Abstract
Intestinal epithelium plays a critical role in host defense against orally acquired pathogens. Dysregulation of this protective barrier is a primary driver of inflammatory bowel diseases (Crohn’s and ulcerative colitis) and also infant gastrointestinal infections. Previously, our lab reported that hyaluronan (HA) isolated from human milk induces the expression of the antimicrobial peptide β-defensin in vivo and protects against Salmonella Typhimurium infection of epithelial cells in vitro. In addition, we demonstrated that commercially available 35 kDa size HA induces the expression of β-defensin, upregulates the expression of tight junction protein zonula occludens-1 (ZO-1), and attenuates murine Citrobacter rodentium infection in vivo. In this current study, we report that HA35 remains largely intact and biologically active during transit through the digestive tract where it directly induces β-defensin expression upon epithelial cell contact. We also demonstrate HA35 abrogation of murine Salmonella Typhimurium infection as well as downregulation of leaky tight junction protein claudin-2 expression. Taken together, we propose a dual role for HA in host innate immune defense at the epithelial cell surface, acting to induce antimicrobial peptide production and also block pathogen-induced leaky gut. HA35 is therefore a promising therapeutic in the defense against bacterially induced colitis in compromised adults and vulnerable newborns.
Keywords: claudin-2, defensin, hyaluronan, IBD, inflammatory bowel disease, Salmonella Typhimurium
Introduction
The development and maintenance of a strong, healthy intestinal tract are vitally important for lifelong health. Dysregulation of this protective barrier can have very serious, even fatal consequences, especially for newborns, the elderly, and immunocompromised patients.1–3 The immune cells and epithelial cells lining the gut stand guard as sentinels, interacting with and responding to changes in the microbial populations that transit through the gut each day. Frank et al. estimated that nearly 35,000 different species of bacteria can be found in the gut.4 Discerning which ones are important, beneficial commensals and which are invading pathogens is a tall task, especially when the stakes are high, as is the case for a newborn child. The colonization of the neonatal gastrointestinal (GI) tract from ≤105 bacteria/ml to concentrations equivalent to those of adults (1011/ml) occurs in just under 1 month.5 Therefore, this virgin gut landscape also presents a window of opportunity for pathogen invasion. The incidence of GI illness involving pathogenic Escherichia coli (E. coli) strains that cause necrotizing enterocolitis (NEC) is greatest within the first few months of life.6 Likewise, Salmonella enterica serovar Typhimurium infections are highest within the first few months of life.7 Failure to quickly recognize and eliminate pathogenic bacteria has been linked to the onset and perpetuation of inflammatory bowel disease (IBD), both Crohn’s disease and ulcerative colitis. Examples of this association to IBD have been found with E. coli,8 C. difficile, and Salmonella enterica serovar Typhimurium.9
Recent initiatives aimed to improve outcomes for maternal and infant health have added support for studying the importance of breast milk during the early days of life. Discerning the individual components of breast milk, on a molecular level, revealed that its contribution goes well beyond simply providing newborn nutrition. Breast milk contains many important factors that drive defense of the newborn at the intestinal interface. In addition to secretory IgA, milk contains a wide array of complex carbohydrates, glycolipids, glycoproteins, mucins, and glycosaminoglycans.10 Recent studies also reveal the presence of antimicrobial peptides and exosomes carrying microRNAs in breast milk.11,12 Some of these molecules serve as prebiotics and probiotics to support beneficial commensal bacteria or directly kill pathogens. However, other molecules exert their effect by regulating specific gene expression, which can enhance the innate immunity. We have identified the glycosaminoglycan hyaluronan (HA) as a component of human breast milk that is present at highest levels directly after delivery, tapering in the months following birth,13 and hypothesize that this HA serves a biological role as a modulator of host defense.
HA is a linear repeating disaccharide composed of alternating units of N-acetyl glucosamine and glucuronic acid. HA is devoid of any protein or chemical modifications.14 In normal tissue, HA is present as large-size polymers (ranging from 106 to 107 Da or from 2500 to 25,000 disaccharide units), where it plays a role in tissue hydration, structural support, and elasticity.15 Our lab was one of the first to identify HA as a natural, early lactation component of human breast milk,13 where its size is less than 500 kDa.16 Interestingly, we demonstrated that HA purified from human breast milk induces the expression of human β-defensin 2 (HβD2) in human intestinal epithelial cells (HT29) in vitro.13 β-defensin is a broad-acting, cationic peptide that can directly kill Salmonella and E. coli species in vitro.11 Importantly, the in vivo biological activity of HA was confirmed by our observation that oral delivery of purified milk HA significantly increased the expression of the murine β-defensin ortholog (MuβD3) in proximal colon epithelium. Investigating whether HA treatment functionally protects against pathogens, we went on to show that human milk HA pretreatment inhibits Salmonella Typhimurium infection of human epithelial cells in vitro.13
Commercially synthesized, highly pure HA is available in a wide range of molecular sizes. Utilizing both in vitro and in vivo studies to compare both large, medium, and small HA, we determined that 35 kDa size HA (HA35) had the highest biological activity with respect to induction of β-defensin. HA35 induced the expression of HβD2 in vitro and, when supplied by oral gavage to mice, HA35 induced the expression of MuβD3 in vivo. β-defensin expression in both systems was dose and time dependent.17 Most recently, we reported that HA35 can improve the tight junction barrier function in vivo by increasing the expression of the tight junction protein zonula occludens-1 (ZO-1) in both transverse and distal regions of the mouse colon. Larger and smaller sized HA were less effective at inducing ZO-1 protein in epithelial cells.18 ZO-1 functions as an adapter molecule that strengthens the connection between the actin cytoskeleton and transmembrane tight junction proteins. HA35 also protected mice from leaky gut resulting from oral administration of dextran sulfate sodium (DSS) through induction of ZO-1. More importantly, HA35 treatments protected mice from oral Citrobacter rodentium infection,18 an attaching and effacing murine pathogen that serves as a model of human enteropathogenic E. coli (EPEC) infection.19
The goals of the present investigation were to test whether oral HA35 reaches the intestinal epithelium to mediate direct effects on epithelium rather than working through an indirect mechanism and to determine whether HA35 also protects from Salmonella Typhimurium infection in vivo. Fluorescence imaging of stained colon tissue revealed HA35 transits through the digestive tract to the colon within hours, and that the expression of β-defensin occurs at the points of HA35 contact with epithelial cells. In addition, immunohistochemistry and direct colony counting demonstrated that mice receiving oral HA35 before and during infection had significant reduction of Salmonella colonization in the cecum as well as reduced dissemination into the mesenteric lymph nodes (MLN) and spleen. Moreover, we also observed that HA35 treatment inhibited Salmonella upregulation of the leaky tight junction protein claudin-2. Taken together, our data support the concept that HA35 is a multifunctional glycan that can concomitantly increase antimicrobial peptide secretion and strengthen tight junction barrier function. We propose that HA35 may be a novel therapeutic molecule that could protect vulnerable populations from pathogenic bacterial infection.
Materials and Methods
Animals
All animal experiments were performed in the Association for Assessment and Accreditation of Laboratory Animal Care–approved (000383) biological resource unit at the Lerner Research Institute (LRI). All animal experiments were approved by the LRI Institutional Animal Care and Use Committee (IACUC) and the LRI Biosafety committee. Six- to 8-week-old adult male C57Bl/6J mice stock 000664 (Jackson Laboratory; Bar Harbor, ME) were fed sterile irradiated Teklad Global Diets chow 2918 (Envigo; Indianapolis, IN) and sterile acidified house tap water (HW) ad libitum in specific pathogen free (SPF) caging with 12-hr light/dark cycle in humidity and temperature controlled rooms. Mice infected with Salmonella were housed in the LRI BSL2 facility under the same housing/food/HW conditions.
