Abstract
Survival of primary brain tumor (glioblastoma) patients is seriously hampered by glioma stem cells (GSCs) that are distinct therapy-resistant self-replicating pluripotent cancer cells. GSCs reside in GSC niches, which are specific protective microenvironments in glioblastoma tumors. We have recently found that GSC niches are hypoxic periarteriolar, whereas in most studies, GSC niches are identified as hypoxic perivascular. The aim of this review is to critically evaluate the literature on perivascular GSC niches to establish whether these are periarteriolar, pericapillary, perivenular, and/or perilymphatic. We found six publications showing images of human glioblastoma tissue containing perivascular GSC niches without any specification of the vessel type. However, it is frequently assumed that these vessels are capillaries which are exchange vessels, whereas arterioles and venules are transport vessels. Closer inspection of the figures of these publications showed vessels that were not capillaries. Whether these vessels were arterioles or venules was difficult to determine in one case, but in the other cases, these were clearly arterioles and their perivascular niches were similar to the periarteriolar niches we have found. Therefore, we conclude that in human glioblastoma tumors, GSC niches are hypoxic periarteriolar and are structurally and functionally look-alikes of hematopoietic stem cell niches in the bone marrow.
Keywords: arterioles, blood vessels, capillaries, glioblastoma, glioma stem cells, microenvironment, niche, therapy resistance, venules
Introduction
Glioblastoma is the most common and most aggressive primary brain tumor with poor patient survival irrespective of treatment. This low survival rate is at least partly caused by glioma stem cells (GSCs).1,2 These self-replicating pluripotent cells reside in GSC niches, which are specific microenvironments in glioblastoma. GSCs are resistant to therapy and may cause tumor recurrence.3–6 Quiescence of GSCs in the niches,7–12 effective DNA damage repair,12–15 effective drug efflux ABC transporter activity,16 and/or Notch signaling13,17 are factors that play a role in their resistance to therapy. It is clear that GSCs need to be eradicated for more effective therapy of glioblastoma.7,8,13,14,18 A promising new therapeutic option is targeting GSCs with hyperthermia in combination with irradiation because multiple DNA repair pathways are sensitive to hyperthermia.19
Since Calabrese et al. introduced in 2007 the perivascular GSC niche,8 this concept of GSC niches has been the most popular. In the perivascular niche, GSCs are located in close association with endothelial cells (ECs) that line blood vessels.4,8,20 In 2015, Hira et al. found that the vessels that are surrounded by GSCs are arterioles and, therefore, introduced the concept of periarteriolar niche.21,22 The two types of niches are very similar. In fact, the only difference is that in the concept of the perivascular niche, no discrimination is made between the types of vessels around which GSC niches are found, be it arteriole, capillary, venule, or lymph vessel, whereas this is an arteriole in the concept of periarteriolar GSC niche. This difference is not trivial because in the concept of perivascular niche, it is often, if not always, assumed that the vessels are capillaries.3,8 A major functional difference between capillaries and arterioles is that capillaries are exchange vessels and arterioles are transport vessels. Morphologically, arterioles have a distinctly larger lumen than capillaries and the wall of arterioles is thicker than that of a capillary (Figs. 1 and 2).
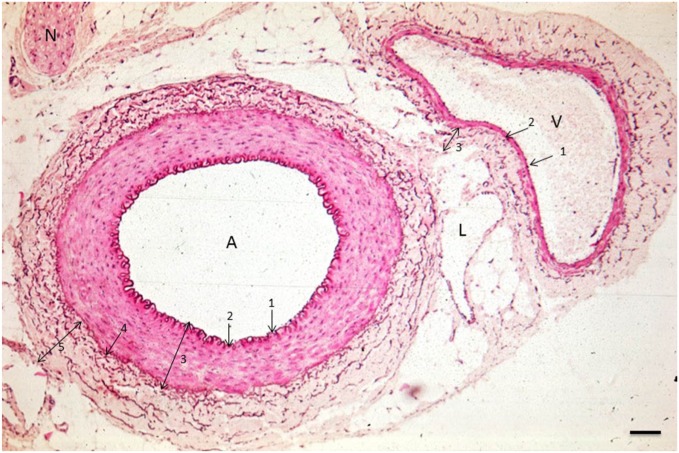
Figure 1.
Image of an arteriole (A), venule (V), and lymph vessel (L) in the human abdominal cavity. 1, tunica intima (endothelial cells); 2, tunica elastica interna; 3, tunica media (smooth muscle cells); 4, tunica elastica externa; 5, tunica adventitia (stroma containing elastic fibers [brown]). N, nerve. Bar = 100 μm.
