Abstract
Background
T cells from HIV+ and aged individuals show parallels in terms of suppressed proliferative activity and interleukin-2 (I1-2) production and an increased number of CD8+ CD28- T cells. In order to compare cytokine production from T cells from these two states, CD4+ and CD8+ T cells from HIV+ aged, and normal young donors (controls) were monitored for cytokine production by flow cytometry, quantitative PCR and ELISA upon activation by PMA and anti-CD3. In addition, the CD8+ T cell subsets CD28+ and CD28- from the HIV+ and the aged groups were evaluated for cytokine production by flow cytometry, and compared with those from young controls.
Results
Flow cytometric analysis indicated that CD8+ T cells from both HIV+ and aged donors showed an increase of approximately 2–3 fold over controls in percentage of cells producing inflammatory cytokines IFN-γ and TNF-α. Similar analysis also revealed that the production of interleukins-4,6 and 10, production was very low (1–2% of cells) and unchanged in these cells. Quantitative PCR also showed a substantial increase (4–5 fold) in IFN-γ and TNF-α mRNA from HIV+ and aged CD8+ T cells, as did ELISA for secreted IFN-γ and TNF-α (2.3–4 fold). Flow cytometric analysis showed that the CD8+ CD28- T cell subset accounts for approximately 80–86% of the IFN-γ and TNF-α production from the CD8+ subset in the aged and HIV+ states. The CD4+ T cell, while not significantly changed in the HIV+ or aged states in terms of IFN-γ production, showed a small but significant increase in TNF-α production in both states.
Conclusions
Our data appear compatible with physiologic conditions existing in HIV+ and aged individuals, i.e. elevated serum levels and elevated CD8+ T cell production of IFN-γ and TNF-α. Thus, the capacity for increased production of cytokines IFN-γ and TNF-α in the aged individual by the dominant CD8+ CD28- subset may have a profound influence on the clinical state by aggravating inflammatory pathologies such as rheumatoid arthritis, and possibly Alzheimer's disease and Crohn's disease. In AIDS, these cytokines may contribute to wasting and cachexia. We theorize that the predominant phenotypic change to the cytotoxic CD8+ CD28- T cell subsets in both the HIV+ and the aged states may reflect a natural "endpoint" in CD8+ T cell differentiation induced after a lifetime of immune activity (toward viruses, etc) in the aged, and after a massive accelerated response to HIV in the HIV-positive individual.
Introduction
Elderly people show an increased incidence of infectious, neoplastic and inflammatory diseases that may be linked to declining functional competence of the peripheral T cells [1-5]. At the cellular level, this decline in immune response is reflected in aged T cell in vitro proliferation, decreased production of interleukin-2 [2,6-8] and 11–2 receptor [7,9,10], and defective progression thought the cell cycle [11,12]. HIV+ infection likewise induces a progressive suppression in proliferation of both CD4+ and CD8+ T cells in vitro that correlates with clinical severity, as well as diminished 11–2 production [13-16]. However, even stronger parallels exist between immune phenomena in aging and AIDS than suppression of T cell proliferation and 11–2 production. There is a relative increase in the memory cell repertoire [17-20], and an increase in absolute counts in the CD8+ CD28-populations in both aged [22] and HIV+ donors [22-25]. The CD8+ CD28- T cell in the HIV+ state appears to be the major cytotoxic T cell (CTL) [26], but curiously, has shortened telemer lengths as well [27].
Previous studies indicate considerable inconsistency regarding production of the immune regulatory cytokine, interferon-γ (IFN-γ) and the preinflammatory cytokine, tumor necrosis factor-α (TNF-α) from T cells of HFV+ and aged individuals. The disparity in IFN-γ expression is reflected in reports of decreased [28-31], normal [21,32] or increased [33-37] synthesis in both aged and HIV+ T cells. Similarly, with TNF-α, there are contradictory reports of decreased [37,38], normal [29], or increased [12,31,39,40] production in the HIV+ and aged T cells. Explanations for the divergent findings can be attributed to many factors including cell preparation, choice of activators, and quantitative methodology. Recently, we showed [25] that IFN-γ production in HIV+-derived CD8+ T cells was strongly stimulated by upon activation anti-CD3 and PMA. Using this mode of activation, we selected flow cytometry for analysis of HIV+ and aged T cells in this study since this method obviates the inherent complications association with experimental variability of T cell purification and activation. Subsequently, the flow cytometric data, based on the percentage of active cells, was supported by cytokine mRNA and ELISA data. We find by all three methods that IFN-γ and TNF-α production is enhanced several fold in CD8+ T cells from aged and HIV+ individuals, and it is the CD8+ CD28- subset, as shown by flow cytometry, which is primarily responsible. These findings assume clinical importance since they may explain in part the chronic and autoimmune pathologies in the elderly, particularly rheumatoid arthritis, Crohn's disease, sepsis, and myelodysplastic syndromes, which are exacerbated by the inflammatory cytokines IFN-γ and TNF-α [41]. Likewise, the elevation of these cytokines, particularly TNF-α potentially may contribute to the deterioration in the HIV+ or AIDS patient by promoting wasting and cachexia [42].
Results
Enhanced IFN-γ and TNF-α production in aged and HIV+ CD8+ T cells
Representative examples of the cytometric profile of aged and HIV+ human T cells (CD4+ and CD8+ and controls (normal young donors) are shown in Fig. 1; the cells producing IFN-γ and TNF-α appear in the upper quadrants. The percentages of either CD4+ or CD8+ T cells producing cytokine (upper quadrant) are shown. In these studies, the peripheral blood mononuclear cells (PBMC) were prepared and activated for 15 hrs with anti-CD3 (plus PMA) and processed with the appropriate fluorescent-labeled antibodies. For 3 color FC analysis, the samples were first gated for the CD3 positive cells thus eliminating B cells, NK cells and macrophages, and then examined for CD8+ and cytokine-containing cells. Based on past studies, the non CD8+ T cells are primarily composed of CD4+ T cells and are designated as such. Controls (not shown) for every sample were also examined for cytokine-producing cells (upper quadrants) in which no activators were added; 0.0–0.6% positive cells were observed. Previously we found [25] that activation by anti-CD3 and PMA was far superior to other activation modes such as Con A plus PMA in stimulating IFN-γ synthesis. Anti-CD3 or PMA alone did not induce cytokine production [25]. In all instances with anti-CD28 as coactivator, the IFN-γ and TNF-α responses were low, rarely exceeding 15% of that produced by PMA/anti-CD3 activation.
Figure 1.
