quaternary centers could be obtained with this kind of easily available chiral catalyst.
Fig. 4.
Synthesis of all four stereoisomers. Unless otherwise noted, the reactions were performed with 1 (0.1 mmol), and cat* or ent-cat* (10 mol%), in DCE (1.0 mL) at 35 °C for 4 days. Isolated yield. The dr value was determined by 1H NMR and HPLC analysis. The ee value was determined by HPLC analysis on chiral stationary phases. cat* = Ni(BF4)2·6H2O/L-PiMe2 (1:1), ent-cat* = Ni(BF4)2·6H2O/ent-L-PiMe2 (1:1)
Stereodivergent synthesis of hyperolactones
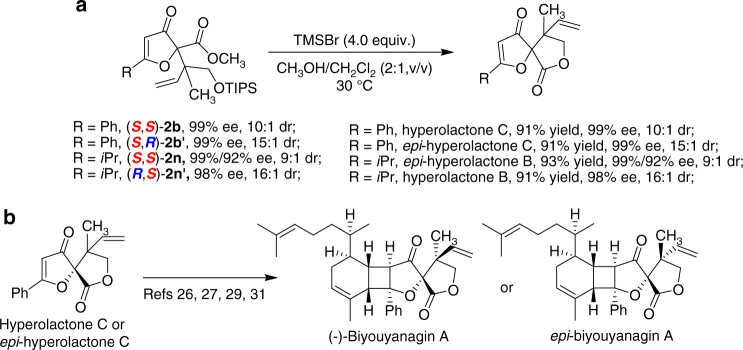
Subsequently, we tried various reagents to realize the deprotection/lactonization transformation of the product 2b and 2n. The conditions, such as TBAF, PPTS, and hydrogen fluoride-pyridine solution proved unsuccessful (For details, see Supplementary Table 8). Delightfully, in the presence of TMSBr reagent, the optically active (S,S)-2b, (S,R)-2b′, (R,S)-2n′ and (S,S)-2n could participate in the transformation well, delivering hyperolactones B, C and epi-hyperolactones B, C in excellent yields with the diastereo- and enantioselectivity maintained (For details, see Supplementary Note 11). Accordingly, the access to natural product (−)-biyouyanagin A and its epimer is possible from the key reaction intermediates26,27,29,31 of hyperolactone C or epi-hyperolactone C (Fig. 5).
Fig. 5.
Stereodivergent synthesis of natural products hyperolactones B, C, (−)-biyouyanagin A, and their epimers. a The deprotection/lactonization transformation of the product 2b, 2b′, 2n and 2n′. b Formal synthesis of (−)-biyouyanagin A and epi-(−)-biyouyanagin A
Proposed stereochemical model
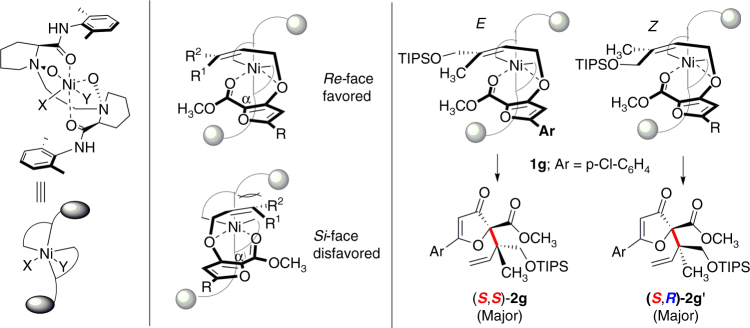
On the basis of our previous works49–51 and the absolute configuration of the product 2g, a possible stereocontrol model was proposed in Fig. 6. The chiral N,N′-dioxide coordinates to the nickel(II) center to form octahedral metal complex in which the chiral ligand occupies four sites. The substrate 1g displays the ancillary solvents or anion and could coordinate to the Lewis acid catalyst via a bidentate manner with the oxygen of ether and carbonyl group of the auxiliary ester group. Due to the steric hindrance between alkene unit and the bulky aniline moiety of ligand, the alkene preferentially approaches α position of furan unit from the Re face. Therefore, the E-alkene substrate 1g affords (S,S)-2g and the Z-alkene substrate 1g′ affords (S,R)-2g′.