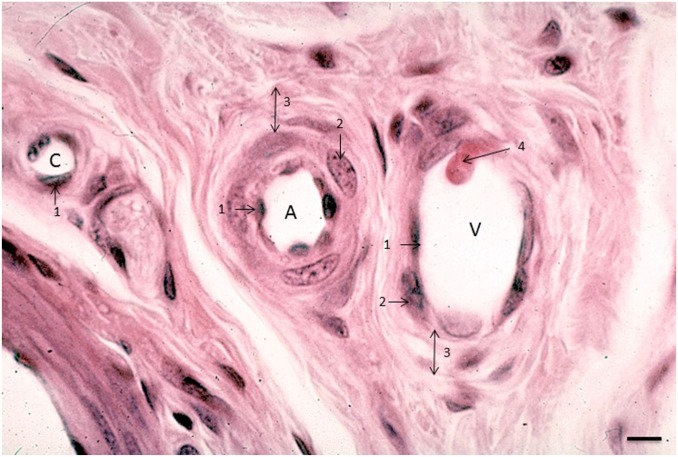
Figure 2.
Image of an arteriole (A), venule (V), and capillary (C) in human lung tissue. 1, endothelial cell; 2, smooth muscle cell; 3, tunica adventitia; 4, leukocyte that adheres to the venular wall in the process of diapedesis. Bar = 5 μm.
The aim of the present review is a critical analysis of the literature on perivascular GSC niches to establish whether a specific type of vessel is the center of a perivascular niche and, if so, which one(s). For that purpose, we briefly discuss the morphology of arterioles, capillaries, venules, and lymph vessels. Then, we discuss the various structural and functional characteristics that are known of perivascular GSC niches and periarteriolar GSC niches. Finally, we analyze, when available, images of perivascular niches in publications on perivascular GSC niches and conclude that GSC niches are found around arterioles in glioblastoma.
Arterioles, Capillaries, Venules, and Lymph Vessels
The four types of vessels that are found in tissues are arterioles, capillaries, venules, and lymph vessels. The larger vessels, arteries and veins, are not discussed here because they do not seem to play a role in GSC niche biology. Figures 1 and 2 show the morphology of these vessel types.23
Capillaries have a lumen with a diameter of 5–10 μm, which is roughly the diameter of an erythrocyte. So, normal capillaries show erythrocytes inside that pass in rolls of single cells. Leukocytes are larger in size, and, therefore, their elasticity and mesenchymal characteristics are needed to pass capillaries. Wider capillaries exist and are called sinuses, and occur, for example, in the bone marrow.23,24 However, the morphological characteristic of sinuses that is different from that of arterioles and venules is their wall consisting of a single EC layer surrounded by pericytes which do not cover the entire endothelium.23
Lymph vessels are much wider than capillaries and lack pericytes in their wall, so the endothelium monolayer is the entire lymph vessel wall23 (Fig. 1).
Arterioles have the thickest wall and a round lumen of 20–130 μm in diameter, whereas the lumen of venules and lymph vessels is not necessarily round23 (Figs. 1 and 2). The lumen of venules is usually larger than those of arterioles of the same size. Figure 1 shows that the arteriolar wall consists of the following layers from luminal to abluminal: endothelium, tunica elastica interna, tunica media (smooth muscle layer), tunica elastica externa, and tunica adventitia (Figs. 1 and 2). Venules have the same layers, but the major difference is the thickness of the tunica media. This smooth muscle cell layer is only a few cell layers thick in the wall of venules.23
Three aspects of the arteriolar wall are relevant for GSC niches. First, arterioles are transport vessels and not exchange vessels,23 meaning that there is no or hardly any oxygen release from the arteriolar lumen to surrounding tissues. This explains the hypoxic areas that express hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) in which we found all periarteriolar niches adjacent to necrotic areas.21,71 Second, members of the Notch family drive the arterial gene program, whereas the orphan receptor COUP-TFII suppresses this program and regulates venous specification. The homeobox gene Prox-1 encodes for the master switch of lymphatic specification.25,26 Expression of Prox-1 is regulated by SOX18, and blood vessel marker expression is downregulated by SOX18 via Prox-1 expression.27 Loss of signaling via Notch, VEGF, and Sonic HedgeHog (Shh) results in loss of arterial identity.28 These signaling pathways are also involved in maintaining the stemness of GSCs. Therefore, the arteriolar wall may be a natural habitat for stem cells and thus for GSCs in glioblastoma tumors. Third, the tunica adventitia of arterioles is not just stroma. It harbors niches for mesenchymal stem cells (MSCs).28–32 MSC niches are localized in the tunica adventitia adjacent to the tunica media.31,32
It can be concluded on the basis of these aspects of arteriolar walls that the place to be for GSCs is their hypoxic periarteriolar niches.
