Highlights
-
•
The sustained-release oxyntomodulin analogue OX-SR increases energy expenditure in rats, measured by indirect calorimetry.
-
•
Blocking the GLP-1 receptor with Ex9-39 does not affect this increase in energy expenditure.
-
•
If activity at the glucagon receptor is blocked, OX-SR no longer increases energy expenditure.
-
•
This shows that OXM increases energy expenditure via the glucagon and not GLP-1 receptors.
Keywords: Oxyntomodulin, Energy expenditure, Receptor, Glucagon, GLP-1
Abstract
The gut hormone oxyntomodulin (OXM) causes weight loss by reducing appetite and increasing energy expenditure. Several analogues are being developed to treat obesity. Exactly how oxyntomodulin works, however, remains controversial. OXM can activate both glucagon and GLP-1 receptors but no specific receptor has been identified. It is thought that the anorectic effect occurs predominantly through GLP-1 receptor activation but, to date, it has not been formally confirmed which receptor is responsible for the increased energy expenditure.
We developed OX-SR, a sustained-release OXM analogue. It produces a significant and sustained increase in energy expenditure in rats as measured by indirect calorimetry. We now show that this increase in energy expenditure occurs via activation of the glucagon receptor. Blockade of the GLP-1 receptor with Exendin 9–39 does not block the increase in oxygen consumption caused by OX-SR. However, when activity at the glucagon receptor is lost, there is no increase in energy expenditure. Glucagon receptor activity therefore appears to be essential for OX-SR’s effects on energy expenditure. The development of future ‘dual agonist’ analogues will require careful balancing of GLP-1 and glucagon receptor activities to obtain optimal effects.
1. Introduction
Oxyntomodulin (OXM) is a 37 amino-acid peptide produced by the neuroendocrine L-cells of the ileum. It consists of the 29 amino acids of glucagon plus an octapeptide tail. So far, no specific OXM receptor has been identified. OXM does activate the glucagon receptor, though less potently than native glucagon due to the octapeptide tail. The same tail allows OXM to activate the GLP-1 receptor, but also less potently than native GLP-1 [1], [2], [3], [4]. Additionally, the octapeptide tail slows clearance of OXM from the circulation when compared to glucagon [5]. OXM is produced by the action of PCSK1 proprotein convertase subtilisin/kexin type 1 on the proglucagon peptide. OXM is co-secreted from the intestine with GLP-1 in response to nutrient intake. Like GLP-1, OXM is an incretin, directly causing insulin release from pancreatic islet cells.
All current available obesity treatments – dietary, pharmacological and surgical – reduce food intake. However, the initial weight loss from the food intake reduction is associated with a fall in energy expenditure, which limits overall weight loss [6], [7], [8]. Conversely, states where energy expenditure is increased, such as thyrotoxicosis or cold exposure, are accompanied by a compensatory hyperphagia, which has a similar limiting effect on weight loss [9], [10]. Short-term studies suggest that administration of exogenous OXM can reduce body weight in humans [4], [5]. The efficacy of OXM, compared to other anti-obesity treatments, arises from its ability to both reduce food intake and increase energy expenditure. By affecting both sides of the energy balance equation, OXM offers a means of causing effective and sustainable weight loss.
The mechanism underlying the anorectic effect of OXM is well established. It is centrally mediated via GLP-1 receptor activation, confirmed by both pharmacological blockade of the GLP-1 receptor, and using GLP-1 receptor knock-out mice [2], [11], [12]. However, the mechanism by which it increases energy expenditure remains controversial, and both the glucagon and GLP-1 receptors have been implicated.
Specific alteration to the OXM peptide has allowed the effect of activating the different receptors to be investigated. Glucagon and OXM have glutamine, a neutral polar residue at position 3 [6]. In contrast, both GLP-1 and its naturally occurring analogue Exendin-4 (Ex-4), have an acidic glutamate residue in this position and are unable to activate the glucagon receptor. Using targeted peptide engineering to substitute the third residue of OXM with glutamic acid (OXMQ3E), the peptide maintains its activity at the GLP-1 receptor, but loses its ability to activate the glucagon receptor. The action of native OXM has been compared to OXMQ3E in mice. Both peptides suppressed food intake to a similar degree, but weight loss was significantly greater in the OXM group. This additional weight loss indirectly suggests that the glucagon activity in OXM increases energy expenditure [13]. Similarly, in a study comparing OXM analogues engineered to have differing potency at the GLP-1 and GCG receptors, peptides with more glucagon action caused greater weight loss in mice despite causing only a small food intake reduction [14]. Together, these studies suggest that glucagon receptor activation by OXM causes increased energy expenditure, however, other potential explanations for the results, such as increased diuresis or faecal excretion, have not been excluded. Moreover, when OXM is administered via an intracerebroventricular infusion, OXM increases intrascapular brown adipose tissue (BAT) sympathetic activation; GLP-1 receptor knockout prevents this. As activation of BAT increases energy dissipation through thermogenesis, this study suggests the GLP-1 receptor has a role in energy expenditure, at least when OXM is given intracerebroventricularly [15].
Contributing to the uncertainty as to which receptor increases energy expenditure is the difficulty of directly measuring the energy expenditure effects of OXM. The studies mentioned above all use surrogate markers of energy expenditure: comparison of weight loss with food intake, or sympathetic nerve activity in BAT. Indeed, no studies to date have shown an increase in oxygen consumption following OXM administration. This has not been fully explained but may reflect the relative insensitivity of most metabolic cages [16], compounded with the short half-life of OXM, which necessitates multiple daily injections of OXM. A significant increase in energy expenditure in rodents has been measured directly with several different OXM analogues [17], [18], [19]. The reproducibility of these results lends credibility to the idea that OXM does affect energy expenditure.
Analogues are increasingly being used to investigate the physiology of peptide hormones, since manipulation of native peptides can increase the half-life and potency of the hormones [17], [19]. We developed a sustained-release OXM analogue, OX-SR. This differs from native OXM by 5 amino acids between residues 16 and 27. These changes allow OX-SR to form a subcutaneous depot, enabling administration as a single daily subcutaneous injection. We directly measured the energy expenditure caused by this analogue within metabolic cages and compared this to the effects of inhibition of both the glucagon and GLP-1 receptors. We were therefore able to effectively determine the relative contribution of these receptors on the energy expenditure effects of OXM.