Biotin Labeling of 35 kDa Hyaluronan
HA (35 kDa; Lifecore Biomedical, Inc., Chaska, MN) was biotinylated as previously described with minor modifications.20 HA35 was dissolved in PBS at 5 mg/ml, and the solution was mixed with biotin-long chain (LC)-hydrazide (Thermo Fisher Scientific; Carlsbad, CA) to a final concentration of 1 mM to yield a maximum of one in 10 carboxyl groups of HA to be biotin-labeled. Freshly prepared N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride [EDAC]; Millipore Sigma; St. Louis, MO) was added to this mixture to a final concentration of 10 mM, and the solution was stirred overnight at room temperature. The reaction was stopped by dialysis of the reaction mixture against PBS at 4C for 24 hr. Dialysis was carried out in a tube with a Mr of 4000, and the resulting biotin-HA35 size was confirmed by gel electrophoresis using streptavidin AlexaFluor 488 (Thermo Fisher Scientific). The final product was diluted to 1.2 mg/ml with HW and stored in aliquots at −80 until supplied to mice by oral gavage.
Immunohistochemistry of Biotinylated HA35 in Mouse Colon
Adult mice (n=2 per time-point) received a single 250 µl gavage of either HW or 1.2 mg/ml (300 µg) of biotinylated HA35. Mice were sacrificed by approved methods, and colons removed intact from cecum to rectum at the following time-points post gavage: 1, 2, 3, 4, 8, and 24 hr. Colons were placed in separated 15 ml conical tubes filled with Histochoice MB #H120 (AMRESCO LLC; Solon, OH) for 24 hr. Following fixation, colons were opened by cutting once longitudinally from cecum end to rectal end. Large fecal pellets were carefully removed by forceps before placing the entire colon in 10 ml PBS. Colons were gently washed by rotation 3× for 1 hr each in 10 ml PBS and final wash in 10 ml PBS for 24 hr at 4C. Colons were immersed for 30 min at 25C in 4.0 ml of streptavidin AlexaFluor 700 #s21383 diluted 1:500 (Thermo Fisher Scientific; Kalamazoo, MI). Colons were protected from light from this step forward. Following removal of streptavidin AlexaFluor 700 solution, colons were washed 3× for 10 min at 25C in 5 ml of PBS with gentle rotation. Stained colons were then imaged (excitation wavelength 700 nm, emission wavelength 750 nm) with the Bruker In-Vivo Xtreme system and Bruker Molecular Imaging Software application (Bruker Corporation; Billerica, MA).
Immunohistochemistry Localization of HA35 and β-defensin in Mouse Colon
Adult mice (n=5) received a single daily gavage of either HW or 300 µg of biotinylated HA35 in 250 µl HW for 5 consecutive days in caging that precluded coprophagy. Approximately 18 hr post-final gavage, colons were removed and fixed in Histochoice MB #H120 (AMRESCO LLC) for 24 hr at room temperature. Tissue was processed, paraffin embedded, and sectioned. Slides containing 5-um-thick paraffin cross sections were deparaffinized by immersing in ClearRite (2 × 3 min), Flex100 (1 min, 2 min), and Flex95 (1 min, 2 min) (Richard-Allan Scientific-Thermo Fisher; Kalamazoo, MI), followed by tap water. Tissues were encircled with a Super HT Pap Pen #195505 (Research Products International; Thermo Fisher, Waltham, MA) and blocked for 45 min in 1× Hanks balanced salt solution (HBSS) without CaCl2 and MgCl2, with phenol red (Cell Services Media Core, LRI; Cleveland, OH) containing 2% fetal bovine serum (BioWhittaker Lonza; Walkersville, MD). Subsequently, antibodies were diluted in this HBSS + 2% FBS blocking buffer, applied to the slides, and they were incubated in a humidified chamber. Detection of biotinylated HA was achieved with streptavidin AlexaFluor 488 (1:500 applied 30 min at 25C; Thermo Fisher Scientific), and detection of β-defensin was achieved by applying rabbit polyclonal anti-β-defensin sc-30116 (Santa Cruz Biotechnology; Santa Cruz, CA) overnight at 4C, followed by goat anti-rabbit AlexaFluor 568 (1:1000) for 1 hr at 25C (Thermo Fisher Scientific). Three 10 min washes with 1× HBSS occurred before application of secondary antibodies and before mounting in VectaShield containing 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Vector Laboratories, Burlingame, CA) for identifying nuclei. Coverslips were affixed with clear nail polish before imaging with a Leica DM5500B upright microscope fitted with a Leica DFC425C camera and LAS software (Leica Microsystems, GmbH; Wetzlar, Germany). Confocal images were captured with a Leica TCS-SP8-AOBS inverted microscope. Images were processed using the Image-Pro Plus software (Media Cybernetics; Rockville, MD) and Photoshop Software (Adobe Systems; San Jose, CA).
Salmonella Infection
The Salmonella enterica serovar Typhimurium strain SL1344 is a well-characterized, invasive mouse and human virulent strain of Salmonella that is streptomycin resistant.21 The infection protocol based on previous work22,23 was modified for HA35 feeding. Salmonella bacterial cultures were cultured and prepared for gavage as described previously.23 To reduce stress influences, 1 week or more before starting the experiment, mice were moved from standard room housing site to the BSL2 barrier housing site. Three days before infection, and continuing through the infection phase (end of day 4), mice received a single daily oral gavage of 300 µg HA35 in 250 µl in HW. HA35 gavage occurred at the same time each day. Twenty-four hr before infection, mice were fasted from food and water for 4 hr before a single 200 µl gavage of 20 mg streptomycin (Sigma Aldrich; St. Louis, MO) in HW. Food and water were returned to the cage following streptomycin gavage. On the day of infection, mice were fasted from food and water for 4 hr before gavage with 100 µl of 1 × 108 cfu Salmonella in log phase. Water was returned to the cage immediately, and food was returned after 2 hr. Ninety-six hr post infection, mice were sacrificed by approved humane methods. Tissues (cecum, colon, MLN, spleen, liver) were collected and divided in half for immunohistochemistry staining (fixation in Histochoice) and half for bacterial load determination by direct plate counts of homogenized tissues. Tissues were weighed and placed in 5 ml of sterile cold PBS before homogenization on ice for 20 sec. Serial dilutions in PBS were prepared (10−1 to 10−12) and spotted (5 µl × 2 spots for each dilution) on duplicate MacConkey agar (BD Difco, Becton Dickinson; Franklin Lakes, NJ) + streptomycin (50 µg/ml) poured in square gridded Fisherbrand FB0875711A Petri plates (Thermo Fisher Scientific). Agar plates were incubated 18 hr at 32C until visually counted by two researchers. Plate counts (cfu/g tissue) are reported as the average number of colonies from four samples of the same dilution divided by the total weight of the tissue.