Perivascular Niches
Perivascular GSC niches are the most frequently described niches. ECs are the essential cell type of perivascular niches that control GSC stemness.8,13,18,33 ECs are derived from hematopoietic stem cells (HSCs) in the bone marrow13 and can be visualized by immunohistochemical staining of markers such as CD31, CD36, and CD34.6,13 GSCs have been reported to express VEGF that promotes angiogenesis.8,20 Angiogenesis is a hallmark of glioblastoma.8,20
In vitro and in vivo studies showed that co-cultures of ECs and GSCs promote expression of stemness proteins, such as Sox2, Olig2, Bmil, and CD133 in GSCs,34 and interactions between ECs and GSCs are responsible for the maintenance of stemness properties of GSCs.8,13 In vitro, basic fibroblast growth factor (bFGF)–conditioned medium of glioblastoma ECs reverted differentiated glioblastoma cells to GSCs.35 CD133-positive GSCs reside closely to ECs.8 Calabrese et al. showed that glioblastoma cells decreased in number after ablation of blood vessels in a mouse xenograft glioma model.8 ECs release nitric oxide (NO) that induces Notch signaling in GSCs expressing nestin and the NO receptor sGC.3,36 NO produced by nitric oxide synthase-2 (NOS2) in GSCs has been shown to play an important role in GSC survival, proliferation, therapy resistance, SOX2 expression,37,38 and poor patient survival39 and is therefore an interesting therapeutic target.
Other cell types of perivascular niches are pericytes and smooth muscle cells.18,30,40 Pericytes are involved in tumor progression.33,40 It has been reported that GSCs can transdifferentiate in ECs and pericytes which means that GSCs can differentiate into other types of cells, seemingly to generate cell types that are needed in their niches.40,41 Cheng et al. demonstrated with in vivo cell lineage tracing that the majority of pericytes in glioblastoma tumors were generated from GSCs. Furthermore, elimination of GSC-derived pericytes disrupted angiogenesis; this suggests that targeting pericytes being part of the microenvironment of GSCs may be an effective therapeutic strategy in glioblastoma.40
The need of GSCs for both ECs and hypoxia in their perivascular niches is explained by the structure of blood vessels that is different from that of the vasculature of healthy brain tissue.42 Blood vessels in glioblastoma are dilated and often leaky.1,5,42 As a consequence, the blood flow is altered which may lead to poor oxygen supply and subsequently a hypoxic microenvironment of GSCs.43,44 Leaky blood vessels can also be explained by the production of NO by nitric oxide synthase-3 (NOS3), which is expressed in ECs. NO inhibits smooth muscle cell proliferation. NOS3 expression is induced by VEGF and is involved in vascular leakage. Proteases such as matrix metalloproteinases (MMPs) are activated by oxidized products of NO that modulate endothelial permeability.45,46
Fibroblasts are also present in perivascular niches. Fibroblasts are responsible not only for extracellular matrix (ECM) synthesis but also for ECM breakdown. For the latter purpose, fibroblasts express MMPs, which are proteases that degrade the ECM.47 Degradation of the ECM is required for invasion and metastasis,48,49 and extracellular expression of MMPs and also cysteine cathepsins has been reported to be associated with invasiveness of glioblastoma.3,42,50–53
VEGF is expressed by, among other cell types, GSCs and ECs. Furthermore, ECs in perivascular niches express Shh,34,54 Delta-like ligand 4 (DLL4),20,55 and Jagged-1 (J1).20 Shh is involved in the activation of the Hedgehog signaling pathway, which controls proliferation of normal stem cells in various organs and also in the GSC niche.34,54 Shh is involved in glioblastoma tumor growth, whereas knock down of DLL4 and J1 in ECs reduced glioblastoma growth in vivo.34 Both ligands activate the Notch signaling pathway, which is another essential stem cell pathway.56 Nestin-positive GSCs express Notch receptors for these ligands and therefore have Notch signaling activity.54,55
Periarteriolar Niches
