Abstract
Hexokinase domain component 1 (HKDC1) is a recently discovered novel protein, which is being promoted as a putative fifth hexokinase. Although the exact role HKDC1 plays in physiology is still unclear, it has been shown to be important during pregnancy in the regulation of glucose homeostasis. In this study, we have comprehensively studied the expression pattern of HKDC1 in the human body. Using human tissue sample, immunohistochemistry imaging was performed. Our studies indicate that the tissues with highest HKDC1 expression were the brush border epithelium of the intestines, parts of the pancreas, and lung alveolar macrophages. Future directions will be to understand the role of this fifth hexokinase in these tissues, with a focus on its relative function as compared with other endogenously expressed hexokinases.
Keywords: comparative IHC, hexokinase, HKDC1
Introduction
Hexokinases (HK) are a family of enzymes that catalyze the phosphorylation of hexose sugars at the very first step of glucose utilization in a wide variety of organisms ranging from bacteria to humans.1 There are several HK isozymes found in mammals: HKI, HKII, HKIII, and HKIV (also called glucokinase [GCK]), each of which has been extensively characterized.2 Although these isozymes have common ancestry and a conserved function in phosphorylation of glucose, they differ in kinetic properties, localization, and tissue expression pattern.1,2 HKI, HKII, and HKIII have a low Km for glucose and phosphorylate it preferentially, but they can also phosphorylate other hexoses.1 However, GCK has a very high Km for glucose, and it only phosphorylates glucose. Phosphorylated glucose is a crucial substrate for glycolysis, a major energy-generating pathway in all organisms, as well as many biosynthetic pathways including the pentose phosphate pathway and glycogen synthesis.2
HK isozymes have a distinct role in glucose metabolism based on their different properties and expression pattern. For example, HKI is an active isozyme expressed relatively ubiquitously, most prominently in the brain, and has a role in the generalized function of glucose catabolism for energy production.2 In contrast, GCK is an HK isozyme that is selectively expressed in tissues that are sensitive to glucose levels in the body such as pancreatic β-cells and hepatocytes, and has a role in whole-body glucose-sensing mechanism.3 Thus, the selective expression of such isozymes in different tissues correlates with their role in determining the pattern of glucose metabolism in mammals.2
Although these four isozymes have been well characterized, phylogenetic analysis of the human genome more recently indicated the existence of a previously uncharacterized HK-like gene in humans.4 This gene, named hexokinase domain containing 1 (HKDC1), was found to have significant homology to HKI, and a comparison with a National Center for Biotechnology Information database of protein expression indicated that HKDC1 was actively expressed, together indicating that it was possibly a novel, functional HK.4 The HKDC1 gene is found on chromosome 10 next to the HKI gene with high similarity in amino acid sequence to HKI, suggesting that both these proteins may have originated from a similar precursor via a gene duplication event in evolution5–7 and that this similarity to HKI may have hindered the discovery of HKDC1 in previous studies.5 The HKDC1 gene contains a 917 residues open reading frame and is conserved across species, and the encoded protein contains a conserved glucose-binding site as well as an ATP-binding site, indicating that it has capability to phosphorylate hexoses.5–6 In a recent study by Guo et al., an assay of HKDC1 purified protein HK activity confirmed that HKDC1 had HK activity in vitro while in vivo activity has also been demonstrated by a gene knockdown study in mice.5 Along with the genetic properties of HKDC1, these data led to the conclusion that HKDC1 could, indeed, be considered a fifth HK.8