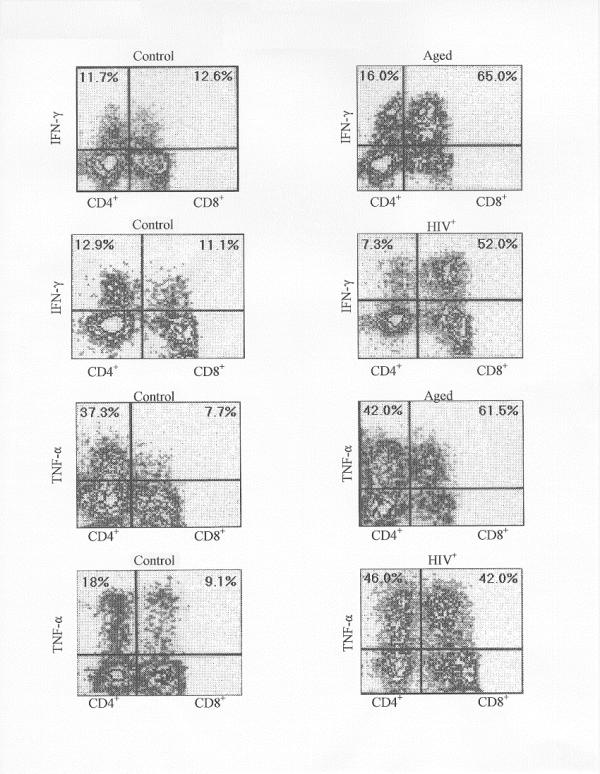
The distribution of CD4+ and CD4+ T cells are shown for typical samples of PBMC from HIV, aged and normal (control) donors as evaluated by flow cytometry. The cells positive for IFN-γ (PE) or TNF-α (PE) distribute vertically above the horizontal marker; the vertical distance is a relative measure of cytokine intensity. The two populations of cells that comprise the CD3 positive population are the CD8+ T cells and the CD4+ T cells (CD3+, CD8-) as shown on the X axis. The percentage of CD4+ or CD8+ T cells which contain cytokine are shown in the upper quadrants. The PBMC were activated for 15 hrs with anti-CD3 and PMA and subsequently treated as described in METHODS. In all cases, nonactivated controls (not shown) were examined; less than 0.5% of cells were cytokine positive.
The production of IFN-γ and TNF-α from aged and HIV+ T cells (CD4+ and CD8+) activated by anti-CD3 and PMA is shown in Table I. The data were derived from the flow cytometric analysis of the CD8+ and CD4+ T cell profiles of forty aged, thirty-two HIV+ and forty-eight normal young donors. Experiments with the HrV+-derived cells were performed separately from those with aged cells with 24 control donors used in each case. The cytokine analysis relies on the intracellular accumulation of cytokines in the Golgi compartment over the 15 hr. activation period induced by either brefeldin A or monensin. Both inhibitors block Golgi function and thus cytokine secretion, the former by inhibition of protein vesicle transport [43], the latter by inhibition of Na+ gradients [44].
Table I.
Percentage of T cell subsets Producing Cytokines
| Incubated with: | |||
| Monensin | Brefeldin | Brefeldin | |
| Aged | IFN-γ | TNF-α | IFN-γ |
| CD4+ | 16.5 ± 7.1 | 50.3 ± 12.6 | 16.6 ± 6.4 |
| (N.S.) | (p < 0.001) | (N.S.) | |
| CD8+ | 42.2 ± 15.6 | 57.6 ± 14.3 | 57.2 ± 12.6 |
| (p < 0.001) | (p < 0.001) | (p < 0.001) | |
| Control1 | |||
| CD4+ | 14.2 ± 5.8 | 35.9 ± 11.2 | 15.8 ± 4.8 |
| CD8+ | 13.1 ± 3.5 | 19.0 ± 6.5 | 17.9 ± 4.7 |
| HIV+ | |||
| CD4+ | 12.7 ± 5.1 | 51.0 ± 14.9 | 14.6 ± 6.2 |
| (N.S.) | (p < 0.01) | (N.S.) | |
| CD8+ | 40.6 ± 16.5 | 35.8 ± 14.5 | 42.3 ± 11.4 |
| (p < 0.001) | (p < 0.001) | (p < 0.001) | |
| Control1 | |||
| CD4+ | 14.5 ± 3.6 | 41.9 ± 9.8 | 16.2 ± 4.9 |
| CD8+ | 16.1 ± 4.7 | 16.1 ± 8.5 | 13.5 ± 6.7 |
The percentage of CD4+ or CD8+ T cells that show a positive production of IFN-γ or TNF-α was determined by the number of cells above the anchor gate (cytokine producing) over the total cell number as described in Methods. The results are from 40 aged, 32 HIV+ and 48 normal donors; and show the mean ± S.D. The data obtained with the aged and HIV+-derived T cells represent experimental sets performed at different times with the same or different control donors (24 controls with each group). In all cases, PBMC were incubated for 15 hrs at 37° with PMA and anti-CD3 and either monensin or brefeldin as described in Methods prior to analysis by the flow cytometry. The p values were calculated with the Student's t test and refers to a statistical comparison of the aged or HIV+ CD8+ T cells and the control cells. 1Controls are young donors with no known medical conditions or diseases. No HIV+ or aged subject was part of the control group in this study.
Flow cytometric analysis of cells from the aged donors activated by anti-CD3 and PMA shows that IFN-γ production is enhanced significantly (approximately 3 fold) in the CD8+ T cell compartment, in terms of percent of producing cells, compared to young control donors (Table I). Even with Con A and PMA activation, the aged CD8+ T cell response was 4 fold enhanced over young controls (28.0% and 7.0% respectively)(data not shown). The response of brefeldin-treated CD8+ cells was somewhat greater than in the monensin-treated cells, for both aged and controls. For the HiV+ group, the number of IFN-γ producing CD8+ T cells was similarly enhanced over normal controls with both types of Golgi inhibitors. Interestingly, although the CD4+ T cell is a target for the HIV, the IFN-γ response is not significantly changed from the normal CD4+ T cell response. Likewise, with the aged CD4+ cells, there is no significant difference with the responses of the cells from young donors.
The changes in TNF-α production in CD8+ T cells from HIV+ and aged individuals are comparable to that for IFN-γ production (Fig. 1 and Table I). Aged CD8+ T cells show a significant increase (approximately 3 fold) over young cells in the percentage producing TNF-α (57.6 versus 19.0%). In the HIV+ cases also, the CD8+ T cells show significantly enhanced TNF-α production (2 fold). However, in contrast to the IFN-γ data, the aged CD4+ T cells are modestly, but significantly increased over the young CD4+ T cells (50.3 versus 35.9%). The CD4+ T cells from HIV+ donors also are slightly more capable of TNF-α production than the normal CD4+ T cells (51.0 versus 42.0%), a difference that is significant. Thus in the aging and HIV+ scenario, it is apparent that the CD8+ T cells show an enhanced capacity for both TNF-α production and IFN-γ production. The CD4+ T cells show a small but significant capacity for TNF-α production in both scenarios.
Comparative analysis of the CD8+ CD28- and CD8+ CD28+ T cells by flow cytometry
For each blood sample, a non-activated control was prepared in which the PBMC were incubated in the absence of activators (anti-CD3 and PMA), and then examined by flow cytometry. Such controls showed no significant cytokine-containing cells, and these samples were used to calculate the resting CD4+/CD8+ distribution and the CD28+/CD28- distribution. As shown in Table III, there is a dramatic phenotypic change in the CD8+ subsets in the aged and HIV states in relation to young healthy controls. In our IFN-γ studies, the CD28- population accounts for 61.3% (aged) to 70.0% (HIV+) of the total CD8+ population. In healthy controls, the CD8+ CD28- subset accounts for only 28–31% of the CD8+ cells. In the TNF-α studies, the CD28- cells are 64.2% (aged) to 68.3% (HIV+) of the total CD8+ population. The TNF-α and IFN-γ studies were carried out separately. These results indicate that in the HIV+ and aged states, the CD28- subset becomes dominant, assuming approximately two-thirds of the total CD8+ cells whereas in the normal healthy state the CD28+ T cell subset accounts for approximately two-thirds. Thus, in terms of cell numbers, the CD28- cells constitute the majority of the CD8+ T cells and explains our previous findings (16,45) that the mitogenic signal arising from coactivation by anti-CD28 was greatly suppressed in CD8+ T cells from HIV+ donors compared to normal donors.