In 2015, Hira et al. were the first to demonstrate periarteriolar niches of GSCs.21 By performing immunohistochemistry on cryostat sections of human glioblastoma, it was demonstrated that CD133-positive and nestin-positive GSCs reside in hypoxic environments surrounding CD31-positive ECs and smooth muscle actin (SMA)-positive smooth muscle cells of arterioles. Hypoxia around arterioles was explained by the fact that arterioles are transport vessels and not exchange vessels, whereas capillaries are the exchange vessels. The niches were always found in close vicinity of necrotic areas, and expression of HIF-1α and VEGF was found around the arterioles in wider areas than the GSC niches.21 Later on, Hira et al. repeated their investigations in a semiquantitative manner in a larger number of human glioblastoma samples and found niches around seven of 335 arterioles and none around 924 venules and 8085 capillaries.71 Apparently, the smooth muscle cells of arterioles are essential as well besides the ECs for GSC niches. We cannot yet rule out the possibility that GSC niches occur around venules as well because the venular vessel walls also contain smooth muscle cells although in lower numbers (see Fig. 1). The study characterized various proteins in periarteriolar GSC niches that appeared to be a replica of HSC niches.24 These proteins include chemoattractant stromal-derived factor-1α (SDF-1α; also known as CXCL12), its receptor C-X-C receptor type 4 (CXCR4), cathepsin K (CatK), CD44, and osteopontin (OPN).21,22,24
Receptor–ligand interactions such as CXCR4–SDF-1α and CD44–OPN are involved in homing of HSCs where SDF-1α and OPN are chemoattractants.24,57,58 SDF-1α, CXCR4, OPN, and CD44 are predominantly expressed in hypoxic conditions in human bone marrow.57 SDF-1α and OPN bind to their receptors CXCR4 and CD44, respectively, in the HSC niche in bone marrow around arterioles.24,59 SDF-1α and OPN are produced by ECs.41 The function of SDF-1α and OPN is to maintain HSCs in their niches via interactions with their receptors.24 CatK is among the highest differentially expressed proteases in glioblastoma as compared with the healthy brain.60,61 CatK can cleave and thereby inactivate SDF-1α in the bone marrow, which leads to the release of HSCs out of HSC niches into the blood circulation.24 An in vitro study demonstrated how inactivation of SDF-1α by CatK inhibits the binding of CXCR4-postive GSCs to SDF-1α.22 Besides the SDF-1α–CXCR4 axis, the OPN–CD44 axis is also associated with homing of GSCs in niches where CD44-positive GSCs are attracted by OPN.4 We hypothesize that the function of the OPN–CD44 axis in glioblastoma is similar to the function of SDF-1α–CXCR4 axis because of the analogy with the HSC niche in human bone marrow. Furthermore, OPN is involved in the recruitment of tumor-associated macrophages (TAMs) which are anti-inflammatory and protumorigenic.62 Hypoxia increases CXCR4 expression of the TAMs that are in turn attracted not only by SDF-1α63 but also by other chemoattractants such as OPN and periostin.62,64 Whether immunomodulators will be effective as anti-glioblastoma therapy is unknown but certainly an aspect to keep in mind for further studies.
Vessel Type in Perivascular GSC Niches
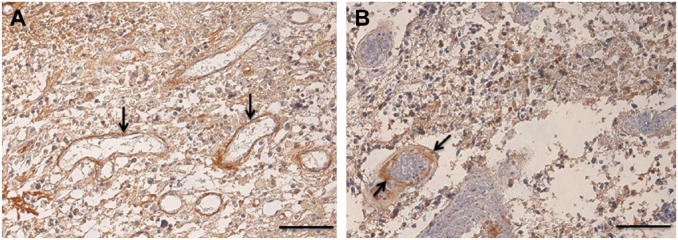
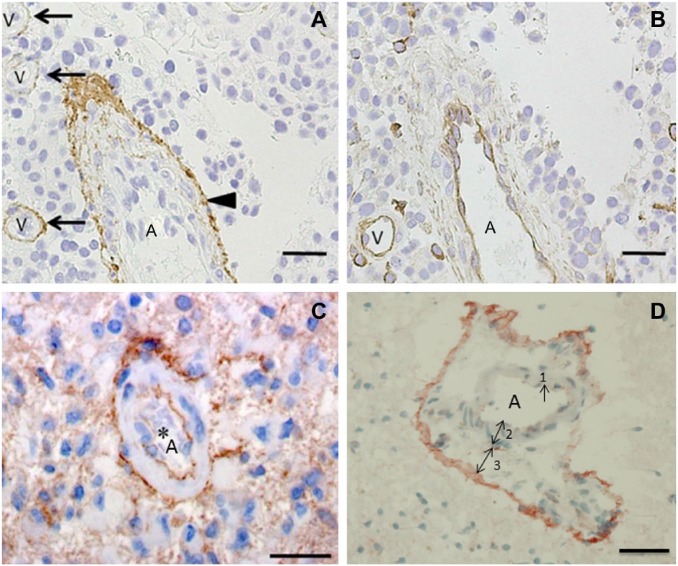
The term perivascular niches does not specify which type of vessel, arteriole, capillary, venule, or lymphatic vessel is localized in the center of the niches. However, it is regularly assumed that perivascular means pericapillary.3,8 A number of studies of perivascular niches have histological or histochemical images.7,65–69 Figures 3–5 show the relevant images. The vessels in the center of the perivascular niches have in all four cases a diameter that is too large and walls that are too thick for capillaries. In none of these four publications, the vessel type is specified and is indicated as blood vessel or vessel. We were not able to discriminate arterioles from venules in one case (Fig. 3A reprinted from Nie et al.65), but in the other cases (Figs. 3B, 4, 5A, B, and 5C, reprinted from Schiffer et al.,69 Motegi et al.,68 and Hermansen et al.,67 respectively), arterioles are shown. Moreover, the CD133 immunostaining in Hermansen et al.67 and Motegi et al.68 is in agreement with the CD133 immunostaining patterns that we found around the tunica adventitia of arterioles21 (Fig. 5D).