Fig. 6.
Proposed stereochemical model. Substrate 1g was selected for the model analysis
Discussion
In summary, we have developed a catalytic asymmetric dearomatization Claisen rearrangement of allyl furyl ethers for the synthesis of chiral γ,δ-unsaturated carbonyl compounds with two adjacent quaternary-quaternary carbon centers. By adjusting the configuration of the catalysts and alkene units, all four possible product stereoisomers could be prepared in excellent yields and stereoselectivities. In addition, the reaction has a broad substrate scope, which may lead to the discovery of new bioactive molecules. Furthermore, the bioactive natural products hyperolactones B, C, and their epimers could be readily acquired in high efficiency, which are also the key intermediates for the synthesis of natural product (−)-biyouyanagin A and its epimer.
Methods
General procedure for asymmetric Claisen rearrangement
N,N′-Dioxide ligand L-PiMe2 or ent-L-PiMe2 or ent-L-PiMe3 (10 mol%), Ni(BF4)2·6H2O (10 mol%) in CH2Cl2 were stirred at 35 °C for 1 h. Then CH2Cl2 was removed in vacuo. Substrate 1 and DCE were added and the resulting mixture was stirred at 35 °C for 4–8 days. After the reaction completed (monitored by TLC), flash column chromatography was carried out to provide the desired product (Petroleum ether/EtOAc = 20:1 to 10:1).
General procedure for deprotection/cyclization reaction
To a solution of the product 2 (0.1 mmol) in CH3OH/CH2Cl2 (1.5 mL, 2:1, v/v), TMSBr (0.4 mmol, 4.0 equiv.) was added, the reaction mixture was stirred at 30 °C for 8–24 h. Then the reaction was quenched by saturated aq. Na2CO3 (1 mL) and diluted with water (1 mL). The product was extracted with ethyl acetate (3 × 10 mL). The combined organic extracts were washed with brine (20 mL) and concentrated in vacuo. The product was purified by column chromatography on silica gel (hexane/EtOAc = 10/1 as eluent).
Data availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC). Crystal data and structure refinement for 3g was displayed in Supplementary Information. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/ under deposition number 1554368. All other data is available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
We appreciate the National Natural Science Foundation of China (Nos. 21432006, 21625205) for financial support. Dedicated to professor George A. Kraus for his initial contribution on the dearomatization Claisen rearrangement of allyl furyl ethers.
Author contributions
H.F.Z. performed experiments and prepared the Supplementary Information and Manuscript. Y.W. and C.R.X. took part in the reaction development and synthesized several substrates. X.X. repeated some experiments. L.L.L. helped with modifying the paper and Supplementary Information. X.H.L. and X.M.F. conceived and directed the project.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04123-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaohua Liu, Email: liuxh@scu.edu.cn.
Xiaoming Feng, Email: xmfeng@scu.edu.cn.