Table III.
Secreted IFN-γ and TNF-α from T Cell Culture*
| Source | Cells | IFN-γ (pg/ml) | TNF-α (pg/ml) |
| Aged | PBMC | 70,800 ± 8,500 (p < 0.001) | 35,400 ± 6,200 (p < 0.005) |
| Control | PBMC | 20,300 ± 3,000 | 16,900 ± 4,100 |
| Aged | CD8+ | 86,300 ± 15,600 (p < 0.001) | 44,200 ± 7,800 (p < 0.005) |
| Control | CD8+ | 24,400 ± 6,200 | 19,400 ± 4,300 |
| HIV+ | CD8+ | 121,000 ± 15,600 (p < 0.001) | 57,200 ≅ 11,500(p < 0.001) |
| Control | CD8+ | 27,300 ± 4,800 | 16,400 ± 4,900 |
PBMC and purified CD8+ T cells were incubated for 66 hrs. as described in Methods, and the media was tested by ELISA. The results are expressed in pg IFN-γ or TNF-α per 105 cells (mean value ± S.D.). The standard deviation is shown and the p valves, determined with Student's t test, compare the IFN-γ or TNF-α secreted by aged or HIV+-derived cells and normal control cells. Eight different aged and HIV+ donors were used, and seven young control donors. In the presence of 5 mM of N-acetylcysteine, the production of both INF-γ and TNF-α was significantly increased in aged, HIV+ and control samples, generally by 1.5 to 2 fold.
Table II.
Comparison of cytokine Production in CD8+ CD28 and CD8+ CD28+ T cells
| Cells derived form | ||||
| HIV+ | Control | Aged | Control | |
| In TNF-α studies | ||||
| % CD28- of the | 68.3 | 36.3 | 64.2 | 27.1 |
| total CD8+ cells | ||||
| % CD28- | 55.2 | 26.8 | 68.0 | 29.5 |
| producing TNF-α | ||||
| % Total TNF-α± | 79.5 | 20.5 | 84.6 | 15.4 |
| In IFN-γ studies | ||||
| % CD28- of the total | 70.0 | 30.7 | 61.3 | 28.0 |
| CD8+ cells | ||||
| % CD28- producing | 59.8 | 21.2 | 62.3 | 35.1 |
| IFN-γ | ||||
| % Total IFN-γ ± | 86.7 | 13.3 | 79.6 | 20.4 |
*Analysis of CD8+ T cells from CD28+ and CD28- subsets in terms of cytokine production was performed by flow cytometry as described in Methods. Twenty HIV+ and aged donors and fifteen control donors were used in the IFN-γ studies; twenty-two and nineteen were used respectively in the TNF-α studies. ± The percent total of TNF-α or IFN-γ refers to the percentage of CD28- cells producing cytokine compared to the total produced by the controls and HIV+ (or aged) CD28- T cells. For example, in the TNF-α studies, 8% of the control CD28- cells produce cytokine (27.1 × 29.5), whereas in the aged state, CD28- cells producing TNF-α is 43.7% (64.2 × 68.0). Thus the TNF-α produced by aged CD28- CD84 T cells is 84.6% compared to 15.4% for controls.
The data (Table III) show that the CD8+ CD28- T cells also account for the increased production of TNF-α and IFN-γ in both the HIV+ and aged states. In these states, the percent of CD28- cells producing each cytokine dominates the normal young controls by a 2:1 to 3:1 margin. Thus not only are the CD28- cells greater in number, but they are proportionately much more active in cytokine production. As a consequence, the data show that the CD28- population accounts for the majority of the cytokine-producing CD8+ T cells (79.6–86.7%) in the HIV+ and aged populations. Typical examples of the CD8+ CD28- profiles are shown in Fig. 2. It is evident that the cells from the HIV+ and aged states have a greater capacity for TNF-α and IFN-γ production compared to the normal control CD28- or CD28+ population.
Figure 2.
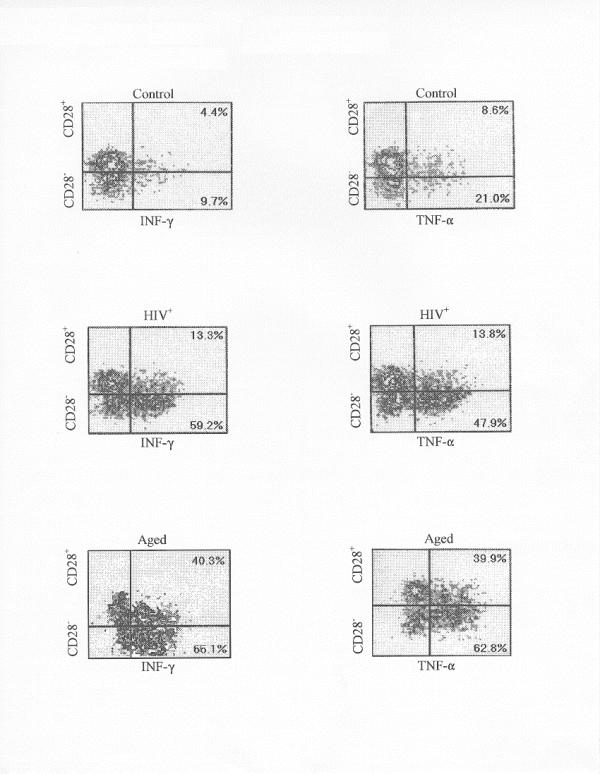
The distribution of CD8+ T cells are shown as obtained from PBMC by four color flow cytometry using anti-CD28-PE and anti-IFN-γ-FITC (or anti-TNF-α-FITC) as described in METHODS. The CD28+ and CD28- cells distribute vertically and the IFN-γ or TNF-α positive cells distribute horizontally according to their intensity. The percentage of the CD8+ CD28+ or CD8+ CD28- T cells which contains cytokine are shown in the right quadrants. Cells were activated with anti-CD3 and PMA as described in METHODS. The controls refers to cells from a normal young donor. The HIV+ and aged samples were derived from typical donors.
Production of other cytokines (I1-2, 4, 6 and 10)
The ability of CD4+ and CD8+ T cells from aged and HIV+ donors to synthesize 11–2, 11–4, 11–6 and 11–10 were also studied by flow cytometry. Only in the case of 11–2 was an appreciable number of cytokine-producing cells observed [25]. For normal young cells at least 15–30% of CD4+ cells were positive for 11–2 while only 3–6% of the CD8+ cells were positive. With HIV+ and aged T cells, there was a significant 11–2 response also, but it was generally smaller than from the young normal control cells (data not shown). The percentage of cells positive for production of 11–4, 11–6 and 11–10 was extremely low. The number of productive cells was often <1% and only rarely exceeded 2% of either the CD4+ or CD8+ population. No significant difference was observed between the control cells and those derived from HIV+ or aged donors.