Considering the fact that HKDC1 was only recently discovered, there are only a few studies that discuss its exact function in physiology and pathophysiology. Some studies do suggest that HKDC1 may play an important role in glucose metabolism during pregnancy, where the energy needs are substantially altered. These studies have shown the presence of various regulatory elements, such as enhancers that promote HKDC1 expression are associated with gestational hyperglycemia during pregnancy in humans.5 Therefore, a more in-depth knowledge of the function of HKDC1 during pregnancy is crucial.5,6 Other studies have shown that HKDC1 was significantly overexpressed in certain types of cancer such as liver, lung, cervical, and colorectal, indicating that it may play a crucial role in tumorigenesis.8,9
The expression pattern of HKDC1 as initially described by Irwin and Tan indicated that HKDC1 was widely expressed, with particularly high levels of expression in the pharynx, thymus, colon, esophagus, and eye.4 Reverse transcriptase-PCR (RT-PCR) analysis of HKDC1 mRNA in various human tissues also indicated that hexokinase domain containing 1 (HKDC1) is broadly expressed, with expression highest in colon, small intestine, trachea, thymus, kidney, and endocrine tissue.7 These data were reconfirmed in a further study, with most appreciable expression found in liver, colon, and kidney, by comparison with a human-tissue-specific RNA-expression database.10 The same study also indicated by immunohistochemistry (IHC) that HKDC1 had restricted expression within high-expressing tissues; in the colon, expression was limited to epithelial cells of the villi, and in the kidney, expression was restricted to the renal tubule excluding the glomeruli.10 Comparison of HKDC1 RNA expression in mice in this study also indicated that the tissue expression pattern of HKDC1 was consistent between human and mouse mRNA.10
Considering the observation that tissue localization of HKs is crucial for their physiological function, we undertook this study to characterize the localization of HKDC1 in human tissues through immunohistochemical analyses. We used IHC to visualize the tissue localization of HKDC1 in a number of human tissues.
Materials and Methods
Tissue Samples
Normal human tissues were obtained from surgical specimens and autopsy cases with an Institutional Review Board approval (#207299). For surgical resection specimens, the normal tissue was sampled far from any pathological region. The tissue sections were fixed in formalin and paraffin embedded.
Immunohistochemistry
The HKDC1 (HPA011956; Sigma, St. Louis, MO) antibody that was used in this study has already been reported by us and others to be specific for human HKDC1 both by IHC and western blot.7,9 This antibody has also been validated and approved for IHC (http://www.proteinatlas.org/ENSG00000156510-HKDC1/antibody#assay_ih_normal).
Immunostaining studies were carried out on paraffin sections of 4 mm thickness that were stained using a Leica Bond Max automated system (Leica; Bannockburn, IL) with the Leica-Refine detection kit. Two separate samples were evaluated for each tissue. After deparaffinization, a heat-induced epitope retrieval was performed with Bond Epitope Retrieval Solution (pH 7.6) for 20 min at 100C. The sections were peroxide blocked for 5 min, then incubated with the primary HKDC1 antibody (1:100 dilution) for 15 min. The tissue sections were further treated by the Leica Polymer and post-polymer detection (each 10 min), DAB for 10 min, and a hematoxylin counterstain for 5 min. Appropriate positive (small intestinal villous epithelium) and negative (small intestinal crypt base epithelium) controls were stained in parallel with each round of IHC. The IHC was reviewed and interpreted by a histopathologist (coauthor, X.D.).
Scoring of Immunostaining
Stained sections were evaluated by a surgical pathologist with a semi-quantitative method.11 For every sample, an HKDC1 expression score was assigned by giving a score ranging from 0 to 9 resulting from the product of intensity score (A) and distribution score (B).
The Intensity Score (A)
Assessment of the immunostains was performed by estimating the intensity of cells with cytoplasmic staining and scored as negative (no staining, 0), weakly (pale staining significantly differing from background, 1), moderately positive (more than pale, with tinctorial quality intermediate between weak and strong, 2), and strongly positive (deep, golden brown staining, 3) and denoted as A as reported previously.11
The Distribution Score (B)
A percentage of positive cells were also evaluated and scored as 0=0%, 1≤30%, 2=30–60%, and 3≥60%, and denoted as B.