Figure 3.
Images of glioma stem cell niches in human glioblastoma tumors immunohistochemically stained for tenascin-C. (A) Tenascin-C is localized in highest amounts around venules (arrows). (B) Tenascin-C is localized in highest amounts around an arteriole (arrow). Reprinted with permission from Nie et al.65 Bar = 100 μm.
Figure 4.
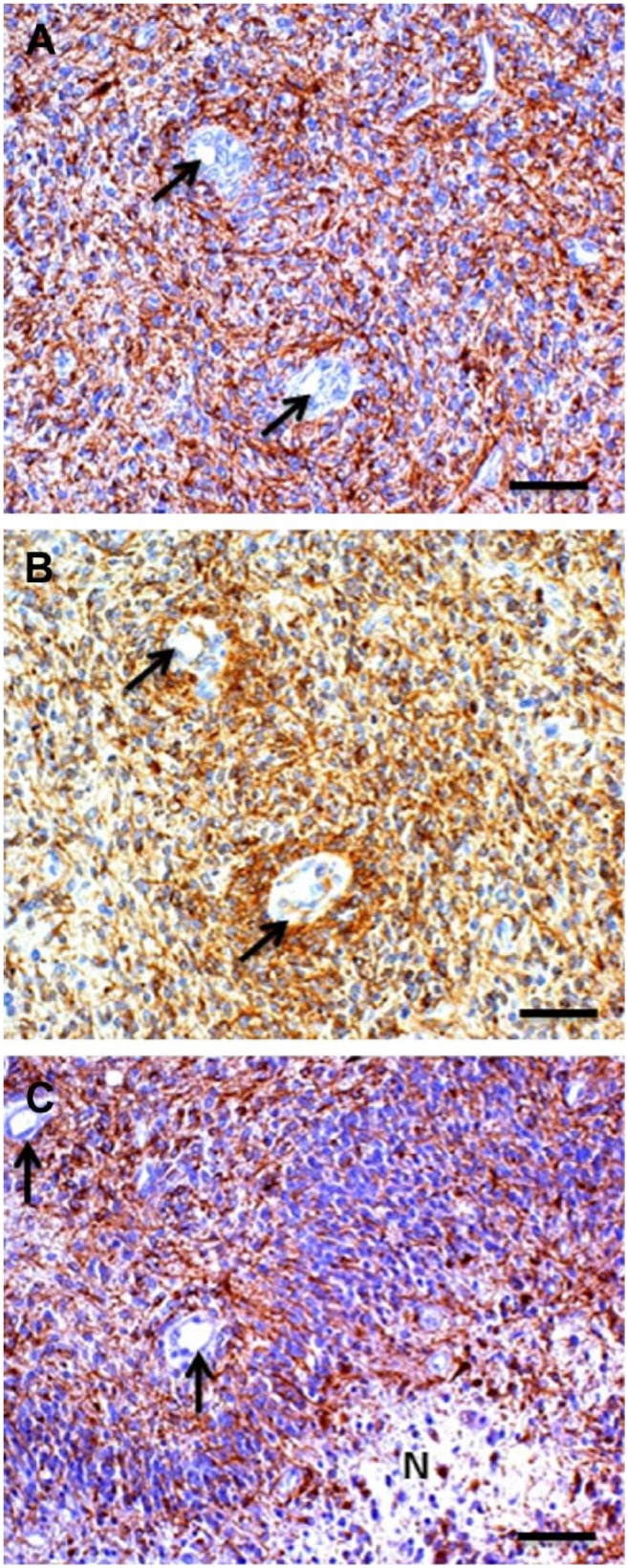
Images of glioma stem cell niches in human glioblastoma showing arterioles (arrows) and staining for glial fibrillary astrocytic protein (GFAP) in red (A, C) and nestin in brown (B). N = necrotic area. Reprinted with permission from Schiffer et al.69 Bar = 50 μm.
Figure 5.
Images of glioma stem cell (GSC) niches in human glioblastoma tumors showing an arteriole (A) and venules (V) immunohistochemically stained for CD133 (arrowhead and arrows). Reprinted with permission from (A, B) Motegi et al.68 and (C) Hermansen et al.67 (D) Periarteriolar GSC niche in a human glioblastoma tumor with CD133 immunostaining adjacent to the tunica adventitia of an arteriole. 1, endothelial cell; 2, tunica media; 3, tunica adventitia. Reprinted with permission from Hira et al.21 Bar = 30 μm (A, B, C); bar = 20 μm (D).