2. Methods
2.1. Peptides
OX-SR and OX-SR-Glu3 were synthesized by Insight Biotechnology Ltd. (Middlesex, UK) using solid phase peptide synthesis (SPPS) methodology and purified by reverse-phase preparative HPLC. Peptide purity was greater than 95%. Throughout, OX-SR and OX-SR-Glu3 were administered in a zinc-based diluent. Oxyntomodulin, GLP-1, glucagon and exendin 9–39 were all purchased from Bachem (Bubendorf, Switzerland). OX-SR-Glu3 has the same peptide structure as the long-acting OXM analogue OX-SR with a substitution of glutamic acid at position 3 to eliminate activity of the peptide at the glucagon receptor [6].
2.2. cAMP accumulation assay
CHO-K1 cells stably over-expressing the human glucagon receptor (hGCGr) were purchased from Invitrogen Life Technologies (Paisley, UK) while CHO cells over-expressing the human GLP-1 receptor were produced in house [7]. cAMP accumulation was measured using a cAMP dynamic 2 assay (Cisbio Assays, Codolet, France), following the manufacturer’s protocol.
2.3. Animal studies
All animal procedures undertaken were approved by the British Home Office conforming to the Animal (Scientific Procedures) Act 1986 (Project Licences 70/7236 and 70/7596). Male Wistar rats (Charles River, Margate, UK) were used throughout. Animals were single-housed in a temperature controlled environment with 12:12 h light:dark cycle, lights on at 0730. To ascertain energy expenditure, a Comprehensive Laboratory Animal Monitoring System (CLAMS − Columbus Instruments, Columbus, Ohio) was used. Animals are placed in metabolic cages, with continuous measurement of oxygen consumption, carbon dioxide production, locomotor activity as well as food intake.
2.4. Pharmacokinetic studies
Intravenous pharmacokinetics of the peptides were measured as previously described [20]. In brief, each peptide was infused through a femoral vein catheter inserted into an anesthetized rat (n = 3). Peptides were infused at a concentration of 30nmol/ml and a rate of 0.3 ml/hr. Regular samples were taken from a jugular vein catheter over a period of 100 min. To determine pharmacokinetics after a subcutaneous injection, rats were given a single subcutaneous dose formulated in zinc of 1.4 μmol/kg of peptide. Blood samples were taken at predetermined intervals over 7 days through tail venesection. Peptide levels were determined from the blood samples using an in-house RIA [21], and pharmacokinetic properties ascertained.
2.5. Pair-feeding study to demonstrate energy expenditure effect of OX-SR
Rats were randomised to three groups (n = 8) by body weight (mean 414 g). Control and peptide groups received daily SC injections of vehicle and OX-SR (40 nmol/kg) for 3 days at 0830 starting on day 0. The pair-fed group received daily injections of saline from day 1, but food intake was restricted to the mean intake of the OX-SR group over the previous 24 h. Food intake and body weight were measured daily at 0830. Heavier animals were used in the pair-feeding study compared to the subsequent CLAMS studies as they have lower variation in their daily food intake, improving the quality of the study when animals are fed to the mean food intake of the treatment group.
2.6. Measurement of energy expenditure following acute administration of OX-SR in CLAMS
Rats were randomised to two groups (n = 16) of equal mean body weight (mean 266 g) and placed in CLAMS metabolic cages. This sample size had been established to allow a 10% increase in energy expenditure to be detected with a significance of p < 0.05, with power of 80%.
24 h after entering the cages, animals received a single subcutaneous injection of vehicle or OX-SR (40 nmol/kg) at 0830. Food intake, oxygen consumption, carbon dioxide production and locomotor activity were measured continuously for 24 h. Body weight was measured 24 h after injection. Throughout, all animals had ad libitum access to chow.
To investigate whether the time of OX-SR administration altered its metabolic effects, the experiment was repeated in a second cohort of rats (mean body weight) but with OX-SR (40 nmol/kg) administered at 1630.
2.7. Effect of blockade of GLP-1 receptor on energy expenditure following administration of OX-SR
Rats were randomised to 4 groups (n = 8) of equal mean body weight (mean 222 g). On day 0, all animals entered CLAMS metabolic cages. Between 1000 and 1200 on day 1, all rats had mini-osmotic pumps (Alzet Osmotic Pumps, Model 1003D, Durect Co., Cupertino, USA) inserted under isofluorane anaesthesia. The pumps delivered either saline or Ex9-39 at 100 nmol/kg/hr. This dose of Ex9-39 had been shown in preliminary studies to eliminate completely the anorectic effect of 0.04 mmol/kg of the long-acting GLP-1 agonist Ex4, a dose which is more potent than 40 nmol/kg of OX-SR. After recovery from anaesthesia, the animals were returned to the CLAMS metabolic cages. At 0830 on day 2, half of the animals with saline pumps, and half of those with Ex9-39 pumps, were given a SC injection of vehicle, while the remaining animals received a SC injection of OX-SR (40 nmol/kg). Metabolic parameters were measured continuously for 24 h. Body weight was measured just before subcutaneous injection, and 24 h later.
2.8. Comparison of energy expenditure following administration of OX-SR and OX-SR-Glu3
Rats were randomised to 3 groups of equal mean body weight (n = 10–11) (mean 318 g). 16 h after entry into CLAMS metabolic cages, at 0830, animals received subcutaneous injections of either vehicle, OX-SR (40 nmol/kg) or OX-SR-Glu3 (40 nmol/kg). Metabolic parameters were measured throughout. Body weight was measured just before the injections, and 24 h later.
2.9. Data analysis
All data are presented as mean ± SEM. P-values are based on two-sided tests with significance set at p < 0.05. Student t-tests, one-way ANOVA with Tukey’s post-hoc tests and two-way ANOVA with Sidak’s multiple comparison tests are used as detailed for each experiment. All analysis was undertaken using Prism 7.0 (GraphPad Software Inc., San Diego, USA).
3. Results
3.1. In vitro cAMP accumulation following peptide administration
At the GLP-1 receptor, the EC50 levels of OXM, OX-SR and OX-SR-Glu3 are all higher than native GLP-1 (Table 1). GLP-1 was 17 x more potent than OXM, 11 x more potent than OX-SR, and 9.5 x more potent than OX-SR-Glu3.