Results
HKDC1 Expression in the Digestive System
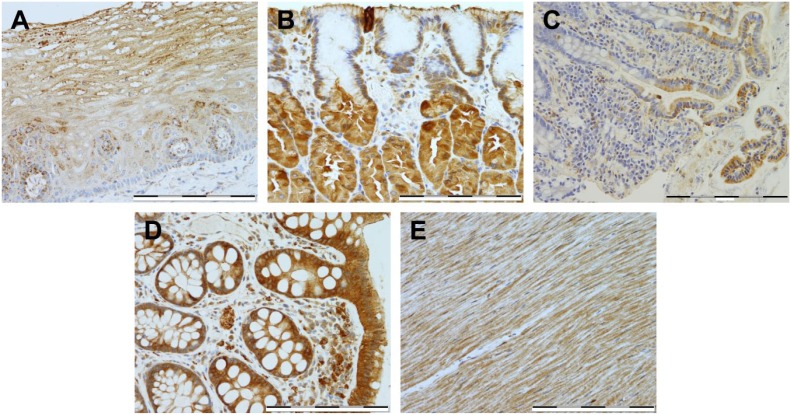
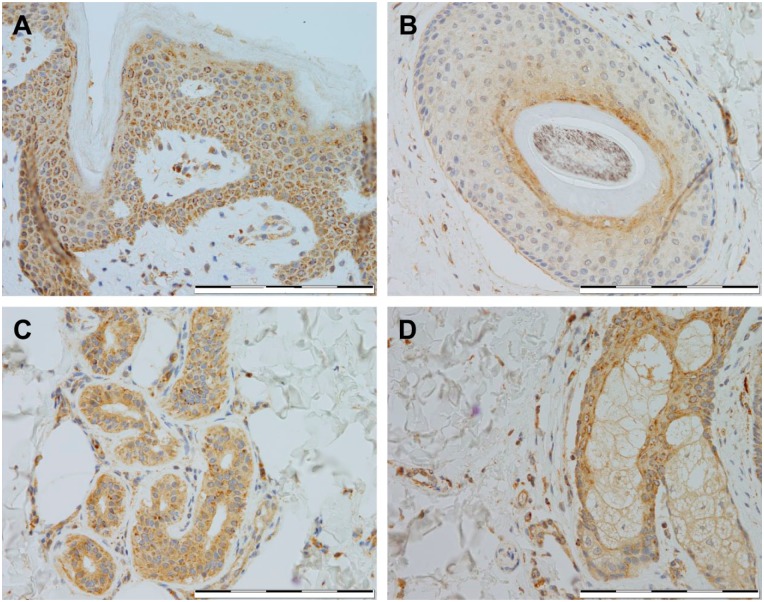
In this study, we have extensively explored the cellular localization of HKDC1 in a variety of tissues, by immunostaining. RNA datasets suggested that some of the most extensive expression of HKDC1 appears in the intestinal tract; therefore, we first explored this organ system for HKDC1 protein expression (Fig. 1). In the esophagus (Fig. 1A), weak granular expression was noted in the basal epithelium, with moderate granular expression seen in the suprabasal squamous epithelium in around 30–60% cells. In the stomach (Fig. 1B), weak expression localized to the basal perinuclear area was seen in the superficial foveolar epithelium, and moderate cytoplasmic expression pattern was seen in the majority of the parietal and chief cells. In the small and large intestines (Fig. 1C and D), strong expression was noted in the brush border and surface epithelium, respectively, but interestingly, expression was absent in the crypt base, in particular, for the small intestine. The intestinal muscularis propria (Fig. 1E) exhibited diffused weak to moderate expression in smooth muscle, but areas of focal strong expression were found to be localized to the myenteric plexus. In sum, the gastrointestinal tract was found to be a locus of high expression for HKDC1 (Table 1).
Figure 1.
Immunostaining of HKDC1 in the digestive system. Shown are representative tissue samples from esophagus (A), stomach (B), small intestine (C), large intestine (D), and intestinal muscularis propria (E). All images were taken at 400× magnification, and scale bar = 200 µm for all images. Abbreviation: HKDC1, hexokinase domain containing 1.
Table 1.