Production of IFN-γ and TNF-α mRNA determined by Quantitative PCR
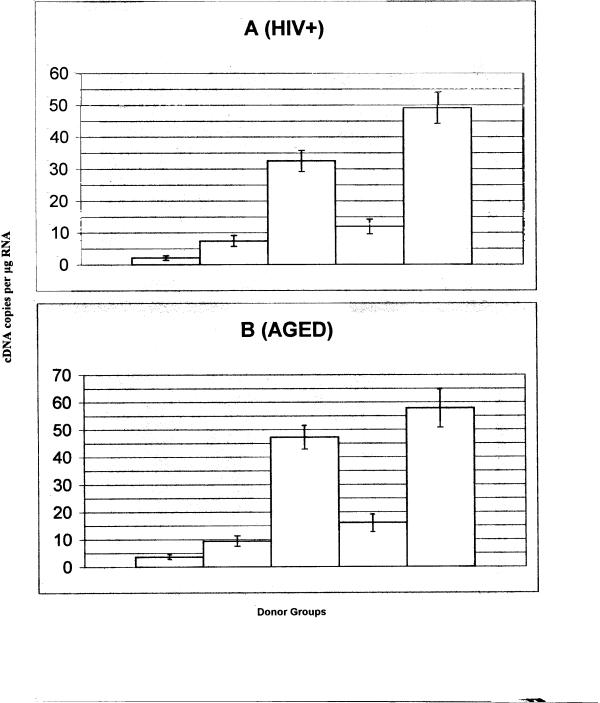
The mRNA isolated from CD8+ T cells from 12 aged, 10 HIV+ and 15 controls were purified and used in these studies. The results, shown in Fig 3 are expressed as IFN-γ (or TNF-α) cDNA copies/ug total RNA. CD8+ T cells were incubated for 3 hrs with PMA and anti-CD3 prior to total RNA extraction. For IFN-γ, CD8+ T cells from both the HIV+ and aged states show a 4.5–5 fold enhancement in cDNA copies (32,000 and 47,000 respectively versus controls of 7,400 and 9,100). Likewise, the TNF-α cDNA copies are enhanced approximately 4 fold in the CD8+ T cells from aged and HIV+ states. In view of the flow cytometric data, we can infer that the enhanced mRNA in the CD8+ T cells derives primarily from the CD8+ CD28- T cells.
Figure 3.
The production of cytokine mRNA determined by quantitative by PCR is shown for (A) HIV+ donor CD8+ T cells and (B) aged donor CD8+ T cells. The cDNA copies per ug RNA from the CD8+ T cells. The cDNA copies per ug RNA from the CD8+ T cells were derived from: 1, nonativated controls; 2, activated controls (INF-γ); 3, activated HIV+ or aged (INF-γ); 4, activated controls (TNF-α); 5, HIV+ or aged (TNF-α).
Secreted IFN-γ and TNF-α determined by ELISA
The PBMC and purified CD8+ T cells from eight donors and seven control donors were incubated for 66 hrs, and the media examined by ELISA. The results, shown in Table III, demonstrate that production of IFN-γ is upregulated over controls, from 3.5–4 fold, for the CD8+ T cells from HIV+ and aged donors. For TNF-α, the increase was also substantial, 2.3–4 fold.
Discussion
Our data show that the HIV+ and aged conditions foster a change in the CD8+ T cell repertoire characterized by expansion of a CD8+ CD28- phenotype capable of enhanced IFN-γ and TNF-α production. These results contrast with other studies concluding that the production of these cytokines was downregulated or unchanged in T cells from HIV+ and aged individuals [21,28-32,37,38]. Reliability in our findings derives from use of flow cytometric analysis which measures de novo synthesis per cell and thus obviates numerous artifacts possibly associated with ELISA or procedures that measure mRNA synthesis. Secondly, similar results were obtained with two different inhibitors of Golgi transport. Thirdly, of the cytokines examined, including 11–4, 11–6 and 11–10, only IFN-γ and TNF-α were enhanced in the HIV+ or aged states. Fourthly, CD4+ T cells did not show a significant change in IFN-γ production, but did show a small but significant increase in TNF-α production in aged and HIV+-derived cells. Fifthly, the mRNA and ELISA studies support the conclusions of the flow cytometric data.
The question arises whether or not our results have physiological relevance since PMA and anti-CD3 was used to elicit the cytokine responses we observed. The question resolves into two parts: are TNF-α and IFN-γ levels increased in the HIV+ and aged states, and do the CD8+ T cells show increased production of these cytokines. There is considerable evidence supporting both parts of this question. Regarding part one, IFN-γ levels are elevated in the sera of HIV+ patients [45]. Serum levels of both TNF-α and IFN-γ were markedly higher in HIV+ patients over controls, but varied depending on the skin disease exhibited by the patient [46]. AIDS patients also showed elevated TNF-α levels in the plasma [47]. Studies of lymphocytes from aged donors showed increase serum levels of TNF-α and IFN-γ that correlate with upregulation of HLA-DR on CD3+-lymphocytes [48].
Regarding part two, there are many studies showing that physiological activation of CD8+ T cells from HIV+ or aged donors prompts increased IFN-γ and/or TNF-α production. HIV+ lymph nodes exhibit a large (6 fold) increase in CD8+ T cells, most of which are involved in IFN-γ production [49]. Amison et al [50] concluded that IFN-γ is secreted by the CD8+ T cell in HIV+ infections. In CD8+ T cells from African HIV+ patients, stimulated with env peptide, both IFN-γ and TNF-α production were increased over controls [52]. PBMC from the elderly (almost healthy donors) showed upregulation of TNF-α in unstimulated cultures [52]. HLA-DR activation of CD8+ CD57+ cells from elderly subjects strongly produced TNF-α [48]. Lipopolysaccharide-activation of T cells from aged donors induced higher levels of TNF-α than controls in two separate studies [53,31]. Finally, in HIV+ patients receiving HIV type 1 antigen, both INF-γ and TNF-α were significantly increased in memory T cells activated by antigen [54].
Thus there appears to be considerable support for the enhanced presence of TNF-α and INF-γ in the HIV+ and the aged states, and for a physiologic role of the CD8+ T cell subset in their production. Our data shows that it is the CD8+ CD28- cell that is primarily responsible. Why the CD8+ CD28- subset should dominated in such apparently diverse conditions as the HIV+ and the aged states is not known. However, the notion that similarities exist between the HIV+ and the aged states with respect to the CD8+ CD28- T cell has been presented previously [55]. It has also been proposed that the CD8+ CD28- T cell in the HIV+ state is derived from the CD8+ CD28+ cells by clonal expansion induced by massive antigenic stimulus (HIV proteins) since both showed the same epitope repertoire [26]. Dolad et al [56] have shown that the CD8+ CD28- cell, not the CD8+ CD28+ cell, comprises the major CTL activity in the HIV+ state, a finding confirmed by Vingerhoets et al [57], who associated cytotoxic T cell activity with the CD8+ CD28- T cell in HIV-infected subjects. Thus, the CD8+ CD28- T cell assumes a crucial role in HIV infection. Likewise in the aged state, we can speculate that chronic viral and bacterial exposure over a lifetime may induce the clonal expansion of the CD8+ CD28- T cell.
There is evidence that the cytotoxic activity of the CD28- cell can lyse and suppress HIV-infected cells [58,59] and thus play a protective role in HIV infection. Perhaps in the aged cell as well, the CD28- memory cell serves a similar protective role against a variety of viruses. The production of IFN-γ and TNF-α can result in strong anti-viral consequences as shown with hepatitis B virus [60], but the cytokines have a dual role since they are associated with inflammatory activity. IFN-γ, in its defensive role against infection, is a foremost factor in converting resting macrophages into the active state [61] where they produce reactive oxidants [62], nitric oxide [63], TNF-α [61], and neopterin. Not only can these factors damage DNA and host tissues but can modulate inflammatory reactions [60] and stimulate apoptosis [64], a major mechanism leading to loss of CD4+ T cells [65]. TNF-α also has the capacity to perturb the clinical condition in AIDS by strongly upregulating the transcription of the HIV genome [66]. In fact, it has been proposed [67] that AIDS is in many ways a TNF-α disease since this cytokine it is a potent activator of NF-kB, and an inducer of HIV genome transcription and apoptosis in HIV-infected T cells. Moreover, the wasting, weight loss, and cachexia associated with AIDS has also been related to TNF-α activity [42]. Thus both IFN-γ and TNF-α have the potential for a double role in the pathogenesis associated with AIDS i.e. antiviral and inflammatory responses.