Hypoxic Perivascular GSC Niches Are Hypoxic Periarteriolar GSC Niches
Since the introduction of perivascular GSC niches in glioblastoma tumors by Calabrese et al., and their assumption that the vessels were capillaries,8 the correctness of this assumption has never been rigidly questioned. The vessels were demonstrated by fluorescence immunohistochemical staining of ECs only with anti-CD34 antibodies.8 These antibodies stain ECs of all types of blood vessels,70 whereas the morphology of the vessel walls cannot be studied in these fluorescence images. The six studies that have been published with light microscopical images of perivascular GSC niches do not analyze the type of vessels and name them blood vessels or vessels without specification.7,65–69 However, the vessels shown in these publications do not have the morphology of capillaries or sinuses. Therefore, we conclude that perivascular GSC niches are more precisely defined as periarteriolar GSC niches.
This conclusion makes sense in a number of ways. First, the hypoxic character of periarteriolar GSC niches can now be explained by the fact that there is no oxygen exchange between oxygenated blood in the lumen of arterioles and surrounding tissues because arterioles are transport vessels. Hypoxia is an essential condition for stem cells to keep their stemness. Second, the hypoxic periarteriolar GSC niche is a copy of hypoxic periarteriolar HSC niches in the bone marrow. Third, the tunica adventitia of arterioles is the place where GSC niches can develop because Notch signaling drives both the arteriolar specification and the stemness of GSCs. We propose here that not only signaling by ECs is essential for GSC niches but also signaling by smooth muscle cells. Whether there is direct signaling by ECs to GSCs or indirect signaling via the smooth muscle cells or both needs further investigations.
Fourth, the tunica adventitia of arterioles contains MSC niches. Therefore, the edge of the tunica adventitia of arterioles in glioblastoma tumors (Fig. 5) is the place to be for GSCs.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: VVVH and CJFN designed the study, wrote the manuscript, gave intellectual input, revised the manuscript, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work. DAA wrote the manuscript, approved the final version of the manuscript, and agreed to be accountable for all aspects of the work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by the Dutch Cancer Society (KWF; UVA 2014-6839).
Contributor Information
Vashendriya V.V. Hira, Cancer Center Amsterdam, Department of Medical Biology at the Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Diana A. Aderetti, Cancer Center Amsterdam, Department of Medical Biology at the Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands
Cornelis J.F. van Noorden, Cancer Center Amsterdam, Department of Medical Biology at the Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Literature Cited
- 1. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. [DOI] [PubMed] [Google Scholar]
- 2. Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–46. [DOI] [PubMed] [Google Scholar]
- 3. Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle. 2010;9:3012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamour V, Henry A, Kroonen J, Nokin MJ, von Marschall Z, Fisher LW, Chau TL, Chariot A, Sanson M, Delattre JY, Turtoi A, Peulen O, Rogister B, Castronovo V, Bellahcene A. Targeting osteopontin suppresses glioblastoma stem-like cell character and tumorigenicity in vivo. Int J Cancer. 2015;137:1047–57. [DOI] [PubMed] [Google Scholar]
- 5. Mao DD, Gujar AD, Mahlokozera T, Chen I, Pan Y, Luo J, Brost T, Thompson EA, Turski A, Leuthardt EC, Dunn GP, Chicoine MR, Rich KM, Dowling JL, Zipfel GJ, Dacey RG, Achilefu S, Tran DD, Yano H, Kim AH. A CDC20-APC/SOX2 signaling axis regulates human glioblastoma stem-like cells. Cell Rep. 2015;11:1809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bjerkvig R, Johansson M, Miletic H, Niclou SP. Cancer stem cells and angiogenesis. Semin Cancer Biol. 2009;19:279–84. [DOI] [PubMed] [Google Scholar]
- 7. Ishii A, Kimura T, Sadahiro H, Kawano H, Takubo K, Suzuki M, Ikeda E. Histological characterization of the tumorigenic “peri-necrotic niche” harboring quiescent stem-like tumor cells in glioblastoma. PLoS ONE. 2016;11:e0147366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- 9. Turaga SM, Lathia JD. Adhering towards tumorigenicity: altered adhesion mechanisms in glioblastoma cancer stem cells. CNS Oncol. 2016;5:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hale JS, Sinyuk M, Rich JN, Lathia JD. Decoding the cancer stem cell hypothesis in glioblastoma. CNS Oncol. 2013;2:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roos A, Ding Z, Loftus JC, Tran NL. Molecular and microenvironmental determinants of glioma stem-like cell survival and invasion. Front Oncol. 2017;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91. [DOI] [PubMed] [Google Scholar]