Table 1.
Comparison of pharmacokinetic and pharmacodynamic features of OXM, OX-SR and OX-SR-Glu3.
| OXM | OX-SR | OX-SR-Glu3 | |
|---|---|---|---|
| cAMP accumulation EC50 (nM) at the glucagon receptor ± SEM [ratio compared to glucagon] | 3.46 ± 1.5 [7.8] | 2.17 ± 0.6 [4.8] | 20.8 ± 2.5 [46] |
| cAMP accumulation EC50 (nM) at the GLP-1 receptor ± SEM [ratio compared to GLP-1] | 136.6 ± 29.1 [17] | 91.2 ± 16.1 [11] | 74.7 ± 31.5 [9.5] |
| Half-life after intravenous administration (mins) | 12.1 | 15.9 | |
| Time to peak plasma concentration after subcutaneous administration (hours) | 0.5 | 3 | |
| Time to undetectable plasma levels after subcutaneous administration (days) | 1 | 6 |
Similarly, at the GCG receptor, the EC50 levels of OXM, OX-SR and OX-SR-Glu3 were higher than native GCG. GCG was 7.8 x more potent than OXM, 4.8 x more potent than OX-SR, and at least 46 x more potent than OX-SR-Glu3.
These results show that while OX-SR is less potent than the cognate hormones at both the glucagon and GLP-1 receptors, it is slightly more potent than OXM by 1.59 x and 1.50 x respectively.
These data also demonstrate that there is minimal activation of the glucagon receptor by OX-SR-Glu3, and also that OX-SR is slightly less potent at the GLP-1 receptor than the Glu3 version, a finding consistent with other studies comparing OXM analogues with the Glu3 switch [22].
3.2. Pharmacokinetic studies
Following the IV infusion, the plasma half-life of OXM and OX-SR were calculated to be 12.1 and 15.9 min respectively. When administered subcutaneously, OXM reached a peak plasma concentration at 30 min, and was undetectable by 24 h; in contrast OX-SR levels continued to rise up to 3 h, and remained at this concentration for 24 h, before slowly falling over the next 6 days (Table 1).
3.3. Pair-feeding study shows OX-SR increases energy expenditure
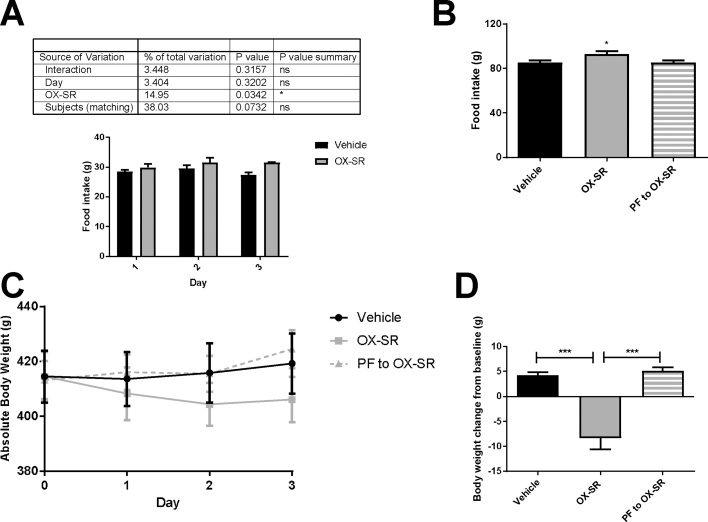
Over 3 days, OX-SR caused hyperphagia, with the animals eating 8% more food than the vehicle group (p < 0.05). Despite this, the OX-SR group lost an average of 8 g body weight (2% of baseline), while the vehicle group gained weight (an average of 4 g). Moreover, the pair-fed group also gained an average of 5 g body weight. This difference was significant for the OX-SR group compared to both other groups (p < 0.001 in both cases). The difference in body weight change between the OX-SR and pair-fed groups could represent an increase in energy expenditure (Fig. 1 A–D).
Fig. 1.
3-day Pair-Feeding Study using OX-SR. Daily food intake (A), cumulative food intake (B), daily absolute bodyweight (C), and final body weight change from baseline (D) in male Wistar rats after 3 days of injections of vehicle, OX-SR (40 nmol/kg) or pair-feeding to OX-SR group. Food and body weight were measured daily at 0830. N = 8. Mean initial body weight in each group was 414 g. Data shown as the mean ± SEM. Statistical analysis carried out using two-way ANOVA for daily food intake with Sidak’s multiple comparisons test, and one-way ANOVA with post hoc tests Tukey’s multiple comparison test for total food intake and body weight change, *p < 0.05, ***p < 0.001.
3.4. Energy expenditure measurement in metabolic cages
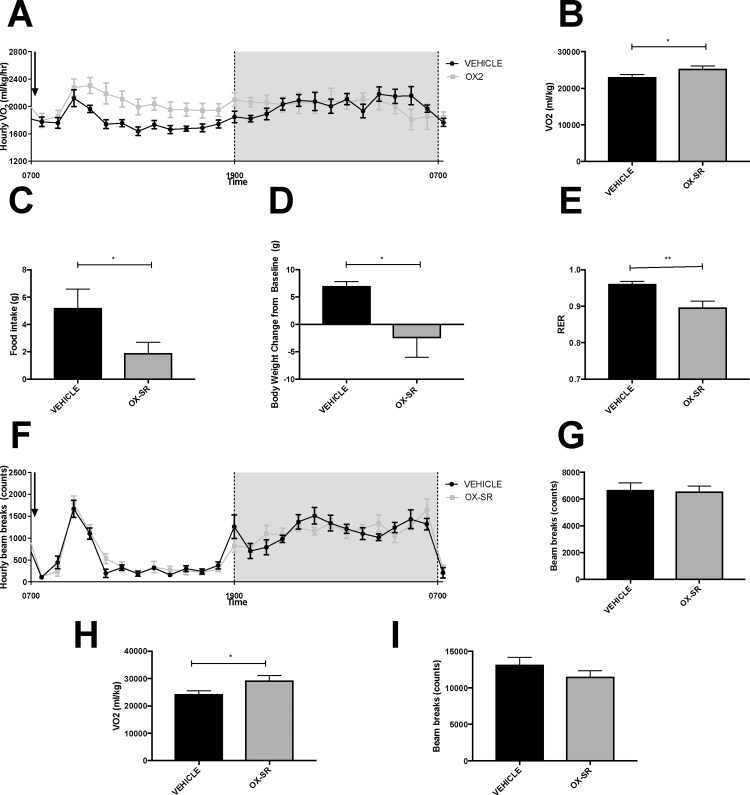
The difference in body weight between the OX-SR and pair-fed groups likely represents a difference in energy expenditure. However, increased diuresis or faecal output could not be excluded as an alternative cause for the enhanced weight loss in the OX-SR group. To confirm that OX-SR was capable of increasing energy expenditure, we examined its effect on oxygen consumption using metabolic cages. A single dose of OX-SR caused a 10% increase in oxygen consumption during the 12 h following OX-SR administration (Fig. 2A-B). There was no significant difference in locomotor activity between groups during the period of increased energy expenditure (Fig. 2F-G) demonstrating that increased physical activity does not explain the enhanced energy expenditure. Food intake was also significantly reduced: it was reduced by over 60% in the 12 h following injection, and by 40% in the 24 h following injection (p < 0.05 and p < 0.01 respectively) (Fig. 2C). Respiratory exchange ratio was significantly reduced by OX-SR from 0.96 to 0.90 for the 12 h following peptide administration (p < 0.01) (Fig. 2E). Over 24 h, the change in body weight differed significantly between the two groups; the vehicle group gained on average 7 g body weight (2.6% of baseline), and the OX-SR group lost 2.6 g of body weight (1% of baseline) (p < 0.05) (Fig. 2D). This loss of weight is due to the reduced food intake and increased energy expenditure.