Based on the AIDS scenario, it is reasonable to propose that chronic viral and bacterial infections suffered by an individual over a lifetime may lead to similar consequences as those occurring in acute HIV infection, i.e., clonal expansion of the CD8+ CD28- subset at the expense of the CD8+ CD28- subset. The data argue that the CD28-cell may serve as a natural endpoint in the differentiation of CD8+ cells, as proposed by Effros et al [27], where aging represents a "normal endpoint" that occurs after many years of immune activity, while AIDS represents an accelerated endpoint due to the increase rate in T cell formation during attempted compensation for CD4+ loss induced by HIV. By virtue of their inflammatory roles, TNF-α and IFN-γ likely contribute to the exacerbation of the inflammatory conditions associated with the aged. For example, autoimmune diseases are more prevalent in the aged, where certain diseases such as arthritis are often associated with chronic debilitation. Interestingly, both IFN-γ and TNF-α are prominent in the pathogenesis of autoimmune diseases in general; the former by activation of local auto antigen presentation to appropriate T cells, and the latter by mediation of inflammatory tissue damage [68]. Apparently TNF-α plays a central role in the chronic release of highly arthritogenic II-1 [69], and thus serves as a major factor in the pathogenesis of rheumatoid arthritis (RA) [69]. Recent studies show that treatment with anti-TNF-α gives significant clinical improvement in RA [70]. Thus our findings in aged individuals of a prominent CD8+ CD28- T cell subset with enhanced ability to produce TNF-α, may assume clinical relevance. It is logical to infer that the increased susceptibility of aged individuals to rheumatoid arthritis and other autoimmune pathologies, may be due to increased TNF-α (and IFN-γ) production by the CD8+ CD28- T cell. It should be noted that the action of IFN-γ on macrophages induces TNF-α production [61], thus amplifying the effects of the latter.
Although IFN-γ production in the HIV+ individual by the CD4+ T cells (TH1) has been emphasized [13,45,49] there appears to be little doubt that the production of IFN-γ by CD8+ CD28- cells may have greater clinical relevance as discussed above on the increased serum levels [45,46]. With TNF-α as well, our data suggest it is likely that the increased serum levels in AIDS [46-48] may derive in part from increased numbers of CD8+ CD28-cells. However, TNF-α levels could also originate from monocytes stimulated by the enhanced IFN-γ production. In any case, the HIV+ state is replete with antagonistic complexities of antiviral effects of IFN-γ and TNF-α on one hand, and their inflammatory effects on the other. Overall, our data presents an intriguing parallel between AIDS and the aging state with regard to their cytokine and CD8+ T cell profiles, and the potential for new insight into aging mechanisms. It is noteworthy that our results are compatible with recent aging studies using gene arrays to measure gene expression in the neocortex and cerebellum of aged and young mice. Lee et al [71] found that in both brain regions, one hallmark of aging was enhanced gene expression of the inflammatory response, including proinflammatory peptides and many interferon-related proteins. Thus the advent of the CD8+ CD28- cell in aging, with its potential for proinflammatory cytokines, could have profound effects not only on autoimmune diseases, but on Alzheimer's disease as well since cytokines may contribute to the neuropathology [72].
Materials and Methods
Materials
Con A, phorbol myristate acetate (PMA), N-acetylcysteine (NAC), brefeldin A and monensin were obtained from Sigma (St. Louis, Mo.), fetal calf serum from Harlan Bioproducts (Madison, Wis.), anti-CD28 and anti-CD3 (4E) from Research Diagnostics (Flanders, N.J.). Purified monoclonal Ab anti-CD4, anti-CD8, anti-CD 14 and anti-CD 19, bound to magnetic beads (BioMag), were obtained from Perseptive Diagnostics (Cambridge, MA). Interleukin 4, 8, 10 and 12 were obtained from the Genzyme Corp.
Study approval
This study was approved by the Institutional Review Board of the Ponce School of Medicine, Ponce, PR.
Blood donors
The HIV+ population (32 donors aged 22–34 years, mean 29.3 years) used in this study had a CD4+ T cell count from 210–500 cells/mm3 and fell in the A2 and B2 classification recommended by the Centers for Disease Control. Most (93%) of the HIV+ donors were receiving the anti-retroviral drug zidovudine. Transmission in the HIV+ donors was approximately 60% from intravenous drug use and 40% from unprotected sexual activity. Blood from forty aged individuals (mean 84.2 years, range 76–98 years) was collected for this study. The controls (48 young Hispanics aged 19–30 years, mean 23.7 years, from Ponce, Puerto Rico), had no serious medical problems. All donors were Hispanic, blood was collected from HIV+ individuals (Hispanic) under the direction of Dr. Harold I. Laroche and Dr. E. Godreau, Center for Diagnosis and Treatment, Ponce. The aged population, recruited from the retirement home Los Diamantes, had no serious medical problems in general. Some had minor arthritis and high blood pressure (medicated); one had Parkinson's disease.
Purified T cells
PBMC were prepared by Hypaque-Ficoll centrifugation of blood diluted 1:1 with PBS (5). For preparation of purified T cells (CD4+ and CD8+) free of macrophages, granulocytes, B cells, and NK cells, the PBMC (1 × 107 cells) were centrifuged, and diluted to 200 ul with 0.1% BSA in PBS. After addition of 10 ul of fetal calf serum and 10 ul of antibody "mix" (T cell negative isolation kit from Dynal Inc), the cells were incubated 10 minutes at 4°, washed in PBS, and suspended in 900 ul PBS with 100 ul of "depletion" beads (Dynal). After 15 minutes at room temperature, the beads are magnetically removed leaving highly purified T cells (CD4+ and CD8+, 98% purified in general). The yield varied from 40–50% based on the starting number of PBMC. For the preparation of purified CD4+ T cells, 2 × 107 purified T cells were incubated for 30 minutes on ice with BioMag mouse anti-human CD8 (IgG1) monoclonal Ab (500 ul) in 3.0 ml RPMI plus 9% FCS (diluted 1:1) for 30 min on ice with rocking. Similarly, to obtain purified CD8+ T cells, the same procedure was followed except BioMag mouse anti-human CD4 (IgG2a) monoclonal Ab (500 ul) was used. The cell suspension was brought to 10 ml with cold RPMI media, and the desired T cell subset was obtained by magnetic removal of the contaminating cells. Flow cytometric analysis indicated that the T cell subsets were 93–98% purified. The yield of control and aged cells varied from 58–89% generally, whereas the yield from HIV+ blood generally fell between 24–68%. Accessory cells were effectively removed during T cell isolation as shown by the failure of mitogen-induced proliferation in the absence of coactivators. All cell purification steps were completed and assays initiated within 10 hrs. after blood donation. The purified CD8+ T cells were used for the ELISA and quantitative PCR studies.