References
- 1.Ito H, Taguchi T. Asymmetric Claisen rearrangement. Chem. Soc. Rev. 1999;28:43–50. doi: 10.1039/a706415b. [DOI] [Google Scholar]
- 2.Nubbemeyer U. Recent advances in asymmetric [3,3]-sigmatropic rearrangements. ChemInform. 2003;7:0961–1008. [Google Scholar]
- 3.Ilardi EA, Stivala CE, Zakarian A. [3,3]-Sigmatropic rearrangements: recent applications in the total synthesis of natural products. Chem. Soc. Rev. 2009;38:3133–3148. doi: 10.1039/b901177n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehbein J, Hiersemann M. Claisen rearrangement of aliphatic allyl vinyl ethers from 1912 to 2012: 100 years of electrophilic catalysis. Synthesis. 2013;45:1121–1159. doi: 10.1055/s-0032-1316869. [DOI] [Google Scholar]
- 5.Abraham L, Czerwonka R, Hiersemann M. The catalytic enantioselective Claisen rearrangement of an allyl vinyl ether. Angew. Chem. Int. Ed. 2001;40:4700–4703. doi: 10.1002/1521-3773(20011217)40:24<4700::AID-ANIE4700>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Marié JC, et al. Enantioselective synthesis of 3,4-chromanediones via asymmetric rearrangement of 3-allyloxyflavones. J. Org. Chem. 2010;75:4584–4590. doi: 10.1021/jo100889c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan JJ, Cheon CH, Yamamoto H. Catalytic asymmetric Claisen rearrangement of enolphosphonates: construction of vicinal tertiary and all-carbon quaternary centers. Angew. Chem. Int. Ed. 2012;51:8264–8267. doi: 10.1002/anie.201203092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YB, et al. Nickel(II)-catalyzed asymmetric propargyl and allyl Claisen rearrangements to allenyl- and allyl-substituted ß-ketoesters. Angew. Chem. Int. Ed. 2014;53:11579–11582. doi: 10.1002/anie.201404643. [DOI] [PubMed] [Google Scholar]
- 9.Li J, et al. Gold(I)/chiral N,N′-dioxide–nickel(II) relay catalysis for asymmetric tandem intermolecular hydroalkoxylation/Claisen rearrangement. Angew. Chem. Int. Ed. 2017;56:885–888. doi: 10.1002/anie.201611214. [DOI] [PubMed] [Google Scholar]
- 10.Fu JK, et al. Gold-catalyzed rearrangement of allylic oxonium ylides: efficient synthesis of highly functionalized dihydrofuran-3-ones. Angew. Chem. Int. Ed. 2013;52:4198–4202. doi: 10.1002/anie.201208305. [DOI] [PubMed] [Google Scholar]
- 11.Uyeda C, Jacobsen EN. Enantioselective Claisen rearrangements with a hydrogen-bond donor catalyst. J. Am. Chem. Soc. 2008;130:9228–9229. doi: 10.1021/ja803370x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaeobamrung J, Mahatthananchai J, Zheng PG, Bode JW. An enantioselective Claisen rearrangement catalyzed by N-heterocyclic carbenes. J. Am. Chem. Soc. 2010;132:8810–8812. doi: 10.1021/ja103631u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linton EC, Kozlowski MC. Catalytic enantioselective Meerwein-Eschenmoser Claisen rearrangement: asymmetric synthesis of allyl oxindoles. J. Am. Chem. Soc. 2008;130:16162–16163. doi: 10.1021/ja807026z. [DOI] [PubMed] [Google Scholar]
- 14.Geherty ME, Dura RD, Nelson SG. Catalytic asymmetric Claisen rearrangement of unactivated allyl vinyl ethers. J. Am. Chem. Soc. 2010;132:11875–11877. doi: 10.1021/ja1039314. [DOI] [PubMed] [Google Scholar]
- 15.Wu HM, Zi WW, Li GG, Lu HJ, Toste FD. Gold(I)-catalyzed desymmetrization of 1,4-dienes by an enantioselective tandem alkoxylation/claisen rearrangement. Angew. Chem. Int. Ed. 2015;54:8529–8532. doi: 10.1002/anie.201503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Fu XN, Chen JW, Zhai HB. Total synthesis of (−)-Jiadifenin. Angew. Chem. Int. Ed. 2012;51:9825–9828. doi: 10.1002/anie.201203176. [DOI] [PubMed] [Google Scholar]
- 17.Akahori Y, Yamakoshi H, Hashimoto S, Nakamura S. Stereoselective synthesis of the CDE ring system of antitumor Saponin Scillascilloside E-1. Org. Lett. 2014;16:2054–2057. doi: 10.1021/ol500657f. [DOI] [PubMed] [Google Scholar]