Fig. 2.
Metabolic Effects of a Single Dose of OX-SR measured by CLAMS Metabolic Cages. Measurements of average hourly oxygen consumption (A), total oxygen consumption over the 12 h (B), total food intake over 12 h (C), change in body weight over 24 h (D), average RER over 12 h (E), hourly beam breaks (F) and total beam breaks over 12 h (G) in 16 male Wistar rats receiving a single injection of vehicle or OX-SR (40 nmol/kg) in CLAMS metabolic cages. Injection was administered at 0830, after 24 h acclimatization to CLAMS cage. Total oxygen consumption over the 12 h (H) and total beam breaks over 12 h (I) in a similar experiment with OX-SR administered at 1630. n = 7-8. Data expressed as mean ± SEM. Statistical analysis of B-E and G-I were undertaken by unpaired t-test. * p < 0.05, **p < 0.01 vs vehicle.
The same pattern of results was seen when OX-SR was administered at 1630. Food intake was halved over the 24 h following OX-SR administration (12.2 g compared to 24.7 g, p < 0.01). The OX-SR group lost, on average, 11.5 g, compared to an increase in body weight of 3.4 g in the control group, in the 24 h following administration (p < 0.01). In the 12 h following the injection, energy expenditure increased by 20% (p < 0.03) in the OX-SR group. Though not statistically significant, movement was overall reduced by 13% in the OX-SR group compared to the control group over the same period. As with the injections at the start of the light phase, RER was significantly reduced in the OX-SR group over the 12 h following injection, from 1.00 to 0.86 (p < 0.001).
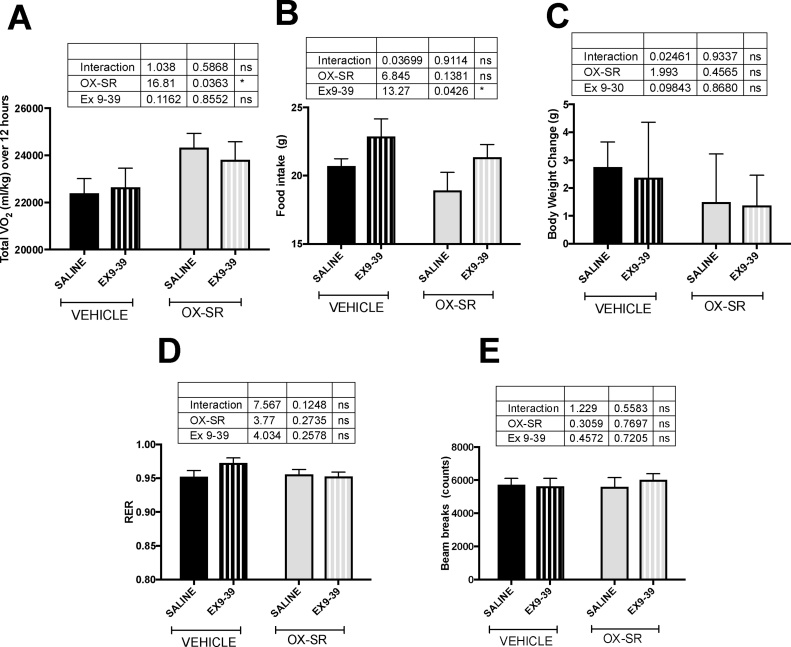
3.5. Energy expenditure following GLP-1 blockade
OX-SR caused an 8% increase in oxygen consumption during the 12 h post-injection, both in those animals infused with Ex 9–39 and in those infused with saline (ordinary 2-way ANOVA, effect of peptide p < 0.05; effect of pump content p = 0.86; interaction p = 0.59) (Fig. 3A). OX-SR did not significantly affect food intake; however, all animals receiving Ex9-39 ate significantly more over 24 h than those with a saline pump (ordinary 2-way ANOVA, effect of peptide p = 0.1; effect of pump content p < 0.05; interaction p = 0.9) (Fig. 3B). This is commensurate with blocking endogenous GLP-1 activity. Though neither Ex 9–39 nor OX-SR had a significant effect on body weight, there was a trend for the OX-SR groups to gain less weight than the vehicle groups (ordinary 2-way ANOVA, effect of peptide p = 0.5; effect of pump p = 0.9; interaction p = 0.9) (Fig. 3C). Locomotor activity did not differ between groups, nor did RER (Fig. 3D-E).
Fig. 3.