Immunostaining Score of HKDC1 Expression for Different Organs.
| Tissue | Intensity | Distribution | Summary Score |
|---|---|---|---|
| 1. Esophageal squamous mucosa | |||
| a. Basal layer | 1 | 3 | 3 |
| b. Suprabasal squamous epithelium | 2 | 3 | 6 |
| 2. Stomach | |||
| a. Superficial foveolar epithelium | 2 | 3 | 6 |
| b. Parietal cells and chief cells | 3 | 3 | 9 |
| 3. Small intestine | |||
| a. Villous brush border | 3 | 3 | 9 |
| b. Deep crypt epithelium | 0 | 3 | 0 |
| 4. Colon | |||
| a. Surface epithelium | 3 | 3 | 9 |
| b. Crypt base epithelium | 1 | 2 | 2 |
| 5. Muscularis propria of GI tract | |||
| a. Smooth muscle | 2 | 3 | 6 |
| b. Myenteric plexus: | |||
| Focal | 3 | 1 | 3 |
| Rest | 1 | 3 | 3 |
| 6. Pancreas | |||
| a. Acinar cells | 0 | 3 | 0 |
| b. Acinar centrocytes | 3 | 3 | 9 |
| c. Interlobular pancreatic duct | 3 | 3 | 9 |
| d. Larger pancreatic duct | 3 | 3 | 9 |
| e. Pancreatic islet cells | 1 | 1 | 1 |
| 7. Liver | |||
| a. Hepatocytes | 0 | 3 | 0 |
| b. Interlobular bile duct | 1 | 1 | 1 |
| 8. Spleen | |||
| a. Red pulp | 1 | 3 | 3 |
| b. White pulp | 1–2 | 1 | 1–2 |
| 9. Adipose tissue | 0–1 | 3 | 0–3 |
| 10. Cardiac muscle | |||
| a. 90% cardiomyocytes | 1 | 3 | 3 |
| b. 10% cardiomyocytes | 2 | 1 | 2 |
| 11. Skin | |||
| a. Epidermis | 1 | 1 | 1 |
| b. Sebaceous gland | 0 | 3 | 0 |
| c. Hair follicle | 1 | 3 | 3 |
| d. Sweat glands | 1 | 3 | 3 |
| e. Sweat gland duct | 2 | 3 | 6 |
| 12. Kidney | |||
| a. Renal tubule | |||
| 90% cells | 1 | 3 | 3 |
| 10% cells | 2 | 1 | 2 |
| Glomerulus | 1 | 1 | 1 |
| 13. Lung | |||
| a. Alveolar lining cells | 1 | 3 | 3 |
| b. Alveolar macrophages | 3 | 3 | 9 |
| c. Bronchial epithelium | 2 | 3 | 6 |
| 14. Prostate | |||
| a. Basal cells | 2 | 3 | 6 |
| b. Gland epithelium | 1 | 3 | 3 |
| c. Stromal cells | 1–2 | 3 | 3–6 |
| 15. Skeletal muscle | 1 | 3 | 3 |
| 16. Testes | 1 | 2 | 3 |
| 17. Thyroid | 2–3 | 3 | 6–9 |
Abbreviations: HKDC1, hexokinase domain containing 1; GI = gastrointestinal.