Immunohistochemical Staining of Salmonella Typhimurium and Claudin-2
Salmonella Typhimurium infection of the mouse terminal ileum and colon was detected in paraffin sections as described above with application of the following antibodies at the indicated dilutions: rabbit polyclonal anti-Salmonella (PA1-7244) 1:1000 at 4C overnight, goat anti-rabbit AlexaFluor 488 1:1000 at 25C for 1 hr (both Thermo Scientific). Claudin-2 was detected in mouse tissue sections with rabbit anti-claudin-2 (PA5-13334) 1:50 at 4C overnight followed by goat anti-rabbit AlexaFluor 488 1:1000 at 25C for 1 hr (both Thermo Scientific). All antibodies were diluted in 1× HBSS + 2% FBS with three 10-min washes in 1× HBSS before and following secondary antibody application. VectaShield + DAPI (Vector Laboratories) was used as the mounting medium under coverslips to mark nuclei. Image capture and processing utilized the same confocal and upright fluorescent microscopy and image processing software systems as described above.
IL-6 in Serum
Terminal mouse blood samples (~500 µl) were collected from Salmonella Typhimurium–infected mice into serum separators #104757 (BD BioSciences; Franklin Lakes, NJ) and centrifuged at 4000 rpm at 25C in a Beckman Sorvall centrifuge fitted with swinging buckets. Serum was collected and average interleukin-6 (IL-6) levels per µl were measured in duplicate samples per mouse utilizing a LegendMax ELISA kit #431307 (BioLegend; San Diego, CA) and analyzed in a SpectroMax ELISA plate reader (Molecular Devices; Sunnyvale, CA).
Statistics
The data collected from experimental mice were analyzed for statistical significance utilizing the GraphPad Prism 5 software (GraphPad Software; La Jolla, CA). Comparison between groups was evaluated using unpaired one-tailed Student’s t-test or Mann–Whitney tests. Error bars represent the standard error of the mean (SEM).
Results
Detection of HA35 During GI Transit
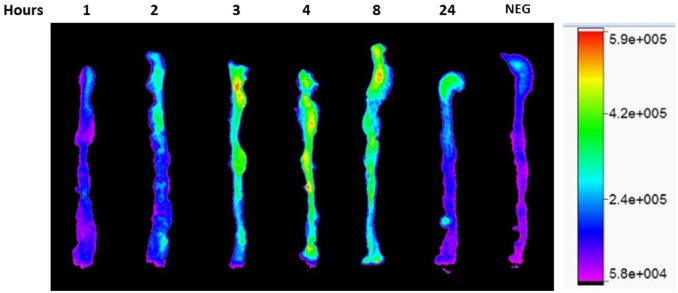
We have previously reported that HA35 induces the expression of β-defensin within the proximal colon of mice receiving HA35 by oral gavage.17 However, we were curious to learn if HA35 remains intact during transit through the gut, especially the colon. To address this question, we labeled HA35 with biotin on every 10th sugar, removed free biotin from the labeling reaction mixture, and then confirmed its size by gel electrophoresis (data not shown). Biotinylated HA35 was then diluted in sterile mouse drinking water to 1.2 mg/ml and then gavaged in one single dose (300 µg in 250 µl) to mice. At 1, 2, 3, 4, 8, and 24 hr post gavage, mouse colons were removed, fixed, and stained for the presence of biotinylated HA35 with fluorescent-tagged streptavidin 700. Figure 1 shows a representative colon (cecum to rectum) from one mouse at each time-point and an untreated control mouse gavaged with water. Biotinylated HA35 appears in the colon starting after 2 hr post gavage and remains at high levels out to 8 hr. By 24 hr post gavage, HA35 is still detectable, with comparatively little remaining in the proximal colon and distal colon regions.
Figure 1.
Biotin-HA35 detection in the colon. Immunohistochemistry of colons removed from mice at the indicated hour time-point following a single 250 µl oral gavage of biotinylated HA35 (300 µg). Following extensive washing, biotin-HA35 was detected with streptavidin 700 (1:500 in PBS) and imaged by the Bruker In Vivo Xtreme system. Signal intensity measured as photons/sec/mm2. Abbreviations: HA35, 35 kDa hyaluronan; NEG, negative control.
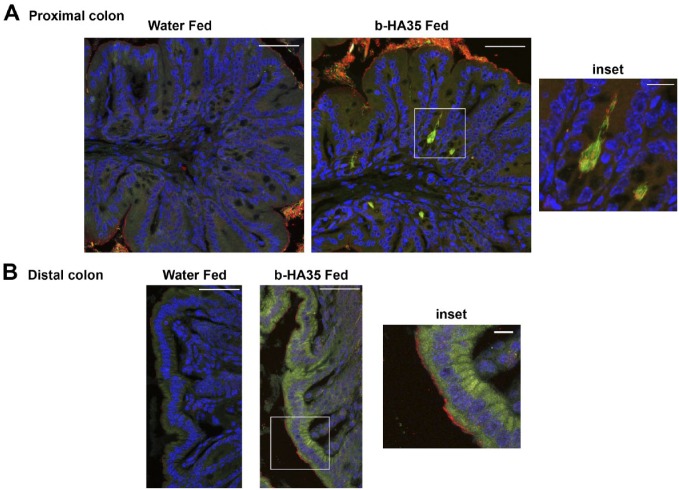
Co-localization of HA35 and β-defensin at the Colon Crypt Epithelial Surface
We previously reported that highly purified HA35 induced HβD2 expression in cultured human colon epithelial cells, and, importantly, expression of the murine β-defensin 2 ortholog (MuβD3) in vivo when supplied as a once daily oral treatment for 5 days in mice.17 In the treated mice, β-defensin expression was highest in the epithelial cells of the proximal colon tissue, and induction was both HA size and dose dependent.17 To determine whether HA35 induced β-defensin directly at the epithelial surface, rather than as a consequence of another biological event during transit through the GI tract, we set out to localize HA35 in the gut. Biotin-HA35 was diluted in sterile mouse drinking water and supplied by oral gavage at the same volume and concentration used in previous in vivo experiments. Figure 2 (Panel A) shows distinct localization of biotin-HA35 at the epithelial cell surface in the crypts of the proximal colon of wild-type C57Bl/6J adult mice, whereas tissue from water-fed control mice lacked green staining. The inset image in Panel A (and supplemental figure 1 and video) more clearly highlights HA35 (green fibrils) in close proximity to β-defensin (red) and co-localization (yellow) at the epithelial cell surface in crypts. In Panel B, biotin-HA35 staining is detected within the distal colon epithelial cells (green) with β-defensin staining at the epithelium surface. Although we do not yet know whether the oral HA35 was somewhat modified in the digestive tract, it is clear that (1) the HA transits to the large intestine without being consumed nor degraded to single sugars; and (2) the starting material remains sufficiently large to remain biologically active, capable of directly inducing β-defensin 2 at the precise points where HA35 contacts the epithelial cell surface. Previous experiments with HA4.7 kDa and HA16 kDa failed to induce β-defensin in epithelial cells in vivo.17 Together, our data suggest that HA35 is not significantly modified from its original size. Attempts to capture sufficient quantities of biotin-HA35 from the mouse feces to quantify HA or conduct comparative sizing have, as yet, been unsuccessful.
Figure 2.