Effect of GLP-1 Receptor Blockade on the Energy Expenditure Effects of OX-SR. Oxygen consumption over 12 h (A), food intake over 24 h (B), body weight change over 24 h (C), average RER over 12 h (D), and total locomotor activity over 12 h (E) following an injection of vehicle or OX-SR (40 nmol/kg), in rats single-housed in CLAMS metabolic cages. Rats previously had subcutaneous mini-osmotic pumps implanted containing saline or EX9-39. Animals had free access to standard laboratory chow throughout. N = 8. Data expressed as mean ± SEM. Statistical analysis undertaken using ordinary 2-way ANOVA with Sidak’s multiple comparison test.
3.6. Comparison of energy expenditure following OX-SR and OX-SR-Glu3 administration
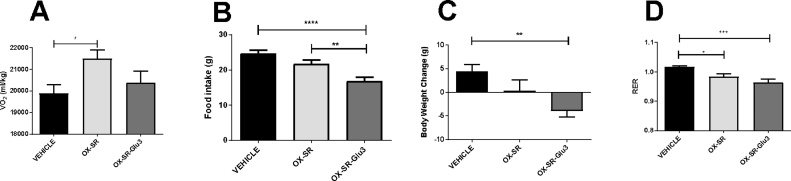
While OX-SR increased oxygen consumption by 8% over 12 h compared to vehicle (p < 0.05), there was no significant increase in oxygen consumption following OX-SR-Glu3 administration (Fig. 4A). This showed that glucagon receptor activity was required for OX-SR to increase energy expenditure. Notably, while OX-SR did not significantly affect food intake, OX-SR-Glu3 suppressed food intake over 24 h compared to both the vehicle and OX-SR groups (Fig. 4B), representing the potent anorectic effect of GLP-1 receptor activation. The vehicle group gained an average of 4 g body weight in the 24 h following subcutaneous injection; both the OX-SR and OX-SR-Glu3 groups gained less weight than the control group, with the difference being significant between vehicle and OX-SR-Glu3 groups (p < 0.01) (Fig. 4C). These results suggest that, acutely, the reduction in food intake has a greater effect on body weight than the increase in energy expenditure. There was no significant difference in locomotor activity between groups. RER was significantly suppressed in both the OX-SR and the OX-SR-Glu3 groups over the 12 h following peptide injection (p < 0.05 and p < 0.001 respectively) (Fig. 4D).
Fig. 4.
Comparison of Energy Expenditure Effects of OX-SR and OX-SR-Glu3. Total oxygen consumption over 12 h (A), food intake over 24 h (B), body weight change over 24 h (C) and average RER over 12 h (D) following an injection in male Wistar rats injected of vehicle, OX-SR (40 nmol/kg) or OX-SR-Glu3 (40 nmol/kg) in CLAMS metabolic cages. Animals had free access to standard laboratory chow throughout. Data expressed as mean ± SEM. n = 9–11. Statistical analysis was performed using one-way ANOVA with post-hoc Tukey’s multiple comparison test.* p < 0.05 ** p < 0.01, *** p < 0.001.
4. Discussion
Peptide analogues are a useful tool in the investigation of hormone physiology. To this end, the OXM analogue OX-SR was developed. OX-SR was slightly more potent at both the glucagon and GLP-1 receptors than OXM. It had a sustained release from a subcutaneous depot, taking 6 days for plasma levels to be undetectable compared to 1 day for the same dose of OXM be cleared. OX-SR and OXM had similar plasma half-lives following IV infusion (within the same order of magnitude),suggesting that OX-SR is cleared at a similar rate to OXM by peptidases such as DPPIV and Neprilysin [8]. These characteristics enabled a single low dose of OX-SR to be administered and its physiological effects measured, without the stress associated with repeat administration of OXM.
A single dose of OX-SR clearly increased energy expenditure, with oxygen consumption increasing by 10% over 12 h. Blocking activity at the GLP-1 receptor with Ex 9–39 did not limit the increase in energy expenditure, but reducing glucagon receptor activity did. This is the first study to directly show that the glucagon receptor is essential for OXM to increase energy expenditure.
As early as 1957, glucagon was shown to increase energy expenditure in rodents in both pair-feeding studies and through directly increasing oxygen consumption [23], [24]. This has more recently been confirmed in man, through indirect calorimetry during glucagon infusions [25], [26], [27]. This contrasts with GLP-1; in man, GLP-1 has been shown to either have no effect on energy expenditure [25], [26], [28], [29], or indeed may suppress energy expenditure [30]. In rodents, the results are also ambiguous, with GLP-1 agonists both transiently increasing [31], [32] and decreasing [33] oxygen consumption.
In these experiments, OX-SR had a variable effect on food intake. Over the 3 days of the pair-feeding study, it increased food intake. In contrast, when administered alone in the metabolic cage experiments, OX-SR reduced food intake. GLP-1 is an established anorectic agent. Consistent with this, OX-SR-Glu3, a GLP-1 receptor agonist, caused a significant reduction in food intake compared to controls, and blocking endogenous GLP-1 with Ex 9–39 caused an increase in food intake. GLP-1 has a dose-dependent anorectic effect, progressively stronger at higher doses [26], [34], [35]. Glucagon also has a dose-dependent effect on food intake, but this is not linear: at low doses, it can cause a hyperphagia, whereas at high doses, it reduces food intake [36], [37]. The increase in food intake at low dose of glucagon is likely an attempt to compensate for the increased energy expenditure; similar hyperphagia is seen in other conditions of increased energy expenditure such as thyrotoxicosis and after beta-3 adrenergic agonist administration [38], [39]. In line with this, it appears that the glucagon activity of OX-SR can counteract the anorectic GLP-1 effect within the peptide. This glucagon activity led to a relative increase in food intake in the OX-SR group compared to the OX-SR-Glu3 group, and an absolute hyperphagia in the OX-SR group in the pair-feeding study. Dose finding studies using OXM analogues have shown that the balance between the anorectic effect of GLP-1 receptor activation, and GCG receptor-mediated energy expenditure is fine and non-linear. Small dose adjustments in OXM analogues can switch them from being anorectic to orexigenic. The balance is also affected by other ’background’ factors such as the age and size of the animal, and the type of cage [9]. These factors together may explain the variability in food intake seen within our experiments. Higher doses of OX-SR could have been used, with a more pronounced GLP-1 effect. This would have produced a more reproducible and significant reduction in food intake; however, this would result in substantial weight loss, which would raise significant welfare concerns. In man, the higher levels of GLP-1 activity are likely to manifest as unacceptable levels of nausea. Therefore the lower dose is more likely to represent a dose that would be acceptable in man.