- 13. Codrici E, Enciu AM, Popescu ID, Mihai S, Tanase C. Glioma stem cells and their microenvironments: providers of challenging therapeutic targets. Stem Cells Int. 2016;2016:5728438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vermeulen L, de Sousa e, Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:e83–9. [DOI] [PubMed] [Google Scholar]
- 15. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. [DOI] [PubMed] [Google Scholar]
- 16. Sultan M, Coyle KM, Vidovic D, Thomas ML, Gujar S, Marcato P. Hide-and-seek: the interplay between cancer stem cells and the immune system. Carcinogenesis. 2017;38:107–18. [DOI] [PubMed] [Google Scholar]
- 17. Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. [DOI] [PubMed] [Google Scholar]
- 18. Liebelt BD, Shingu T, Zhou X, Ren J, Shin SA, Hu J. Glioma stem cells: signaling, microenvironment, and therapy. Stem Cells Int. 2016;2016:7849890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oei AL, Vriend LE, Krawczyk PM, Horsman MR, Franken NA, Crezee J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;33:419–27. [DOI] [PubMed] [Google Scholar]
- 20. Sharma A, Shiras A. Cancer stem cell-vascular endothelial cell interactions in glioblastoma. Biochem Biophys Res Commun. 2016;473:688–92. [DOI] [PubMed] [Google Scholar]
- 21. Hira VV, Ploegmakers KJ, Grevers F, Verbovsek U, Silvestre-Roig C, Aronica E, Tigchelaar W, Turnsek TL, Molenaar RJ, Van Noorden CJ. CD133+ and nestin+ glioma stem-like cells reside around CD31+arterioles in niches that express SDF-1alpha, CXCR4, osteopontin and cathepsin K. J Histochem Cytochem. 2015;63:481–93. [DOI] [PubMed] [Google Scholar]
- 22. Hira VV, Verbovsek U, Breznik B, Srdic M, Novinec M, Kakar H, Wormer J, der Swaan BV, Lenarcic B, Juliano L, Mehta S, Van Noorden CJ, Lah TT. Cathepsin K cleavage of SDF-1alpha inhibits its chemotactic activity towards glioblastoma stem-like cells. Biochim Biophys Acta. 1864;2016:594–603. [DOI] [PubMed] [Google Scholar]
- 23. Kierszenbaum AL. Histology and cell biology: an introduction to pathology. St. Louis, MI: Mosby; 2002. [Google Scholar]
- 24. Hira VVV, Van Noorden CJF, Carraway HE, Maciejewski JP, Molenaar RJ. Novel therapeutic strategies to target leukemic cells that hijack compartmentalized continuous hematopoietic stem cell niches. Biochim Biophys Acta. 1868;2017:183–98. [DOI] [PubMed] [Google Scholar]
- 25. Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. [DOI] [PubMed] [Google Scholar]
- 26. Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–7. [DOI] [PubMed] [Google Scholar]
- 27. Mancini ML, Terzic A, Conley BA, Oxburgh LH, Nicola T, Vary CP. Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev Dyn. 2009;238:2479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–88. [DOI] [PubMed] [Google Scholar]
- 29. Appaix F, Nissou MF, van der Sanden B, Dreyfus M, Berger F, Issartel JP, Wion D. Brain mesenchymal stem cells: the other stem cells of the brain? World J Stem Cells. 2014;6:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ergun S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal. 2011;15:981–95. [DOI] [PubMed] [Google Scholar]
- 33. Fidoamore A, Cristiano L, Antonosante A, d’Angelo M, Di Giacomo E, Astarita C, Giordano A, Ippoliti R, Benedetti E, Cimini A. Glioblastoma stem cells microenvironment: the paracrine roles of the niche in drug and radioresistance. Stem Cells Int. 2016;2016:6809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan GN, Yang L, Lv YF, Shi Y, Shen LL, Yao XH, Guo QN, Zhang P, Cui YH, Zhang X, Bian XW, Guo DY. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J Pathol. 2014;234:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fessler E, Borovski T, Medema JP. Endothelial cells induce cancer stem cell features in differentiated glioblastoma cells via bFGF. Mol Cancer. 2015;14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palumbo P, Miconi G, Cinque B, Lombardi F, La Torre C, Dehcordi SR, Galzio R, Cimini A, Giordano A, Cifone MG. NOS2 expression in glioma cell lines and glioma primary cell cultures: correlation with neurosphere generation and SOX-2 expression. Oncotarget. 2017;8:25582–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, Goldman SA, Bredel M, McLendon RE, Sloan AE, Hjelmeland AB, Rich JN. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tran AN, Boyd NH, Walker K, Hjelmeland AB. NOS expression and NO function in glioma and implications for patient therapies. Antioxid Redox Signal. 2017;26:986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. [DOI] [PubMed] [Google Scholar]
- 42. Herrera-Perez M, Voytik-Harbin SL, Rickus JL. Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng Part A. 2015;21:2572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pistollato F, Abbadi S, Rampazzo E, Persano L, Della Puppa A, Frasson C, Sarto E, Scienza R, D’Avella D, Basso G. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28:851–62. [DOI] [PubMed] [Google Scholar]