HA35 induces β-defensin expression at colon epithelial cell surface. Co-localization of biotinylated HA35 (green) and β-defensin (red), co-localization (yellow) was detected in proximal colon (Panel A) or distal colon (Panel B) cross sections from mice fed water or 300 µg of biotinylated HA35 (b-HA35). Immunohistochemical staining with streptavidin 488 for HA35 and rabbit anti-murine β-defensin, goat anti-rabbit AlexaFluor 568 was performed as described in the “Materials and Methods” section. Nuclei were stained with VectaShield containing DAPI (blue). Image captured with Leica confocal and Image-Pro Plus software. Panel A scale bars are 50 µm and inset 5 µm. Panel B scale bars are 50 µm and inset 10 µm. Abbreviations: HA35, 35 kDa hyaluronan; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
HA35 Protects Mice From Salmonella Typhimurium Infection In Vivo
In a previous study, we demonstrated that human milk HA protected human colon epithelial cells from Salmonella Typhimurium infection in vitro.13 To test whether HA35 could protect mice from Salmonella Typhimurium infection in vivo, we orally gavaged mice with HA35 (without biotin modification) and challenged them with Salmonella Typhimurium. The Salmonella infection protocol, based on previously published methods,22,23 was adapted for HA35 intervention. Figure 3 illustrates the timeline for the infection protocol. Mice received HA35 (300 µg, once daily) 3 days before, and continuing through infection with Salmonella enterica serovar Typhimurium SL1344 strain. One day before oral gavage delivery of the bacteria, mice received a single streptomycin gavage to reduce host commensal flora competition. Small intestine, cecum, colon, spleen, MLN, and liver tissue, as well as serum, were collected on day 4 for either immunohistochemistry staining or viable bacterial counts. The number of viable Salmonella bacteria present in each tissue was determined by colony counting on day 5, 24 hr after tissue homogenization and direct plating on MacConkey agar supplemented with streptomycin.
Figure 3.
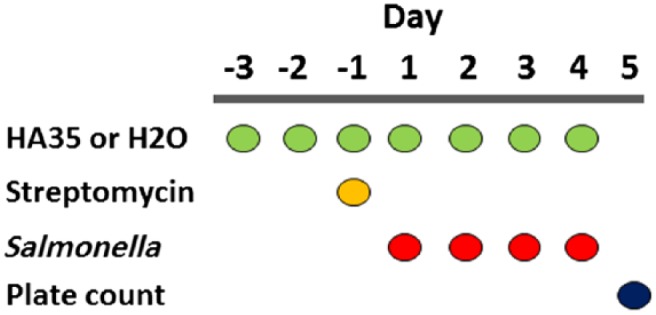
Salmonella infection protocol timeline. The course of events detail administration of HA35 (300 µg once daily) or water 3 days before, and continuing throughout Salmonella enterica serovar Typhimurium strain SL1344 infection (1 × 108 on day 1) in c57Bl/6 mice (n=5). Streptomycin (20 µg) is provided 24 hr before infection (day −1). All tissue collection, homogenization, and plating occur on day 4 with direct plate colony counts (cfu) recorded on day 5. Abbreviation: HA35, 35 kDa hyaluronan.
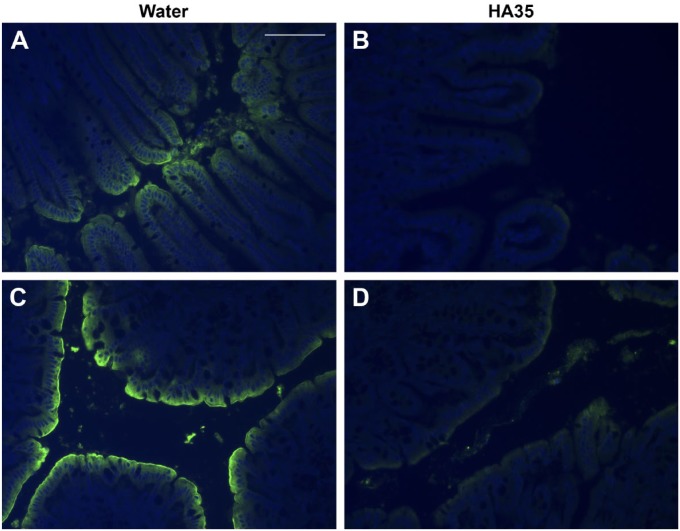
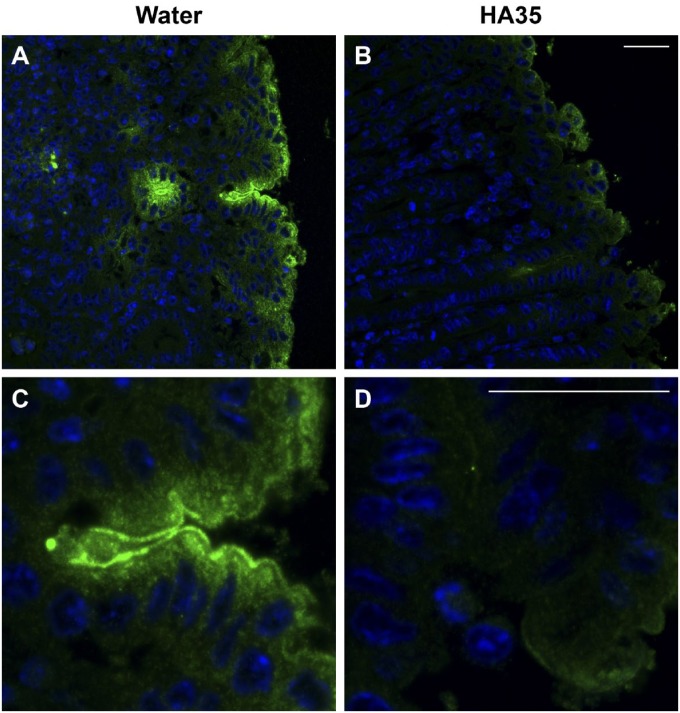
Immunohistochemical staining of Salmonella Typhimurium in the small intestine, cecal, and colon tissues from HA35-treated mice reveals dramatically reduced colonization when compared with water control mice (Fig. 4). Both terminal ileum and proximal colon tissues contain high levels of Salmonella Typhimurium staining all along the epithelial cell layer in water-treated control mice (Panels A and C). These same tissues in HA35-treated mice (Panels B and D) exhibit only low levels of Salmonella staining at the epithelial cell surface. Because the cecum is the preferred intestinal colonization site of Salmonella,24 we utilized high-resolution, confocal microscopy to detect Salmonella Typhimurium in these tissues. Figure 5A and C (high magnification) reveal high levels of Salmonella colonization of the cecum in water-treated control mice compared with HA35-treated mice (Fig. 5B and D; high magnification). Comparing Fig. 5C and D reveals the relatively extensive Salmonella penetration deep into cecal epithelial barrier in the water-treated mice that is absent in the HA35-treated mice.
Figure 4.
HA35 inhibits Salmonella Typhimurium infection in vivo. The presence of Salmonella Typhimurium in intestinal and colon sections isolated from Salmonella infected mice treated orally with water (Panels A and C) or HA35 (300 µg daily; Panels B and D) was detected with rabbit anti-Salmonella antibody, followed by goat-anti-rabbit AlexaFluor 488 (green). Nuclei were stained with DAPI (blue). Panels A and B: terminal ileum; Panels C and D: proximal colon. Images were captured with a Leica upright fluorescent microscope and Image-Pro Plus software. Scale bar is 50 µm. Abbreviations: HA35, 35 kDa hyaluronan; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
Figure 5.