Glucagon also has a dose-dependent effect on energy expenditure, with increased energy expenditure at higher doses [25], [26]. Whether a similar dose response on energy expenditure is seen with oxyntomodulin and its analogues remains to be determined; though this would be expected given the glucagon activity, it may be that the mechanisms that suppress energy expenditure after GLP-1 administration become active and lower the overall energy expenditure effect. Understanding the relationship between the doses of GLP-1 and glucagon on energy expenditure will be important in optimising the energy expenditure effects of any OXM analogues developed to treat obesity.
OX-SR did not increase locomotor activity in rats. Other studies using OXM analogues showed no change in physical activity in rodents [17], [18]. Indeed, when administered before the onset of the dark period, when rodents are most active, there was tendency for locomotor activity to be suppressed despite a significant increase in energy expenditure. When glucagon is administered to rodents, there is also increased energy expenditure but no change in locomotion, suggesting that both OXM and glucagon increase energy expenditure by upregulating energy-demanding metabolic processes [40]. These animal results contrast with the study by Wynne et al. in obese humans using native OXM [5] where there was no difference in resting energy expenditure following saline or OXM administration, but OXM did cause a significant increase in physical activity related energy expenditure. The difference in these results may reflect the environment in which the studies occurred: Wynne et al.’s participants were in a ‘free-living’ environment, whereas animals in metabolic cages have little scope for activity as the cages are small. It would be pertinent to see the effect of OXM and related analogues on animal given the opportunity to undertake greater voluntary activity, such as in a running wheel.
Multiple mechanisms have been suggested by which glucagon can increase energy expenditure although none have been conclusively proven to be responsible. Both enhanced gluconeogenesis and enhanced protein turnover secondary to hyperglucagonaemia have been suggested as the reason for the increased metabolic rate in people with diabetes [41], [42], [43]. It has also been suggested that glucagon enhances energy expenditure via increased brown adipose tissue activation. In vitro, glucagon enhances oxygen uptake, heat production and fatty acid release in brown adipocytes [44], [45]. In vivo, it increased oxygen consumption in rats while enhancing blood flow to brown adipose tissue, it increased GDP binding to mitochondria in BAT and increased BAT weight [46], [47], [48]. However, these are all indirect measures of BAT activity, and more recently, a study using thermal imaging has shown no increase in BAT thermogenesis in man during a glucagon infusion, despite a contemporaneous increase in energy expenditure [27]. Further studies are therefore required to determine how glucagon increases energy expenditure. With regards to OXM, few mechanisms for its increase in energy expenditure have been posited. Though ICV OXM has been shown to increase sympathetic nerve discharge to BAT and increased UCP-1 levels, there is no evidence that this leads to enhanced oxygen consumption or clinically relevant weight loss. There is no data on protein turnover following oxyntomodulin administration, and the data on gluconeogenesis is conflicting, with some studies showing increased expression of gluconeogenic enzymes following OXM analogue administration, and other studies showing no changes [14], [15], [49].
Dual and even triple agonist therapies combining GLP-1, GIP and glucagon receptor activities are being actively trialled for obesity and diabetes [17], [50], [51], [52]. Many other drugs which increase energy expenditure have been withdrawn as treatments for obesity due to side effects (for example dinitrophenol for its hepatic toxicity; amphetamines and levothyroxine for their cardiovascular side effects). To develop safe and efficacious treatments for obesity, it is essential to understand how these dual/triple receptor agonists increase energy expenditure, therefore further mechanistic studies in this field are essential.
Funding
The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, an FP7- HEALTH- 2009-241592 EuroCHIP grant and is supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the author(s) and not necessarily those of the funders, the NHS, the NIHR or the Department of Health. R. Scott is also funded by the Wellcome Trust.
Contributor Information
R. Scott, Email: rebecca.scott22@nhs.net.
S.R. Bloom, Email: s.bloom@imperial.ac.uk.
References
- 1.Baldissera F.G.A., Holst J.J., Knuhtsen S., Hilsted L., Nielsen O.V. Oxyntomodulin (Glicentin-(33–69)) – pharmacokinetics, binding to liver-cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small-intestine of pigs. Regul. Pept. 1988;21(1-2):151–166. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 2.Dakin C.L., Gunn I., Small C.J., Edwards C.M.B., Hay D.L., Smith D.M., Ghatei M.A., Bloom S.R. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 3.Druce M.R., Minnion J.S., Field B.C.T., Patel S.R., Shillito J.C., Tilby M., Beale K.E.L., Murphy K.G., Ghatei M.A., Bloom S.R. Investigation of structure-activity relationships of oxyntomodulin (Oxm) using oxm analogs. Endocrinology. 2009;150(4):1712–1721. doi: 10.1210/en.2008-0828. [DOI] [PubMed] [Google Scholar]
- 4.Wynne K., Park A.J., Small C.J., Patterson M., Ellis S.M., Murphy K.G., Wren A.M., Frost G.S., Meeran K., Ghatei M.A., Bloom S.R. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects – a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 5.Wynne K., Park A.J., Small C.J., Meeran K., Ghatei M.A., Frost G.S., Bloom S.R. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int. J. Obes. 2006;30(12):1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 6.Thivel D., Brakonieki K., Duche P., Beatrice M., Yves B., Laferrere B. Surgical weight loss: impact on energy expenditure. Obes. Surg. 2013;23(2):255–266. doi: 10.1007/s11695-012-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaime T.J., Balich L.L., Acevedo G.B., Cave M., Sim S.H., Parada S.H., Silva J.R., Barnett D.B. Effect of calorie restriction on energy expenditure in overweight and obese adult women. Nutr. Hosp. 2015;31(6):2428–2436. doi: 10.3305/nh.2015.31.6.8782. [DOI] [PubMed] [Google Scholar]