HKDC1 Expression in Exocrine and Endocrine Organs
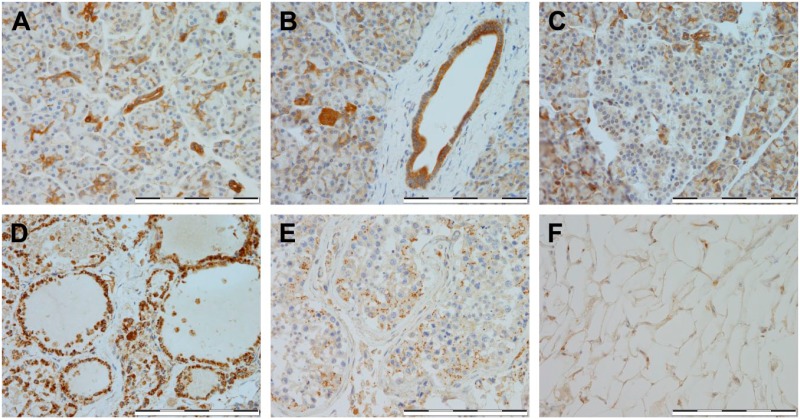
The HKDC1 expression was also evaluated in the pancreatic exocrine cells. The pancreatic interlobular ducts, as well as acinar centrocytes (Fig. 2A and B), exhibited strong HKDC1 expression, however, pancreatic acinar cells (Fig. 2A and B) exhibited minimal or negative expression. For the endocrine organs, we analyzed tissue samples from the pancreas, thyroid, testes, and adipose tissue. Even though the pancreatic interlobular ducts and acinar centrocytes (Fig. 2A and B) showed strong HKDC1 expression, pancreatic islets (Fig. 2C) exhibited minimal or negative expression. In the thyroid (Fig. 2D), moderate to strong expression was seen in the follicular epithelium. HKDC1 expression in the testes was localized to areas of weak expression in spermatocytes (Fig. 2E). There was negative HKDC1 expression in adipose tissue (Fig. 2F).
Figure 2.
Immunostaining of HKDC1 in exocrine and endocrine organs. Shown are representative for pancreas (A–C), thyroid (D), testis (E), and adipose (F). All images were taken at 400× magnification, and scale bar = 200 µm for all images. Abbreviation: HKDC1, hexokinase domain containing 1.
HKDC1 Expression in Other Abdominal Organs
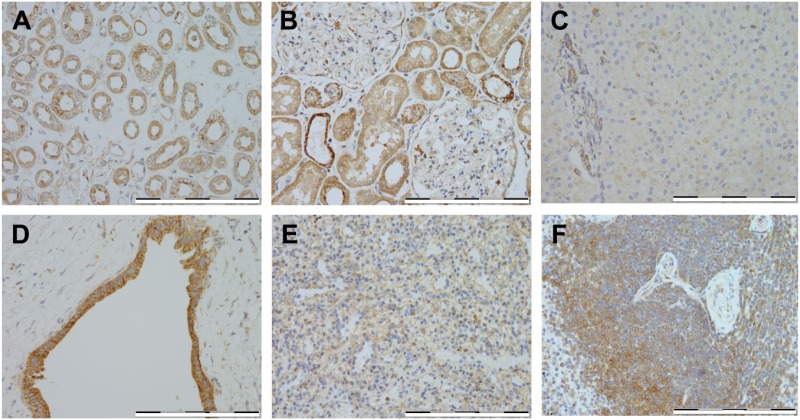
In the kidney, expression was weakly positive in the renal tubules (Fig. 3A), and areas of focal weak positive expression were also noted in the glomeruli (Fig. 3B). In the liver, more than 60% of the hepatocytes exhibited negative staining for HKDC1; however, the interlobular bile duct showed weakly positive staining in less than 30% of the cells (Fig. 3C and D; Table 1). In the spleen, minimal to weak expression was seen (Fig. 3E and F), and areas of focal weak expression were seen in a small proportion of the spleen lymphocytes (Fig. 3E and F).
Figure 3.
Immunostaining of HKDC1 in other abdominal organs. Shown are representative for kidney (A–B), liver (C–D), and spleen (E–F). All images were taken at 400× magnification, and scale bar = 200 µm for all images. Abbreviation: HKDC1, hexokinase domain containing 1.