HA35 inhibits Salmonella Typhimurium infection of mouse cecum. Immunofluorescent staining and confocal imaging were utilized to localize Salmonella Typhimurium (green) in the cecum of infected mice treated orally with water (Panels A and C) or HA35 (300 µg daily; Panels B and D). Nuclei stained blue (DAPI). Scale bars are 25 µm. Abbreviations: HA35, 35 kDa hyaluronan; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
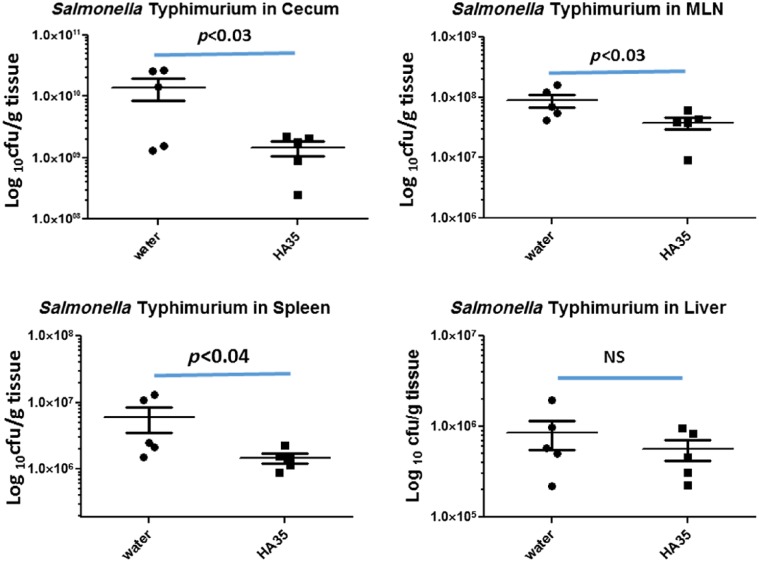
To quantify the Salmonella burden in the cecum of both experimental groups, we determined the colony forming units per gram of tissue (cfu/g). Cecal contents were removed before weighing and homogenization in PBS. Serial dilutions were plated onto MacConkey agar supplemented with streptomycin to reduce the contribution of the confounding commensal bacteria. Importantly for this assay, the Salmonella strain we used is specifically resistant to streptomycin. We observed that HA35 treatment reduced the bacterial load in the cecum by approximately one log compared with water alone (1.40 × 1010 cfu/g in water treated vs. 1.46 × 109 cfu/g in HA35 treated, p<0.03; Fig. 6). To investigate whether HA35 also reduced Salmonella dissemination beyond the gut, we determined the cfu/g of MLN, spleen, and liver tissues. Although HA35 treatment did not achieve one log-fold reduction in the MLN or spleen, it was still a statistically significant reduction in these organs compared with the water control (MLN: 8.94 × 107 water vs. 3.84 × 107 HA35, p<0.03; spleen: 6.02 × 107 water vs. 1.46 × 107, p<0.04 HA35). HA35 treatment reduced the Salmonella burden in the liver, but not to a significant degree: 8.5 × 105 water vs. 5.6 × 105, p=0.2.
Figure 6.
Salmonella Typhimurium burden is reduced in HA35-treated mice. Tissues (cecum, spleen, and liver) and MLN isolated from water or HA35-treated Salmonella infected mice were homogenized in PBS, serially diluted, and plated on MacConkey agar containing streptomycin overnight. Colonies were visually counted and normalized to input tissue weight (mg). Statistical significance (p) was determined by one-tailed Student’s t-test. Error = SEM. Abbreviations: HA35, 35 kDa hyaluronan; MLN, mesenteric lymph nodes; SEM, standard error of the mean; NS, not significant.
We next examined other common physiological markers that are associated with infection in the Salmonella model, including circulating levels of inflammatory cytokines and weight loss. Salmonella Typhimurium infection is known to elicit IL-6 release from multiple cells including intestinal epithelium.25 Therefore, we measured levels of IL-6 in the serum of water-treated and HA35-treated, Salmonella infected mice and found that HA35 treatment led to an overall reduction in serum IL-6 levels in the group, however, not to a significant level (p=0.07), likely due to the variability among animals (Fig. 7). C57Bl/6 mice on the protocol we used have also been shown to lose an appreciable amount of weight during a 4-day Salmonella Typhimurium infection.26 Although both groups of mice lost weight in our study, the HA35 group was partially protected (Fig. 8). Comparison of the final weight to the mouse initial weight revealed a significant difference between the groups. Water-treated mice lost on average 12.6 ± 1.55% total body weight while HA35-treated mice lost an average of 8.6 ± 1.1%, p<0.05.
Figure 7.
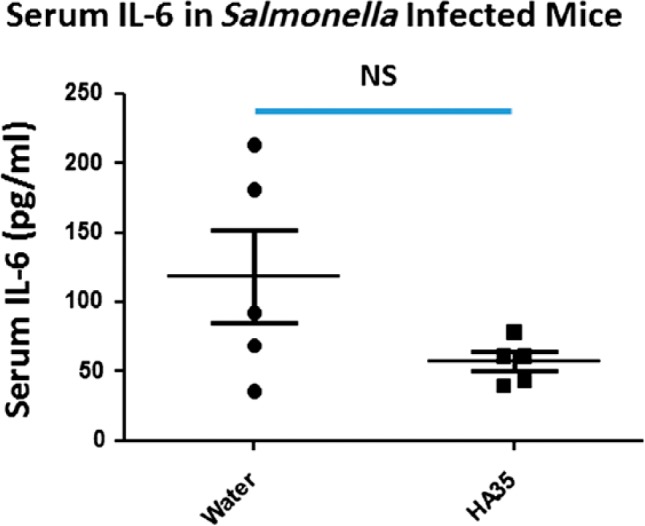
HA35 treatment reduces circulating IL-6 levels. Serum isolated from Salmonella infected mice was analyzed by ELISA for the presence of circulating IL-6. Each data point represents the average of duplicate measurements from the same mouse. A one-tailed Student’s t-test was used to determine significant differences between groups. Abbreviations: HA35, 35 kDa hyaluronan; IL-6, inflammatory cytokine; NS, nonsignificant.
Figure 8.
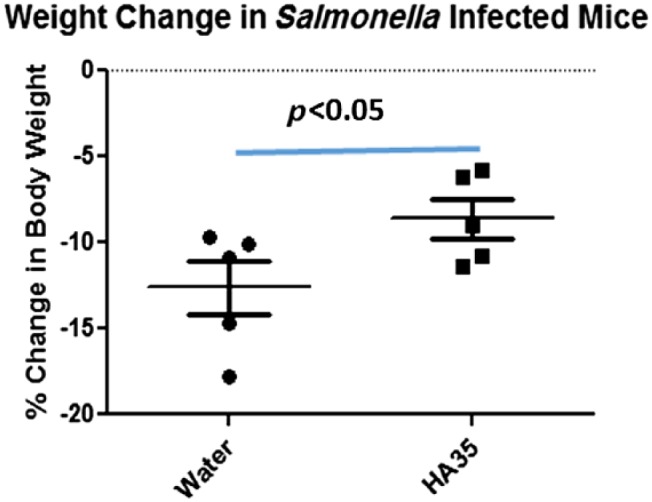
HA35 prevents weight loss in Salmonella Typhimurium–infected mice. Percent weight change was recorded as (final weight – initial weight day) / initial weight day × 100. A one-tailed Student’s t-test was used to detect significance between the groups. Error bars = SEM. Abbreviations: HA35, 35 kDa hyaluronan; SEM, standard error of the mean.
Together, the data from this series of in vivo experiments suggest that oral HA35 pretreatment protects mice from Salmonella colonization and translocation as well as from the downstream physiological consequences of infection.