- 8.van Can J., Sloth B., Jensen C.B., Flint A., Blaak E.E., Saris W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 2014;38(6):784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weetman A.P. Medical progress: graves' disease. New Engl. J. Med. 2000;343(17):1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 10.Lee P., Smith S., Linderman J., Courville A.B., Brychta R.J., Dieckmann W., Werner C.D., Chen K.Y., Celi F.S. Temperature-Acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63(11):3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dakin C.L., Small C.J., Batterham R.L., Neary N.M., Cohen M.A., Patterson M., Ghatei M.A., Bloom S.R. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145(6):2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 12.Baggio L.L., Huang Q.L., Brown T.J., Drucker D.J. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 13.Kosinski J.R., Hubert J., Carrington P.E., Chicchi G.G., Mu J., Miller C., Cao J., Bianchi E., Pessi A., SinhaRoy R., Marsh D.J., Pocai A. The glucagon receptor is involved in mediating the body weight-lowering effects of oxyntomodulin. Obesity. 2012;20(8):1566–1571. doi: 10.1038/oby.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day J.W., Gelfanov V., Smiley D., Carrington P.E., Eiermann G., Chicchi G., Erion M.D., Gidda J., Thornberry N.A., Tschop M.H., Marsh D.J., SinhaRoy R., DiMarchi R., Pocai A. Optimization of co-agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO-rodents. Biopolymers. 2012;98(5):443–450. doi: 10.1002/bip.22072. [DOI] [PubMed] [Google Scholar]
- 15.Lockie S.H., Heppner K.M., Chaudhary N., Chabenne J.R., Morgan D.A., Veyrat-Durebex C., Ananthakrishnan G., Rohner-Jeanrenaud F., Drucker D.J., DiMarchi R., Rahmouni K., Oldfield B.J., Tschoep M.H., Perez-Tilve D. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012;61(11):2753–2762. doi: 10.2337/db11-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speakman J.R. Measuring energy metabolism in the mouse-theoretical, practical, and analytical considerations. Front. Physiol. 2013;4 doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson S., Konkar A., Hornigold D., Trevaskis J., Jackson R., Fredin Fritsch, Jansson-Löfmark R., Naylor J., Rossi A., Bednarek M., Bhagroo N., Salari H., Will S., Oldham S., Hansen G., Feigh M., Klein T., Grimsby J., Maguire S., Jermutus L., Rondinone C., Coghlan M. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metabol. 2016;5 doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y.L., Ford H.E., Druce M.R., Minnion J.S., Field B.C.T., Shillito J.C., Baxter J., Murphy K.G., Ghatei M.A., Bloom S.R. Subcutaneous oxyntomodulin analogue administration reduces body weight in lean and obese rodents. Int. J. Obes. 2010;34(12):1715–1725. doi: 10.1038/ijo.2010.110. [DOI] [PubMed] [Google Scholar]
- 19.Lynch A., Pathak N., Pathak V., O'Harte F.P.M., Flatt P.R., Irwin N., Gault V.A. A novel DPP IV-resistant C-terminally extended glucagon analogue exhibits weight-lowering and diabetes-protective effects in high-fat-fed mice mediated through glucagon and GLP-1 receptor activation. Diabetologia. 2014;57(9):1927–1936. doi: 10.1007/s00125-014-3296-7. [DOI] [PubMed] [Google Scholar]
- 20.Christakis I., Scott R., Minnion J., Cuenco J., Tan T., Palazzo F., Bloom S. Measuring the pharmacokinetic properties of drugs with a novel surgical rat model. J. Invest. Surg. 2017;30(3):162–169. doi: 10.1080/08941939.2016.1231856. [DOI] [PubMed] [Google Scholar]
- 21.Ghatei M.A., Uttenthal L.O., Christofides N.D., Bryant M.G., Bloom S.R. Molecular forms of human enteroglucagon in tissue and plasma – plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J. Clin. Endocrinol. Metabol. 1983;57(3):488–495. doi: 10.1210/jcem-57-3-488. [DOI] [PubMed] [Google Scholar]
- 22.Price S.L., Minnion J.S., Bloom S.R. Investigating the glucagon receptor and glucagon-Like peptide 1 receptor activity of oxyntomodulin-like analogues in male wistar rats. Curr. Ther. Res.-Clin. Exp. 2015;77:111–115. doi: 10.1016/j.curtheres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salter J.M., Davidson I.W., Best C.H. The pathologic effects of large amounts of glucagon. Diabetes. 1957;6(3):248–252. doi: 10.2337/diab.6.3.248. [DOI] [PubMed] [Google Scholar]
- 24.Davidson I.W.F., Salter J.M., Best C.H. Calorigenic action of glucagon. Nature. 1957;8150:1124. doi: 10.1038/1801124a0. [DOI] [PubMed] [Google Scholar]
- 25.Tan T.M., Field B.C.T., McCullough K.A., Troke R.C., Chambers E.S., Salem V., Maffe J.G., Baynes K.C.R., De Silva A., Viardot A., Alsafi A., Frost G.S., Ghatei M.A., Bloom S.R. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131–1138. doi: 10.2337/db12-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cegla J., Troke R.C., Jones B., Tharakan G., Kenkre J., McCullough K.A., Lim C.T., Parvizi N., Hussein M., Chambers E.S., Minnion J., Cuenco J., Ghatei M.A., Meeran K., Tan T.M., Bloom S.R. Co-infusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63(11):3711–3720. doi: 10.2337/db14-0242. [DOI] [PubMed] [Google Scholar]
- 27.Salem V., Izzi-Engbeaya C., Coello C., Thomas D.B., Chambers E.S., Comninos A.N., Buckley A., Win Z., Al-Nahhas A., Rabiner E.A., Gunn R.N., Budge H., Symonds M.E., Bloom S.R., Tan T.M., Dhillo W.S. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes. Metabol. 2016;18(1):72–81. doi: 10.1111/dom.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt J.B., Gregersen N.T., Pedersen S.D., Arentoft J.L., Ritz C., Schwartz T.W., Holst J.J., Astrup A., Sjodin A. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am. J. Physiol.-Endocrinol. Metabol. 2014;306(11):E1248–E1256. doi: 10.1152/ajpendo.00569.2013. [DOI] [PubMed] [Google Scholar]