- 18.Akahori Y, Yamakoshi H, Sawayama Y, Hashimoto S, Nakamura S. Synthesis of chiral building blocks for oxygenated Terpenoids through a simultaneous and stereocontrolled construction of contiguous quaternary stereocenters by an Ireland-Claisen rearrangement. J. Org. Chem. 2014;79:720–735. doi: 10.1021/jo402537u. [DOI] [PubMed] [Google Scholar]
- 19.Uyeda C, Rötheli AR, Jacobsen EN. Catalytic enantioselective Claisen rearrangements of O-allyl ß-ketoesters. Angew. Chem. Int. Ed. 2010;49:9753–9756. doi: 10.1002/anie.201005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aramaki Y, Chiba K, Tada M. Spirolactones, hyperolactone A-D from hypericum Chinense. Phytochemistry. 1995;38:1419–1421. doi: 10.1016/0031-9422(94)00862-N. [DOI] [Google Scholar]
- 21.Crockett SL, Schühly W, Belaj F, Khan IA. Hyperolactone C. Acta Crystallogr. Sect. E. 2004;60:o2174–o2176. doi: 10.1107/S1600536804026819. [DOI] [Google Scholar]
- 22.Tanaka N, et al. Biyouyanagin A, an anti-HIV agent from Hypericum chinense L. var. salicifolium. Org. Lett. 2005;7:2997–2999. doi: 10.1021/ol050960w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichinari D, Ueki T, Yoshihara K, Kinoshita T. First total synthesis of (±)-hyperolactone A. Chem. Commun. 1997;0:1743–1744. doi: 10.1039/a704159d. [DOI] [Google Scholar]
- 24.Ueki T, Ichinari D, Yoshihara K, Morimoto Y, Kinoshita T. Synthesis and absolute configuration of (+)-hyperolactone B. Tetrahedron Lett. 1998;39:667–668. doi: 10.1016/S0040-4039(97)10713-4. [DOI] [Google Scholar]
- 25.Ueki T, et al. Synthesis of hyperolactones A and C. J. Heterocycl. Chem. 2001;38:165–172. doi: 10.1002/jhet.5570380124. [DOI] [Google Scholar]
- 26.Nicolaou KC, Sarlah D, Shaw DM. Total synthesis and revised structure of biyouyanagin A. Angew. Chem. Int. Ed. 2007;46:4708–4711. doi: 10.1002/anie.200701552. [DOI] [PubMed] [Google Scholar]
- 27.Nicolaou KC, et al. Total synthesis, revised structure, and biological evaluation of biyouyanagin A and analogues thereof. J. Am. Chem. Soc. 2008;130:11114–11121. doi: 10.1021/ja802805c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgson DM, Erickson D, Angrish SP, Kloesges J, Lee CH. Consecutive alkene cross-metathesis/ oxonium ylide formation-rearrangement: synthesis of the anti-HIV agent hyperolactone C. Org. Lett. 2008;10:5553–5556. doi: 10.1021/ol802334y. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaou KC, et al. Design, synthesis, and biological evaluation of a biyouyanagin compound library. PNAS. 2011;108:6715–6720. doi: 10.1073/pnas.1015258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgson DM, Man S. Synthesis of the anti-HIV agent (−)-hyperolactone C by using oxonium ylide formation–rearrangement. Chem. Eur. J. 2011;17:9731–9737. doi: 10.1002/chem.201101082. [DOI] [PubMed] [Google Scholar]
- 31.Du C, Li LQ, Li Y, Xie ZX. Construction of two vicinal quaternary carbons by asymmetric allylic alkylation: total synthesis of hyperolactone C and (−)-biyouyanagin A. Angew. Chem. Int. Ed. 2009;48:7853–7856. doi: 10.1002/anie.200902908. [DOI] [PubMed] [Google Scholar]
- 32.Wu YY, Du C, Hu CC, Li Y, Xie ZX. Biomimetic synthesis of hyperolactones. J. Org. Chem. 2011;76:4075–4081. doi: 10.1021/jo102511x. [DOI] [PubMed] [Google Scholar]
- 33.Kraus GA, Wei JQ. A direct synthesis of hyperolactone C. J. Nat. Prod. 2004;67:1039–1040. doi: 10.1021/np0498962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafroth MA, Zuccarello G, Krautwald S, Sarlah D, Carreira EM. Stereodivergent total synthesis of Δ9 Tetrahydrocannabinols. Angew. Chem. Int. Ed. 2014;53:13898–13901. doi: 10.1002/anie.201408380. [DOI] [PubMed] [Google Scholar]