HA35 Inhibits Salmonella-Induced Claudin-2
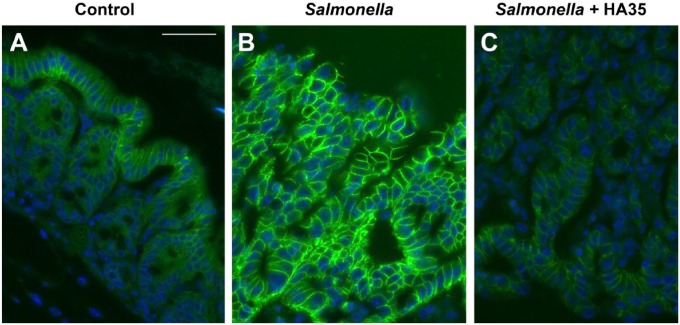
Dysregulation of the tight junction barrier in the gut has been associated with increased incidence of IBDs including Crohn’s and ulcerative colitis as well as NEC in newborns. Several enteric pathogens interfere with the tight junction complexes to gain entry into their host.27 Salmonella Typhimurium is one such example. Salmonella Typhimurium has been shown to strongly increase the expression of the leaky tight junction protein claudin-2 in vivo and in crypt-derived murine intestinal organoids in vitro.25,28 Claudin-2 knockdown in epithelial cells abrogated Salmonella infection, implicating this tight junction protein as being paramount for effective host invasion.28 Because HA35 significantly inhibited Salmonella Typhimurium infection and spreading through the intestinal epithelium in mice, we set out to discern whether claudin-2 expression was concomitantly reduced. Therefore, immunohistochemistry was performed on colon tissue sections from mice treated with HA35 during Salmonella infection. Figure 9 clearly demonstrates that claudin-2 levels are highly elevated in the proximal colon during Salmonella infection (water control: Panel B) compared with uninfected mice (Panel A). Importantly, oral HA35 treatment inhibited Salmonella-induced claudin-2 expression (HA35: Panel C vs. control water: Panel B). Interestingly, HA35 decreased claudin-2 staining to levels below what we observed in uninfected healthy mice (HA35: Panel C vs. uninfected control: Panel A).
Figure 9.
HA35 inhibits leaky tight junction protein claudin-2 expression. Uninfected (Panel A) and Salmonella infected water-treated (Panel B) or HA35-treated (Panel C) proximal colon sections were stained with rabbit anti-claudin-2 antibody and goat-anti-rabbit AlexaFluor 488 (green). Nuclei were stained with VectaShield + DAPI (blue). Slides were imaged by Leica confocal microscopy and Image-Pro software. Scale bar is 25 µm. Abbreviations: HA35, 35 kDa hyaluronan; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride.
Discussion
In this article, we have provided evidence that oral administration of HA35 provides protection against Salmonella Typhimurium infection in a preclinical mouse model, reducing the overall intestinal bacterial burden as well as translocation across the epithelial barrier. In addition, in this model, HA35 treatment results in partial protection from the systemic consequences of infection, reducing total weight loss and the levels of circulating inflammatory cytokine IL-6 in the serum of HA35-treated mice. We have recently shown that HA35 increases ZO-1 tight junctions and reduces intestinal permeability,18 and here we demonstrate, additionally, that HA35 also mediates decreased expression of the permeability increasing or “leaky” junctional protein claudin-2. Claudin-2 is a transmembrane protein that naturally functions to permit ion and water flow into and out of the cell, which is important for normal intestinal functioning, but can also be dysregulated during infection and inflammation.29 Although HA35 downregulation of claudin-2 expression alone may be insufficient to block Salmonella infection, we hypothesize that it may serve as a backup mechanism to prevent dissemination of Salmonella that have escaped the initial impact of HA35-induced β-defensin at the primary site of infection.
Salmonella Typhimurium is a gram negative bacterial pathogen that possesses several virulence mechanisms that can disrupt the intestinal barrier function to gain access and persist in the host. For example, Salmonella intracellularly invades host cells, and almost doubles the expression of claudin-2 mRNA and protein levels upon infection of crypt-derived mouse intestinal organoids in vitro, and increases IL-6 secretion.25 Human infection caused by food-borne Salmonella enteritidis and by multidrug-resistant strains of Salmonella enterica serovar Typhimurium (S. typhimurium) has increased rapidly in Europe, Africa, and in the United States, most prevalently in Gulf Coast states.7,30,31 Interestingly, IBD development also is elevated in southern states by a 2:1 ratio compared with northern states.32 Whether one of these conditions provides an opportunity for the other remains to be seen. Nevertheless, because elevated claudin-2 protein level is also a hallmark of ulcerative colitis and Crohn’s disease,33 further investigation of employing HA35 as a therapeutic agent for the treatment of IBD, EPEC, and non-typhoidal Salmonella infection is warranted. Of note, we have previously reported that oral gavage of HA35 also protects mice from Citrobacter rodentium infection, the murine model for EPEC,18 bacteria that infect their host via transcellular mechanism, different from Salmonella Typhimurium.
Worldwide, the second leading cause of death in children under five is diarrheal illness.34 In the United States, enteric bacterial infections account for significant morbidity and mortality in this population, and in underdeveloped countries, the incidence of infection and its consequences are even more severe. In 2013, the World Health Organization concluded that diarrhea is a leading cause of malnutrition in chil-dren under five. Disruption of the microbiota during malnutrition persists long after therapeutic intervention and predisposes children to further infection.35 In the United States, the main bacterial culprits in this young population are Salmonella, Campylobacter, and Shigella, and among children in their first year of life, Salmonella is the most prevalent.36 In particular, infections from non-typhoidal Salmonella enterica serovar Typhimurium have increased globally, as well as in the United States.37 Therefore, the development of a highly purified, easily deliverable, non-immunogenic, low toxicity, protective substance that strengthens the body’s innate defenses may be a welcome therapeutic platform for children at risk of enteric infection as well as the immunocompromised patients and the elderly.
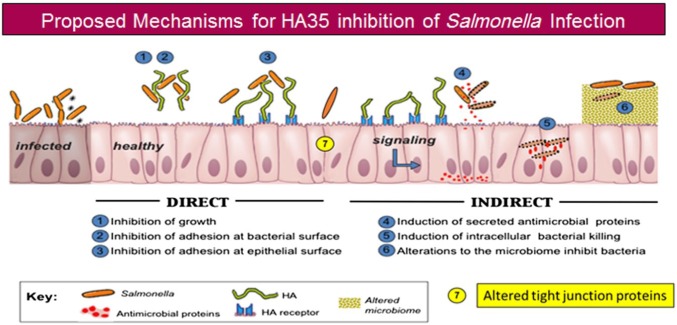
With discovery that HA is a naturally occurring component of breast milk that is present at the highest levels immediately after birth,13 we set out to discern its function. Other milk glycans, especially the human milk oligosaccharides, have nutritive as well as protective roles.38 A growing body of literature indicates that HA, especially smaller sized polymers, acts as a damage associated molecular pattern molecule or danger signal.39 We hypothesized that HA would have protective roles in the GI tract that would be relevant for a newborn. Figure 10 outlines several potential mechanisms that are under consideration in our laboratory. Initially, we found that milk HA induced HβD2 in the HT29 human epithelial cell model and caused death of the intracellular pathogen Salmonella Typhimurium in an in vitro infection model. Importantly, we also observed increased in vivo expression of the mouse ortholog of HβD2, MuβD3, in the colon epithelium of animals ingesting milk HA. Although investigating milk HA, we also began to examine commercially available, highly purified HA to mimic the effects of the human product. Not only would this provide evidence that the effects we observed were definitely HA-specific but also could identify a source for potential therapeutic use. Synthetic HA is nontoxic, nonimmunogenic, and has been Food and Drug Administration (FDA) approved for a variety of uses from cosmetic to eye surgery, joint therapy, and wound healing. We observed that 35 kDa size HA induced the highest level of β-defensin in vitro (as applied to cultured human cells) as well as in vivo (delivered orally to mice) compared with all of the other sizes we tested: (HA4.7 kDa, HA16 kDa, HA74 kDa, and HA200 kDa).17 Although milk HA required both CD44 and TLR4 receptors for its β-defensin inducing activity, HA35 signaling was TLR4-dependent alone.13 Curiously, subsequent analysis of the size of HA contained in milk revealed that most of the HA is approximately 500 kDa, with only about 5% in the HA35 size range.16 If, as our data with purified HA suggest, HA of around 35 kDa is the active form, possibly the larger milk HA is degraded in the intestine in a process that requires CD44, thus explaining the requirement for this receptor for milk HA effects. We have observed, using histochemical methods, that murine intestinal epithelium possess hyaluronidase 2 (unpublished data). Hyaluronidase 2 is thought to cooperate with CD44 in a canonical degradation pathway to degrade large HA rapidly to a preferred 50 kDa fragment.40 As mentioned, our attempts to recapture HA from feces to address this point have been technically unsuccessful so far. Additional studies from the Stenson group also correlated HA size to intestinal protection, although in their study, HA750 kDa was delivered by intraperitoneal injection rather than gavage. Adult mice were protected from colon shortening that results during DSS colitis and, similar to milk HA, both CD44 and TLR4 are required for 750 kDa HA-induced colon growth in neonate mice.41
Figure 10.