HKDC1 Expression in the Other Tissues
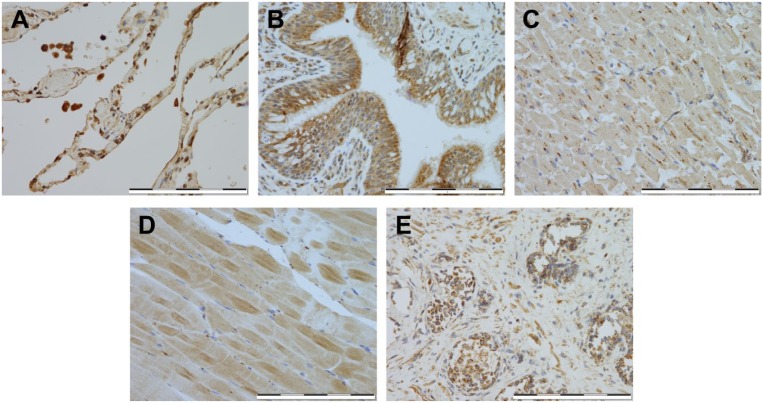
In the lung (Fig. 4A and B), strong expression was seen in alveolar macrophages, and weak expression in the alveolar lining epithelium. Moderate expression was localized to the apical surface of the bronchial epithelium (Fig. 4A and B). In cardiac muscles (Fig. 4C), staining for HKDC1 revealed weak expression with areas of focal moderate expression. There was minimal HKDC1 expression in the majority of the skeletal muscle cells (Fig. 4D). In the prostate tissue, >60% basal cells had moderately positive staining, the gland epithelium stained weakly positive, and the stromal cells ranged from weak to moderate staining for HKDC1 (Fig. 4E).
Figure 4.
Immunostaining of HKDC1 in other tissues. Shown are representative for lung (A–B), cardiac muscle (C), skeletal muscle (D), and prostate (E). All images were taken at 400× magnification, and scale bar = 200 µM for all images. Abbreviation: HKDC1, hexokinase domain containing 1.
HKDC1 Expression in the Integumentary System
In organs of the integumentary system, there was weak positive HKDC1 expression in the epidermis and hair follicles (Fig. 5A and B). Sweat glands displayed weakly positive expression (Fig. 5C), while there was moderately positive expression in the ducts of the sweat glands (Fig. 5C). There was negative expression in the sebaceous glands (Fig. 5D).
Figure 5.
Immunostaining of HKDC1 in integumentary organs. Shown are representative for skin epidermis (A), hair follicle (B), sweat gland (C), and sebaceous gland (D). All images were taken at 400× magnification, and scale bar = 200 µm for all images. Abbreviation: HKDC1, hexokinase domain containing 1.
Discussion
In our studies, we found that HKDC1 expression was strongest in the brush border, surface epithelium, and the myenteric plexus of the small and large intestines; the acinar centrocytes and interlobular ducts of the pancreas; and the alveolar macrophages in the lungs (Table 1). Strong to moderate expression was also seen in the thyroid follicular epithelium. While the degree of expression does not fully indicate level of the role of protein in tissue, it is a good indication, and provides directions as we explore the role of this recently identified HK (Table 1).
HKDC1 was uniquely discovered as a gene related to glucose metabolism during pregnancy. Despite bearing a close resemblance to another HK, HKI, in sequence and homology, there is a vast difference in the tissue expression patterns of these two HK isozymes. HKI is ubiquitously and constitutively expressed and plays a major role in glucose metabolism; however, HKDC1 expression is found in some tissues, possibly indicating that it is poised to play a more regulatory role in metabolism. For instance, the expression of HKDC1 is increased during pregnancy to meet the increased energy demands of gestation and where the loss of HKDC1 has been shown to cause glucose intolerance.5–7,10 We speculate that HKDC1 may play more of a regulatory role similar to GCK in tissues it is highly expressed in. Further studies are warranted to study the role of HKDC1 during pregnancy, specifically, and metabolism, more generally.
Existing data suggest that this novel fifth HK, HKDC1, is involved in global glucose utilization and is particularly important during pregnancy. Studies also show that there may exist a strong correlation between HKDC1 over-expression and cancer in some tissues. It is expected that rigorous future evaluation of these questions will provide valuable new insights into HKDC1-mediated metabolic changes. More broadly, the results from this study give valuable aid to researchers by proving tissue expression pattern of HKDC1 in some physiologically important organs and will help as we unravel the role of HKDC1 in both physiological and pathophysiological states.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors have contributed to this article as follows: MWK, XD, and SJC conducted the experiments and wrote the manuscript; MC helped in preparing the manuscript; BTL conceived the idea of the work and prepared the manuscript, along with MWK, XD, and SJC; and all authors have read and approved the manuscript as submitted.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: B.T.L. is supported by the National Institutes of Health under award number R01DK104927-01A1, The University of Chicago DR&TC (P30DK020595), and Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, VA merit (grant 1I01BX003382-01-A1).