- 35.Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 36.Lin LL, Feng XM. Catalytic strategies for diastereodivergent synthesis. Chem. Eur. J. 2017;23:6464–6482. doi: 10.1002/chem.201604617. [DOI] [PubMed] [Google Scholar]
- 37.Zhan G, Du W, Chen YC. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 2017;46:1675–1692. doi: 10.1039/C6CS00247A. [DOI] [PubMed] [Google Scholar]
- 38.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 39.Schindler CS, Jacobsen EN. A new twist on cooperative catalysis. Science. 2013;340:1052–1053. doi: 10.1126/science.1238769. [DOI] [PubMed] [Google Scholar]
- 40.Huo XH, He R, Zhang X, Zhang WB. An Ir/Zn dual catalysis for enantio- and diastereodivergent α-allylation of α-hydroxyketones. J. Am. Chem. Soc. 2016;138:11093–11096. doi: 10.1021/jacs.6b06156. [DOI] [PubMed] [Google Scholar]
- 41.Jiang XY, Beiger JJ, Hartwig JF. Stereodivergent allylic substitutions with aryl acetic acid esters by synergistic iridium and Lewis base catalysis. J. Am. Chem. Soc. 2017;139:87–90. doi: 10.1021/jacs.6b11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz FA, Dong VM. Stereodivergent coupling of aldehydes and alkynes via synergistic. J. Am. Chem. Soc. 2017;139:1029–1032. doi: 10.1021/jacs.6b10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denmark SE, Ghosh SK. The first catalytic, diastereoselective, and enantioselective crossed-aldol reactions of aldehydes. Angew. Chem., Int. Ed. 2001;40:4759–4762. doi: 10.1002/1521-3773(20011217)40:24<4759::AID-ANIE4759>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Kim HY, Li JY, Kim S, Oh K. Stereodivergency in catalytic asymmetric conjugate addition reactions of glycine (ket)imines. J. Am. Chem. Soc. 2011;133:20750–20753. doi: 10.1021/ja2100356. [DOI] [PubMed] [Google Scholar]
- 45.Molinaro C, et al. Catalytic, asymmetric, and stereodivergent synthesis of non-symmetric β,β-diaryl-α-amino acids. J. Am. Chem. Soc. 2015;137:999–1006. doi: 10.1021/ja511872a. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Liu XF, Deng L. Dual-function cinchona alkaloid catalysis: catalytic asymmetric tandem conjugate addition-protonation for the direct creation of nonadjacent stereocenters. J. Am. Chem. Soc. 2006;128:3928–3930. doi: 10.1021/ja060312n. [DOI] [PubMed] [Google Scholar]
- 47.Mechler M, Peters R. Diastereodivergent asymmetric 1,4-addition of oxindoles to nitroolefins by using polyfunctional nickel-hydrogen-bond-azolium catalysts. Angew. Chem. Int. Ed. 2015;54:10303–10307. doi: 10.1002/anie.201502930. [DOI] [PubMed] [Google Scholar]
- 48.Engl OD, Fritz SP, Wennemers HD. Stereoselective organocatalytic synthesis of oxindoles with adjacent tetrasubstituted stereocenters. Angew. Chem. Int. Ed. 2015;54:8193–8197. doi: 10.1002/anie.201502976. [DOI] [PubMed] [Google Scholar]
- 49.Liu XH, Lin LL, Feng XM. Chiral N,N′-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 2011;44:574–587. doi: 10.1021/ar200015s. [DOI] [PubMed] [Google Scholar]
- 50.Liu XH, Lin LL, Feng XM. Chiral N,N′-dioxide ligands: synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014;1:298–302. doi: 10.1039/c3qo00059a. [DOI] [Google Scholar]
- 51.Liu XH, Zheng HF, Xia Y, Lin LL, Feng XM. Asymmetric cycloaddition and cyclization reactions catalyzed by chiral N,N′-dioxide-metal complexes. Acc. Chem. Res. 2017;50:2621–2631. doi: 10.1021/acs.accounts.7b00377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC). Crystal data and structure refinement for 3g was displayed in Supplementary Information. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/ under deposition number 1554368. All other data is available from the corresponding author upon reasonable request.