Proposed mechanisms for HA35 inhibition of Salmonella Typhimurium infection. Abbreviation: HA35, 35 kDa hyaluronan.
In the present study, we have also provided important evidence that orally administered HA35 survives transit through the digestive tract, reaches the colon within several hours, and retains β-defensin inducing activity. Although we have not ruled out that the HA may be slightly modified, HA35 was not degraded to a non-biologically active size (16 kDa, 4.7 kDa, or smaller) by enzymes or oxidative damage before reaching its destination. Interestingly, histochemistry reveals that the HA35 associates with the epithelium surface in the proximal colon, yet some portion appears to be internalized in the distal colon epithelium. We do not yet know what these regional differences suggest for host defense but hopefully our ongoing investigation with intestinal organoids will provide us with future insights. Other groups have shown that 87–99% of radiolabeled HA can be recovered in the feces of rats following oral administration of a high molecular mass 1100–1500 kDa HA,42 although no HA sizing was performed in these studies. While this HA1500 kDa would presumably not elicit β-defensin expression based on our work, it does support our finding that HA is stable throughout the digestive tract. In addition, we have recently shown that oral HA35 modulates resident liver macrophages (Kupffer cells) in a murine alcohol damage model, acting to normalize the usually hyper-responsiveness of these cells.43 HβD2 expression has also been demonstrated in the intrahepatic biliary tree and in bile ducts of patients with primary sclerosing cholangitis.44,45 This suggests that if HA35 is able to transit to other organs, it might prove beneficial in those sites as well to protect against bacterial infection.
The new frontier of concomitantly studying microbiome, genome, exposome associations with disease also provokes the question: What does HA35 do to the gut microbiome? Our investigations on this topic are in the early phases but the relevance for Salmonella infection is high as one documented mechanism of Salmonella colonization is interference with the host microbiome.27 We previously reported that feeding the ubiquitous food preservative maltodextrin to mice significantly increased colonization by Salmonella Typhimurium and E. coli.46 Therefore, exploring the use of HA35 to improve gut barrier and microbiome selection/stability in the face of pathogen attack or “Western” diet may prove very beneficial for at-risk populations. The results of this study suggest a multifunctional role for HA35 in innate host defense. HA35 retains biological activity during GI transit to induce antimicrobial peptide β-defensin expression and, at the same time, strengthen the intestinal tight junction barrier by inhibiting claudin-2. Oral HA35 treatment results in the inhibition of Salmonella Typhimurium infection and translocation, likely the result of inducing multiple epithelial defense mechanisms.
Supplementary Material
Acknowledgments
We thank Diane Mahovlic in the Lerner Research Institute (LRI) histology core for exceptional tissue processing, and Judy Drazba, John Peterson, and Eric Diskin in the LRI Imaging core for assistance with all image capture by confocal microscopy. We thank Craig Homer for assistance growing Salmonella Typhimurium. We thank the LRI Biological Resource Unit and veterinarians for expert animal care and training. We thank members of the LRI Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC) for reviewing our protocols. This work utilized the LeicaSP8 confocal microscope that was purchased with the funding from National Institutes of Health Shared Instrument Grant (SIG) (1S10OD019972-01). This work utilized the LeicaSP5 confocal/multiphoton microscope that was purchased with partial funding from an National Institutes of Health SIG grant (1S10RR026820-01).
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SPK and CADLM designed the study, analyzed data, and wrote the manuscript; DRO collected tissue samples and image data; ACP prepared biotinylated 35 kDa hyaluronan; KPN and CM assisted in the murine Salmonella infection experiments and provided bacterial strains; and CADLM obtained funding for the project.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by (all to CADLM) National Institutes of Health HD061918, R21AA024387; Programs of Excellence in Glycosciences Grant HL107147; Cleveland Clinic Innovators Research Fund.
ORCID iD: C McDonald  http://orcid.org/0000-0002-6745-9487
http://orcid.org/0000-0002-6745-9487
Contributor Information
Sean P. Kessler, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, Ohio.
Dana R. Obery, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio
Kourtney P. Nickerson, Division of Pediatric Gastroenterology and Nutrition, Mucosal Immunology and Biology Research Center, Massachusetts General Hospital, Boston, Massachusetts Department of Pediatrics, Harvard Medical School, Boston, Massachusetts.
Aaron C. Petrey, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, Ohio.
Christine McDonald, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio; Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, Ohio.
Carol A. de la Motte, Department of Pathobiology, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio; Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, Ohio.