- 29.Tan T., Behary P., Tharakan G., Minnion J., Al-Najim W., Albrechtsen N.J.W., Holst J.J., Bloom S.R. The effect of a subcutaneous infusion of GLP-1, OXM, and PYY on energy intake and expenditure in obese volunteers. J. Clin. Endocrinol. Metabol. 2017;102(7):2364–2372. doi: 10.1210/jc.2017-00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flint A., Raben A., Rehfeld J.F., Holst J.J., Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int. J. Obes. 2000;24(3):288–298. doi: 10.1038/sj.ijo.0801126. [DOI] [PubMed] [Google Scholar]
- 31.Osaka T., Endo M., Yamakawa M., Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26(9):1623–1631. doi: 10.1016/j.peptides.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Hwa J.J., Ghibaudi L., Williams P., Witten M.B., Tedesco R., Strader C.D. Differential effects of intracerebroventricular glucagon-like peptide-1 on feeding and energy expenditure regulation. Peptides. 1998;19(5):869–875. doi: 10.1016/s0196-9781(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 33.Larsen P.J., Fledelius C., Knudsen L.B., Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50(11):2530–2539. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 34.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turton M.D., Oshea D., Gunn I., Beak S.A., Edwards C.M.B., Meeran K., Choi S.J., Taylor G.M., Heath M.M., Lambert P.D., Wilding J.P.H., Smith D.M., Ghatei M.A., Herbert J., Bloom S.R. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 36.Hell N.S., Timo-Iaria C. Increase of food intake induced by glucagon in the rat. Physiol. Behav. 1985;34(1):39–44. doi: 10.1016/0031-9384(85)90074-5. [DOI] [PubMed] [Google Scholar]
- 37.Geary N., Smith G.P. Selective hepatic vagotomy blocks pancreatic glucagons satiety effect. Physiol. Behav. 1983;31(3):391–394. doi: 10.1016/0031-9384(83)90207-x. [DOI] [PubMed] [Google Scholar]
- 38.Himmshagen J., Cui J.Y., Danforth E., Taatjes D.J., Lang S.S., Waters B.L., Claus T.H. Effect of CL-316, 243, a thermogenic beta-3-agonist, on energy balance and brown and white addipose tissues in rats. Am. J. Physiol. 1994;266(4):R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 39.Desouza C.J., Hirshman M.F., Horton E.S. The beta-3 specific agonist CL-316243, decreases body fat and enhances insulin-stimulated glucose disposal in non-obese rats. Diabetes. 1995;44:A191. [Google Scholar]
- 40.Atrens D.M., Menendez J.A. Glucagon and the paraventricular hypothalamus −modulation of energy balance. Brain Res. 1993;630(1–2):245–251. doi: 10.1016/0006-8993(93)90663-8. [DOI] [PubMed] [Google Scholar]
- 41.Nair K.S., Halliday D., Garrow J.S. Increased energy expenditure in poorly controlled type-1 (insulin dependent) diabetic patients. Diabetologia. 1984;27(1):13–16. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- 42.Charlton M.R., Nair K.S. Role of hyperglucagonemia in catabolism associated with type 1 diabetes – effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998;47(11):1748–1756. doi: 10.2337/diabetes.47.11.1748. [DOI] [PubMed] [Google Scholar]
- 43.Bogardus C., Taskinen M.R., Zawadzki J., Lillioja S., Mott D., Howard B.V. Increased resting metabolic rates in obese subjects with non-insulin-dependent diabetes mellitus and the effect of sulfonylurea therapy. Diabetes. 1986;35(1):1–5. doi: 10.2337/diab.35.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Joel C.D. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J. Biol. Chem. 1966;241(4):814–821. [PubMed] [Google Scholar]
- 45.Kuroshima A., Yahata T. Thermogenic responses of brown adipocytes to noradrenaline and glucagon in heat-acclimated adn cold-acclimated rats. Jpn. J. Physiol. 1979;29(6):683–690. doi: 10.2170/jjphysiol.29.683. [DOI] [PubMed] [Google Scholar]
- 46.Heim T., Hull D. The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J. Physiol. 1966;187(2):271–283. doi: 10.1113/jphysiol.1966.sp008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Billington C.J., Briggs J.E., Link J.G., Levine A.S. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am. J. Physiol. 1991;261(2):R501–R507. doi: 10.1152/ajpregu.1991.261.2.R501. [DOI] [PubMed] [Google Scholar]
- 48.Billington C.J., Bartness T.J., Briggs J., Levine A.S., Morley J.E. Glucagon stimulation fo brown adipose tissue growth and thermogenesis. Am. J. Physiol. 1987;252(1):R160–R165. doi: 10.1152/ajpregu.1987.252.1.R160. [DOI] [PubMed] [Google Scholar]
- 49.Pocai A., Carrington P.E., Adams J.R., Wright M., Eiermann G., Zhu L., Du X., Petrov A., Lassman M.E., Jiang G., Liu F., Miller C., Tota L.M., Zhou G., Zhang X., Sountis M.M., Santoprete A., Capito E., Chicchi G.G., Thornberry N., Bianchi E., Pessi A., Marsh D.J., SinhaRoy R. Glucagon-like peptide 1/Glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C., Chabenne J., Zhang L., Habegger K.M., Fischer K., Campbell J.E., Sandoval D.L., Seeley R.J., Bleicher K., Uhles S., Riboulet W., Funk J., Hertel C., Belli S., Sebokova E., Conde-Knape K., Konkar A., Drucker D.J., Gelfanov V., Pfluger P.T., Muller T.D., Perez-Tilve D., DiMarchi R.D., Tschop M.H. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015;21(1):27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 51.Finan B., Clemmensen C., Zhu Z.M., Stemmer K., Gauthier K., Muller L., De Angelis M., Moreth K., Neff F., Perez-Tilve D., Fischer K., Lutter D., Sanchez-Garrido M.A., Liu P., Tuckermann J., Malehmir M., Healy M.E., Weber A., Heikenwalder M., Jastroch M., Kleinert M., Jall S., Brandt S., Flamant F., Schramm K.W., Biebermann H., Doring Y., Weber C., Habegger K.M., Keuper M., Gelfanov V., Liu F., Kohrle J., Rozman J., Fuchs H., Gailus-Durner V., de Angelis M.H., Hofmann S.M., Yang B., Tschop M.H., DiMarchi R., Muller T.D. Chemical hybridization of glucagon and thyroid hormone optimises therapeutic impact for metabolic disease. Cell. 2016;167(3):843–857. doi: 10.1016/j.cell.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Jall S., Sachs S., Clemmensen C., Finan B., Neff F., DiMarchi R.D., Tschop M.H., Muller T.D., Hofmann S.M. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol. Metabol. 2017;6(5):440–446. doi: 10.1016/j.molmet.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]