Flow cytometric analysis (FC)
Prior to FC analysis, PBMC (3.3 × 105) suspended in 600 ul media were incubated at 37°C overnight in 6% CO2 with anti-CD3 (1:500 dilution of acetates fluid) and PMA (2.5 ng/ml) as described previously [25]. Either monensin (1 uM) or brefeldin A (1 ug/ml) was used in all cases to block Golgi function and allow cytokine accumulation with the cell. For analysis of CD4+ and CD8+ T cells, the PBMC (activated and non activated controls) were treated as described by the PharMingen (Flow Cytometry and Cell Analysis Catalog (1998) "Immunofluorescent Staining of Intracellular Cytokines for Flow Cytometric Analysis", pp415-417). Briefly, PBMCs were washed twice with staining buffer (PBS plus 0.5% bovine serum albumin), and then fixed by suspending in cold 0.5% paraformaldehyde (PFA). After incubation for 20 min at 4°, the cells were centrifuged, decanted and resuspended in 4% PFA for 30 min at 4°. Cells were then washed with 1 ml of staining buffer, and incubated 30 min at 4° with 50 ul permeabilization buffer (0.1% saponin, PBS, 0.1% azide). To the cell pellet, 3 ul of phycoerythrin (PE) conjugated mouse anti-human IFN-γ or TNF-α monoclonal Ab (Pharmingen Inc.) was added and incubated 30 min at 4°. The cells were then washed with 1 ml permeabilization buffer, spun down, and gently resuspended. Then 10 ul of fluorescein isothiocyanate (FITC) mouse anti-CD8 (Leu-2a) (Becton-Dickinson), and 10 ul peridinin chlorophyll (PerCP) mouse anti-CD3 (Leu-4) (Becton-Dickinson) were added, followed by incubation in the dark for 20 min at room temperature. After washing with 1 ml of staining buffer, the cells were fixed with 600 ul of 0.5% PFA for approximately 1 day and then analyzed with a FACSCalibur flow cytometer (Becton-Dickinson) equipped with a FL4 channel.
As an alternative to FC analysis of activated PBMC described above, we also studied activated CD4+ and CD8+ T cells purified as described in the previous sections. These studies we carried out in order to completely eliminate the possibility, however remote, that our FC procedure did not discriminate between the CD8+ T cells and NK cells, which have low density CD8+ receptors. However, our FC procedure used a sufficiently high "anchor gate" to examine only the high density CD8+ receptors which are uniquely characteristic of the CD8+ T cell. This is accomplished by using an "anchor gate" which is sufficiently high. As expected, our analysis of CD8+ T cells using either the purified cells or the PBMC were in complete agreement in 5 experiments in which they were compared, i.e., both showed the same increased production of IFN-γ and TNF-α from HIV+ derived or aged CD8+ T cells.
Analysis of CD4+ and CD8+ T cells
Four-color cytometric analysis was performed with a Power-Macintosh 7600 computer with Cellquest software. Statistical analysis was performed on data collected from 10,000 events (data points) per cell sample. To distinguish t cell subsets, a gate was drawn around lymphocytes by forward scatter and side scatter (region 1 (R1)). T cells were identified as a CD3 (FL3) positive population (R2), thereby excluding CD3- populations such as B cells, macrophages, and NK cells. In order to analyze T cell subsets (CD4+ and CD8+ cells) for each cytokine production, contour plots on CD8+ (FL1)/ cytokine (FL2) of logical gate 1 events (R1 + R2) were obtained. The event numbers and percentages of each quadrant event were recorded. For simplicity, the CD3+ CD8- T cell subset are assumed to be CD4+ and labeled as such in Fig. 1. In our experience, the CD3+ CD4+ comprise 95% or more of the CD3+ CD8- T cell subset in normal blood. In HIV+ blood, a few CD4+ T cells may be masked by soluble gp-120, but this would not affect the CD3+ CD8+ population.
Analysis of CD28+ and CD28- T cells using four-color cytometry
For this study, PBMC were treated as described above except that anti-human CD3+ (FL4) coupled to allophycocyanin (APC) was used along with anti-IFN-γ FITC (FL1), anti-CD28 PE (FL2) and anti-CDS PerCP (FL3). Region 1 (R1) was drawn to identify lymphocytes by forward scatter and side scatter, and R2 was drawn to identify T cells (FL4 positive). Logical gate 1 (R1+R2) was used to identify CD4 (FL3 negative) and CD8 (FL3 positive) subsets that were designated as R3 and R4 respectively. Logical gate 2 (R1+R2+R3) events were analyzed for cytokine production by CD28+(FL2) or CD28- subpopulations by plotting two dimensional contour plots. Logical gate 3 (R1+R2+R4) events were similarly analyzed.
Competitive PCR
The competitive PCR was performed using both IFN-γ mRNA and TNF-α mRNA detection kits from BioSource Corp. The mRNA was highly purified from purified CD8+ cells activated for 3 hrs, with the Ambion RNAqueous kit for total RNA, and the Micro Poly (A) pure kit for mRNA. Generally the mRNA obtained was near 2% of total RNA. The cDNA was obtained by use of reverse transcriptase (Invitrogen). PCR was performed in 100 ul final volume containing: 1) 3 ul of IFN-γ primer; 2) 18 ul 10X reaction buffer; 3) 25 ul 200 uM NTP's; 4) 44.5 ul H2O; 5) 5 ul cDNA; 6) 5 ul Internal Calibration Standard (ICS) DNA and 0.5 ul Taq polymerase. Components were gently mixed and centrifuged, then heated 2 min at 95°. Thirty cycles were performed with a Perkin Elmer 9600 thermocycler as follows: 1 min at 95°, 1 min at 55°, 1 min at 72°. The reaction was next heated at 72° for 10 min following by cooling at 4°. A 15 ul sample from each PCR reaction was analyzed on 2% agarose gels containing 0.5 ug/ml ethidium bromide along with molecular weight standards to determine the fragment size. Bands were visualized under UV light and photographed using Polaroid film.
After cDNA amplification, 25 ul of PCR mix was removed and transferred to a microcenterfuge tube. The same volume of denaturation solution (alkaline pH) was added to the tube and incubated for 10 min at room temperature. The material was neutralized by the addition of 450 ul of hybridization solution prior to addition to the ICS or IFN-γ oligonucleotide capture wells (OCW). In order to acquire quantitative results the denatured amplicons were analyzed at 1/20, 1/100, 1/200 dilution in hybridization buffer.
Following hybridization, unbound material was removed by washing the wells four times with 200 ul diluted wash buffer; 100 ul of streptavidin – HPR was then added to each well and incubated for 30 min at room temperature. The wells were washed again, and 100 ul of stabilized chromogen (substrate) was added. After a 30 minute incubation in the dark, the reaction was stopped by addition of 100 ul stop solution and the observance was measured at 450 nm. The optical density (OD) in each well is proportional to the amount of amplicon present, which can be related to the copy number of either ICS or IFN-γ in the original PCR reaction. The total OD is calculated as sample OD multiplied by the amplicon dilution. The number of copies of IFN-γ in each PCR reaction are calculated from the ratio of total OD for the IFN-γ specific well to the total OD for the ICS well times the and input copies of the ICS.
ELISA
The IFN-γ and TNF-α secreted by CD8+ T cells into culture media was quantitated using an Elisa kit from either BioSource (Corp.) or Research Diagnostics. The CD8+ T cells were incubated at 37° for 66 hours in precisely the same way as for mitogenic assay except volumes were doubled and 50,000 cells were used. Samples were run in duplicate, the results were highly reproducible (S.D. <15% for any given assay) and sensitive. However, sensitivity was not important since with most samples it was necessary to dilute the culture fluid by 1:20 or more. The procedure was followed as recommended by the manufacturer. The standard curves for IFN-γ and TNF-α, which intersected the origin, was generally linear up to 500 pg/ml for recombinant IFN-γ and 400 pg/ml for TNF-α.