Literature Cited
- 1. Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3(1–2):e1000707. doi: 10.1080/21688370.2014.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mabbott NA. A breakdown in communication? understanding the effects of aging on the human small intestine epithelium. Clin Sci (Lond). 2015;129(7):529–31. doi: 10.1042/CS20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shim S, Jang HS, Myung HW, Myung JK, Kang JK, Kim MJ, Lee SB, Jang WS, Lee SJ, Jin YW, Lee SS, Park S. Rebamipide ameliorates radiation-induced intestinal injury in a mouse model. Toxicol Appl Pharmacol. 2017;329:40–7. doi: 10.1016/j.taap.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 4. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen R, Scott KP, Khan S, Martin JC, Berry SH, Stevenson M, Okpapi A, Munro MJ, Hold GL. First-pass meconium samples from healthy term vaginally-delivered neonates: an analysis of the microbiota. PLoS ONE. 2015;10(7):e0133320. doi: 10.1371/journal.pone.0133320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore SA, Nighot P, Reyes C, Rawat M, McKee J, Lemon D, Hanson J, Ma TY. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg. 2016;51(12):1907–13. doi: 10.1016/j.jpedsurg.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boore AL, Hoekstra RM, Iwamoto M, Fields PI, Bishop RD, Swerdlow DL. Salmonella enterica infections in the United States and assessment of coefficients of variation: a novel approach to identify epidemiologic characteristics of individual serotypes, 1996-2011. PLoS ONE. 2015;10(12):e0145416. doi: 10.1371/journal.pone.0145416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McPhee JB, Small CL, Reid-Yu SA, Brannon JR, Le Moual H, Coombes BK. Host defense peptide resistance contributes to colonization and maximal intestinal pathology by Crohn’s disease-associated adherent-invasive Escherichia coli. Infect Immun. 2014;82(8):3383–93. doi: 10.1128/IAI.01888-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137(2):495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10. Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 11. Baricelli J, Rocafull MA, Vazquez D, Bastidas B, Baez-Ramirez E, Thomas LE. β-defensin-2 in breast milk displays a broad antimicrobial activity against pathogenic bacteria. J Pediatr (Rio J). 2015;91(1):36–43. doi: 10.1016/j.jped.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 12. Liao Y, Du X, Li J, Lonnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. 2017;61(11):1–11. doi: 10.1002/mnfr.201700082. [DOI] [PubMed] [Google Scholar]
- 13. Hill DR, Rho HK, Kessler SP, Amin R, Homer CR, McDonald C, Cowman MK, de la Motte CA. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J Biol Chem. 2013;288(40):29090–104. doi: 10.1074/jbc.M113.468629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Day AJ, Sheehan JK. Hyaluronan: polysaccharide chaos to protein organisation. Curr Opin Struct Biol. 2001;11(5):617–22. [DOI] [PubMed] [Google Scholar]
- 15. Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 16. Yuan H, Amin R, Ye X, de la Motte CA, Cowman MK. Determination of hyaluronan molecular mass distribution in human breast milk. Anal Biochem. 2015;474:78–88. doi: 10.1016/j.ab.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill DR, Kessler SP, Rho HK, Cowman MK, de la Motte CA. Specific-sized hyaluronan fragments promote expression of human beta-defensin 2 in intestinal epithelium. J Biol Chem. 2012;287(36):30610–24. doi: 10.1074/jbc.M112.356238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim Y, Kessler SP, Obery DR, Homer CR, McDonald C, de la Motte CA. Hyaluronan 35kDa treatment protects mice from Citrobacter rodentium infection and induces epithelial tight junction protein ZO-1 in vivo. Matrix Biol. 2016. doi: 10.1016/j.matbio.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhinder G, Sham HP, Chan JM, Morampudi V, Jacobson K, Vallance BA. The Citrobacter rodentium mouse model: studying pathogen and host contributions to infectious colitis. J Vis Exp. 2013(72):e50222. doi: 10.3791/50222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang B, Yang BL, Goetinck PF. Biotinylated hyaluronic acid as a probe for identifying hyaluronic acid-binding proteins. Anal Biochem. 1995;228(2):299–306. [DOI] [PubMed] [Google Scholar]
- 21. Wray C, Sojka WJ. Experimental Salmonella typhimurium infection in calves. Res Vet Sci. 1978;25(2):139–43. [PubMed] [Google Scholar]
- 22. Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71(5):2839–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nickerson KP, Homer CR, Kessler SP, Dixon LJ, Kabi A, Gordon IO, Johnson EE, de la Motte CA, McDonald C. The dietary polysaccharide maltodextrin promotes Salmonella survival and mucosal colonization in mice. PLoS ONE. 2014;9(7):e101789. doi: 10.1371/journal.pone.0101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coburn B, Li Y, Owen D, Vallance BA, Finlay BB. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect Immun. 2005;73(6):3219–27. doi: 10.1128/IAI.73.6.3219-3227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang YG, Wu S, Xia Y, Sun J. Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol Rep. 2014;2(9):1–11. doi: 10.14814/phy2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren Z, Gay R, Thomas A, Pae M, Wu D, Logsdon L, Mecsas J, Meydani SN. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J Med Microbiol. 2009;58(Pt 12):1559–67. doi: 10.1099/jmm.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17(3):173–83. doi: 10.1016/j.micinf.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 28. Zhang YG, Wu S, Xia Y, Sun J. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS ONE. 2013;8(3):e58606. doi: 10.1371/journal.pone.0058606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3(1–2):e977176. doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cito F, Baldinelli F, Calistri P, Di Giannatale E, Scavia G, Orsini M, et al. Outbreak of unusual Salmonella enterica serovar Typhimurium monophasic variant 1,4 [5],12:i:-, Italy, June 2013 to September 2014. Euro Surveill. 2016;21(15):1–10. doi: 10.2807/1560-7917.ES.2016.21.15.30194. [DOI] [PubMed] [Google Scholar]
- 31. Crump JA, Heyderman RS. A perspective on invasive salmonella disease in Africa. Clin Infect Dis. 2015;61 Suppl 4:S235–40. doi: 10.1093/cid/civ709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166–9. doi: 10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- 33. Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23 Suppl 2:S146–50. doi: 10.1111/j.1440-1746.2008.05405.x. [DOI] [PubMed] [Google Scholar]
- 34. Black RE, ousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 35. Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA, Jr, Ahmed T, Gordon JI. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcus R. New information about pediatric foodborne infections: the view from FoodNet. Curr Opin Pediatr. 2008;20(1):79–84. doi: 10.1097/MOP.0b013e3282f43067. [DOI] [PubMed] [Google Scholar]
- 37. Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, Angulo FJ, Devleesschauwer B; World Health Organization Foodborne Disease Burden Epidemiology Reference Group. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015;91(11):619–22. doi: 10.1016/j.earlhumdev.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 39. Soroosh A, Albeiroti S, West GA, Willard B, Fiocchi C, de la Motte CA. Crohn’s disease fibroblasts overproduce the novel protein KIAA1199 to create proinflammatory hyaluronan fragments. Cell Mol Gastroenterol Hepatol. 2016;2(3):358–68.e4. doi: 10.1016/j.jcmgh.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riehl TE, Santhanam S, Foster L, Ciorba M, Stenson WF. CD44 and TLR4 mediate hyaluronic acid regulation of Lgr5+ stem cell proliferation, crypt fission, and intestinal growth in postnatal and adult mice. Am J Physiol Gastrointest Liver Physiol. 2015;309(11):G874–87. doi: 10.1152/ajpgi.00123.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balogh L, Polyak A, Mathe D, Kiraly R, Thuroczy J, Terez M, Janoki G, Ting Y, Bucci LR, Schauss AG. Absorption, uptake and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J Agric Food Chem. 2008;56(22):10582–93. doi: 10.1021/jf8017029. [DOI] [PubMed] [Google Scholar]
- 43. Saikia P, Bellos D, McMullen MR, Pollard KA, de la Motte C, Nagy LE. MicroRNA 181b-3p and its target importin alpha5 regulate toll-like receptor 4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology. 2017;66(2):602–15. doi: 10.1002/hep.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang C, Lleo A, Kananurak A, Grizzi F, Tsuneyama K, Invernizzi P, Bevins CL, Bowlus CL. Human beta-defensin 2 in primary sclerosing cholangitis. Clin Transl Gastroenterol. 2017;8(3):e80. doi: 10.1038/ctg.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harada K, Ohba K, Ozaki S, Isse K, Hirayama T, Wada A, Nakanuma Y. Peptide antibiotic human beta-defensin-1 and -2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology. 2004;40(4):925–32. doi: 10.1002/hep.20379. [DOI] [PubMed] [Google Scholar]
- 46. Nickerson KP, Chanin R, McDonald C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes. 2015;6(1):78–83. doi: 10.1080/19490976.2015.1005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.