- 44. Rodriguez FJ, Orr BA, Ligon KL, Eberhart CG. Neoplastic cells are a rare component in human glioblastoma microvasculature. Oncotarget. 2012;3:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Figueroa XF, Gonzalez DR, Martinez AD, Duran WN, Boric MP. ACh-induced endothelial NO synthase translocation, NO release and vasodilatation in the hamster microcirculation in vivo. J Physiol. 2002;544:883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turunen SP, Tatti-Bugaeva O, Lehti K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim Biophys Acta. 2017;1864(11 Pt A):1974–88. [DOI] [PubMed] [Google Scholar]
- 48. Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S. Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 2000;157:177–84. [DOI] [PubMed] [Google Scholar]
- 49. Herold-Mende C, Mueller MM, Bonsanto MM, Schmitt HP, Kunze S, Steiner HH. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer. 2002;98:362–9. [DOI] [PubMed] [Google Scholar]
- 50. Lankelma JM, Voorend DM, Barwari T, Koetsveld J, Van der Spek AH, De Porto AP, Van Rooijen G, Van Noorden CJ. Cathepsin L, target in cancer treatment? Life Sci. 2010;86:225–33. [DOI] [PubMed] [Google Scholar]
- 51. Breznik B, Motaln H, Vittori M, Rotter A, Lah Turnsek T. Mesenchymal stem cells differentially affect the invasion of distinct glioblastoma cell lines. Oncotarget. 2017;8:25482–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lah TT, Duran Alonso MB, Van Noorden CJ. Antiprotease therapy in cancer: hot or not? Expert Opin Biol Ther. 2006;6:257–79. [DOI] [PubMed] [Google Scholar]
- 53. Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, Moin K, Blum G, Mattingly RR, Gillies RJ, Sloane BF. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. 2013;15:1125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang M, Song T, Yang L, Chen R, Wu L, Yang Z, Fang J. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res. 2008;27:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang JF, Chen Y, Qiu XX, Tang WL, Zhang JD, Huang JH, Lin GS, Wang XF, Lin ZX. The vascular delta-like ligand-4 (DLL4)-Notch4 signaling correlates with angiogenesis in primary glioblastoma: an immunohistochemical study. Tumour Biol. 2016;37:3797–805. [DOI] [PubMed] [Google Scholar]
- 57. Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55:496–505. [DOI] [PubMed] [Google Scholar]
- 59. Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Verbovsek U, Van Noorden CJ, Lah TT. Complexity of cancer protease biology: cathepsin K expression and function in cancer progression. Semin Cancer Biol. 2015;35:71–84. [DOI] [PubMed] [Google Scholar]
- 61. Verbovsek U, Motaln H, Rotter A, Atai NA, Gruden K, Van Noorden CJ, Lah TT. Expression analysis of all protease genes reveals cathepsin K to be overexpressed in glioblastoma. PLoS ONE. 2014;9:e111819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, Zhang J, Xu S, Li T, Zhang P, Gao X, Qiu W, Liu Q, Li G. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7:80521–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Owen JL, Mohamadzadeh M. Macrophages and chemokines as mediators of angiogenesis. Front Physiol. 2013;4:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nie S, Gurrea M, Zhu J, Thakolwiboon S, Heth JA, Muraszko KM, Fan X, Lubman DM. Tenascin-C: a novel candidate marker for cancer stem cells in glioblastoma identified by tissue microarrays. J Proteome Res. 2015;14:814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reinhard J, Brosicke N, Theocharidis U, Faissner A. The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int J Biochem Cell Biol. 2016;81:174–83. [DOI] [PubMed] [Google Scholar]
- 67. Hermansen SK, Nielsen BS, Aaberg-Jessen C, Kristensen BW. miR-21 is linked to glioma angiogenesis: a co-localization study. J Histochem Cytochem. 2016;64:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Motegi H, Kamoshima Y, Terasaka S, Kobayashi H, Houkin K. Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology. 2014;34:378–85. [DOI] [PubMed] [Google Scholar]
- 69. Schiffer D, Mellai M, Annovazzi L, Caldera V, Piazzi A, Denysenko T, Melcarne A. Stem cell niches in glioblastoma: a neuropathological view. Biomed Res Int. 2014;2014:725921. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70. Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, Greaves MF, Denekamp J, Ruiter DJ. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990;62:690–6. [PubMed] [Google Scholar]
- 71. Hira VVV, Wormer JR, Kakar H, Breznik B, van der Swaan B, Hulsbos R, Tigchelaar W, Tonar Z, Khurshed M, Molenaar RJ, Van Noorden CJF. Periarteriolar glioblastoma stem cell niches express bone marrow hematopoietic stem cell niche proteins. J Histochem Cytochem 2018. doi: 10.1369/0022155417749174. [DOI] [PMC free article] [PubMed] [Google Scholar]