Acknowledgments
Acknowledgment
We wish to thank Miss Veronica Rodríguez for excellent technical help, and Dr. Carlos Ríos-Bedoya of the RCMI Epidemiology and Biostatistics Program. This work was supported by NIH Research Grants S06 GM 08239 (MBRS) and 5 G 12RR03050 (RCMI).
Contributor Information
Edward H Eylar, Email: eheylar@yahoo.com.
Carmen E Lefranc, Email: eheylar@yahoo.com.
Yasuhiro Yamamura, Email: yy11@coqui.net.
Ineabely Báez, Email: eheylar@yahoo.com.
Sol Luis Colón-Martinez, Email: slcolonmartinez@yahoo.com.
Nayra Rodriguez, Email: yy11@coqui.net.
T B Breithaupt, Email: tbreit@uomhs.com.
References
- Miller RA. The Aging Immune System: Primer and Prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Gillis S, Kosak R, Durante M, Weksler MA. Immunological studies of aging. J Clin Invest. 1981;67:937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- Thoman ML, Weigle WO. The cellular and subcellular bases of immunosenescence. Adv Immunol. 1989;46:221–261. doi: 10.1016/s0065-2776(08)60655-0. [DOI] [PubMed] [Google Scholar]
- Eylar EH, Molina F, Quiñones C, Zapata M, Kessler M. Aging: Deficient mitogenic response of old rhesus monkey T cells. AGING: Immunol and Infectious Disease. 1989;14:219–226. [Google Scholar]
- Miller RA, Stutman O. Decline, in aging mice, of the anti-2,4,6-trinitrophenyl (TNP) cytotoxic T cell response attributable to loss of Lyt-2-, interleukin 2-producing helper cell function. Eur J Immunol. 1981;11:751–756. doi: 10.1002/eji.1830111004. [DOI] [PubMed] [Google Scholar]
- Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schnider EL. Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest. 1988;81:1096–1102. doi: 10.1172/JCI113422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar EH, Báez I, Vasquez A, Colón-Martínez SL, Rodriguez J, Kessler M, Breithaupt TB. N-acetylcysteine (NAC) strongly enhances the proliferative and interleukin-2 response of aged rhesus CD4+ and CD8+ T cells. AGING: Immunol and Infec Dis. 1996;6:141–151. [Google Scholar]
- Negoro S, Hara H, Miyata S, Saiki O, Tanaka T, Yoshizaki K, Igarashi T, Kishimoto S. Mechanisms of age-related decline in antigen-specific T cell proliferative response: IL-2 receptor expression and recombinant IL-2 induced proliferative response of purified Tac-positive T cells. Mech Aging Dev. 1986;36:223–241. doi: 10.1016/0047-6374(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Vie H, Miller RA. Decline, with age, in the portion of mouse T cells that express 11–2 receptors after mitogen stimulation. Mech Aging Dev. 1986;33:313–322. doi: 10.1016/0047-6374(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Beckman I, Dimopoulos K, Xu XN, Bradley J, Henschke P, Ahem M. T cell activation in the elderly: evidence for specific deficiencies in T cell accessory cell interaction. Mech Aging Dev. 1990;51:265–276. doi: 10.1016/0047-6374(90)90076-R. [DOI] [PubMed] [Google Scholar]
- Song L, Kim YH, Chopra RK, Proust JJ, Nagel JE, Nordin AA, Adler WH. Age-related defects in T cell activation and proliferation. Exp Gerontol. 1993;28:313–321. doi: 10.1016/0531-5565(93)90058-L. [DOI] [PubMed] [Google Scholar]
- Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T-helper-cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J din Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T-helper-cell dysfunction in asymptomatic individuals infected with HIV. J Clin Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar EH, Rivera-Quiñones C, Larroche H, Yamamura Y. Proliferative response of CD4+ and CD8+ T-cell subsets from Hispanics with HIV+ and AIDS: the superantigen hypotheses. J AIDS. 1994;7:124–128. [PubMed] [Google Scholar]
- Eylar EH, Baez I, Vazquez A, Colón-Martínez SL, Yamamura Y. Suppressed proliferative response and interleukin-2 production in hispanic HIV+ and AIDS T-cell subsets. Cell Mol Biol. 1995;41:S25–S33. [PubMed] [Google Scholar]
- DePaoli P, Battisstin S, Santini GF. Age-related changes in human lymphocyte subsets: progressive reduction of the CD4 CD45R (suppressor inducer) population. Clin Immunol Immunopathol. 1988;48:290–296. doi: 10.1016/0090-1229(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concavalin A. Eur J Immunol. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- Ernst DN, Weigle WO, Noonan DJ, McQuitty DN, Hobbs MV. The age-associated increase in INF-γ synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993;151:575–587. [PubMed] [Google Scholar]
- Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28- T cells in healthy aging people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choremi-Papadapoulou H, Vigils V, Gargadianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J AIDS. 1994;7:245–253. [PubMed] [Google Scholar]
- Vingerhoets JH, Vanham GI, Kestens LL, Penne GG, Colebunders RL, Vandenbmaene MJ, Goeman J, Gigase PL, DeBoer M, Ceuppens JL. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin Exp lmmunol. 1995;100:425–433. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnoni D, Prati E, Malacarne F, Gorla R, Airo P, Cattaneo R. The primary response to HIV is characterized by an expansion of activated CD8+ CD28- cells. AIDS. 1996;10:104–106. doi: 10.1097/00002030-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Eylar EH, Lefranc C, Báez I, Colon SL, Yamamura Y, Rodríguez N, Yano N, Breithaupt TB. Enhanced interferon-γ by CD8+ CD28- lymphocytes from HIV+ patients. J Clinical Immunol. 2000. [DOI] [PubMed]
- Fiorentino S, Dalod M, Olive D, Guillet JG, Gomard E. Predominant involvement of CD8+ CD28- lymphocytes in human immunodeficiency virus-specific cytotoxic activity. J Virol. 1996;70:2022–2026. doi: 10.1128/jvi.70.3.2022-2026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R, Allsopp R, Chiu C, Hausner M, Hirji K, Wang L, Harley Villeponteau B, West M, Giorgi Shortened telomers in the expanded CD28- CD8+ cell subsets in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Murray HW, Rubin BY, Masur H, Roberts RB. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Gauchat JF, de Weck AL, Stadler BM. Decreased cytokine messenger RNA levels in the elderly. AGING: Immunol Infect Disease. 1988;1:191. [Google Scholar]
- Caruso C, Candore G, Cigna D, Dilorenzo G, Sireci G, Dieli F, Salerno A. Cytokine Production Pathway In the elderly. Immunol Res. 1996;15:84–90. doi: 10.1007/BF02918286. [DOI] [PubMed] [Google Scholar]
- Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Aging Dev. 1998;102:199–209. doi: 10.1016/S0047-6374(97)00153-X. [DOI] [PubMed] [Google Scholar]
- Weifeng C, Shulin L, Xiaomeni G, Xuewen P. The capacity of lymphokine production by peripheral blood lymphocytes from aged humans. Immunol Invest. 1986;15:575. doi: 10.3109/08820138609026697. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Corssarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Saxena RK, Saxena QB, Adler WH. Lectin-induced cytotoxic activity in spleen cells from young and old mice: age-related changes in types of effector cells, lymphokine production and response. Immunology. 1988;64:457–461. [PMC free article] [PubMed] [Google Scholar]
- Caruso A, Canaris AD, Licenziati S, Cantalamessa A, Folghera S, Lonati MA, dePanfilis G, Garotta G, Turano A. CD4+ and CD8+ lymphocytes of patients with AIDS synthesize increased amounts of interferon-gamma. J Acquir Immune Defic Syndr Hum Retroviral. 1995;10:462–470. doi: 10.1097/00042560-199512000-00010. [DOI] [PubMed] [Google Scholar]
- Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- Chang HN, Wang SR, Chiang SC, Teng WJ, Cheng ML, Tsai JJ, Huang DF, Lin HY, Tsai YY. The Relationship Of Aging To Endotoxin Shock And To Production Of TNF-alpha. J Gerontol Biol Sci Med Sci. 1996;51:220–222. doi: 10.1093/gerona/51a.5.m220. [DOI] [PubMed] [Google Scholar]
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ, Rowland-Jones SL. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–76. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Uthgenanf D, Dodt C, Nunninghoff D, Ringvolt E, Wagner T, Fehm H. Cytokine production and lymphocyte subpopulation in aged humans. Mech Aging Dev. 1995;84:113–126. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- Vyakarnam A, Meatar P, Meager A, Kelly G, Stanley B, Weller I, Beverly P. Altered production of tumor necrosis factors alpha and beta and interferon gamma by HIV-infected individuals. Clin Exp Immunol. 1991;84:109–115. doi: 10.1111/j.1365-2249.1991.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A. Anti-TNF therapies in rheumatoid arthritis, Chrom's disease, sepsis, and myclodysplastic syndromes. Microsc Res Tech. 2000;50:229–235. doi: 10.1002/1097-0029(20000801)50:3<229::AID-JEMT6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Kotler DP. Pathophysiology of the AIDS wasting syndrome. AIDS Clin Rev. 1992:191–224. [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Yokota S, Takasuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- Eylar EH, Báez I, Lefranc CE, Colón-Martínez SL, Rodríguez V, Yamamura Y, Rodríguez N. The proliferative response of HIV+ T-cells (CD4+ and CD8+) are severely suppressed via CD28 coactivation. Cell Mol Biol. 1997;43:989–993. [PubMed] [Google Scholar]
- Fuchs D, Hausen A, Reibnegger G, Werner-Felmayer G, Dierich MP, Wachter H. Interferon-γ concentrations are increased in sera from individuals infected with human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1989;2:158–162. [PubMed] [Google Scholar]
- Breuer-McHam JN, Marshall GD, Lewis DE, Duvic M. Distinct serum cytokines in AIDS-related skin diseases. Viral-Immunol. 1998;11:215–220. doi: 10.1089/vim.1998.11.215. [DOI] [PubMed] [Google Scholar]
- Heijligenberg R, Romijn JA, Godfried MH, Endert E, Sauerwein HP. In vitro production of cytokines in whole blood versus plasma concentrations of cytokines in AIDS. AIDS Res Hum Retroviruses. 1998;14:123–127. doi: 10.1089/aid.1998.14.123. [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan SE, Alexander HD. CD69, CD25, and HLA-DR activation antigen statement on CD3+ lymphocytes and relationship to serum TNF-α, IFN-γ, and sIL-2R levels in aging. Exp Gerontol. 1999;34:79–93. doi: 10.1016/S0531-5565(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Emilie D, Peuchmaur M, Malliot MC, Brousse N, Delfraissy JF, Dormont J, Galanaud P. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J din Invest. 1990;86:148–159. doi: 10.1172/JCI114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameisen JC, Estaquier J, Idziorek T. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol Rev. 1994;142:9–51. doi: 10.1111/j.1600-065x.1994.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S, Fabiani M, Ferrante P, Clerici M. Immune activation in HIV-infected individuals. Italian-Ugandan AIDS cooperation program. AIDS. 1998;12:2387–2396. doi: 10.1097/00002030-199818000-00007. [DOI] [PubMed] [Google Scholar]
- My'sliwska J, Bryl E, Foerster J, My'sliwski A. The upregulation of TNF-α production is not a generalized phenomenon in the elderly between their sixth and seventh decades of life. Mech Ageing Dev. 1999;107:1–14. doi: 10.1016/S0047-6374(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Wallis RS, Lederman HM, Spritzeler J, Devers JL, Georges D, Weinberg A, Sthen S, Lederman MM. Measurement of induced cytokines in AIDS clinical trials using whole blood: a preliminary report. ACTG Inducible Cytokines Focus Group. AIDS Clinical Trials Group. Clin Diag Lab Immunol. 1998;5:556–560. doi: 10.1128/cdli.5.4.556-560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maino VC, Suni MA, Wormsley SB, Carlo DJ, Wallace MR, Moss RB. Enhancement of HIV type 1 antigen-specific CD4+ T cell memory in subjects with chronic HIV type 1 infection receiving an HIV type 1 immunogen. AIDS Res Hum Retroviruses. 2000;16:539–547. doi: 10.1089/088922200308954. [DOI] [PubMed] [Google Scholar]
- Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–454. doi: 10.1016/S0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- Dolad M, Sinet M, Deschemin JC, Fiorentino S, Venet A, Guillet JG. Altered ex vivo balance between CD28+ and CD28 cells within HIV-specific CD8+ T cells of HIV-seropositive patients. Eur J Immunol. 1999;29:38–44. doi: 10.1002/(SICI)1521-4141(199901)29:01<38::AID-IMMU38>3.3.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Vingerhoets JH, Vanham GL, Kestens LL, Penne GG, Colebunders RL, Vandenbruaene MJ, Goeman J, Giase PL, De Boer M, Ceuppens JL. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin Exp Immunol. 1995;100:425–433. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, Walker BD. Suppression of HIV-1 replication by CD8+ cells: Evidence for HLA class I restricted triggering of cytolytic and non cytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Kalams SA, Rosenzweig M, Trocha A, Koziel M, Walker BD, Johnson RP. Efficient lysis of HIV-1 infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/S1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- Billiau A. Interferon-γ: Biology and Role in Pathogenesis. Advances in Immunology. 1996;62:61–107. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- Nathan C, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–681. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo R, Shapiro D, Le J, Huang S, Aguet M, Vilcek J. Generation of nitric oxide and induction of major histocompatibility complex class II antigen in macrophages from mice lacking the interferon gamma receptor. Proc Natl Acad Sci USA. 1993;90:6626–6630. doi: 10.1073/pnas.90.14.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Janaway CA., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self tolerance. J Exp Med. 1990;172:1753–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai C, Kornbluth RS, Pauza CD, Richman DD, Carlson DA. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Kinter A, Justenment JS. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Sci Acad USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T, Kobayashi N, Yamamoto N. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease. AIDS. 1991;5:1405–1417. doi: 10.1097/00002030-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Elliott MJ, Woody JN, Maini RN. Anti-Tumor Necrosis Factor-α Therapy of Rheumatoid Arthritis. Advances in Immunology. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- Pettipher ER, Higgs GA, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the sinovial joint. Prot Natl Acad Sci USA. 1986;83:8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Bijl H, Woody JN. Repeated therapy with monoclonal antibody to tumor necrosis factor a (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344:1125–1128. doi: 10.1016/S0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Lee Cheol-Koo, Weindruch R, Prolla T. Gene expression profile of aging brain in mice. Nature Gen. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Rogers J. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17:681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]