ORCID iD: MW Khan  https://orcid.org/0000-0002-4398-8798
https://orcid.org/0000-0002-4398-8798
Contributor Information
Md. Wasim Khan, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Illinois at Chicago, Chicago, Illinois.
Xianzhong Ding, Department of Pathology, Section of Hepatology, Loyola University Chicago, Maywood, Illinois.
Scott J. Cotler, Department of Medicine, Loyola University Chicago, Maywood, Illinois
Michael Clarke, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Illinois at Chicago, Chicago, Illinois.
Brian T. Layden, Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Illinois at Chicago, Chicago, Illinois; Jesse Brown Veterans Affairs Medical Center, Chicago, Illinois.
Literature Cited
- 1. Cárdenas ML, Cornish-Bowden A, Ureta T. Evolution and regulatory role of the hexokinases. Biochim Biophys Acta. 1998;1401(3):242–64. [DOI] [PubMed] [Google Scholar]
- 2. Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–57. [DOI] [PubMed] [Google Scholar]
- 3. Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5(3):171–6. [DOI] [PubMed] [Google Scholar]
- 4. Irwin DM, Tan H. Molecular evolution of the vertebrate hexokinase gene family: identification of a conserved fifth vertebrate hexokinase gene. Comp Biochem Physiol Part D Genomics Proteomics. 2008;3(1):96–107. [DOI] [PubMed] [Google Scholar]
- 5. Guo C, Ludvik AE, Arlotto ME, Hayes MG, Armstrong LL, Scholtens DM, Brown CD, Newgard CB, Becker TC, Layden BT, Lowe WL, Reddy TE. Coordinated regulatory variation associated with gestational hyperglycaemia regulates expression of the novel hexokinase HKDC1. Nat Commun. 2015;6:6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayes MG, Urbanek M, Hivert MF, Armstrong LL, Morrison J, Guo C, LP Lowe, DA Scheftner, A Pluzhnikov, DM Levine, CP McHugh, CM Ackerman, L Bouchard, D Brisson, BT Layden, D Mirel, KF Doheny, MV Leya, RN Lown-Hecht, AR Dyer, BE Metzger, TE Reddy, NJ Cox, Lowe WL, Jr.; HAPO Study Cooperative Research Group. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes. 2013;62(9):3282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ludvik AE, Pusec CM, Priyadarshini M, Angueira AR, Guo C, Lo A, Hershenhouse KS, Yang GY, Ding X, Reddy TE, Lowe WL, Jr, Layden BT. HKDC1 is a novel hexokinase involved in whole-body glucose use. Endocrinology. 2016;157(9):3452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li GH, Huang JF. Inferring therapeutic targets from heterogeneous data: HKDC1 is a novel potential therapeutic target for cancer. Bioinformatics. 2014;30(6):748–52. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Huang S, Wang H, Wu J, Chen D, Peng B, Zhou Q. High expression of hexokinase domain containing 1 is associated with poor prognosis and aggressive phenotype in hepatocarcinoma. Biochem Biophys Res Commun. 2016;474(4):673–9. [DOI] [PubMed] [Google Scholar]
- 10. Hayes MG, Urbanek M, Hivert MF, Armstrong LL, Morrison J, Guo C, LP Lowe, DA Scheftner, A Pluzhnikov, DM Levine, CP McHugh, CM Ackerman, L Bouchard, D Brisson, BT Layden, D Mirel, KF Doheny, MV Leya, RN Lown-Hecht, AR Dyer, BE Metzger, TE Reddy, NJ Cox, Lowe WL., Jr. Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes. 2013;62(9):3282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–8. [DOI] [PubMed] [Google Scholar]